Abstract
The adhesive proteins secreted by marine mussels form a natural glue that cures rapidly to form strong and durable bonds in aqueous environments. These mussel adhesive proteins contain an unusual amino acid, 3,4-dihydroxy-L-phenylalanine (DOPA), which is largely responsible for their cohesive and adhesive strengths. In this study, we incorporated DOPA into diblock and triblock polymers and developed a membrane contact experiment to assess the adhesive interactions of these materials with TiO2 and tissue surfaces. In a typical experiment a micrometer-thick DOPA-functionalized elastomeric membrane is attached to the end of a cylindrical glass tube. Application of a positive pressure to the tube brings the membrane into contact with the surface of interest. The negative pressure needed to separate the membrane from the substrate is a measure of the strength of the adhesive interaction. The test confirms previous results obtained with TiO2 substrates. Because the membrane geometry is well suited for rough or chemically heterogeneous surfaces, it is ideal for studies of tissue adhesion. DOPA was found to give strong adhesion to tissue surfaces, with the strongest adhesion obtained when the DOPA groups were oxidized while in contact with the tissue surface.
Keywords: Block Copolymers, DOPA, Marine Mussel Mimetics, Membrane, Tissue Adhesion
1. INTRODUCTION
Bioadhesives that are able form strong and durable bonds in wet environments, where most adhesives do not perform well, are of great interest. Adhesive materials inspired by marine mussel adhesive proteins (MAPs) are particularly intriguing in this respect. When secreted, these liquid MAPs harden and form water-resistant bonds within a few seconds [1,2]. A range of different MAPs have been isolated from the blue mussel, Mytilus edulis, referred to as Mefp-1 to Mefp-5. These proteins all contain a modified amino acid, 3,4-dihydroxy-L-phenylalanine (DOPA) [3]. Mefp-3 and Mefp-5 both contain large amounts of DOPA (up to 30 mol%) [4] and are found to concentrate at the interface between the adhesive and the substrate. Evidence suggests that DOPA plays an important role in determining both the cohesive and adhesive properties of the secreted liquid adhesive [5–7]. The DOPA catechol can easily oxidize into quinone, which then undergoes crosslinking reactions that are responsible for the cohesive strength of these materials [8–11]. Although the adhesive role is not completely understood, catechol groups are capable of forming hydrogen bonds, metal-ligand complexes, Michael-type addition compounds, and quinhydrone charge-transfer complexes [1]. MAPs and their synthetic analogs have also been shown to have useful mucoadhesive properties [6,12,13].
There have been several efforts to mimic the water-resistant adhesive properties of MAPs by the incorporation of DOPA into synthetic polymers [5,6,14–18]. First generation DOPA mimetic adhesives targeted the crosslinking reactions that occur during DOPA oxidation to form a gel network. DOPA, however, is believed to lose its strong adhesive properties due to oxidation [7]. This conjecture has been confirmed for metallic surfaces recently by the single molecule AFM experiments of Lee et al. [19]. Furthermore, the use of DOPA oxidizing reagents (such as NaIO4 and H2O2) [7] may complicate the future in vivo applications of these materials. Therefore, the second generation mussel mimetic systems target alternative gelation mechanisms that do not rely on DOPA oxidation, such as temperature [12] and UV exposure [20]. Although neither of these systems requires oxidizing reagents for gel formation, the crosslinking reactions used to form strong chemical gels can be inhibited either by oxygen, or by the DOPA itself. This can lead to formation of a weak boundary layer that will affect the adhesive properties.We recently reported an alternative method to chemically crosslinked gels, where we incorporated DOPA into self-assembling poly(methyl methacrylate-b-methacrylic acid-b-methyl methacrylate) (PMMA-PMAA-PMMA) triblock copolymer gels, which form by physical association of the PMMA end blocks [21]. Under aqueous conditions PMMA-PMAA-PMMA triblock copolymers self-assemble to form strong hydrogels, where hydrophobic PMMA end groups form spherical aggregates acting as physical crosslinks. Although we showed that these DOPA mimetic hydrogels had strong adhesion on TiO2 surfaces, the strength of the gel/titania interface was limited by the cohesive strength of the gel [21]. In our previous work, we used an indentation set-up with a rigid flat punch to ensure uniform contact. This limited our ability to study the adhesive inter-actions of the hydrogel with the soft substrates such as tissue. This method also requires a large amount of polymer solution to prepare gels with the correct geometry. To overcome these limitations we use a membrane geometry (Fig. 1a), which requires a very small amount of the DOPA-containing polymer solution to prepare flexible samples suitable for quantifying adhesion with both soft and hard material surfaces. The membrane geometry also provides an enhanced sensitivity to adhesion [22,23].
