Introduction
The National Heart, Lung and Blood Institute's Pediatric Circulatory Support Program (PCSP) was established to fund the development of novel circulatory support devices for children with medically refractory heart failure.1 Prior to this, developers of circulatory support devices found little incentive to enter the pediatric market due to the small patient numbers that are generally insufficient to justify the significant costs required to develop these devices. Due to the lack of availability of new devices for circulatory support of pediatric patients, extracorporeal membrane oxygenation (ECMO), which had first been used clinically in the 1960's, remained the most commonly utilized modality to support these critically ill children during the next 40 years.2 The most attractive feature of ECMO for pediatric circulatory support is its ability to be used in even the smallest infants and neonates. However, ECMO support is characterized by thromboembolic complications and sepsis in a significant percentage of patients.3 Perhaps most importantly, ECMO has generally only been suitable for short-term support limiting its usefulness as a bridge to transplantation, while the size and extracorporeal configuration of the system components usually limit its use to the intensive care unit setting and preclude ambulation and rehabilitation during support.
In recognition of the limitations of the existing devices for pediatric mechanical circulatory support and the limitations for device companies' entry into the pediatric market, the NHLBI established the PCSP to “…perform basic and applied research to develop novel circulatory assist devices or other bioengineered systems for infants and children with congenital and acquired cardiovascular disease who experience cardiopulmonary failure and circulatory collapse.”4 Because the PCSP was a development program for new devices to address a broad goal (circulatory support for pediatric heart failure) rather than for a specific design and because funding various promising approaches would improve the chances of successfully achieving the program's objectives, proposals were solicited under a Broad Agency Announcement (BAA). BAAs are used, as in this case, when proposals with varying scientific or technical approaches are anticipated. After receipt, review, and assessment of the proposals submitted in response to the BAA, contracts totaling more than $20 million were awarded by the NHLBI in the spring of 2004 to five contractors to develop a family of pediatric circulatory support devices (Table 1). Funding support for the PCSP concluded in 2009. In 2006, a summary report was published that described these devices and the development goals of their respective programs as the PCSP was initiated.1 Because the PCSP was a development program involving different devices, the design processes, the in vitro and in vivo tests and analyses, and the goals for those tests varied between contractors. However, to make the most of the program, contractors participated in monthly conference calls, attended and presented their progress at annual meetings, and shared quarterly progress reports. These activities provided a means for each of the contractor teams to learn from and leverage the progress made by the other contractors and, as appropriate, modify their approach and improve their device. The present report provides a summary of progress for each of the contractors at the conclusion of this program, including a sampling of the some of the test results and analyses. The report concludes with the plans for achieving clinical application of these and other promising pediatric circulatory support devices.
Table 1.
NHLBI Pediatric Circulatory Support Program Contractors and Devices.
| Program | Device |
|---|---|
| University of Pittsburgh | PediaFlow Pediatric VAD |
| Cleveland Clinic | PediPump |
| Ension, Inc. | Pediatric Cardiopulmonary Support System (pCAS) |
| Jarvik Heart, Inc. | Pediatric Jarvik 2000 |
| Pennsylvania State University | Pediatric VAD (PVAD) |
The PediaFlow™ Pediatric VAD (The University of Pittsburgh)
A consortium led by the University of Pittsburgh undertook an ambitious program which relied on fundamental bioengineering principles to develop, de-novo, a miniature blood pump specifically intended for the youngest heart failure patients. Through the PCSP, the consortium produced the PediaFlow™ PF3, a miniaturized magnetically levitated (mag-lev) blood pump (Fig. 1). With a flow rate range between 0.3 -1.5 LPM and a volume approximately that of a AA cell battery (Table 2), the clinical PediaFlow VAD has been developed to meet the PCSP's goal to provide circulatory support for neonates, infants, and children less than 25 kg who experience cardiac failure and circulatory collapse due to congenital and/or acquired cardiovascular disease.
Figure 1.
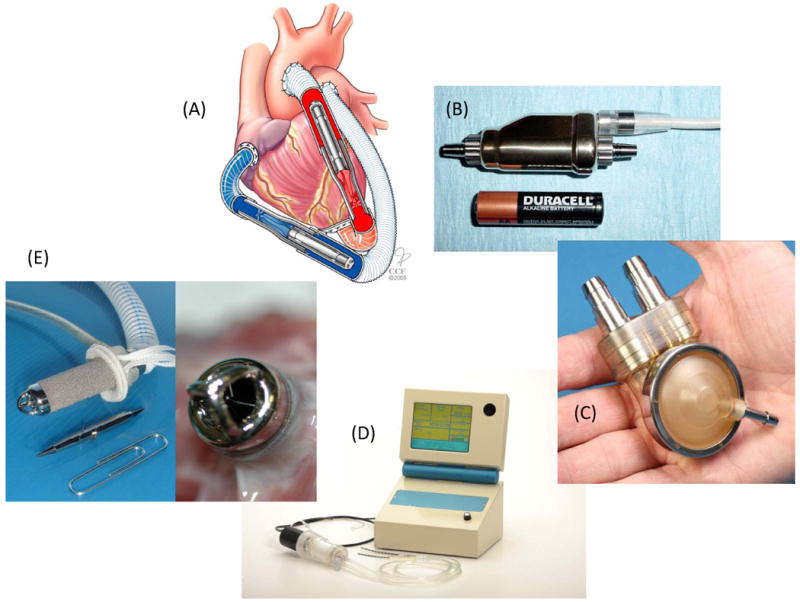
The family of devices developed under the Pediatric Circulatory Support Program: (A) The PediPump Ventricular Assist Device showing application for biventricular support, (B) The PediaFlow Ventricular Assist System, (C) the Penn State Infant VAD, (D) Ension's pCAS system with prototype controller console, and (E) the infant size Jarvik 2000 showing thrombus-free bearings after five weeks in lamb animal model.
