Abstract
Context: No consensus exists for management of adults with congenital adrenal hyperplasia (CAH) due to a paucity of data from cohorts of meaningful size.
Objective: Our objective was to establish the health status of adults with CAH.
Design and Setting: We conducted a prospective cross-sectional study of adults with CAH attending specialized endocrine centers across the United Kingdom.
Patients: Participants included 203 CAH patients (199 with 21-hydroxylase deficiency): 138 women, 65 men, median age 34 (range 18–69) years.
Main Outcome Measures: Anthropometric, metabolic, and subjective health status was evaluated. Anthropometric measurements were compared with Health Survey for England data, and psychometric data were compared with appropriate reference cohorts.
Results: Glucocorticoid treatment consisted of hydrocortisone (26%), prednisolone (43%), dexamethasone (19%), or a combination (10%), with reverse circadian administration in 41% of patients. Control of androgens was highly variable with a normal serum androstenedione found in only 36% of patients, whereas 38% had suppressed levels suggesting glucocorticoid overtreatment. In comparison with Health Survey for England participants, CAH patients were significantly shorter and had a higher body mass index, and women with classic CAH had increased diastolic blood pressure. Metabolic abnormalities were common, including obesity (41%), hypercholesterolemia (46%), insulin resistance (29%), osteopenia (40%), and osteoporosis (7%). Subjective health status was significantly impaired and fertility compromised.
Conclusions: Currently, a minority of adult United Kingdom CAH patients appear to be under endocrine specialist care. In the patients studied, glucocorticoid replacement was generally nonphysiological, and androgen levels were poorly controlled. This was associated with an adverse metabolic profile and impaired fertility and quality of life. Improvements in the clinical management of adults with CAH are required.
Adult patients with congenital adrenal hyperplasia (CAH) have poor subjective health status and only a minority of CAH adults receives regular review by endocrine specialists.
Congenital adrenal hyperplasia (CAH) is the commonest genetic endocrine disorder (1). Mutations in the 21-hydroxylase gene, a key enzyme in cortisol and aldosterone synthesis, account for 95% of cases (2,3). 21-Hydroxylase deficiency has an incidence of one in 12,000 live births for severe mutations causing classic CAH, which manifests in the neonatal period or during childhood, one in 2500 for milder mutations causing nonclassic CAH manifesting during adolescence or early adulthood (2,3). In CAH, failure in cortisol synthesis results in reduced cortisol feedback and consequently increased pituitary ACTH release, which promotes oversecretion of 17-hydroxyprogesterone (17OHP), progesterone, and adrenal androgens. This results in androgen excess that clinically manifests with disordered sex development in girls, precocious pseudopuberty, and short stature and in adulthood with hirsutism in females and fertility problems in both sexes.
Glucocorticoid replacement in CAH aims both to replace cortisol and prevent ACTH-driven androgen excess. This is challenging, because therapy aiming to normalize ACTH and reduce androgen levels frequently results in excess glucocorticoid exposure with associated complications including short stature, obesity, hypertension, and an adverse metabolic profile (4,5). Striking the balance between too much and too little glucocorticoid treatment is especially difficult because currently available glucocorticoid formulations cannot replicate the physiological circadian rhythm of cortisol secretion (6,7,8).
CAH is a life-long chronic disorder. In childhood, treatment focuses on issues of gender assignment, genital surgery, and optimization of growth and pubertal development (2,3). Priorities change with increasing age (4,5,9), typically focusing on fertility in early adult life and prevention of metabolic syndrome and osteoporosis in middle and older age, respectively.
Children with CAH are managed using established guidelines (10) recommending a multidisciplinary team approach including a pediatric endocrinologist, geneticist, urologist, gynecologist, psychologist, and specialist nurses. No such consensus exists for the management of adults with CAH. There is a paucity of data from cohorts of meaningful size, with previous reports focused on relatively small numbers of patients under the care of single treatment centers (11,12,13,14). Therefore we performed a large, multicenter cross-sectional study assessing the subjective and objective health status of adults with CAH in the United Kingdom.
