Abstract
Purpose of Review
Individuals homozygous for a deletion in the CCR5 gene (CCR5Δ32) are almost completely resistant to HIV-1 infection. A recent report that transplantation of hematopoietic stem/progenitor cells (HSC) from a CCR5Δ32 homozygous donor effectively cured an HIV patient has increased interest in the development of strategies that could be used to recreate this phenotype using a patient’s own cells. This review will focus on recent developments to disrupt CCR5 expression in both autologous T cells and HSC.
Recent Findings
CCR5 expression in HIV-1 target cells can be suppressed by RNA-based gene suppression technologies such as RNA interference, or completely eliminated by zinc finger nuclease (ZFN)-mediated gene disruption. ZFNs bind specifically to a DNA sequence and generate a double-stranded DNA break, whose subsequent repair by the cell’s error-prone non-homologous end-joining pathway can lead to permanent disruption of the gene’s open reading frame. Recent developments in humanized mouse models have facilitated pre-clinical studies that have demonstrated the ability of CCR5-targeted ZFNs to suppress HIV-1 in vivo, when used to modify human T cells or HSC. The same CCR5 ZFNs are now being evaluated in a phase I clinical trial of ex vivo expanded autologous T cells.
Summary
CCR5 gene knockout in T cells or HSC by ZFNs effectively suppresses the replication of CCR5-tropic strains of HIV-1 in animal models. ZFNs are currently being evaluated in a phase I clinical trials using ex vivo expanded T cells and HSC targeted therapies are under development.
Keywords: CCR5, zinc finger nucleases, hematopoietic stem cells, T cells
Introduction
Chemokine receptor 5 (CCR5) is the major co-receptor molecule used by HIV-1 to enter cells [1]. The CCR5Δ32 allele, which produces a prematurely truncated form of the molecule, is associated with profound resistance to HIV-1 infection in homozygotes and better disease outcome in heterozygotes [2,3]. These observations have driven the development of anti-HIV drugs that disrupt the virus-CCR5 interaction, including the first-in-class approved drug, Maraviroc [4*,5].
In complementary approaches, gene therapy strategies to downregulate or eliminate expression of CCR5 are also being developed. The potential of such therapies was given a boost by the recent report that a patient who received an hematopoietic stem/progenitor cell (HSC) transplantation from a CCR5Δ32 donor was able to eliminate all traces of HIV-1 from his body, despite discontinuing antiviral therapy [6**]. While the presence of CCR5-negative donor derived T cells in the recipient was expected to have some anti-HIV effect, the surprising degree of success of this treatment – effectively a cure – indicates that the generation of CCR5-negative cells in an HIV patient may actually be curative.
The morbidity and mortality associated with allogeneic (donor) HSC transplantation, as well as the difficulty of identifying CCR5Δ32 donors with suitable HLA compatibility, mean that this is not practical as a treatment for HIV infection. However, it is possible to mimic this phenotype in autologous (patient’s own) cells through use of variety of gene transfer and gene modification tools, including RNA interference to knockdown CCR5 expression and zinc finger nucleases (ZFNs) to permanently disrupt the CCR5 open-reading frame. The target cells for such modifications are both HSC and mature CD4+ T lymphocytes. This review will focus on recent developments in the use of CCR5-targeted ZFNs that are showing promise in both pre-clinical studies and early phase clinical trials.
CCR5 as an anti-HIV target and the “Berlin patient”
The identification of CCR5 as the major HIV-1 co-receptor provided an explanation for the previously noted HIV-resistance of certain groups of frequently exposed, but uninfected individuals, who were found to carry two copies of the CCR5Δ32 allele [2]. These observations have been subsequently exploited in the development of drugs that target the virus-CCR5 interaction [5], as well as anti-CCR5 monoclonal antibodies [7].
Although CCR5 inhibitors are proving clinically useful [*4], their performance so far did not predict the recent report of a ‘cured’ HIV patient who received an HSC transplantation from a CCR5Δ32 donor [6**]. The patient, a 42yr old HIV-positive man living in Berlin, underwent allogeneic HSC transplantation as part of his treatment for acute myeloid leukemia. His physician screened the German database of bone marrow donors and was able to identify a suitable matched donor who was also homozygous for CCR5Δ32, and the patient received a transplant from this donor, with recurrence of the leukemia requiring a second round of chemotherapy and HSC transplantation from the same donor. Strikingly, at 3 ½ years follow up, no HIV could be detected in the patient [Gero Hütter, pers.comm]. Although other mechanisms are possible, this clinical result suggests that the donor CCR5-negative HSC, and the HIV-resistant T cells that developed from them in vivo, were ultimately able to suppress or prevent HIV replication.