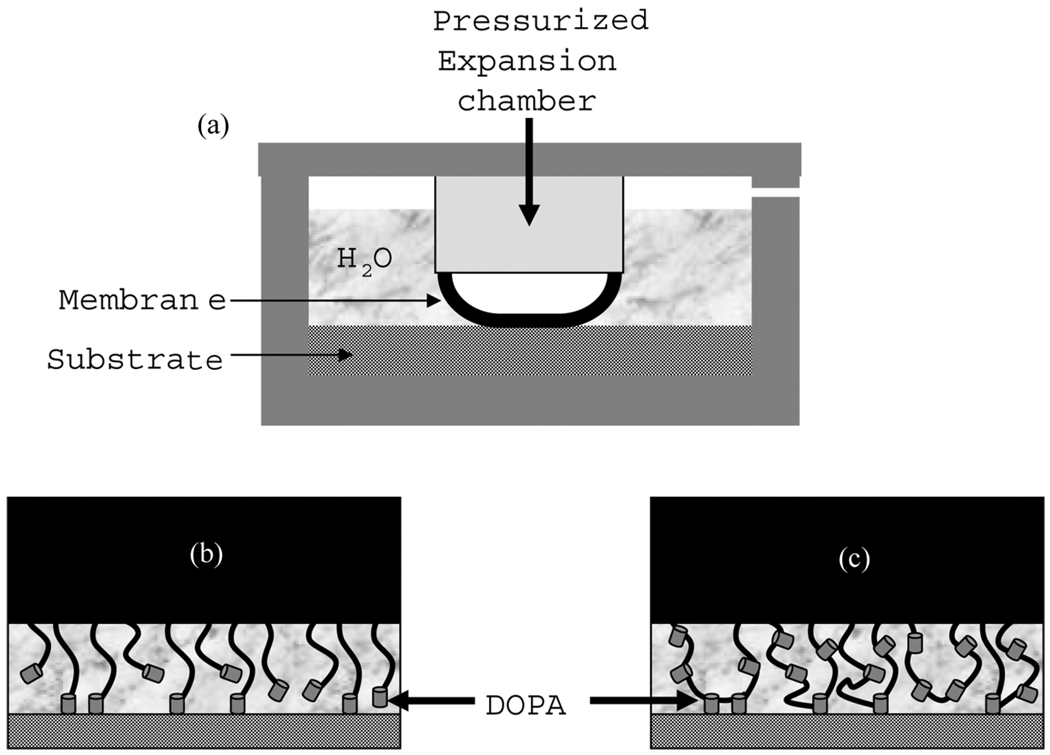
FIGURE 1.
(a) Schematic drawing of the membrane inflation apparatus for assessing adhesion and schematic illustrations of the interfacial structure for membranes modified with (b) the PS-PEO-DOPA copolymer and the (c) DOPA20 acrylic triblock copolymer.
In this study, we have prepared DOPA-modified polymer membranes and have assessed their underwater adhesive properties by using the membrane inflation method. We have synthesized both poly(styrene-b-ethylene oxide)-tert-butyloxycarbonyl-DOPA (PS-PEO-Boc-DOPA) diblock and DOPA-modified PMMA-PMAA-PMMA triblock copolymers. The PS-PEO-Boc-DOPA was tested with a poly(styrene-b-isoprene-b-styrene) (SIS) supporting membrane and the DOPA-modified PMMA-PMAA-PMMA membranes were supported by a hydrophobic poly(methyl methacrylate-b-n-butyl acrylate-b-methyl methacrylate) (PMMA-PnBA-PMMA) membrane, to aid in structural integrity and help with attachment to the end of the inflation chamber.
2. EXPERIMENTS
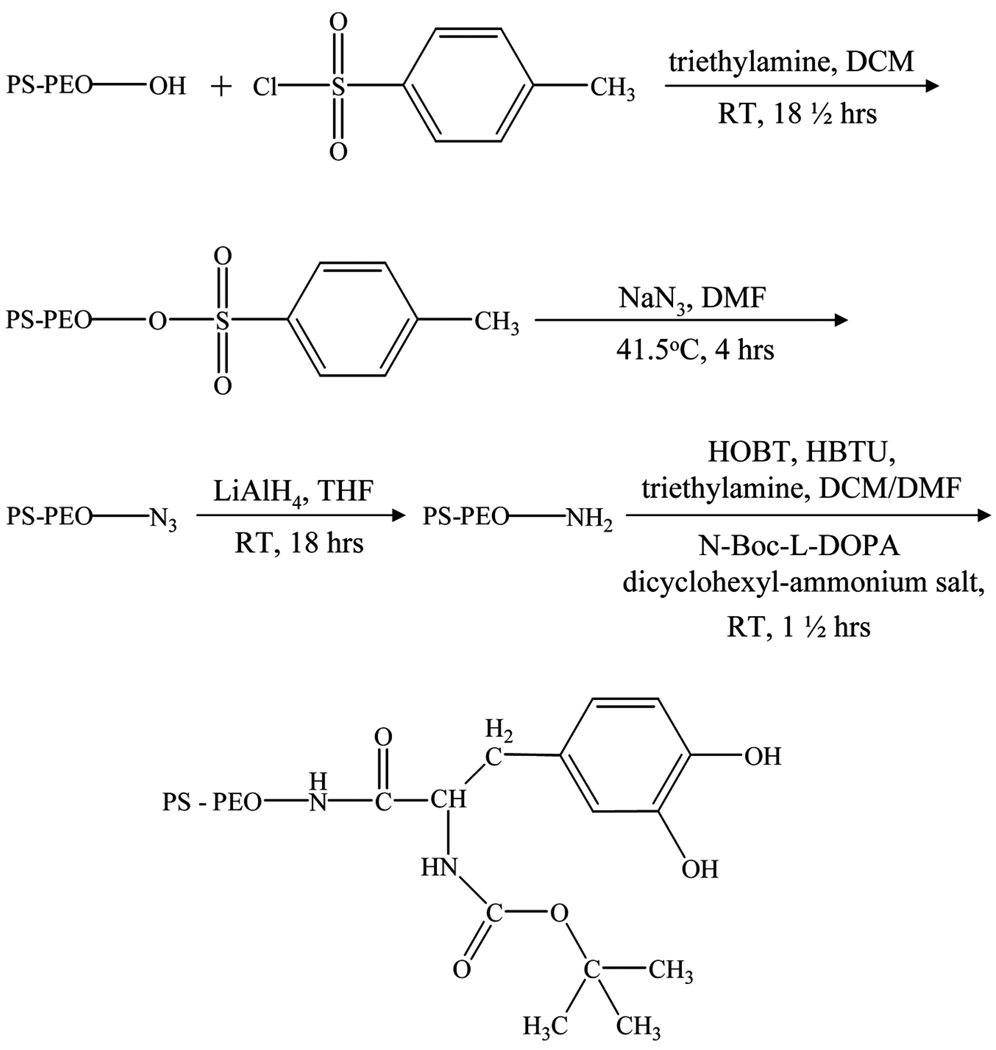
Synthesis of PS-PEO-DOPA
Boc-DOPA terminated PS-PEO was synthesized by following the reaction path shown in Fig. 2. The hydroxyl terminus of a PS-PEO diblock copolymer (3.8–4.8 kg/mol, Polymer Source, Montreal, Canada) was converted to an amine group [24]. Once the amine terminus was obtained the Boc-DOPA end-cap was attached through carbodiimide coupling chemistry [20,25]. In this procedure P-toluene sulfonyl chloride (215mg) and triethylamine (0.25 mL) were dissolved in dichloromethane (5 mL) and placed in an air-evacuated and nitrogen-filled flask. PS-PEO-OH (1 g) dissolved in dichloromethane (5 mL) was then added into the flask and the solution was stirred at room temperature for 18 h. The PS-PEO-tosylate was precipitated into hexane at −30°C and vacuum dried. Sodium azide (80 mg) was dissolved in dimethyl formamide (5 mL) in a nitrogen-filled flask and preheated to 415°C for 25 min. PS-PEO-tosylate (1 g) dissolved in dimethyl formamide (5 mL) was added and reacted at 41.5°C for 4 h. The resultant PS-PEO-azide was precipitated into hexanes at −30°C and vacuum dried. PS-PEO-azide (0.75 g) was dissolved in tetrahydro-furan (13 mL) and placed in a nitrogen filled flask, and 1 mL of 1 M LiAlH4 solution in THF was added and stirred at room temperature for 18 h. The tetrahydrofuran was evaporated and the resulting products were dissolved in dichloromethane. These products were washed in water, 5 wt% aqueous potassium carbonate, and water. The organic phase was dried with excess magnesium sulfate which was then filtered out. The PS-PEO-NH2 was precipitated into hexane at −30°C and vacuum dried.