Patients and Methods
Patient recruitment
Leading up to this study, the Society for Endocrinology sent a questionnaire to all United Kingdom centers providing specialist endocrine care (n = 30). Replies were received from 27 centers (90%), indicating active involvement in the care of CAH adults, with approximately one third of consultants looking after either fewer than 10, 10–20, or more than 20 adult CAH patients. All centers were asked whether they were interested to participate in a nationwide audit. Seventeen centers, including all that reported more than 20 adult CAH patients under their care, agreed to participate. The United Kingdom Congenital Adrenal Hyperplasia Adult Study Executive (CaHASE) was formed and the study protocol approved by West Midlands MREC (MREC/03/7/086) and registered with www.ClinicalTrials.gov (NCT00749593). All centers contacted adult patients (18 yr or older) with a confirmed diagnosis of CAH currently under their care; recruitment started August 2004 and ended July 2007. All participants gave written informed consent.
Procedures
Participants attended the research unit of their respective center after an overnight fast and having taken their regular medication, followed by medical history, physical examination, and fasting blood sampling with the patient seated after being erect. After breakfast, patients completed psychometric questionnaires.
Physical examination included blood pressure (three seated and one standing, separated by 5 min), height, weight, waist circumference, presence and degree of stretch marks, and in female patients hirsutism and in males testicular volume (orchidometer) and palpation for masses.
Biochemical assessment included renal and liver function, fasting plasma glucose, serum insulin, cholesterol (total, low-density lipoprotein, and high-density lipoprotein), triglycerides, plasma renin activity, and steroid hormones including 17OHP, androstenedione, testosterone, and SHBG. The median (interquartile range) for the time from intake of last glucocorticoid dose until blood sampling was 3.75 (2.25–12.5) hours. Measurements were performed at local laboratories and results recorded as either below, within, or above the local reference range, apart from 17OHP, androstenedione, and plasma renin where absolute values were compared with previously recommended target ranges for patients with CAH and adrenal insufficiency (1,5). All laboratories participate in the United Kingdom National External Quality Assessment Service scheme for quality control of steroid immunoassays. Serum insulin was measured centrally using an ultrasensitive ELISA (DRG Instruments, Marburg, Germany) and used for calculation of homeostasis model assessment of insulin resistance (15). Bone mineral density (BMD) assessed by dual x-ray absorptiometry and testicular ultrasound scans performed within the last 5 yr were recorded.
Psychometric evaluation was carried out employing self-administered questionnaires, including three validated questionnaires for the assessment of subjective health status [36-item short-form health survey (SF-36) (16,17)], mood [Hospital Anxiety and Depression Scale (HADS) (18)], and sexuality [five-item version of the International Index of Erectile Function (IIEF-5) in men (19) and the nine-item short form of the McCoy Female Sexuality Questionnaire (MFSQ-9) in women (20)] and a disease-specific questionnaire (CAH Wellbeing) designed as a four-level Likert scale for this study (see Supplemental Appendix, published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org).
Statistical analysis
Data from anonymized clinical case record forms were entered into a central database. Differences between subgroups were tested using t tests for comparison of means of continuous variables and χ2 tests for categorical variables. Sex-specific comparisons of anthropometric and blood pressure data with the general population were made using age-weighted data for CAH participants and data from Health Surveys for England (HSE) (2006 data for anthropometric comparison and 2003 data for blood pressure comparisons because blood pressure data were not available in the 2006 survey) (21,22).