A potentially synergistic role of the ablative regimen that was used, possibly depleting long-lived latent reservoirs in primitive stem/precursor cells, has not been ruled out as a contributing factor in the success of this treatment, although the previous experience of HIV-infected individuals undergoing allogeneic HSC transplantation from donors not selected to be CCR5-negative is that HIV-1 is not eliminated [8–11]. The Berlin patient received high dose chemotherapy to treat his leukemia that consisted of amsacrine, fludarabine, cytarabine and cyclophosphamide. He was also treated with total body irradiation, both to enhance the chemotherapeutic regimen and to eradicate his own HSC and immune system and thereby facilitate engraftment of the allogeneic HSC. Finally, the patient was treated with several agents that are used to prevent graft versus host disease, including antithymocyte globulin (ATG), cyclosporine and mycophenolate mofetil. It is possible that the direct effects of these cytotoxic agents could have reduced or eliminated cellular HIV-1 reservoirs. ATG is particularly immunosuppressive, containing polyclonal antibodies directed against all known lymphocyte subsets [12], so that this agent in particular could have helped to eliminate the HIV reservoir in this patients. Such an effect of ATG would not have been previously appreciated since this potent immunosuppression has rarely, if ever, been intentionally given to patients with chronic HIV-1 infection.
Finally, it is possible that innate or acquired immunity delivered by the donor immune system may have contributed to the elimination of residual HIV reservoirs. The patient had graft versus host disease, and it is possible that an allogeneic immune response directed against host lymphocytes had a purging effect on the HIV reservoir in lymphocytes.
Gene therapy strategies to reduce CCR5 expression
The gene therapy toolbox contains a number of ways in which CCR5 expression can be inhibited in a cell, and thereby mimic a CCR5-negative cell. These can act at the level of RNA, through RNA interference, ribozymes or antisense approaches, or by protein sequestration following CCR5 intrabody expression [13]. Improvements in humanized mouse models that support the generation of human T cells in vivo [14] are driving analyses of their relative efficacies. Recent reports have highlighted the potential of the RNA interference approach to down-regulate CCR5 expression, including the possibility of exploiting in vivo delivery of siRNAs through the use of T cell targeted nanoparticles [15, 16**]. Alternatively, RNA interference can be achieved through the stable expression of shRNAs targeting CCR5 from lentiviral vectors. Transduction of such vectors into human CD34+ HSC allowed HIV resistance to be conferred on both macrophages derived in vitro from the transduced cells [17], as well as T cell progeny that differentiated in vivo in a BLT mouse model [*18]. A targeting strategy to deliver lentiviral vectors expressing an anti-CCR5 shRNA specifically to CCR5+ cells in vivo has been demonstrated using a PBMC transplanted mouse [*19].
Other RNA approaches to CCR5 gene knockdown include CCR5 ribozymes, which are one component of a triple-target RNA-based lentiviral vector therapy that is being delivered to CD34+ HSC from AIDS lymphoma patients undergoing chemotherapy and autologous HSC transplantation as a lymphoma treatment [**20]. The presence of cells expressing the vector at 24 months post-transplantation demonstrates the utility of this approach. This AIDS lymphoma patient population represents a unique cohort with which to evaluate HSC-based anti-HIV gene therapies, since their CD34+ HSC are mobilized and harvested prior to chemotherapy. This both provides an opportunity to engineer HSC with anti-HIV properties, as well as increasing the chances of engraftment of the modified cells following the fully ablative conditioning regimen.
All of the strategies described above face the challenge of achieving and maintaining sufficient levels of anti-CCR5 activity. In addition, in the case of lentiviral vector approaches, there remains the risk of these integrating vectors causing insertional mutagenesis. More recently, methods to achieve permanent CCR5 gene disruption without requiring continuous expression or delivery of a therapeutic gene have been described through the use of ZFNs, which may therefore overcome some of these limitations.
