Abstract
OBJECTIVE
To assess the biochemical outcome after radical prostatectomy (RP) specifically for men aged 30–39 years, as previous studies suggest that prostate cancer in young men might be more aggressive.
PATIENTS AND METHODS
From a large (15 899) database of RPs (1975–2007) we identified 42 men aged 30–39, 893 aged 40–49, 4085 aged 50–59, 3766 aged 60–69, and 182 men aged ≥70 years old. The clinical characteristics and treatment outcomes were compared between men aged 30–39 years and older men.
RESULTS
Among the men in their thirties, 81% had organ-confined disease in the RP specimen, vs 62% of men aged ≥40 years. At a mean follow-up of 5 years, there was biochemical progression in 4.8% of men in their thirties and 16.1% of men age ≥40 years (P = 0.055). The corresponding 5-year biochemical progression-free survival estimates were 95% for men in their thirties and 83% for men aged ≥40 years (P = 0.045). On multivariate analysis, increasing age was a significant independent predictor of biochemical progression.
CONCLUSION
Contrary to earlier reports, in the present study men in their thirties did not have more aggressive disease. Instead, they had more favourable pathological features and progression-free survival rates than their older counterparts. After controlling for other prognostic variables on multivariate analysis, being in the fourth decade was independently associated with a lower risk of biochemical progression. These results suggest that early aggressive treatment for these patients with a long life-expectancy is associated with favourable long-term biochemical outcomes.
Keywords: prostate cancer, radical prostatectomy, age, thirty, treatment outcomes
INTRODUCTION
In 2007, there will be 218 890 new cases of prostate cancer diagnosed and an estimated 27 050 prostate cancer-related deaths in the USA [1]. Prostate cancer has traditionally been regarded as a disease of older men, but according to data from the Surveillance, Epidemiology, and End Results (SEER) database, the earliest cancer-related deaths are in men aged 35–44 years [2].
Several previous studies compared the tumour features and treatment outcomes between younger men and those aged >50 years [3,4]. However, these studies included few or no men aged <40 years, and overall there are limited published data specifically addressing the subgroup of men in their fourth decade.
Our institution previously reported on 87 men aged <40 years who had a prostate biopsy, of whom 16 ultimately had a radical prostatectomy (RP) [5]. Contrary to earlier reports suggesting that young men might have more aggressive disease, in these few patients there did not appear to be a uniformly poor prognosis. In the present study, our goal was to re-examine these results in a larger population of men in their thirties, and to compare their treatment outcomes with older groups, controlling for known prognostic variables.
PATIENTS AND METHODS
From June 1975 to August 2007, 15 899 men with a documented age had a RP at Johns Hopkins Hospital, including 55 (0.4%) aged 30–39, 1496 (9.4%) aged 40–49, 7295 (45.9%) aged 50–59, 6705 (42.2%) aged 60–69, and 348 (2.2%) aged ≥70 years. Of these men, three, 45, 218, 289 and 18 in the respective decades received hormonal or radiation therapy before RP and were excluded. Of the remaining 15 326 patients, follow-up information was available for 8968, who formed the final study population. The study was approved by the institutional review board and complied with the Health Insurance Portability and Accountability Act. All participants provided informed consent.
The clinical and pathological features were compared among the different age decades using the chi-square and Fisher’s exact tests. Biochemical progression was defined as one PSA level of ≥0.2 ng/mL after RP. Biochemical progression-free survival (PFS) curves were created using the Kaplan-Meier method, and were compared using the log-rank test. Finally, Cox proportional hazards regression was used for multivariate analysis.
RESULTS
The final study population included 42 (0.5%), 893 (10.0%), 4085 (45.6%), 3766 (42.0%) and 182 (2.0%) in the respective decades. Table 1 shows the clinical and pathological characteristics of all men, stratified by age decade. In the RP specimen there were significant differences between the age groups in the proportion with organ-confined disease, positive surgical margins, capsular penetration, seminal vesicle invasion and lymph node metastases.
TABLE 1.