Table 2.
NHLBI Pediatric Circulatory Support Program Device Characteristics
| Parameter | PediaFlow PF3 | PediPump | Jarvik 2000 | Ension pCAS* | Penn State Pediatric VAD | ||
|---|---|---|---|---|---|---|---|
| Infant | Child | Infant | Child | ||||
| Diameter (mm) | 19.6 | 10.5 | 10.5 | 18 | 76 | NA | NA |
| Length (mm) | 60 | 64.5 | 52 | 59 | 190 | NA | NA |
| Total Volume (cc) | 16.6 | 5 | 4 | 12 | 96 | 66 | 150 |
| Total Mass (grams) | 50 | 16.5 | 11 | 35 | 843 | 67.5 | 75 |
| Priming Volume (cc) | 2.3 | 0.6 | 1 | 4 | 105 | 10 | 25 |
| Maximum Speed (RPM) | 15000 | 15000 | 40000 | 16000 | 3000 | NA | NA |
| Maximum Flow Rate (lpm) | 1.5 | 3.0 | 3 | 3 | 3.0 | 1.6 | 3.3 |
The pCAS system is an ECMO device.
The first two designs (the PF1 and PF2 models) focused on demonstrating feasibility and improving the hemodynamic characteristics, particularly to increase the peak flow rate to 2.0 LPM against a pressure head of 80 mmHg. This was accomplished by the development of an optimized 4-pole motor that provided the required torque. Operation was demonstrated to be stable at pump outputs between 0.25 L/min to 2.0 L/min against a pressure load of 80 mmHg. Progress was also made in reducing the size, profile and weight of the PediaFlow pump. Through design optimization, the PF2 pump occupied approximately half of the volume (35.5 cc) associated with the housings for the PF1 pump (63.6cc). As designed, the PF2 pump would be implantable in patients ≥10kg, but problematic for implantation in the smallest patients.
The PF3 design represents the latest enhancements of the PediaFlow VAD. The system overcame size limitations of the earlier PF1 and PF2 designs by being uniquely configured for supercritical operation at rotational speeds beyond those associated with resonance frequencies. This modification resulted in dramatic size reduction and, consequently, the PF3 system takes less than half the volume of the previous versions. As a result, the PediaFlow pediatric VAD is capable of full implantation in the left upper quadrant behind the left rectus abdominus muscle in the smallest patients (<5kg).
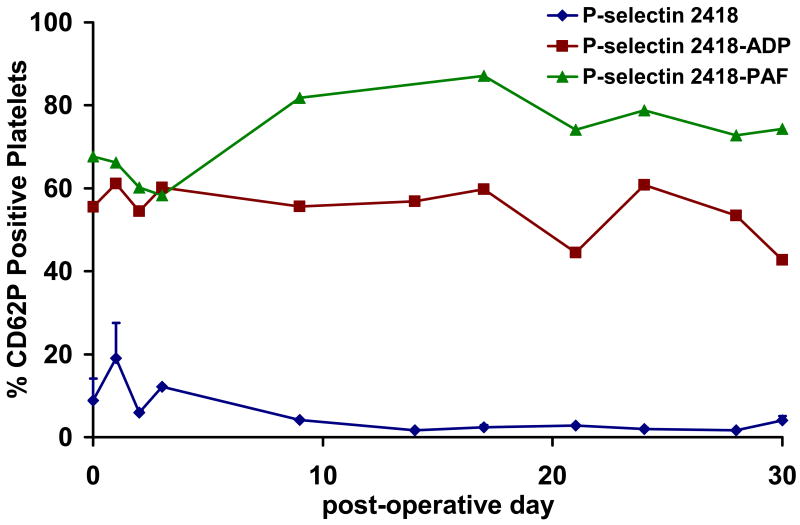
During the program, nine in vivo ovine PediaFlow implant studies ranging from acute (6 hours) to chronic (30 days and 72 days, electively terminated) were conducted. Throughout each of the chronic studies, rheological and biocompatibility studies were completed and the animal physiologic status and serum biochemistry were monitored. Nearly all measured hemodynamic, biochemical, and biocompatibility values either fell within the literature reference range for these parameters in sheep or demonstrated a nearly identical temporal course as observed in “sham” control studies. Assays were specifically developed to quantify circulating activated ovine platelets and these assays have been utilized in each PediaFlow implant5-7. Figure 2 demonstrates platelet activation in a PF2 implant before and following stimulation with adenosine diphosphate (ADP) and platelet activating factor (PAF). Platelet activation rose modestly after surgery and quickly returned to baseline. Platelets could be stimulated to high activation levels with ADP and PAF, signifying conserved platelet function during the implant. This temporal response was similar to the platelet activation results in the “sham” control studies and PF3 implants suggesting attractive platelet biocompatibility7.
Figure 2.
Platelet activation in lamb animal model using an earlier version of the PediaFlow VAS following stimulation of blood with 20 μM ADP and 10 μM PAF. Platelet function with implanted PediaFlow VAS appears to be conserved as evidenced that platelets could be stimulated to high activation levels with ADP and PAF and similar results were observed in sham studies.