Reference data for SF-36 scores were obtained from John Brazier (University of Sheffield, Sheffield, UK) comprising a representative random sample of 14,430 subjects from the United Kingdom population aged between 18 and 79 yr. HADS reference data (n = 2081) were obtained from Andreas Hinz (University of Leipzig, Leipzig, Germany). For every CAH patient, 20 (SF-36) or five (HADS) sex- and age-matched controls were randomly selected from the respective reference samples for comparison. Adjustment for age and sex was performed by transformation of score values from patients and controls into age-adjusted (decade) and sex-adjusted z-scores, with subsequent comparison by Mann-Whitney U test. Analyses were performed using Stata version 10 (College Station, TX) and SPSS version 13.0 (SPSS Inc., Chicago, IL). Statistical significance was assumed when P < 0.05.
Results
Patient cohort
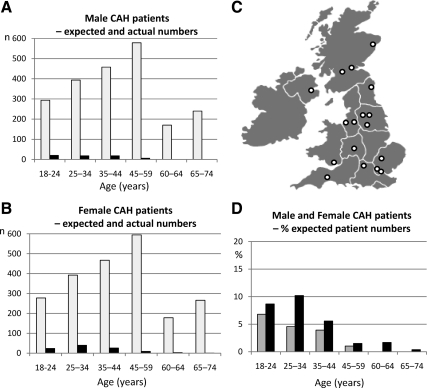
A total of 373 adult CAH patients were contacted, and 203 (54%) agreed to participate. Based on United Kingdom population data (Office of National Statistics) and a presumed classic CAH prevalence of one in 12,000 (2,3), we would expect 3591 adults with classic CAH between ages of 18 and 74 yr (1778 men and 1813 women). Thus, the number of patients recruited represents only a small fraction of the expected number, with overall capture rates as low as 5 and 3% for female and male classic CAH patients, respectively (Fig. 1). The incidence of nonclassic CAH is much greater but variable between populations and is unknown in the United Kingdom; however, recruitment for these patients was very much lower than for patients with classic CAH.
Figure 1.
Expected and recruited number of CAH patients in the United Kingdom. Age distribution of recruited male (A) and female (B) CAH patients from 17 endocrine specialist centers across the United Kingdom (C) in comparison with the expected number of classic CAH patients based on United Kingdom population data (Office of National Statistics) and an assumed incidence of one in 12,000 newborns. A and B show absolute numbers (white bars, normal population; black bars, CAH patients); D shows percentage of the expected patient number per age group (gray bars, males; black bars, females).
In the cohort of 203 patients, 199 had 21-hydroxylase deficiency, three 11β-hydroxylase deficiency, and one 3β-hydroxysteroid dehydrogenase deficiency. To preserve homogeneity, subsequent analysis focused on the 199 patients with 21-hydroxylase deficiency [134 women with a median age of 34 (range 18–69) years and 65 men with a median age of 32 (18–56) years]. Subgroup-specific age distribution in the 21-hydroxylase deficiency patients was as follows: women with classic CAH (n = 103), 33 (18–66) years; women with nonclassic CAH (n = 34), 43 (22–69) years; men with classic CAH (n = 62), 32 (18–56) years; and men with nonclassic (n = 3), 19, 36, and 36 yr. Ethnic origin was 91% white European, 3% Asian (Pakistani), 1.5% African-Caribbean, and 4.5% other.
Diagnosis had been established in the majority during the neonatal period, either due to ambiguous genitalia (34%) or salt-losing crisis (39%). Other primary manifestations included failure to thrive (5%), precocious pseudopuberty (9.5%), tall stature (3.5%), hirsutism (9.5%), oligo-/amenorrhea (7.5%), and family screening (8%).
Glucocorticoid therapy (Table 1)
Table 1.