Zinc finger nucleases allow permanent gene knockout
ZFNs are engineered proteins that contain two linked domains, a DNA binding zinc finger protein fused to the endonuclease domain of a type 1 restriction enzyme [21] (Fig. 1). The zinc finger protein domain is an artificial array of zinc finger peptides that confer sequence-specific DNA binding properties. This occurs because 3–4 residues towards the tip of each zinc finger peptide make contact with 3 or 4 base pairs of DNA, and altering the contact residues changes the preference of the finger for a DNA sequence. Linking of multiple zinc fingers extends the length of a DNA sequence that is recognized, and since ZFNs are dimerized via their endonuclease domain, this further extends the length of a targeted sequence. In this way, a ZFN pair can be engineered to target a sequence that is unique in the human genome.
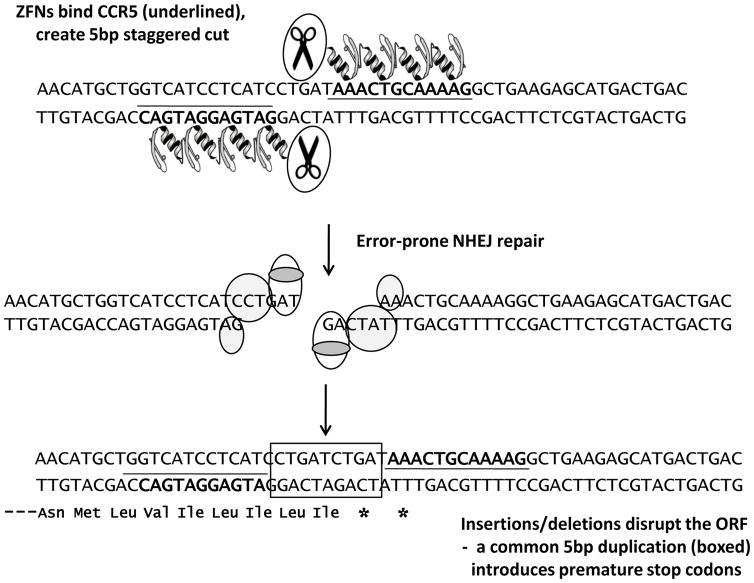
Figure 1. Gene knockout by zinc finger nucleases.
Schematic showing recognition of a specific CCR5 sequence by two ZFNs that subsequently cleave the DNA. Repair by the NHEJ pathway leads to a variety of insertions or deletions at the break site, including the frequent (25%) 5bp duplication [**22,**23] that is depicted and which introduces two premature stop codons in the ORF.
ZFNs can be considered to act like designer restriction enzymes, cutting the DNA at the bound target sequence (Fig. 1). Following this cleavage event, the double-stranded break created is repaired, in mammalian cells, predominantly by the error-prone non-homologous end-joining (NHEJ) pathway. This typically generates a series of small deletions or additions at the break site that can subsequently disrupt the open reading frame (ORF). A pair of ZFNs that target a specific DNA sequence towards the N-terminus of the CCR5 coding sequence have been described [24,**22] and it is known that a particularly common outcome of NHEJ repair of lesions created by this pair is a 5bp duplication at the target site [**22,**23]. This insertion introduces two adjacent stop codons in the ORF that result in premature termination (Fig. 1).
A key feature of ZFN gene editing is that the ZFNs are only required to be expressed during a very short window; once the double stranded break is created, the host repair pathways take over and the ensuing gene editing that occurs following NHEJ repair creates a permanent gene knockout. ZFNs can be delivered to a wide variety of human cells using standard gene delivery techniques and vector systems that produce only transient expression are both effective and may even be preferable. These include adenovirus vectors, non-integrating lentiviral vectors and nucleofection of plasmid DNA [**22,**23, 24].