FIGURE 2.
Reaction path for the modification of PS-PEO-OH into PS-PEO-NH2 and PS-PEO-DOPA.
PS-PEO-NH2 (200 mg), N-Boc-L-DOPA dicyclohexyl-ammonium salt (17 mg), HOBT, a peptide coupling agent (12 mg), and triethylamine (0.2 mL) were dissolved in a 50:50 (v/v) mixture of dichloromethane and dimethyl formamide (10 mL) and placed in a nitrogen-filled flask. HBTU, a peptide coupling agent (14 mg) dissolved in a 5:2 dichloromethane/dimethyl formamide (v/v) mixture was then added. The coupling reaction was carried out for 90 minutes at room temperature. The products were washed in a saturated sodium chloride solution, 6% aqueous sodium bicarbonate solution, dilute HCl solution, and deionized water. The organic phase was dried with excess magnesium sulfate which was then removed by filtration. The PS-PEO-DOPA was precipitated into hexane at −30°C and vacuum dried.
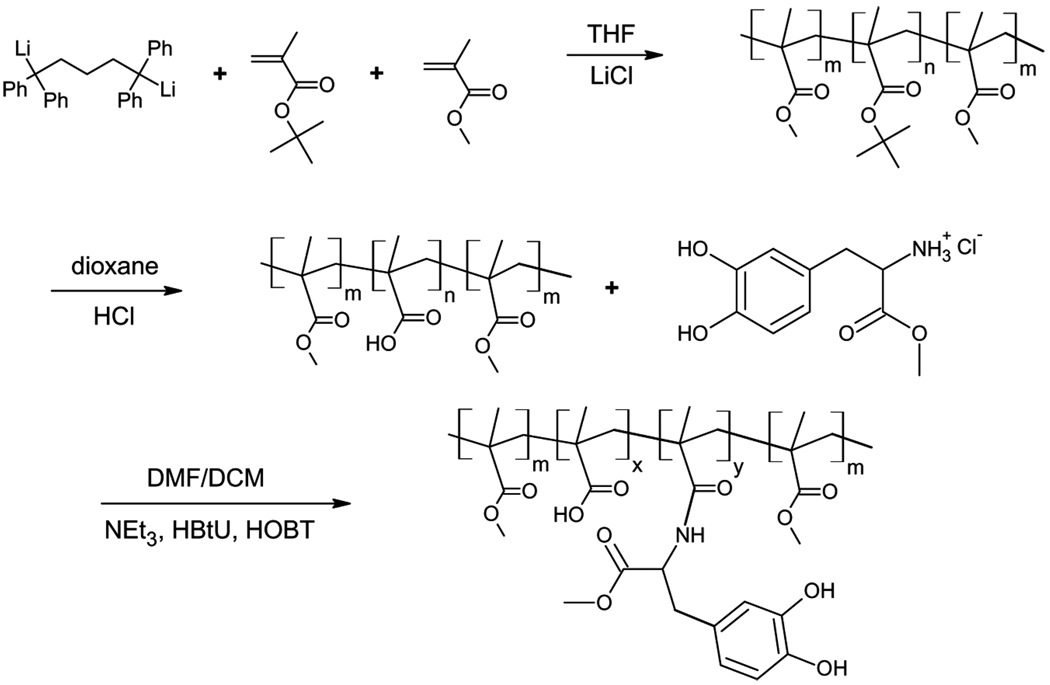
Synthesis of DOPA Incorporated PMMA-PMAA-PMMA
Poly(methyl methacrylate-b-methacrylic acid-b-methyl methacrylate) (PMMA-PMAA-PMMA) triblock copolymer was synthesized by sequential anionic polymerization of tert-butyl methacrylate (tBMA) and methyl methacrylate (MMA) from a difunctional initiator, followed by hydrolysis of the PtBMA midblock to form poly (methacrylic acid) (PMAA) [26]. The resulting polymer had a poly-dispersity of 1.1, PMMA block degrees of polymerization of 370, and a PMAA block degree of polymerization of 1450. DOPA was incorporated into the PMAA midblock of the triblock copolymer by following carboimide chemistry [21] through the reaction path shown in Fig. 3. In brief, 1 g of the triblock copolymer (7.3 mmol methacrylic acid), 15 mmol DOPA methyl ester hydrochloride, 15 mmol HOBT, and 1 mmol HBTU were vacuum degassed in the reaction flask. The flask was then flushed with nitrogen gas and a DMF/DCM (50:50) solvent mixture (40 mL) was injected into the flask through a rubber septum. The system was stirred for at least 15 min to fully dissolve all the reagents. Triethylamine (7.3 mmol) was then injected into the mixture. A faint yellow color was observed as the reaction proceeded. The solution was stirred for 10 h. The solution was then precipitated in hexane and was dried under vacuum overnight. The amount of DOPA incorporated into the PMAA midblock was measured to be 20 mol% via UV vis and NMR [21]. This DOPA-containing polymer is referred to as DOPA20. The precursor polymer with no DOPA is referred to as DOPA00.