The PediPump™ (Cleveland Clinic)
The initial objectives of Cleveland Clinic's development program were to create an implantable VAD that would be small enough to allow implantation in newborns, yet capable of providing hemodynamic support for children up to 25 kg. The resulting PediPump is a novel pediatric VAD based on a design for transcatheter applications in adults that appeared suitable for the pediatric application due to its small size and hemodynamic targets for support (Fig. 1). The PediPump employs a mixed-flow rotary pump with a rotor that is suspended on passive magnetic bearings as in the initial design for the adult transcatheter pump.8-11 Primary blood flow is powered by a three-blade impeller, exits through the annulus containing the outflow stator blades and passes over the motor, which provides motor cooling. Absence of seals in this design removes the need for external purge flows and reduces wear making the pump suitable for prolonged periods of support.
The PediPump has undergone incremental design improvements throughout the PCSP support period, while retaining the same fundamental pump design. These included refinements to the pump's magnetic bearing design, motor design, wash-flow path, axial touch-point design, and electrical/thermal insulation. The most recent version of the PediPump, the Mark IV, includes improvements in design and manufacture of the magnetic bearings which allows reduction in the axial dimension of the pump that now measures 64.5 mm in length by 10.5 mm in diameter.
The PediPump development program also sought to address anticipated anatomic fit issues of VAD implantation in children. Initial work in this program created on-screen 3D reconstructions from clinically obtained computed tomography (CT) and magnetic resonance imaging (MRI) studies.12 This approach to anatomic 3D reconstructions was subsequently used in a sheep model to aid in planning for device fitting prior to the initiation of the in vivo testing program for the PediPump described below.13, 14 Subsequent studies have combined digital renderings of the PediPump with CT and MRI derived 3D studies to perform virtual fitting, whereby various approaches to implantation can be evaluated on-screen.15 (Fig. 3) This approach should be particularly useful as an aid to preoperative planning in the smallest pediatric patients where anatomic fit will be most difficult, as well as in patients with extreme anatomic variants often encountered in congenital heart disease.
Figure 3.
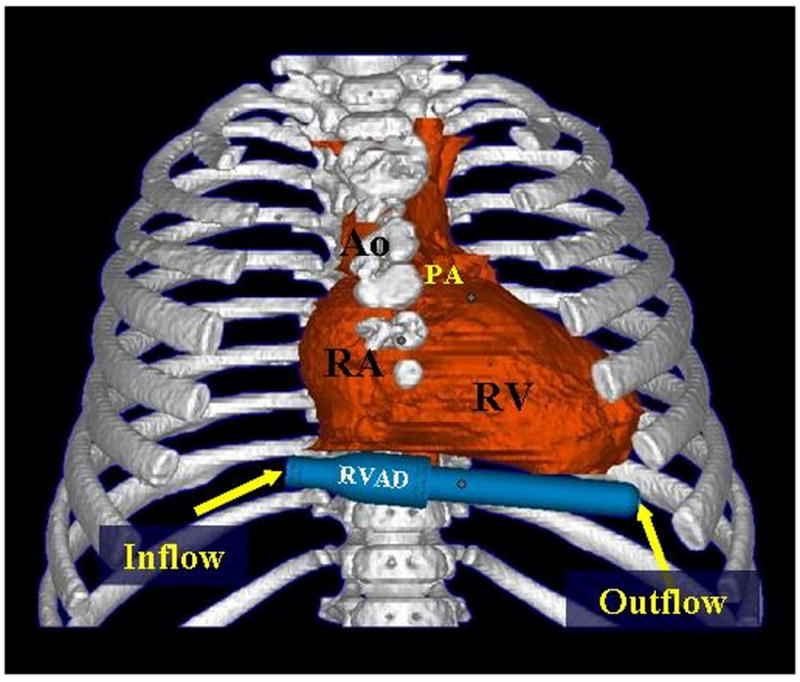
Model of the PediPump in a right ventricular assist device configuration virtually positioned on the diaphragmatic surface of the heart in a 3-dimensional reconstruction derived from a computerized tomography scan of an 8-month old patient with tetralogy of Fallot. (Ao, aorta; PA, pulmonary arter; RA, right atrium; RV, right ventricle). (Reproduced with permission from [15].
Animal testing of the PediPump began with five six-hour acute studies in a sheep model that were used to develop the implant procedures and evaluate basic performance including in vivo hemolysis and deposition.16 Nine chronic implants have been performed with a number of these animals surviving to completion of the target 30-day period of study. Implantation was technically easy and surviving animals have tolerated subsequent recovery well. Hemodynamic performance of the PediPump was stable throughout the period of chronic in vivo support in all animals and appeared to be suitable for the physiologic envelope initially targeted for the development program with flows averaging 1.7-2.0 L/min with a pressure rise of 50-60 mmHg. Importantly, minimal hemolysis has been observed during the entire in vivo testing program. Some animals early in the chronic support series developed a “cap” of deposition on the rear rotor touch point. This was addressed by a redesign of the rear touch point as well as a change to more biocompatible materials in this region of the pump. In summary, progress in the PediPump development program has created an important foundation of pre-clinical data as the PediPump program progresses to clinical trials in its next stage of development.
Pediatric Cardiopulmonary Assist System (Ension, Inc.)
Ension's pediatric cardiopulmonary assist system (pCAS) has been developed as a next-generation ECMO system. The pCAS system improves upon the current state of ECMO by offering improved blood compatibility, reduced surface area and priming volume, rapid deployment, and a simplified system configuration that facilitates parent-child bonding and patient transport.