CCR5 knockout in T cells
ZFN engineering can be used directly with the mature target cells that HIV-1 infects, or applied to the precursor stem/progenitor cells that give rise to these cells. Delivery of ZFNs to T cells has been shown to be especially effective using adenovirus vectors. In preclinical studies, Perez and colleagues [**] reported that adenovirus vector delivery of a CCR5-targeted ZFN pair led to disruption of ~50% of CCR5 alleles in populations of primary human CD4+ T cells. They further demonstrated that the ZFN treatment generated HIV-resistant primary CD4+ T cells that expanded stably in HIV-infected cultures for several weeks, resulting in enrichment of ZFN-generated CCR5-modified cells in the population upon long-term exposure to virus (>50 days). In addition, when the cells were transplanted into an immunodeficient NOD/LtSz-scid IL2R gamma null (NSG) mouse followed by infection with a CCR5-tropic strain of HIV-1, the ZFN-modified T cells preferentially expanded, so that the proportion of modified cells present at the end of the experiment was > 2–3 fold higher in the HIV-infected mice. In other experiments, at 50 days post-infection, they found that mice receiving the ZFN-modified cells had increased numbers of CD4 cells and a statistically significant reduction in viral load in their peripheral blood (P<0.001) compared to mice given control human T cells. These data suggest that, in the presence of HIV-1, ZFN-modified T cells have a selective advantage compared to control, unmodified human T cells. As expected, the suppression of HIV-1 infection was also shown to be specific for CCR5-tropic HIV-1, since the CCR5-modified cells remained susceptible to infection with CXCR4-tropic strains of HIV-1. Thus, mature post-thymic CD4+ cells modified by CCR5-specific ZFNs phenocopy the HIV-1 resistance that has been reported in patients with the naturally occurring CCR5Δ32 allele [2,3].
CCR5 knockout in HSC
Disruption of the CCR5 gene in HSC could potentially create a more long-lasting effect than T cell modifications, with the added advantage that CCR5-negative cells would be generated in all of the lineages that HIV-1 infects, including T cells and macrophages. However, manipulation of HSC is not a straightforward process, since the cells are difficult to maintain in culture without differentiation or loss of viability. An early report described the use of non-integrating lentiviral vectors to deliver ZFNs to CD34+ HSC, but achieved only low efficiency [24]. Alternate delivery methods may be better suited to this cell type and nucleofection of ZFN plasmid DNA has proved to be particularly effective, resulting in an average disruption rate of 17% of the CCR5 alleles [**23]. Importantly, such ZFN-modified HSC retained their “stemness”, being able to engraft NSG mice with the same efficiency as unmanipulated HSC [**23]. In addition, the extent of CCR5 disruption observed in the HSC prior to engraftment was maintained in the mature human cells that differentiated in vivo in the mice, and also persisted through secondary transplantations, indicating that the ZFNs were able to modify true SCID-repopulating stem cells. Finally, sequence analysis of the CCR5 gene in gut mucosal lymphocytes revealed a highly diverse population of gene disruption signatures at the ZFN target site, which is consistent with the modification of a polyclonal population of HSC.
When mice previously transplanted with ZFN-modified HSC were challenged with a CCR5-tropic strain of HIV-1, viral loads in the animal’s peripheral blood were initially high and their human CD4+ T cell levels dropped at similar rates to control animals receiving unmodified HSC. However, by 6 weeks post-infection, the viral loads in the ZFN-treated animals started to decline and their human CD4+ T cell levels had recovered to pre-infection levels. At necropsies performed at 10 or 12 weeks post-infection, HIV-1 was undetectable in the ZFN cohort by quantitative PCR of gut mucosa samples. FACS analysis of the human cells that persisted in the gut mucosa after HIV-1 challenge showed a profound selection for CCR5-negative human cells in the ZFN mice, consistent with a selective advantage of such cells [**23]. A major finding from this work is therefore that HIV infection itself can play an important role in the selection of the CCR5-negative cells, so that even if such cells are only in a minority, they are able to replenish and stabilize T cell populations that would otherwise be destroyed by HIV-1.
Clinical trials of CCR5 targeted ZFNs
Combination antiretroviral therapy has dramatically improved the survival of patients with HIV infection and reduced the frequency of opportunistic infections and cancers. However, its use is associated with significant toxicities, mainly metabolic, that could impact the long-term clinical outcomes of patients, and have a profound impact on their quality of life [25]. In addition, HIV infection presents a significant economic burden, since the discounted, lifetime medical care costs of HIV-1 infection in the US were recently estimated at $278,078 to $316,149 (2005 dollars) for one individual [26]. Such realities are driving the development of eradiation therapies with the goal of long-term, drug-free control of HIV-1
Which patient populations could benefit from treatment with CCR5-knockout autologous CD4+ T cells, and eventually HSC? One scenario is to use this as a salvage therapy in patients with multidrug resistant (MDR) HIV infection. Such patients have high rates of intercurrent infections and hospitalizations, and are at substantial risk for death, with mortality rates of 5.5 per 100 person-years and a 3-year mortality risk of 15.3% [27]. A second patient population that may benefit from the HSC modification are individuals already undergoing autologous HSC transplantation during treatment for malignancies, such as the cohort of AIDS lymphoma patients at City of Hope (Duarte, California), undergoing lentiviral vector therapy [**20].