FIGURE 3.
Anionic polymerization of tBMA and MMA in the presence of a difunctional initiator followed by hydrolysis of the PtBMA midblock to PMAA and incorporation of DOPA to the PMAA midblock.
Membrane Fabrication
The supporting membrane of poly(styrene-isoprene-styrene) (SIS, Exxon VR4111, ExxonMobie Chemical, Maelieleu, Germany, 10.6k–96.8k–10.6 kg/mol) was created from a 10 wt.% solution in toluene, which was spin-coated onto the surface of sodium chloride crystals (International Crystal Laboratories, Garfield, NJ, USA) to produce a 1.2 µm-thick film. These membranes were then floated onto the surface of a water bath and attached to the end of glass cylindrical tube. Langmuir layers were created through drop-wise addition of 5 µL of a 0.1mg/mL solution in chloroform of either the PS-PEO-NH2 or the PS-PEO-DOPA copolymers onto the surface of a buffer solution. This produced Langmuir layers with a dry thickness hdry = 1.0nm, measured by ellipsometry The membrane-covered glass tube was lowered through the Langmuir layer adhering it to the surface of the membrane. If air was trapped between the membrane and the buffer surface, the membrane was slowly extracted until the air was released and intimate contact could be made.
DOPA-modified acrylic triblock membranes were prepared by spin coating the DOPA00 or DOPA20 acrylic triblock copolymer from 10 wt.% DMSO solutions onto a sodium chloride crystal. The triblock copolymer film was then floated onto distilled water, where it was swollen to equilibrium. Due to low moduli of the swollen PMMA-PMAA-PMMA triblock copolymers [26], we used a hydrophobic supporting membrane of PMMA-PnBA-PMMA (166 kg/mole, Kuraray Co. Ltd., Tokyo, Japan). This supporting membrane was spun-cast from a 10 wt.% toluene solution, floated on distilled water, and transferred to the end of the cylindrical membrane. The swollen DOPA00 or DOPA20 layer was then carefully transferred on top of the supporting membrane. The thicknesses of the two layers in this experiment (PMMA-PMAA-PMMA and PMMA-PnBA-PMMA) were each approximately 1 µm.
Membrane Inflation Experiments
A schematic diagram of the membrane inflation system is given in Fig. 1a. The details of the set-up have been explained previously by Flory et al. [23]. In brief, the membrane attached end of the cylindrical expansion chamber (radius = 3 mm) was immersed in water. The chamber was then connected to a syringe pump (New Era 1000, New Era Pump Systems, Wantagh, NY, USA) and inflated with a volumetric flow rate of ~2 mL/h in order to expand the membrane into contact with the substrate. The substrate was either a TiO2-coated quartz crystal Maxtek/Inficou, Syracuse, NY, USA or hairless pig skin (split-thickness porcine dermal tissue) obtained from Brennen Medical (St. Paul, MN, USA). The distance between the chamber and substrate was 1 mm for TiO2 substrate and 3 mm for pig skin. The pump was stopped after the membrane was expanded on the substrate. After 20 min of delay time, the air in the chamber was withdrawn at 2 mL/h in order to pull the membrane away from the substrate surface. The pressure difference across the membrane was measured by a differential pressure transducer (MKS Baratron, Andover, MA, USA). Contact area images, either from side or from top, were collected by video imaging.
3. RESULTS
The schematics of the DOPA-modified diblock and triblock copolymer membranes used in this study are given in Figs. 1b and 1c, respectively. PS-PEO-DOPA-modified membranes were prepared by forming a Langmuir layer of this polymer from chloroform solution. When the chloroform was dried the film was transferred to a SIS membrane, which was previously attached to the end of the inflation chamber. Control experiments were performed on PS-PEO-NH2-modified membranes. DOPA-modified PMMA-PMAA-PMMA membranes were prepared directly by floating the spun-cast polymer solution on water. The film was then transferred onto PMMA-PnBA-PMMA-supporting membrane which was previously transferred to the end of the inflation chamber. Our previous studies on bulk gels showed that DOPA oxidizes to a less adhesive form when kept in alkaline buffer solutions [21]. In order to eliminate these effects in this study, experiments were done in a pH 7.4 buffer where the DOPA was stable against oxidation for at least several hours.