The pCAS system (Fig. 1) consists of a pump-oxygenator, controller console, and ancillary components such as venous and arterial cannulae and an arterial bubble trap. It is a compact, integrated device consisting of an impeller directly integrated to a blood oxygenator using a turning diffuser. The system is designed to provide partial or complete cardiopulmonary support to patients from 2 kg to 25 kg. Though the period of intended use is two weeks for a single disposable blood-contacting pCAS pump-oxygenator, extended support is accommodated through pump-oxygenator change-out typically taking less than five minutes.
Blood-contacting surfaces of the pump-oxygenator incorporate Ension's Bioactive Surface (EBS). This surface has been specifically engineered to be stable, bioactive, cost-effective, permeable to oxygen and carbon dioxide, and applicable to a variety of fiber materials such as microporous polypropylene and polymethylpentene. A bioactive surface is also applied to the blood tubing and cannulae yielding a circuit with “tip-to-tip” bioactive surface modification. Cannulation can be via neck vessels in infants, femoral vessels in children with sufficient size, or chest cannulation in surgical patients. The pCAS pump-oxygenator was designed for use with a variety of different cannulae (from 8 Fr. to 22 Fr.) due to the wide range of clinical preferences and practices and patient sizes.
The touch screen-based pCAS control console has been designed to provide simple and reliable operation. Control console functions include display and regulation of pump speed, flow, and other operational parameters, monitoring the system for fault conditions.
Development of the pCAS system has been guided by risk management, reliability, and human factors analyses as well as functionality, biocompatibility, and clinical requirements. Fabrication processes have been established, refined, and validated as part of the product development and prototype production processes. Over the five-year period of development, design iterations of the pCAS pump-oxygenator and controller console have been fabricated and tested extensively both in vitro and in vivo. In vitro data have demonstrated the pCAS pump-oxygenator's ability to achieve required blood flow rates and pressures and to exchange oxygen and carbon dioxide with minimal blood damage. Ension has utilized two different animal models for in vivo evaluations. Acute in vivo system testing in piglets ranging from 7.9 to 11.6 kg has demonstrated that the pCAS system provides appropriate hemodynamic support for the small patients in the target population using appropriately sized cannula. Specifically, blood flow rates of up to 1.2 lpm and oxygenation saturation levels of 100% were achieved using 10 Fr. venous and 8 Fr. arterial cannula. Three-day studies in calves have demonstrated improved blood compatibility.
The Jarvik 2000 Infant and Child VADs (Jarvik Heart, Inc.)
Jarvik Heart, Inc. has developed both child size and infant size intraventricular blood pumps generally patterned after the Jarvik 2000 adult model (Fig. 1). The final design pumps are very small, provide flow from ¼ - 3 L/min., and have not caused hemolysis, platelet activation, or pump thrombosis in lamb implants.
During the first three years of the five year NIH development program, pump stoppage due to bearing thrombus was a major barrier to success. This was first observed when several pin-in-sleeve bearing designs seized during chronic animal studies. The bearing thrombus problem was overcome only following the development of a new type of miniature ceramic blood immersed bearing, called the cone bearing (Fig. 4). During the program 21 animal implants intended for long-term survival were conducted. Using the original bearings with the child and infant models, 75% (9 of 12 pumps implanted) failed due to bearing seizure from thrombus or bearing fracture, usually within three weeks. With the new cone bearing design, animals survived with elective termination up to 70 days. No pumps failed due to bearing problems with the new bearings, and only 1 of 9 implant cases had thrombus within the pump. However, this was secondary to infection and thrombosis of the outflow graft near the flow probe. Thus the cone bearings were successful in all cases and the pump was thrombus-free in 89% of the implants. Accelerated durability testing of the cone bearings for the child size pump for more than three years is ongoing with no failures and measured bearing surface wear of only one micron/5 years.
Figure 4.

The cone bearing developed for the Infant and Child Jarvik 2000 VADs. The novel design eliminated thrombosis in the bearing during chronic animal studies.
The infant size Jarvik 2000 heart is the smallest implantable blood pump. The size of an AAA battery, it is small enough to implant in the ventricular apex of 3.5 kg newborns, yet it has flow capacity over 3L/min - sufficient for children up to 25 kg. Animal implants have shown that the pump remains free of thrombus and operates at low power (3-5 Watts) without causing hemolysis. The pump incorporates all of the requirements for a long-term implant, including welded hermetic sealing of the motor stator and rotor magnet, miniature hermetic feed-throughs for the power cables, a very long life conical ceramic bearing design, and pacemaker type high flex life power cables from the external controller to the implanted blood pump.
Work is underway to complete a new control system having important user friendly features, which is expected to be especially useful to support children living at home.
The Penn State Pediatric VAD (Penn State University-Hershey Medical Center)
The Penn State Pediatric VAD is a pneumatically-actuated pulsatile pump (Fig. 1) based on the design of the Pierce-Donachy adult VAD (Thoratec PVAD™ developed at Penn State. The Penn State Pediatric VAD has been developed in two sizes: a 12 ml stroke volume Infant VAD with 6 and 8 mm cannulae, and a 25 ml stroke volume Child VAD with 8 and 10 mm cannulae 17. The pneumatic pulsatile VADs are flexible in their application, and may be utilized for, left, right, or bi-ventricular support. Pump filling is detected by measuring the driveline airflow, enabling a full-to-empty automatic rate control, resulting in an increase in pump rate with increasing inlet pressure. This automatic preload sensitivity allows maximum flow and ventricular unloading to be achieved while limiting excessive inlet suction, and provides safe left atrial pressure control in biventricular support. The pump is designed with the option of paracorporeal placement or implantation. The duration of support in excess of one year is expected to be achieved with this design. Improvements to the devices during the program include a new Björk-Shiley Monostrut (BSM) custom valve (manufactured in–house), a re-designed diaphragm, and better seals.