Glucocorticoid treatment in the 21-hydroxylase deficiency patients in the United Kingdom CaHASE cohort (n = 199)
| Glucocorticoid treatment | Male classic CAH (n = 62) | Female classic CAH (n = 103) | Female nonclassic CAH (n = 31) |
|---|---|---|---|
| Hydrocortisone only | |||
| n (%) | 24 (39%) | 21 (20%) | 5 (16%) |
| Median dose (range) (mg/d) | 25 (10–60) | 20 (10–32.5) | 20 (10–25) |
| Administration (od/bd/tds) (n = 51) | 13/61/26% | ||
| Reverse circadian administration (n = 51) | 11% | ||
| Prednisolone only | |||
| n (%) | 18 (29%) | 50 (49%) | 17 (55%) |
| Median dose (range) (mg/d) | 7.5 (2.5–10) | 6 (2.5–10) | 5 (1–7.5) |
| Administration (od/bd/tds) (n = 88) | 24/75/1% | ||
| Reverse circadian administration (n = 88) | 60% | ||
| Dexamethasone only | |||
| n (%) | 15 (24%) | 17 (17%) | 5 (16%) |
| Median dose (range) (mg/d) | 0.5 (0.25–0.75) | 0.375 (0.25–0.75) | 0.25 (0.25–0.5) |
| Administration (od/bd/tds) (n = 37) | 78/22/0% | ||
| Reverse circadian administration (n = 37) | 44% | ||
| Combination of glucocorticoid preparationsa | 4 (6%) | 14 (14%) | 2 (6%) |
| No glucocorticoids | 1 (1%) | 2 (6%) | |
| Reverse circadian glucocorticoid administration | 21 (34%) | 51 (50%) | 11 (35%) |
| Fludrocortisone | |||
| n (%) | 51 (82%) | 74 (72%) | 3 (10%) |
| Median dose (range) (μg/d) | 125 (10–500) | 150 (50–500) | 50 (50–250) |
Overall, 20 of 199 patients received combined glucocorticoid treatment: hydrocortisone plus prednisolone (n = 11), hydrocortisone plus dexamethasone (n = 4), prednisolone plus dexamethasone (n = 4), hydrocortisone plus prednisolone plus dexamethasone (n = 1). od, Once daily; bd, twice daily; tds, three times daily.
Among women with classic CAH, 48% received prednisolone, 17% dexamethasone, and 20% hydrocortisone. Females with nonclassic CAH showed a similar distribution (55% prednisolone, 16% dexamethasone, and 16% hydrocortisone), whereas men were more frequently treated with hydrocortisone (37%). Hydrocortisone and prednisolone were mostly administered twice daily (61 and 75%, respectively), whereas dexamethasone was administered once daily in 78%. Combined treatment with two or three different glucocorticoid preparations was most frequent in females with classic CAH (14 vs. 6% in both men with classic and women with nonclassic CAH). Females with classic CAH were also most likely to receive reverse circadian glucocorticoid administration (50 vs. 34 and 35% in male classic and female nonclassic CAH patients, respectively). Glucocorticoid doses varied widely, with some patients receiving high doses that we consider likely to cause signs and symptoms of glucocorticoid excess.
Eighty-two percent of men with classic CAH, 72% of women with classic CAH, and 10% of female nonclassic CAH patients received mineralocorticoid replacement with fludrocortisone. Of those treated with fludrocortisone, 65% had plasma renin levels outside the therapeutic target range, i.e. the upper half of the normal reference range (1); 12% had low or suppressed renin, and 53% had too high renin levels. About one third of patients without mineralocorticoid replacement had raised renin levels.
Serum steroid hormones (Fig. 2)
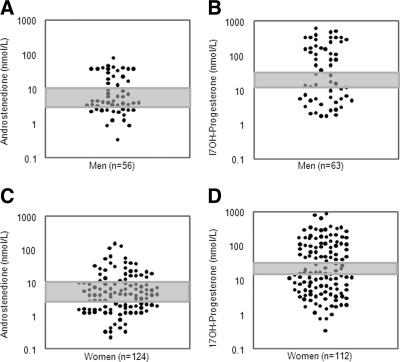
Figure 2.
Serum levels of the androgen precursor androstenedione (A, C) and the glucocorticoid precursor 17OHP (B, D) in male and female CAH patients sampled in the morning after intake of the usual glucocorticoid morning dose. The shaded areas represent recommended target range (2). Note the logarithmic scale used for representing the serum hormone concentrations.