The therapeutic benefit of CCR5 knockout T cells is currently being evaluated in a phase I clinical trial sponsored by Sangamo Biosciences (Richmond, California), in conjunction with investigators at the University of Pennsylvania. The protocol, titled “Pilot test of adoptive transfer of CCR5 deleted CD4 T cells using ZFNs to introduce HIV resistance” is evaluating the safety and feasibility of autologous CD4+ T cell infusions modified by CCR5-specific ZFNs delivered with an adenovirus vector (designated SB-728). The principal investigator for this trial is Dr. Pablo Tebas, and details of the protocol design can be found at clinicaltrials.gov NCT00842634. An overview of the clinical trial is shown in Fig. 2. Three cohorts of patients are being enrolled on the protocol: 1) patients with MDR HIV infection; 2) patients with well controlled HIV infection who have reconstituted their immune system to normal CD4 levels, and 3) patients with HIV infection who have drug-responsive HIV infection but have failed to reconstitute peripheral blood CD4+ cells. This last cohort of patients have increased mortality rates [28] and may have a specific benefit from reconstitution with CCR5 knockout CD4+ T cells. The clinical trial is scheduled to be completed in 2011.
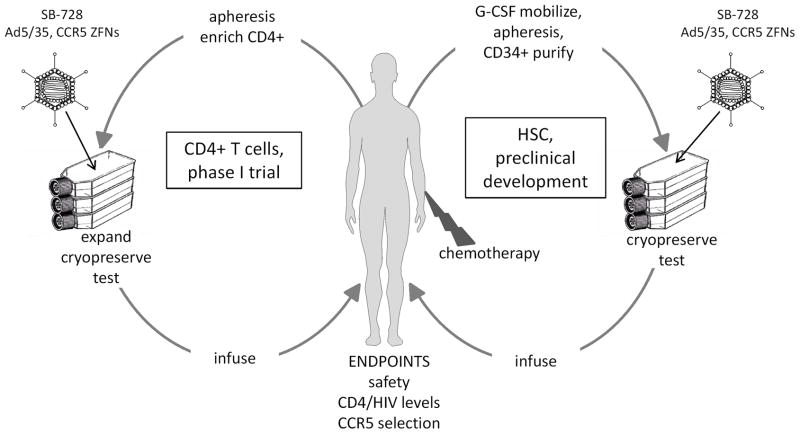
Figure 2. Clinical applications of CCR5 ZFNs.
CCR5 knockout in autologous T cells or HSC can be achieved by transduction with an Ad5/35 vector (SB-728) expressing left and right ZFNs, linked by a self-cleaving protease 2A sequence. In an ongoing phase I clinical trial, the CCR5 modified T cells are expanded ex vivo using antibodies to CD3 and CD28 for approximately 10 days and adoptively transferred to the patient (protocol NCT00842634). Also depicted is a possible clinical trial in AIDS lymphoma patients using autologous CD34+ HSC, purified from peripheral blood following G-CSF mobilization. Chemotherapy is given as part of the lymphoma treatment and also assists the engraftment of the modified HSC.
Conclusions and implications
The CCR5 co-receptor is an important target for HIV-1 therapies. It is a cellular protein that cannot be mutated, in contrast to viral targets that rapidly mutate in response to immune and drug pressures. In addition, the observation of naturally occurring HIV-resistance in CCR5Δ32 individuals, as well as the proof of principle of the “Berlin patient”, suggests that the acquisition of CCR5-negative cells post-infection has the potential to be curative. Technologies are now available to both knock down and knock out CCR5 expression. The positive results from pre-clinical studies using ZFNs are driving the development of autologous treatments for patients that aim to replicate the phenotype of CCR5Δ32 individuals and allow patients to control HIV-1 in the absence of drugs. At the same time, complimentary approaches are also being developed using RNA interference to target CCR5. It will be interesting to see whether a knock out is better than a knock down.
Acknowledgments
Funding:
Paula Cannon - National Institutes of Health P01 HL73104, the California HIV/AIDS Research Program ID07-CHLA-133 and the California Institute for Regenerative Medicine DR1-01490.