The primary design objective has been to produce a device with a low thrombogenic risk. This is especially challenging because the fluid mechanics in small-scale devices favor low wall shear rates and have a higher propensity for clot formation, relative to adult-sized devices. Significant efforts have been made in understanding the spatial and temporal properties of blood flow in the pump and cannulae with special attention on the valves using Particle Image Velocimetry (PIV)18 and a viscoelastic blood analog19. Wall shear rates were calculated from the data to quantify wall washing (Fig. 5). The BSM valve was selected because the results revealed that it provided a stronger and more sustained rotational flow due to the major orifice inlet jet as well as lower outlet valve regurgitation than the Carbomedics valve which was also evaluated20. PIV was also used to evaluate the effect of reduced flow rate during weaning21.
Figure 5.
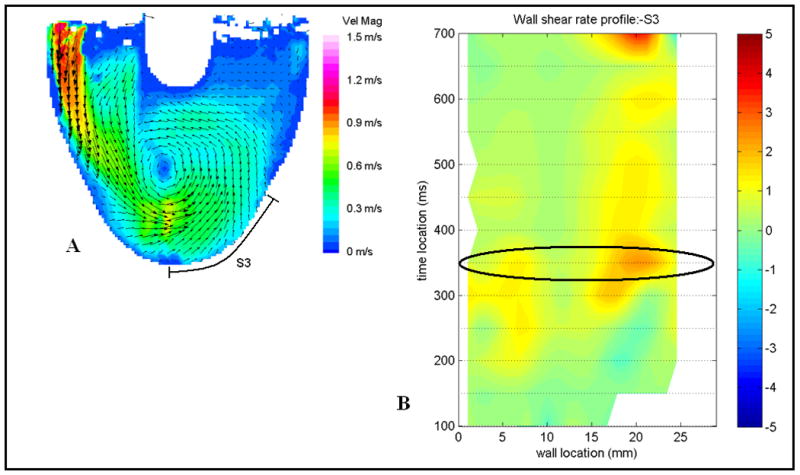
An example of a flow map (A) and the corresponding wall shear rate map (B). The section highlighted and labeled S3 is the surface shown in the wall shear map in B. An example wall shear rate (profile B) where positive shear is defined in the clockwise direction and negative shear is in the counter-clockwise direction. The oval highlights the time step shown in the flow map A. The shear rate is normalized by the threshold shear of 500 s-1, so that 1 to -1 on the shear map is equivalent to 500 s-1 to -500 s-1.
The Penn State investigators made substantial progress in developing animal models for studies of pediatric VADs. Chronic animal studies of the infant pump were completed in 11 lambs and 4 goats. One acute study, 3 surgical shams, and 4 hematologic control studies were also completed. A major challenge in the early studies was atelectasis and respiratory failure, not related to VAD function, which required protocol improvements22, 23. A stable animal model in the 20-25 kg lamb was subsequently achieved. This model demonstrated healthy survival to elective termination at 4 weeks in 5 of the 7 most recent studies (duration 5 to 41 days; mean 26.1 days).
The results from the animal studies are promising. Hemolysis was not evident in animal testing and in vitro hemolysis testing with bovine blood demonstrated a normalized index of hemolysis similar to adult pulsatile VADs. Likewise, thromboembolism was also not evident in the animal testing. In the one group of animals (the “aPTT group” (n=4)), unfractionated heparin was titrated to achieve a therapeutic aPTT of 2 times normal, which is a common approach in humans6. As shown in Figure 6, thromboelastography (TEG) in this group indicated hypo-coagulability despite a therapeutic aPTT, raising concern that the in vivo testing protocol was less challenging (in terms of thromboembolism) than the expected clinical use. Therefore, a reduced heparin series “TEG group” (n=3) in which heparin was titrated to achieve a target TEG R-time of 2 times normal was initiated. (TEG-based protocols are used in some centers24). Figure 6 reveals that the heparin activity, as measured by antifactor Xa, was not detectable in the reduced heparin TEG group. No thromboembolism was observed in either group. The kidneys were grossly normal except for cortical depressions and fibrosis consistent with old infarcts in two cases (TEG group), and a recent renal infarct in one animal with a cecal abscess and fever (aPTT group). These results are generally consistent with previous pre-clinical studies of successful adult VADs, even at the low levels of heparin anticoagulation.
Figure 6.
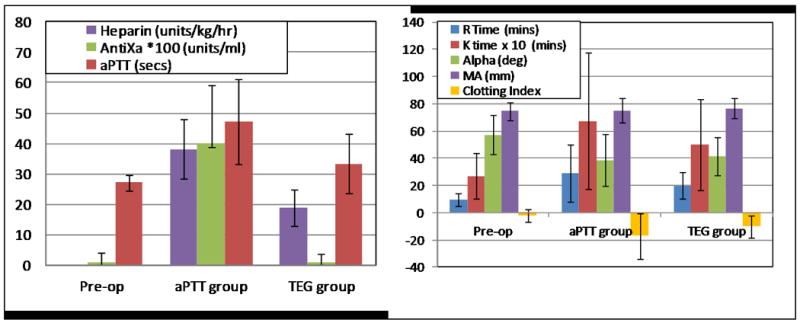
Left: The heparin dose in the TEG group (reduced heparin protocol) was approximately one half that of the aPTT group, and circulating heparin (antifactor Xa) was barely detectable in the TEG group. The aPTT and TEG groups were significantly different (p < .001) for the 3 parameters shown. Antifactor Xa in the TEG group was not significantly different than pre-op levels. Right:TEG R, K, and clotting index were statistically different (p < .001) between the aPTT and TEG groups.