Adhesion of PS-PEO-DOPA Membranes
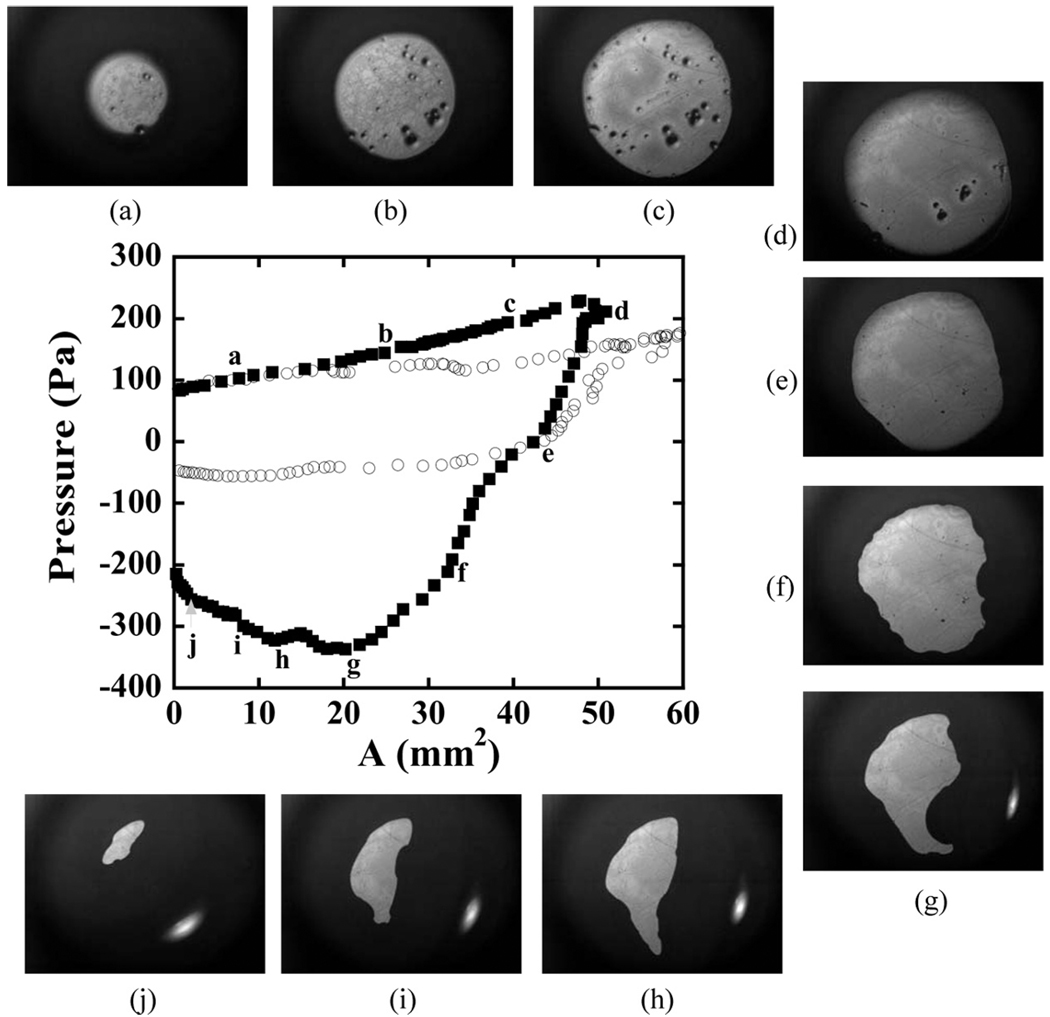
Membrane inflation experiments were conducted with both the amine and the Boc-DOPA terminus in a pH 7.4 buffer solution. The pressure curves for these two trials are given in Fig. 4, which illustrates the effect of the DOPA group on the adhesion to a titanium oxide surface. Two features illustrate the result that the DOPA-functional polymer adheres much more strongly to the titanium surface than the amine functional control. The first of these is the maximum negative pressure needed to remove the membrane from the surface. The NH2 was removed with a release pressure of 56 Pa, while 340 Pa was needed to remove the DOPA-functionalized polymer. The second feature is the shape of the contact during withdrawal of the membrane. When adhesion is present, membrane pull-off is no longer axially symmetric, but adopts a more complicated geometry. This result can be partially attributed to lateral variations in the adhesive response, although mechanical instabilities similar to those observed in adhesive contact of thin films might also be playing a role [27]. This lack of axial symmetry makes it more difficult to apply the type of quantitative analysis of the adhesive strength that has been developed previously [23]. For this reason we use the release pressure itself to compare the adhesiveness of the different membranes.
FIGURE 4.
Pressure applied to a SIS membrane modified with PS-PEO-NH2 (open symbols) and PS-PEO-DOPA (filled symbols) for membrane contact with a TiO2 substrate. Contact images are shown for the PS-PEO-DOPA experiment at the points indicated by the letters.
Repetitions of these contact experiments were conducted with the same membrane/substrate combination. For the amine functional polymers, the membrane release behavior for subsequent releases was nearly identical to the behavior obtained for the first contact. Subsequent contacts of the membrane functionalized with PS-PEO-DOPA showed reductions in the magnitude of the release pressure, however. There are two potential reasons for this behavior: (1) Some of the Langmuir layer might have been pulled from the membrane surface, due to the strong adhesion between the titanium and Boc-DOPA; and (2) some oxidation may have occurred to the DOPA due to its prolonged exposure to the slightly basic environment. Oxidation of DOPA when it is not in contact with the substrate is known to reduce the adhesion [21].
Adhesion of DOPA-Modified PMMA-PMAA-PMMA Membranes
The adhesive interactions of the DOPA-modified PMMA-PMAA-PMMA triblock copolymer with titanium and pig skin were also investigated. The release pressure at which the membrane was completely separated from the substrate was used as an indication of adhesiveness, where a higher magnitude of the negative release pressure is indicative of higher adhesion.