Testosterone levels within the reference range were found in only 55% of classic and 62% of female nonclassic CAH patients, and only 36% of patients in both groups had normal levels of androstenedione. Suppressed androstenedione (<3 nmol/liter) and testosterone levels (<0.5 nmol/liter) were found in 38 and 16% of the female classic and in 44 and 10% of female nonclassic CAH patients, respectively. By contrast, increased androstenedione (>11 nmol/liter) and testosterone (>2.5 nmol/liter) levels were found in 27 and 30%, respectively, of female classic and in 15 and 28% of female nonclassic CAH patients. In males, androstenedione levels were suppressed (<3.0 nmol/liter) in 29% and elevated in 35% of patients. There was a significant correlation between 17OHP and androstenedione measurements (r = 0.621; P < 0.001).
Serum 17OHP was much more variable than the androgens and outside the previously recommended target range (12–36 nmol/liter) (2) in the majority of patients. Forty-five percent of female classic and 41% of female nonclassic CAH patients had 17OHP levels lower than 12 nmol/liter, and 43 and 35%, respectively, had 17OHP levels higher than 36 nmol/liter. Serum 17OHP levels showed similar variability in male CAH patients, with 37% having levels under 12 nmol/liter and 52% more than 36 nmol/liter.
Clinical characteristics and anthropometric data (Table 2)
Table 2.
Age-weighted comparison of anthropometric and blood pressure data for the 21-hydroxylase deficiency United Kingdom CaHASE cohort patients (n = 199) with the normal United Kingdom population based on HSE data
| Variable | CAH | HSE | P, CAH vs. HSE | P, CAH subgroup comparison |
|---|---|---|---|---|
| Height (cm) | ||||
| Male classic (n = 61) | 162 | 176 | <0.0001 | |
| Female classic (n = 103) | 152 | 162 | <0.0001 | <0.0001a |
| Female nonclassic (n = 31) | 157 | 162 | 0.0001 | 0.003b |
| BMI (kg/m2) | ||||
| Male classic (n = 61) | 27.2 | 27.2 | 1.0 | |
| Female classic (n = 103) | 32.9 | 26.7 | <0.0001 | 0.064a |
| Female nonclassic (n = 31) | 29.4 | 26.7 | 0.0064 | 0.24b |
| Waist circumference (cm) | ||||
| Male classic (n = 57) | 96.7 | 94.4 | 0.24 | |
| Female classic (n = 99) | 99.5 | 85.9 | <0.0001 | 0.94a |
| Female nonclassic (n = 31) | 92.6 | 85.9 | 0.0043 | 0.36b |
| Sitting systolic blood pressure (mm Hg) | ||||
| Male classic (n = 61) | 126 | 130 | 0.04 | |
| Female classic (n = 102) | 124 | 124 | 1.0 | 0.21a |
| Female nonclassic (n = 30) | 123 | 124 | 0.73 | 0.57b |
| Sitting diastolic blood pressure (mm Hg) | ||||
| Male classic (n = 61) | 77.5 | 75.4 | 0.16 | |
| Female classic (n = 102) | 77.9 | 73.7 | 0.0002 | 0.96a |
| Female nonclassic (n = 30) | 74.4 | 73.7 | 0.54 | 0.48b |
Classic male vs. classic female CAH patients.
Classic female vs. nonclassic female CAH patients.
In comparison with age- and sex-matched HSE data, CAH patients were significantly shorter, on average 14 cm in men and 8 cm in women. Compared to population-based controls, female patients had a greater waist circumference but male patients did not differ from controls. Both men and women with CAH had higher BMI than HSE participants. Women with classic CAH were shorter and had higher BMI than women with nonclassic CAH. Overall, 41% of CAH patients had a BMI in the obese range (≥30.0 kg/m2), and 37% were overweight (≥25.0 and <30.0 kg/m2). After adjusting for age distribution to allow comparison with participants of 16–64 yr of age in the HSE 2006, prevalence of obesity was 37.1% in men with classic CAH (compared with 23.3% for men in HSE 2006) and 52.2 and 34.9%, respectively, in women with classic and nonclassic CAH (compared with 26.5% for women in HSE 2006).