Discussion
Extraordinary progress in the field of pediatric circulatory support has been made over the past five years since the inception of the PCSP. When the program began, the only options for very young children with cardiopulmonary failure and circulatory collapse were conventional ECMO and, under emergency use granted by the FDA, the Berlin Heart EXCOR Pediatric VAD. Since then, a number of substantial advances in the field of pediatric mechanical circulatory support have occurred (see Table 3).25 In the spring of 2004, MicroMed received a Humanitarian Device Exemption from the FDA for use of the DeBakey VAD Child in pediatric patients slightly larger than those in the PCSP program.26 In 2006, tracking of outcomes for pediatric patients who received chronic circulatory support using FDA-approved devices began in INTERMACS.27 In 2007, Berlin Heart initiated a clinical trial of its EXCOR Pediatric VAD in the United States. This was a substantial step towards providing a reliable option for long-term circulatory support of children of any size in the United States.28 Also during the past five years, important new medical conferences devoted specifically to this area were established.
Table 3.
Important Advances in Pediatric Circulatory Support (2004-2010).
| Advance | Date |
|---|---|
| NHLBI Pediatric Circulatory Support Program launched | March 2004 |
| MicroMed DeBakey VAD Child receives Humanitarian Device Exemption | April 2004 |
| 1st International Conference on Pediatric Mechanical Circulatory Support Systems | May 2005 |
| Pediatric INTERMACS begins enrollment | June 2006 |
| Berlin Heart VAD U.S. Clinical Trial | December 2007 |
| PumpKIN Contracts Awarded | January 2010 |
The PCSP has contributed significantly to this “golden era” in pediatric mechanical circulatory support. A palpable sense of momentum was created when this program was initiated in the spring of 2004. Since then, as is evident in this update, the five participating contractors have made outstanding technical advancements towards the ultimate goal of providing safe and effective devices for circulatory support in infants, neonates, and young children with advanced, severe heart disease. The size and flow characteristics of the devices (Table 2) and the various in vitro and in vivo test results reveal that the goals of the PCSP have been, in large part, achieved. The science and technologies developed through the program, such as the animal models, new assays, virtual fitting methodologies, and the novel bearings and biocompatible coatings, are poised to benefit the broader fields of mechanical circulatory support, cardiovascular devices, and pediatric therapeutics. The developments through the program have been disseminated through the 75 scientific publications and the 247 presentations at national and international meetings from the contracting centers during the program which, in turn, have significantly impacted the scientific knowledge base. That knowledge base extends across the disciplines of biomedical engineering, physiology, and perfusion sciences as applied to pediatric mechanical circulatory support. Furthermore, while the program has generated considerable excitement in the clinical fields of pediatric cardiology and cardiac surgery, it has also generated substantial interest in the field of adult heart failure because, if successful, the PCSP devices could be placed using minimally invasive procedures while sufficiently augmenting cardiac output in adults.
The progress achieved during the PCSP has come through a great effort which, in turn, has resulted in substantial lessons learned during the program. As evidenced through the developments in the program, such as novel bearings, modified device designs, and animal models, the contractors quickly learned that the goals would not be simply achieved by miniaturization of adult size devices and easily applying the experience with those devices. The PCSP also revealed that groups of contractors can work together synergistically and cooperatively while developing potentially competitive products. The program also demonstrated that, despite extraordinary dedication, experience, and skills of the contract teams and significant federal support, development programs like these take substantial time and that this was only one of many steps to be made before the devices will be available for clinical use.
The use and availability of pediatric circulatory support devices is poised to grow over the next 5-10 years. In October, 2008, NHLBI issued a request for proposals for the Pumps for Kids, Infants, and Neonates (PumpKIN) program. The program was designed to provide support to contractors to perform the work necessary to receive Investigational Device Exemptions (IDEs) from the FDA for pediatric circulatory support devices within 30 months from the initiation of the PumpKIN program. The required activities include pre-clinical tests and analyses, development and documentation of manufacturing processes and procedures, and collaboration to develop an IDE clinical study. Awards for the pre-clinical phase were made in January, 2010. Three of the five contractors which comprised the NHLBI Pediatric Circulatory Support Program (Ension, Jarvik Heart, and University of Pittsburgh) received the PumpKIN contract program awards. A fourth PumpKIN award was made to the University of Maryland, Baltimore, which is partnering with Levitronix, LLC on efforts on another promising compact ECMO device known as the pediatric pump-lung (PediPL). In order to help facilitate the timely approval of PumpKIN IDEs, pre-IDE applications were submitted by the four PumpKIN contractors to the FDA in April, 2010, as required by the NHLBI contract. A second RFP for a PumpKIN Data and Clinical Coordinating Center to develop, manage, and oversee the clinical trial(s) involving the devices will be issued and awarded at a future date. NHLBI envisions the award to be made so that the clinical trial can begin in January, 2013.29
Footnotes
Disclosure Statement: Dr. Borovetz is a full-time faculty member at The University of Pittsburgh which licenses IP to WorldHeart, Inc. Dr. Borovetz does not receive any proceeds from the licenses. Dr. Gartner is the President and retains ownership interest in Ension, Inc. Dr. Jarvik is the President and a shareholder of Jarvik Heart, Inc. Dr Weiss is a consultant for Thoratec Corporation.