The clinical and pathological features of the patients, stratified by age decade
| Age, years | |||||
|---|---|---|---|---|---|
| Variable | 30–39 | 40–49 | 50–59 | 60–69 | ≥70 |
| Preoperative PSA level, ng/mL | |||||
| Mean (SD) | 6.8 (10.7) | 6.9 (6.7) | 7.3 (5.9) | 7.9 (7.4) | 8.8 (7.6) |
| Median (range) | 4.0 (0.3–66) | 5.4 (0.2–79) | 5.8 (0.1–68) | 6.2 (0.1–151) | 7.0 (0.7–49.9) |
| n (%): | |||||
| Biopsy Gleason score | |||||
| ≤6 | 36 (85.7) | 744 (84.3) | 3242 (79.8) | 2757 (74) | 126 (70.4) |
| 7 | 4 (9.5) | 123 (13.9) | 697 (17.2) | 798 (21.5) | 40 (22.3) |
| 8–10 | 2 (4.8) | 16 (1.8) | 123 (3.0) | 166 (4.5) | 13 (7.3) |
| Clinical stage | |||||
| ≤T1c | 26 (61.9) | 523 (58.8) | 2543 (62.3) | 2202 (58.7) | 95 (53.7) |
| T2 | 16 (38.1) | 353 (39.7) | 1492 (36.6) | 1517 (40.4) | 82 (46.3) |
| T3 | 0 | 13 (1.5) | 44 (1.1) | 30 (0.8) | 0 |
| D0 | 0 | 0 | 0 | 2 (0.1) | 0 |
| Organ-confined | 34 (81.0) | 616 (69.1) | 2654 (65.2) | 2133 (56.8) | 90 (49.7) |
| Positive surgical margins | 2 (4.8) | 100 (11.2) | 536 (13.1) | 607 (16.1) | 38 (20.9) |
| Capsular penetration | 8 (19.1) | 272 (30.5) | 1386 (33.9) | 1587 (42.1) | 87 (47.8) |
| Seminal vesicle invasion | 1 (2.4) | 43 (4.8) | 224 (5.5) | 274 (7.3) | 21 (11.6) |
| Lymph node metastases | 1 (2.4) | 32 (3.6) | 124 (3.0) | 146 (3.9) | 8 (4.4) |
Table 2 compares men in their thirties with the combined group of all men aged ≥40 years. Before RP, the two groups had a similar clinical stage, biopsy Gleason score, and proportion of men with a PSA level of ≥10 ng/mL. In the RP specimen, men in their thirties were significantly more likely to have organ-confined disease (P = 0.011) and less likely to have capsular penetration (P = 0.014).
TABLE 2.
A comparison of characteristics between men aged 30–39 years and older men
| Variable, n (%) | 30–39 | ≥40 | P |
|---|---|---|---|
| Preop. PSA level ≥ 10 ng/mL | 6 (14.6) | 1619 (18.9) | 0.488 |
| Biopsy Gleason score ≥ 7 | 6 (14.3) | 1976 (22.1) | 0.178 |
| Clinical stage ≥ T2 | 16 (38.1) | 3533 (39.6) | 0.810 |
| Organ-confined | 34 (81.0) | 5493 (61.7) | 0.011 |
| Positive surgical margins | 2 (4.8) | 1281 (14.4) | 0.079 |
| Capsular penetration | 8 (19.1) | 3332 (37.3) | 0.014 |
| Seminal vesicle invasion | 1 (2.4) | 562 (6.3) | 0.519 |
| Lymph node metastases | 1 (2.4) | 310 (3.5) | 1.0 |
| Biochemical progression | 2 (4.8) | 1438 (16.1) | 0.055 |
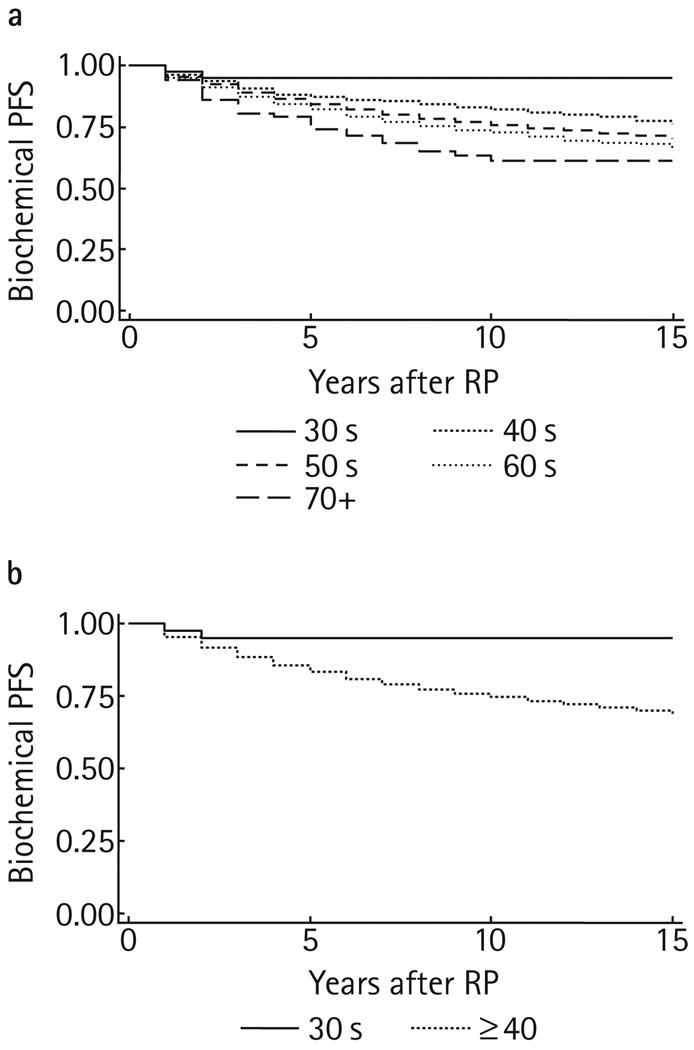