The presence of stretch marks was common in both sexes but more prevalent in women (62%) than in men (38%). In females, 73% showed no or only mild signs of hirsutism, 22% had moderate hirsutism, and in 5% hirsutism was graded severe.
Systolic blood pressure was slightly but significantly lower in men with CAH than in population-based HSE controls. Systolic blood pressure in women did not differ from HSE data, but women with classic CAH had significantly higher diastolic blood pressure than age- and sex-matched HSE controls.
Biochemical markers of metabolic risk
Total fasting cholesterol levels were higher than 5 mmol/liter in 46% of patients (male classic 36%, female classic 48%, female nonclassic 59%), and low-density lipoprotein cholesterol was higher than 3 mmol/liter in 39% of patients (male classic 35%, female classic 37%, female nonclassic 60%). Decreased high-density lipoprotein cholesterol (<1.0 and <1.2 mmol/liter in men and women, respectively) was found in 14% of male classic, 8% of female classic, and 7% of female nonclassic patients.
Fasting plasma glucose was increased above 5.6 mmol/liter in 8% of the patients (male classic 6%, female classic 7%, female nonclassic 13%). Insulin resistance as defined by homeostasis model assessment of insulin resistance values below 2.5 was found in 29% of all patients (male classic 36%, female classic 28%, female nonclassic 24%).
Bone mineral density
BMD data were available for 77 participants. Mean ± sd lumbar and femoral BMD z-scores were −0.34 ± 1.47 and −0.25 ± 1.01, respectively. The respective prevalences of osteopenia (BMD T-score ≤ −1.0 to −2.5) and osteoporosis (BMD T-score ≤ −2.5) were 39.2 and 6.8% at the lumbar spine (L1–L4) and 28.8 and 1.4% at the femoral neck.
Female fertility
At birth, 17 of the 138 females with CAH with confirmed 46,XX karyotype had been assigned male gender; 15 underwent gender reassignment, whereas two permanently live as men. Sixty-nine percent of women (92 of 134) had undergone genital reconstruction surgery; 43% underwent more than one operation, and 23% had surgery in adulthood. In the majority (62%), a combination of clitoral and vaginal surgery had been performed, and isolated clitoral and vaginal procedures had been undertaken in 24 and 14%, respectively.
Menarche occurred spontaneously in 80% of female patients at a median age of 13 (range 9–25) years. At the time of the study, 80 of 101 premenopausal women were not taking oral contraceptives; 45% of them had irregular menses (24% oligomenorrhea and 21% amenorrhea). Overall, 35% of women had sought pregnancy (classic, 25%; nonclassic, 68%). Twenty-eight of 47 patients reported 46 live births, including six conceived after fertility treatment. The success rate of CAH women seeking pregnancy was 54% in the classic and 67% in the nonclassic forms of CAH.
Male fertility
Twenty-four of 65 men (37%) had sought fertility and 16 of 24 (67%) had been successful, reporting 25 live births, including two conceived after fertility treatment. Testicular adrenal rest tumors (TARTs) were found in 11 of 16 patients (69%) investigated with ultrasound; only four were palpable at clinical examination. An additional four men had previously undergone unilateral orchidectomy, with histopathology reporting benign Leydig cell tumor, Leydig cell hyperplasia, and Leydig cell nodular hyperplasia. Regarding fertility in the patients found to have TARTs, seven never tried, one tried for 3 yr without success, one is trying and still under treatment, and two patients tried with success, achieving one and two live births, respectively.