References
- 1.Baldwin JT, Borovetz HS, Duncan BW, Gartner MJ, Jarvik RK, Weiss WJ, Hoke TR. The National Heart, Lung, and Blood Institute Pediatric Circulatory Support Program. Circulation. 2006;113:147–155. doi: 10.1161/CIRCULATIONAHA.105.571422. [DOI] [PubMed] [Google Scholar]
- 2.Duncan BW. Pediatric Mechanical Circulatory Support in The United States: Past, Present, and Future. ASAIO J. 2006;52:525–529. doi: 10.1097/01.mat.0000237588.44529.d1. [DOI] [PubMed] [Google Scholar]
- 3.Loforte A, Delmo Walter EM, Stiller B, Huebler M, Alexi-Meskishvili V, Boettcher W, Berger F, Hetzer R. Extracorporeal Membrane Oxygenation for Intraoperative Cardiac Support in Children with Congenital Heart Disease. Interact CardioVasc Thorac Surg. 2010;10:753–758. doi: 10.1510/icvts.2009.220475. [DOI] [PubMed] [Google Scholar]
- 4.BAA NHLBI-HV-04-01; Broad Agency Announcement for The Pediatric Circulatory Support Program. 2002. [Google Scholar]
- 5.Johnson CA, Jr, Snyder TA, Woolley JR, Wagner WR. Flow Cytometric Assays for Quantifying Activated Ovine Platelets. Artif Organs. 2008;32:136–145. doi: 10.1111/j.1525-1594.2007.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson CA, Jr, Vandenberghe S, Daly AR, Woolley JR, Snyder ST, Verkaik JE, Ye SH, Borovetz HS, Antaki JF, Wearden PD, Kameneva MV, Wagner WR. Biocompatibility Assessment of the First Generation PediaFlow Pediatric Ventricular Assist Device. Artif Organs. 2010 doi: 10.1111/j.1525-1594.2010.01023.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson CA, Jr, Wearden P, Kocyildirim DE, Maul TM, Woolley JR, Ye SH, Strickler EM, Borovetz HS, Wagner WR. Platelet Activation in Ovines Undergoing Sham Surgery or Implant of the Second Generation PediaFlow™ Pediatric Ventricular Assist Device. Artif Organs. doi: 10.1111/j.1525-1594.2010.01124.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan BW, Dudzinski DT, Gu L, Mielke N, Noecker AM, Kopcak MW, Fukamachi K, Cingoz F, Ootaki Y, Smith WA. The PediPump: Development Status of a New Pediatric Ventricular Assist Device: Update II. ASAIO J. 2006;52:581–587. doi: 10.1097/01.mat.0000235821.63492.c4. [DOI] [PubMed] [Google Scholar]
- 9.Duncan BW, Dudzinski DT, Noecker AM, Kopcak MW, Fukamachi K, Ootaki Y, Chen HM, Chapman PA, Smith WA. The Pedipump: Development Status of a New Pediatric Ventricular Assist Device. ASAIO J. 2005;51:536–539. doi: 10.1097/01.mat.0000178211.70743.99. [DOI] [PubMed] [Google Scholar]
- 10.Duncan BW, Lorenz M, Kopcak MW, Fukamachi K, Ootaki Y, Chen HM, Chapman PA, Davis SJ, Smith WA. The PediPump: A New Ventricular Assist Device for Children. Artif Organs. 2005;29:527–530. doi: 10.1111/j.1525-1594.2005.29088.x. [DOI] [PubMed] [Google Scholar]
- 11.Weber S, Dudzinski DT, Gu L, Mielke N, Casas F, Noecker AM, Saeed D, Ootaki Y, Fukamachi K, Smith WA, Duncan BW. The PediPump: a Versatile, Implantable Pediatric Ventricular Assist Device-Update III. ASAIO J. 2007;53:730–733. doi: 10.1097/MAT.0b013e318159822c. [DOI] [PubMed] [Google Scholar]
- 12.Noecker AM, Chen JF, Zhou Q, White RD, Kopcak MW, Arruda MJ, Duncan BW. Development of Patient-Specific Three-Dimensional Pediatric Cardiac Models. ASAIO J. 2006;52:349–353. doi: 10.1097/01.mat.0000217962.98619.ab. [DOI] [PubMed] [Google Scholar]
- 13.Cingoz F, Fukamachi K, Ootaki Y, Kamohara K, Akiyama M, Ootaki C, Kopcak MW, Liu J, Noecker A, Dudzinski D, Smith WA, Duncan BW. Cleveland Clinic Pedipump Lamb Cadaver Fitting Studies. Artif Organs. 2007;31:405–408. doi: 10.1111/j.1525-1594.2007.00400.x. [DOI] [PubMed] [Google Scholar]
- 14.Noecker AM, Cingoz F, Ootaki Y, Liu J, Kuzmiak S, Kopcak MW, Fukamachi K, Duncan BW. The Cleveland Clinic Pedipump: Anatomic Modeling and Virtual Fitting Studies in a Lamb Model. ASAIO J. 2007;53:716–719. doi: 10.1097/MAT.0b013e31805fe98b. [DOI] [PubMed] [Google Scholar]