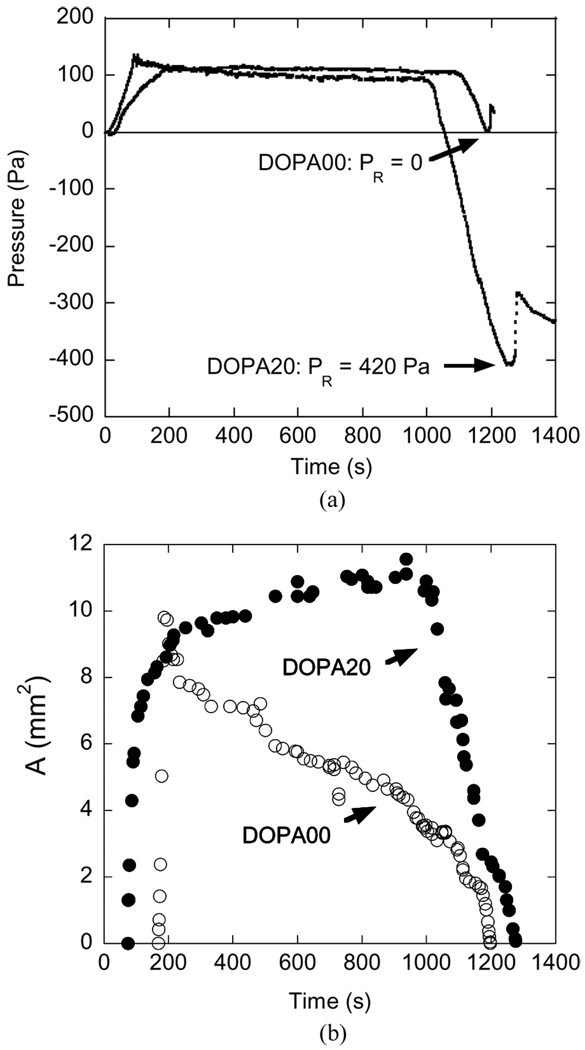
The time dependence of the applied pressure for membranes functionalized with DOPA00 and DOPA20 and brought into contact with a TiO2 surface is shown in Fig. 5a. In these experiments, the membrane was held in contact with the substrate for about 20 min at an applied pressure of 100 Pa. During this period the membrane pressure did not change, but the contact area increased for the DOPA-TiO2 contact, as shown in Fig. 5b. When the pressure was reduced, the contact was lost at a release pressure, PR of ~0 Pa, which also indicates a weak adhesive interaction between PMAA and TiO2. For these materials the elastic tension in the membrane itself is sufficient to detach the membrane from the substrate. For membranes modified with the DOPA20 triblock copolymer, a negative release pressure of 420 Pa was needed in order to completely remove the membrane from the TiO2 surface. These results indicate that the presence of DOPA in the PMAA midblock enhances the adhesion of methacrylic membranes to TiO2 surfaces, a result that was obtained previously with a more traditional punch geometry [21].
FIGURE 5.
Time dependence of (a) the membrane pressure and (b) contact area for the acrylic membranes functionalized with DOPA00 or DOPA20 tri-block copolymers, inflated into contact with a TiO2 substrate.
In order to study the potential use of these materials as skin adhesives, we also used hairless pig skin as a substrate material. The pig skin was cut into the desired size and attached to a glass slide using Super Glue®, and then immersed in distilled water. The membrane was then inflated into contact with the tissue. Membrane pressure data are shown in Fig. 6 for both DOPA-modified (DOPA20) and unmodified PMMA-PMAA-PMMA membranes. The DOPA00-functionalized membrane was released from the tissue surface at almost zero pressure, indicating a poor interaction between the methacrylic acid and the tissue. In the case of the DOPA-modified membrane, a release pressure of ~50 Pa was obtained. As with the TiO2 substrates, the release pressure decreased for subsequent contacts of the same membrane/substrate pair.
FIGURE 6.
Measured values of the membrane pressure for contact between methacrylic membranes and hairless pig skin.
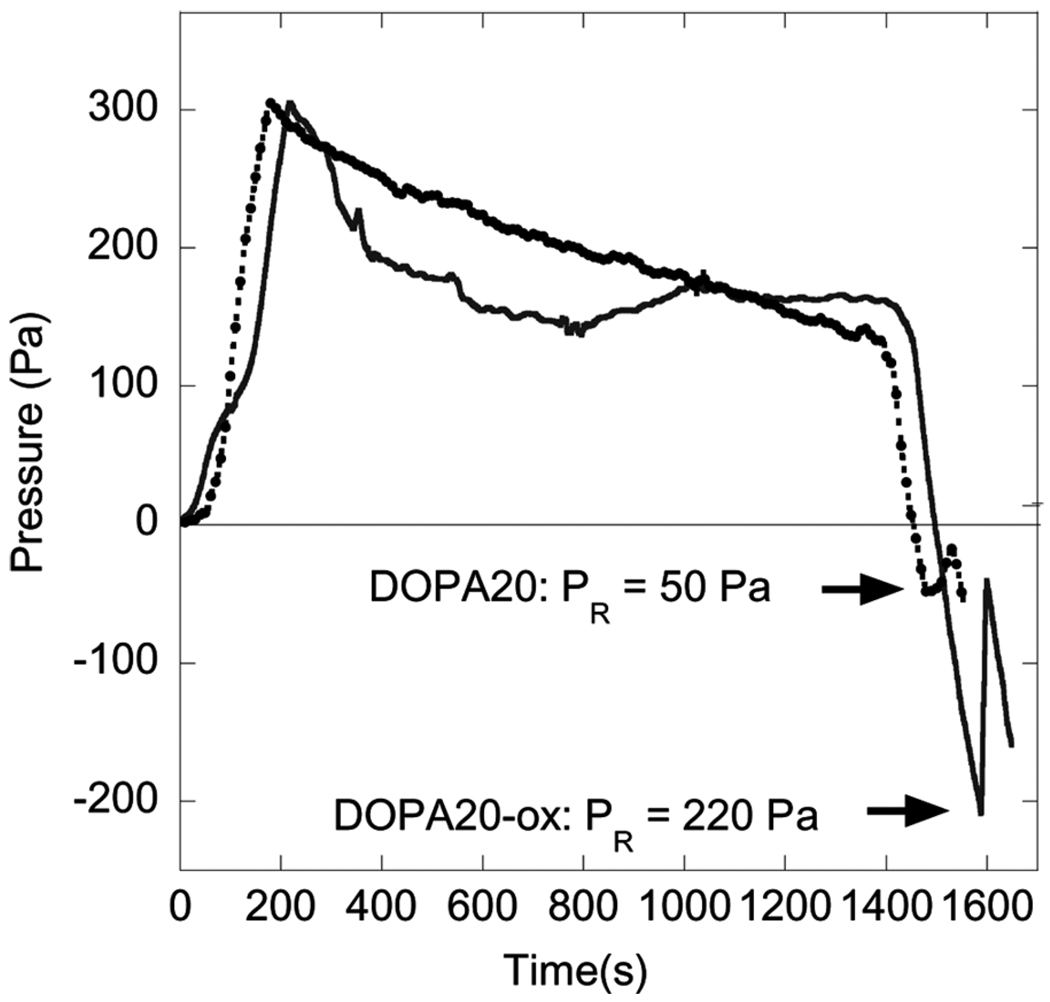