Quality of life analysis
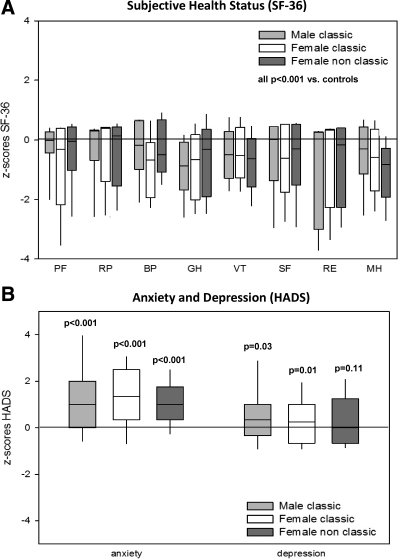
Subjective health status was significantly impaired across all eight SF-36 domains in the three CAH subgroups, with most prominent differences to age- and sex-matched controls for the domains general health, vitality, and role limitations due to emotional problems (Table 3 and Fig. 3A). The HADS questionnaire revealed increased anxiety scores for all three CAH subgroups and increased depression scores in patients with classic CAH (Table 4 and Fig. 3B).
Table 3.
Subjective health status according to the SF-36 scale in patients with 21-hydroxylase deficiency (n = 148)
| SF-36 dimensions | PF | RP | BP | GH | VT | SF | RE | MH |
|---|---|---|---|---|---|---|---|---|
| Male classic CAH (n = 47/62, 76%) | ||||||||
| Median (IQR) | 95 (90/100) | 100 (75/100) | 84 (62/100) | 62 (47/72) | 60 (40/70) | 88 (62/100) | 100 (33/100) | 76 (64/88) |
| Matched controls (n = 940) | ||||||||
| Median (IQR) | 100 (95/100) | 100 (100/100) | 100 (78/100) | 80 (67/92) | 70 (55/80) | 100 (89/100) | 100 (100/100) | 84 (72/92) |
| P (CAH vs. controls) | <0.001 | 0.005 | 0.009 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 |
| Female classic CAH (n = 75/103, 73%) | ||||||||
| Median (IQR) | 85 (60/100) | 100 (50/100) | 70 (41/84) | 60 (37/77) | 50 (35/70) | 75 (50/100) | 100 (33/100) | 68 (48/84) |
| Matched controls (n = 1500) | ||||||||
| Median (IQR) | 100 (95/100) | 100 (100/100) | 100 (67/100) | 77 (65/92) | 65 (50/80) | 100 (89/100) | 100 (100/100) | 84 (68/92) |
| P (CAH vs. controls) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Female nonclassic CAH (n = 26/31, 84%) | ||||||||
| Median (IQR) | 88 (64/100) | 88 (25/100) | 67 (51/100) | 62 (36/78) | 43 (25/60) | 69 (50/100) | 84 (33/100) | 64 (43/72) |
| Matched controls (n = 520) | ||||||||
| Median (IQR) | 100 (95/100) | 100 (100/100) | 100 (78/100) | 77 (67/87) | 70 (55/80) | 100 (89/100) | 100 (100/100) | 88 (76/92) |
| P (CAH vs. controls) | <0.001 | <0.001 | 0.001 | 0.004 | <0.001 | <0.001 | <0.001 | <0.001 |
For every patient, 20 sex- and age-matched controls were selected from the normative United Kingdom SF-36 reference cohort (n = 14,470). Differences were analyzed by Mann Whitney U test. Data are given as median and interquartile range (IQR, i.e. 25th − 75th percentile). The lower the score, the worse is the perceived impairment. PF, Physical functioning; RP, role limitations due to physical problems; BP, bodily pain; GH, general health; VT, vitality; SF, social functioning; RE, role limitations due to emotional problems; MH, mental health.
Figure 3.