- 15.Saeed D, Ootaki Y, Noecker A, Weber S, Smith WA, Duncan BW, Fukamachi K. The Cleveland Clinic Pedipump: Virtual Fitting Studies in Children Using Three-Dimensional Reconstructions of Cardiac Computed Tomography Scans. ASAIO J. 2008;54:133–137. doi: 10.1097/MAT.0b013e31815b4495. [DOI] [PubMed] [Google Scholar]
- 16.Saeed D, Weber S, Ootaki Y, Mielke N, Ootaki C, Akiyama M, Horai T, Catanese J, Fumoto H, Dessoffy R, Dudzinski DT, Gu L, Casas F, Smith WA, Fukamachi K, Duncan BW. Initial Acute In Vivo Performance of the Cleveland Clinic PediPump Left Ventricular Assist Device. ASAIO J. 2007;53:766–770. doi: 10.1097/MAT.0b013e3181569c1a. [DOI] [PubMed] [Google Scholar]
- 17.Connell JM, Khalapyan T, Myers JL, Rosenberg G, Weiss WJ. Anatomic Fit Assessment for the Penn State Pediatric Ventricular Assist Device. ASAIO Journal. 2007;53:687–691. doi: 10.1097/MAT.0b013e318155741d. [DOI] [PubMed] [Google Scholar]
- 18.Manning KB, Wivholm BD, Yang N, Fontaine AA, Deutsch S. Flow behavior within the 12-cc Penn State Pulsatile Pediatric Ventricular Assist Device: An Experimental Study of the Initial Design. Artif Organs. 2008;32:442–452. doi: 10.1111/j.1525-1594.2008.00565.x. [DOI] [PubMed] [Google Scholar]
- 19.Long JA, Undar A, Manning KB, Deutsch S. Viscoelasticity of Pediatric Blood and its Implications for the Testing of a Pulsatile Pediatric Blood Pump. ASAIO J. 2005;51:563–566. doi: 10.1097/01.mat.0000180353.12963.f2. [DOI] [PubMed] [Google Scholar]
- 20.Cooper B, Roszelle B, Long T, Deutsch S, Manning KB. The 12 cc Penn State Pulsatile Ventricular Assist Device: Fluid Dynamics Associated with Valve Selection. J Biomech Eng. 2008;130:041019-1–14. doi: 10.1115/1.2939342. [DOI] [PubMed] [Google Scholar]
- 21.Roszelle BN, Cooper BT, Long TC, Deutsch S, Manning KB. The 12 cc Penn State Pulsatile Pediatric Ventricular Assist Device: Flow Field Observations at a Reduced Beat Rate with Application to Weaning. ASAIO J. 2008;54:325–331. doi: 10.1097/MAT.0b013e3181695cfe. [DOI] [PubMed] [Google Scholar]
- 22.Carney EKT, Connell J, Myers J, Clark J, Wilson R, Stark P, Weiss W. Intraoperative Respiratory Management in Lambs Undergoing PVAD Implantation. ASAIO J. 2008;54:62A. [Google Scholar]
- 23.Carney E, Litwak K, Weiss W. Animal Models for Pediatric Circulatory Support Device Pre-clinical Testing: National Heart, Lung, and Blood Institute Pediatric Assist Device Contractor's Meeting Animal Models Working Group. ASAIO J. 2009;55:6–9. doi: 10.1097/MAT.0b013e318198e11c. [DOI] [PubMed] [Google Scholar]
- 24.Drews T, Stiller B, Hubler M, Weng Y, Berger F, Hetzer R, Drews T, Stiller B, Hubler M, Weng Y, Berger F, Hetzer R. Coagulation Management in Pediatric Mechanical Circulatory Support. ASAIO J. 2007;53(5):640–645. doi: 10.1097/MAT.0b013e3181492929. [DOI] [PubMed] [Google Scholar]
- 25.Duncan BW. Pediatric Mechanical Circulatory Support: A New Golden Era? Artif Organs. 2005;29:925–926. doi: 10.1111/j.1525-1594.2005.00161.x. [DOI] [PubMed] [Google Scholar]
- 26.Fraser CD, Jr, Carberry KE, Owens WR, Arrington KA, Morales DL, Heinle JS, McKenzie ED. Preliminary Experience with the MicroMed DeBakey Pediatric Ventricular Assist Device. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006:109–114. doi: 10.1053/j.pcsu.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Holman WL, Kormos RL, Naftel DC, Miller MA, Pagani FD, Blume E, Cleeton T, Koenig SC, Edwards L, Kirklin JK. Predictors of Death and Transplant in Patients with a Mechanical Circulatory Support Device: a Multi-Institutional Study. J Heart Lung Transplant. 2009;28:44–50. doi: 10.1016/j.healun.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Rockett SR, Bryant JC, Morrow WR, Frazier EA, Fiser WP, McKamie WA, Johnson CE, Chipman CW, Imamura M, Jaquiss RD. Preliminary Single Center North American Experience with the Berlin Heart Pediatric EXCOR Device. ASAIO J. 2008;54:479–482. doi: 10.1097/MAT.0b013e318184e200. [DOI] [PubMed] [Google Scholar]
- 29.NHLBI . RFP NHLBI-HV-09-14; Request for Proposals for the NHLBI Pumps for Kids, Infants, and Neonates (PumpKIN) Program. 2008. [Google Scholar]