Carl June - National Institutes of Health 1U19AI082628
Contributor Information
Paula Cannon, Email: pcannon@usc.edu, Molecular Microbiology & Immunology, University of Southern California Keck School of Medicine, 2011 Zonal Avenue, HMR502, Los Angeles, CA 90033, (323) 442 1510 (office).
Carl June, Email: cjune@exchange.upenn.edu, Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, Room 554 BRB II/III, 421 Curie Blvd, Philadelphia, PA. 19104-6160, (215)-573-3269 phone.
References
- 1.Wu L, Gerard NP, Wyatt R, et al. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;6605:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 2.Samson M, Libert F, Doranz BJ, et al. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;6593:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 3.Chalmet K, Van Wanzeele F, Demecheleer E, et al. Impact of Delta 32-CCR5 heterozygosity on HIV-1 genetic evolution and variability--a study of 4 individuals infected with closely related HIV-1 strains. Virology. 2008;379:213–222. doi: 10.1016/j.virol.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 4*.Hardy WD, Gulick RM, Mayer H, et al. Two-Year safety and virologic efficacy of Maraviroc in treatment-experienced patients with CCR5-tropic HIV-1 infection: 96-week combined analysis of MOTIVATE 1 and 2. J Acquir Immune Defic Syndr. 2010 Aug 11; doi: 10.1097/QAI.0b013e3181ee3d82. [Epub ahead of print]. Results from 2 year follow up of over 1,000 treatment-experienced patients enrolled in 2 prospective studies of Maraviroc safety and efficacy. Durable anti-HIV responses were observed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westby M, van der Ryst E. CCR5 antagonists: host-targeted antiviral agents for the treatment of HIV infection, 4 years on. Antivir Chem Chemother. 2010;20:179–192. 6. doi: 10.3851/IMP1507. [DOI] [PubMed] [Google Scholar]
- 6**.Hütter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. Case study report of an HIV-1 infected AML patient who received a stem cell transplantation from a homozygous CCR5Δ32 donor. No HIV-1 could be detected in the patient at 20 months follow-up, despite discontinuation of anti-retroviral drugs. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson JM, Lalezari JP, Thompson MA, et al. Phase 2a study of the CCR5 monoclonal antibody PRO 140 administered intravenously to HIV-infected adults. Antimicrob Agents Chemother. 2010;54:4137–4142. doi: 10.1128/AAC.00086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avettand-Fenoel V, Mahlaoui N, Chaix ML, et al. Failure of bone marrow transplantation to eradicate HIV reservoir despite efficient HAART. AIDS. 2007;21:776–777. doi: 10.1097/QAD.0b013e3280b01836. [DOI] [PubMed] [Google Scholar]
- 9.Kamp C, Wolf T, Bravo IG, et al. Decreased HIV diversity after allogeneic stem cell transplantation of an HIV-1 infected patient: a case report. Virol J. 2010;7:55. doi: 10.1186/1743-422X-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolf T, Rickerts V, Staszewski S, et al. First case of successful allogeneic stem cell transplantation in an HIV-patient who acquired severe aplastic anemia. Haematologica. 2007;92:e56–8. doi: 10.3324/haematol.11394. [DOI] [PubMed] [Google Scholar]
- 11.Woolfrey AE, Malhotra U, Harrington RD, et al. Generation of HIV-1-specific CD8+ cell responses following allogeneic hematopoietic cell transplantation. Blood. 2008;112:3484–3487. doi: 10.1182/blood-2008-05-157511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rebellato LM, Gross U, Verbanac KM, Thomas JM. A comprehensive definition of the major antibody specificities in polyclonal rabbit antithymocyte globulin. Transplantation. 1994;57:685–694. doi: 10.1097/00007890-199403150-00010. [DOI] [PubMed] [Google Scholar]
- 13.Swan CH, Buhler B, Steinberger P, et al. T-cell protection and enrichment through lentiviral CCR5 intrabody gene delivery. Gene Ther. 2006;13:1480–1492. doi: 10.1038/sj.gt.3302801. [DOI] [PubMed] [Google Scholar]