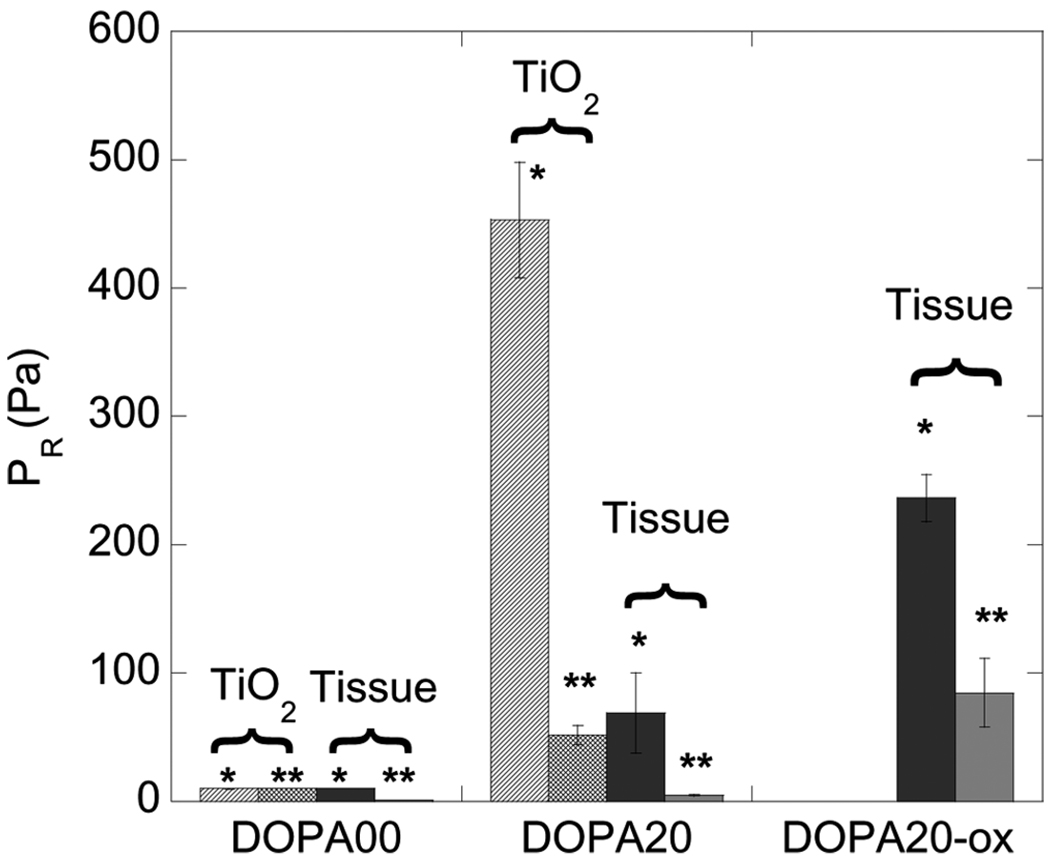
Finally, we have done complementary experiments where we oxidize DOPA while in contact with the tissue. In these experiments a DOPA-modified PMMA-PMAA-PMMA membrane was oxidized by injecting concentrated periodate (NaIO4) into the chamber while in contact with the pig skin. The negative release pressure in this case was ~220 Pa, which is much greater than the release pressure obtained for the case where this contact-oxidation procedure was not utilized. Results for all of the adhesion tests, which were each repeated three times, are summarized in Fig. 7. Results obtained for the first and second contacts for a given membrane/substrate pair are differentiated on this plot. These results indicate that the best adhesive performance is obtained by oxidizing the DOPA while it is in contact with the substrate of interest, a process that is presumed to result in covalent bond formation with the tissue surface [19].
FIGURE 7.
Membrane release pressure for first contact (*) and second contact (**) for DOPA20 membranes inflated into contact with TiO2 and tissue substrates. Error bars refer to standard deviation for at least three sets of samples. Contact times of 20 min were used for both the first and second contact in each experiment.
4. CONCLUSIONS
We have investigated the adhesive properties of DOPA by using two model membrane systems including an end-functionalized PS-PEO diblock copolymer and a PMMA-PMAA-PMMA triblock copolymer with DOPA groups distributed along the water-soluble, PMAA midblock. DOPA-functionalized elastomeric membranes were formed by transferring these polymers to thin, hydrophobic membranes made from elastomeric materials that are compatible with the DOPA-functionalized block copolymers. The adhesive properties of these membranes in contact with TiO2 and tissue surfaces were investigated with a membrane inflation technique that is ideally suited for adhesion measurements against soft, non-uniform substrates like tissue surfaces. Our results can be summarized as follows:
Non-circular contact on membrane withdrawal observed for DOPA-functional membranes is indicative of strong adhesion between DOPA and both TiO2 and tissue surfaces. The contacts on withdrawal for membranes without DOPA were mostly circular, with release pressures close to zero.