At a mean follow-up of 5.1 years (median 3, range 1–25), 1440 (16.1%) men had biochemical progression. This included two (4.8%), 103 (11.5%), 607 (14.9%), 684 (18.2%) and 44 (24.2%) in the respective decades. Figure 1 shows the Kaplan-Meier PFS curves, stratified by age group. The 5-year PFS rate (95% CI) was 94.8 (80.8–98.7)% for men in their thirties, compared to 83.3 (82.3–84.2)% for older men (P = 0.045). Only five men in their thirties had a follow-up extending to 10 years after RP, and there was no biochemical progression among the group, leading to a flattening in the Kaplan-Meier curve. Among 1973 men aged ≥40 years with 10-year follow-up data, the 10-year PFS rate was 73 (71.0–74.9)%.
FIG. 1.
Kaplan-Meier PFS curves, stratified by age.
Table 3 shows the results of the multivariate analysis for predicting biochemical progression. After adjusting for the preoperative PSA level, biopsy Gleason score, clinical stage and adjuvant therapy, age 30–39 years remained independently associated with a lower risk of biochemical progression.
TABLE 3.
The multivariate model for predicting biochemical progression using clinical variables
| Variable | Odds ratio (95% CI) | P |
|---|---|---|
| Age (30–39 vs >40) | 4.3 (1.1–17.0) | 0.041 |
| Biopsy Gleason score | 2.6 (2.4–2.8) | <0.001 |
| Preop. PSA level (<10 vs ≥10 ng/mL) | 2.5 (2.2–2.8) | <0.001 |
| Clinical stage | 1.7 (1.5–1.8) | <0.001 |
| Adjuvant therapy | 1.0 (0.6–1.6) | 0.951 |
DISCUSSION
Based on data from 2001 to 2003, 17% of men in the USA will develop prostate cancer in their lifetime [1]. However, the risk is correlated directly with age. From birth until age 39 years, only one in 10 373 men will develop prostate cancer, compared to one in 39 aged 40–59, one in 14 aged 60–69 and one in seven aged ≥70 years. According to the SEER database, 0.1%, 1.3%, 6.6%, 20.8%, 41.8% and 29.3% of prostate cancer deaths occur in men aged 35–44, 45–54, 55–64, 65–74, 75–84 and ≥85 years, respectively [2].
Because prostate cancer is relatively rare in men aged <40 years there are few data on the pathological features and treatment outcomes in younger men. Furthermore, most of the previously published studies only reported the statistics for men aged <50 years as a group, presumably because there were insufficient men in their thirties to report on this group separately. For example, Khan et al. [3] previously compared the outcomes of RP between 341 men aged ≤50 years and 2556 older men at a mean follow-up of 5.7 years. In that series, the younger men had significantly more favourable pathology features in the RP specimen. Moreover, the 5-, 10- and 15- year PFS rates for men aged <50 years were 88%, 81% and 69%, respectively. These rates were similar to men aged 50–59, slightly better than men aged 60–69 and significantly better than men aged ≥70 years.