- 14.Denton PW, Garcia JV. Novel humanized murine models for HIV research. Curr HIV/AIDS Rep. 2009;6:13–19. doi: 10.1007/s11904-009-0003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar P, Ban HS, Kim SS, et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell. 2008;134:577–586. doi: 10.1016/j.cell.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16**.Kim SS, Peer D, Kumar P, et al. RNAi-mediated CCR5 silencing by LFA-1-targeted nanoparticles prevents HIV infection in BLT mice. Mol Ther. 2010;18:370–376. doi: 10.1038/mt.2009.271. Nanoparticles carrying siRNA against CCR5 were targeted to leucocytes via an antibody to the LFA-1 integrin. Selective uptake by T cells and macrophages was demonstrated. Humanized BLT mice treated with the nanoparticles had reduced HIV loads and CD4+ T cell loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang M, Kamata M, Chen KN, et al. Inhibition of HIV-1 infection by a unique short hairpin RNA to chemokine receptor 5 delivered into macrophages through hematopoietic progenitor cell transduction. J Gene Med. 2010;12:255–265. doi: 10.1002/jgm.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.Shimizu S, Hong P, Arumugam B, et al. A highly efficient short hairpin RNA potently down-regulates CCR5 expression in systemic lymphoid organs in the hu-BLT mouse model. Blood. 2010;115:1534–1544. doi: 10.1182/blood-2009-04-215855. Lentiviral vectors expressing an anti-CCR5 shRNA were used to transduce human CD34+ HSC. The cells showed no adverse effects and were able to engraft a BLT mouse model. Splenocytes that differentiated in vivo from the transduced HSC were HIV resistant when challenged ex vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19*.Anderson JS, Walker J, Nolta JA, et al. Specific transduction of HIV-susceptible cells for CCR5 knockdown and resistance to HIV infection: a novel method for targeted gene therapy and intracellular immunization. J Acquir Immune Defic Syndr. 2009;52:152–161. doi: 10.1097/QAI.0b013e3181b010a0. CCR5+ cells were targeted in vitro and in vivo in a humanized mouse using lentiviral vectors conjugated to a CCR5 monoclonal antibody. The vectors delivered a CCR5 specific shRNA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20**.DiGiusto DL, Krishnan A, Li L, et al. RNA-based gene therapy for HIV with lentiviral vector-modified CD34(+) cells in patients undergoing transplantation for AIDS-related lymphoma. Sci Transl Med. 2010;2:36ra43. doi: 10.1126/scitranslmed.3000931. Describes a clinical trial of genetically modified autologous HSC in a cohort of AIDS lymphoma patients undergo ablative chemotherapy. The HSC were modified using lentiviral vectors expressing three anti-HIV genes, including a CCR5 ribozyme. Gene marked cells were observed at 24 months post-transplantation. Some evidence to indicate that viremia drives increased gene marking, suggesting a protective effect of the therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urnov FD, Rebar EJ, Holmes MC, et al. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010;9:636–646. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- 22**.Perez EE, Wang J, Miller JC, et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol. 2008;26:808–816. doi: 10.1038/nbt1410. First report that ZFN-modified human T cells can suppress HIV-1 replication. Adenovirus vector delivery of CCR5-specific ZFNs to human T cells resulted in the disruption of ~50% of the CCR5 alleles and produced a corresponding anti-HIV effect in cell culture and a T cell humanized mouse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23**.Holt N, Wang J, Kim K, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol. 2010;28:839. doi: 10.1038/nbt.1663. The paper describes efficient ZFN editing of a gene (CCR5) in HSC. CCR5-negative progeny that developed in vivo from the modified HSC in a humanized mouse model where able to potently suppress HIV-1 replication and protect human T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lombardo A, Genovese P, Beausejour CM, et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat Biotechnol. 2007;5:1298–1306. doi: 10.1038/nbt1353. [DOI] [PubMed] [Google Scholar]
- 25.Tebas P. Interventions for body habitus changes associated with HIV infection and its treatment. Curr HIV/AIDS Rep. 2007;4:86–92. doi: 10.1007/s11904-007-0013-x. [DOI] [PubMed] [Google Scholar]
- 26.Schackman BR, Gebo KA, Walensky RP, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Medical Care. 2006;44:990–997. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- 27.Ledergerber B. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. The Lancet. 2004;364:51–62. doi: 10.1016/S0140-6736(04)16589-6. [DOI] [PubMed] [Google Scholar]
- 28.Grabar S, Moing V, Goujard C, et al. Clinical outcome of patients with HIV-1 infection according to immunologic and virologic response after 6 months of highly active antiretroviral therapy. Annals of Internal Medicine. 2000;133:401–410. doi: 10.7326/0003-4819-133-6-200009190-00007. [DOI] [PubMed] [Google Scholar]