The magnitude of the negative release pressure is a useful metric of the adhesive strength in cases where the non-circular contact areas complicate the application of a more quantitative analysis.
DOPA also gives enhanced adhesion to tissue surfaces, with the strongest effect observed when the DOPA-containing polymers are oxidized while in contact with the tissue.
ACKNOWLEDGMENTS
This work was supported by the Northwestern University Materials Research Center through the NSF through the MRSEC program (DMR-0520513) and by the NIH (R01 DE14193). The authors also acknowledge Dr. Anny Flory and Dr. Bruce Lee for a variety of helpful discussions.
Footnotes
One of the Collection of papers honoring J.Herbert Waite, the recipient in February 2009 of The Adhesion Society Award for Excellence in Adhesion Science, Sponsored by 3M.
REFERENCES
- 1.Waite JH. International Journal of Adhesion and Adhesives. 1987;7(1):9–14. [Google Scholar]
- 2.Waite JH. Annals of the New York Academy of Sciences. 1999;875:301–309. doi: 10.1111/j.1749-6632.1999.tb08513.x. [DOI] [PubMed] [Google Scholar]
- 3.Waite JH. The Journal of Biological Chemistry. 1983;258(5):2911–2915. [PubMed] [Google Scholar]
- 4.Papov VV, Diamond TV, Biemann K, Waite JH. J. Biol. Chem. 1995;270(34):20183–20192. doi: 10.1074/jbc.270.34.20183. [DOI] [PubMed] [Google Scholar]
- 5.Yu M, Deming TJ. Polymeric Materials Science and Engineering. 1998;79:248–249. [Google Scholar]
- 6.Yu M, Deming TJ. Macromolecules. 1998;31(15):4739–4745. doi: 10.1021/ma980268z. [DOI] [PubMed] [Google Scholar]
- 7.Yu M, Hwang J, Deming TJ. Journal of the American Chemical Society. 1999;121(24):5825–5826. [Google Scholar]
- 8.Haemers S, Koper GJM, Frens G. Biomacromolecules. 2003;4(3):632–640. doi: 10.1021/bm025707n. [DOI] [PubMed] [Google Scholar]
- 9.Burzio LA, Waite JH. Biochemistry. 2000;39(36):11147–11153. doi: 10.1021/bi0002434. [DOI] [PubMed] [Google Scholar]
- 10.Waite JH, Qin X. Biochemistry. 2001;40(9):2887–2893. doi: 10.1021/bi002718x. [DOI] [PubMed] [Google Scholar]
- 11.Monahan J, Wilker JJ. Langmuir. 2004;20(9):3724–3729. doi: 10.1021/la0362728. [DOI] [PubMed] [Google Scholar]
- 12.Huang K, Lee BP, Ingram DR, Messersmith PB. Biomacromolecules. 2002;3(2):397–406. doi: 10.1021/bm015650p. [DOI] [PubMed] [Google Scholar]
- 13.Schnurrer J, Lehr CM. Intl J. Pharmaceutics. 1996;141:251–256. [Google Scholar]
- 14.Tatehata H, Mochizuki A, Ohkawa K, Yamada M, Yamamoto H. Journal of Adhesion Science & Technology. 2001;15(9):1003–1013. [Google Scholar]
- 15.Yamamoto H, Kuno S, Nagai A, Nishida A, Yamauchi S, Ikeda K. International Journal of Biological Macromolecules. 1990;12(5):305–310. doi: 10.1016/0141-8130(90)90019-7. [DOI] [PubMed] [Google Scholar]
- 16.Deming TJ. Curr. Opin. Chem. Biol. 1999;3(1):100–105. doi: 10.1016/s1367-5931(99)80018-0. [DOI] [PubMed] [Google Scholar]
- 17.Deming TJ, Yu M, Hwang J. Polymeric Materials Science and Engineering. 1999;80:471–472. [Google Scholar]
- 18.Dalsin JL, Hu B-H, Lee BP, Messersmith PB. Journal of the American Chemical Society. 2003;125(14):4253–4258. doi: 10.1021/ja0284963. [DOI] [PubMed] [Google Scholar]
- 19.Lee H, Scherer NF, Messersmith PB. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(35):12999–13003. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee BP, Dalsin JL, Messersmith PB. Biomacromolecules. 2002;3(5):1038–1047. doi: 10.1021/bm025546n. [DOI] [PubMed] [Google Scholar]
- 21.Guvendiren M, Messersmith PB, Shull KR. Biomacromolecules. 2008;9(1):122–128. doi: 10.1021/bm700886b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brass DA, Shull KR. Langmuir. 2006;22(22):9225–9233. doi: 10.1021/la061793r. [DOI] [PubMed] [Google Scholar]
- 23.Flory AL, Brass DA, Shull KR. Journal of Polymer Science Part B-Polymer Physics. 2007;45:3361–3374. [Google Scholar]
- 24.Fallais I, Devaux J, Jerome R. Journal of Polymer Science Part A-Polymer Chemistry. 2000;38(9):1618–1629. [Google Scholar]
- 25.Chao CY, Carvajal D, Szleifer I, Shull KR. Langmuir. 2008;24(6):2472–2478. doi: 10.1021/la702743v. [DOI] [PubMed] [Google Scholar]
- 26.Guvendiren M, Shull KR. Soft Matter. 2007;3:619–626. doi: 10.1039/b615412c. [DOI] [PubMed] [Google Scholar]
- 27.Webber RE, Shull KR, Roos A, Creton C. Physical Review E: Statistical, Nonlinear, and Soft Matter Physics. 2003;68(2–1):021805/021801–021805/021811. doi: 10.1103/PhysRevE.68.021805. [DOI] [PubMed] [Google Scholar]