Similar results were reported by Freedland et al. [4], who compared the outcomes of RP between 88 men aged <50 years and 1665 older men treated at five equal-access centres from 1988 to 2002. Using a Cox proportional hazards model, an age of ≤50 years was a significant independent predictor of a longer time to biochemical recurrence, after controlling for PSA level, pathological tumour features and the year of surgery.
Other studies failed to show any difference in outcomes between men aged <50 years with prostate cancer and older men. For instance, Twiss et al. [6] reported the pathology features and functional outcomes in 66 men aged <50 years (3% were <40 years old) and 724 older men who had RP by one surgeon. In that study, the mean preoperative PSA level, biopsy Gleason score and clinical stage were similar between the groups. Likewise, on the final pathology there were no significant differences in pathological stage, percentage of cancer, positive surgical margins, or prostatectomy Gleason score.
In the present study, men in their thirties had significantly more favourable pathological tumour features and a lower risk of biochemical progression. Moreover, on multivariate analysis controlling for differences in other clinical variables, increasing age was significantly associated with a greater risk of progression. These results are encouraging, particularly considering the long life-expectancy in these patients.
In the Scandinavian Prostate Cancer Group Study no. 4 [7], RP was associated with a significantly higher cancer-specific survival rate than was watchful waiting primarily in men aged <65 years. Taken together, these findings suggest that definitive treatment (e.g. surgery or radiation therapy) is warranted and likely to be associated with favourable long-term cancer control outcomes for young men diagnosed with prostate cancer.
There are several limitations of the present study: because this is a surgical series, there is a selection bias; men in their thirties who had RP at our institution might not reflect the full spectrum of prostate cancer seen in this age group. Lead-time and length-time biases are also possible. However, selection bias might have been greatest in the oldest men, which could have led to an underestimate of the protective benefit associated with younger age.
Also, to our knowledge this represents the largest separate report of RP outcomes for men in their thirties. That notwithstanding, there were relatively few men and the subgroup in their thirties had more limited follow-up information. Thus, additional studies are needed to further examine the long-term outcomes of prostate cancer therapy in this growing population of patients.
In conclusion, contrary to historical accounts of more aggressive prostate cancer in young men, we found that men in their thirties had more favourable RP pathology and a better 5-year PFS rate than men aged ≥40 years. Controlling for other prognostic variables in a multivariate model, men in their thirties continued to have a lower risk of progression than older patients. These results suggest that surgical management is a viable option for men diagnosed with prostate cancer in their thirties, as it is associated with favourable long-term cancer control outcomes in selected patients.
ACKNOWLEDGEMENTS
Financial disclosures: Supported by the National Institutes of Health/National Cancer Institute SPORE Grant #3P50CA058236.
Abbreviations
- RP
radical prostatectomy
- PFS
progression-free survival
- SEER
Surveillance, Epidemiology, and End Results
Footnotes
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.American Cancer Society. [Accessed 5 May 2007];Cancer Facts and Figures. Available at: http://www.cancer.org/downloads/STT/CAFF2007PWSecured.pdf.
- 2.Anonymous. Age Distribution of Deaths by Site, 2000–2004. [Accessed 28 July 2007];Surveillance, Epidemiology and End Results (SEER) Program. Available at: http://seer.cancer.gov/csr/1975-2004/results_single/sect_01_table.12-2pgs.pdf.
- 3.Khan MA, Han M, Partin AW, Epstein JI, Walsh PC. Long-term cancer control of radical prostatectomy in men younger than 50 years of age: update. Urology. 2003;62:86–91. doi: 10.1016/s0090-4295(03)00404-7. [DOI] [PubMed] [Google Scholar]
- 4.Freedland SJ, Presti JC, Jr, Kane CJ, et al. Do younger men have better biochemical outcomes after radical prostatectomy? Urology. 2004;63:518–522. doi: 10.1016/j.urology.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 5.Ruska KM, Partin AW, Epstein JI, Kahane H. Adenocarcinoma of the prostate in men younger than 40 years of age: diagnosis and treatment with emphasis on radical prostatectomy findings. Urology. 1999;53:1179–1183. doi: 10.1016/s0090-4295(99)00020-5. [DOI] [PubMed] [Google Scholar]
- 6.Twiss C, Slova D, Lepor H. Outcomes for men younger than 50 years undergoing radical prostatectomy. Urology. 2005;66:141–146. doi: 10.1016/j.urology.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 7.Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352:1977–1984. doi: 10.1056/NEJMoa043739. [DOI] [PubMed] [Google Scholar]