Subjective health in status in CAH according to SF-36 (A) and HADS (B) questionnaires and shown for male classic (n = 65), female classic (n = 103), and female nonclassic (n = 31) CAH patients with box plots representing median and interquartile ranges; whiskers represent the 5th and 95th percentiles. A z-score of 0 represents the median of the normal reference population; the lower the SF-36 z-scores and the higher the HADS z-scores, the worse is self-perceived wellbeing and mood. SF-36 dimensions: PF, physical functioning; RP, role limitations due to physical problems; BP, bodily pain; GH, general health; VT, vitality; SF, social functioning; RE, role limitations due to emotional problems; MH, mental health.
Table 4.
Anxiety and depression scores as assessed by HADS in patients with congenital adrenal hyperplasia (n = 129), compared with normative data
| Male classic CAH (n = 33/62, 51%) | Age- and sex- matched controls (n = 165) | Female classic CAH (n = 65/103, 63%) | Age- and sex- matched controls (n = 325) | Female nonclassic CAH (n = 31/31, 100%) | Age- and sex- matched controls (n = 155) | |
|---|---|---|---|---|---|---|
| HADS anxiety score | ||||||
| Median (IQR) | 6.5 (3.3–8.0) | 3.0 (2.0–4.3) | 9.0 (6.0–12.5) | 4.0 (2.0–6.0) | 8.0 (5.0–11.0) | 4.0 (2.0–7.0) |
| Pa | <0.001 | <0.001 | <0.001 | |||
| HADS depression score | ||||||
| Median (IQR) | 2.0 (1.0–5.5) | 2.0 (0.8–4.0) | 5.0 (1.0–7.0) | 2.0 (1.0–5.0) | 4.0 (1.5–9.0) | 3.0 (1.0–6.0) |
| Pa | 0.397 | <0.001 | 0.086 |
For every patient, five sex- and age-matched controls were selected from the normative group (n = 2043). Data are given as mean ± sem, median, and interquartile range (IQR, 25th–75th percentile). The higher the score, the worse is the perceived impairment of mood.
P for comparison CAH subgroup vs. sex- and age-matched controls.
The IIEF-5 questionnaire was completed by 85% of male CAH patients. Results revealed erectile dysfunction in 41% (severe 10%, moderate 5%, mild to moderate 9%, and mild 17%). Only 70% of female patients returned a completed McCoy sexuality questionnaire, and responders reported a high incidence of lack of vaginal lubrication and pain during intercourse (Table 5). Of note, this did not differ between female classic and nonclassic and whether women had genital surgery or not. There was a slight but significantly more pronounced lack of genital lubrication in women without previous genital surgery (P = 0.026) (Table 5).
Table 5.
Dimensions of sexual function as assessed by the nine-item short form of the McCoy Female Sexuality Questionnaire (MFSQ-9) in women with 21-hydroxylase deficiency, comparing classic vs. nonclassic CAH women and women with and without genital corrective surgery
| Response rate | Classic CAH women (n = 74/103, 72%) | Nonclassic CAH women (n = 25/31, 81%) | CAH women with genital surgery (n = 71/92, 77%) | CAH women without genital surgery (n = 28/42, 67%) |
|---|---|---|---|---|
| Are you satisfied with your present frequency of sexual activity? (1, extremely unsatisfied; 4, neither unsatisfied nor satisfied; 7, extremely satisfied) | ||||
| Mean ± sem | 3.75 ± 0.20 (n = 86) | 4.32 ± 0.35 (n = 25) | 3.78 ± 0.21 (n = 81) | 4.17 ± 0.32 (n = 30) |
| Pa | 0.180 | 0.327 | ||
| How often did you have sexual thoughts or fantasies during the last month? (1, never; 3, once a week; 5, once a day; 7, more than 10 times a day) | ||||
| Mean ± sem | 3.63 ± 0.19 (n = 88) | 3.26 ± 0.26 (n = 27) | 3.74 ± 0.19 (n = 81) | 3.09 ± 0.25 (n = 34) |
| Pa | 0.304 | 0.054 | ||
| How enjoyable is sex for you? (1, not at all enjoyable; 4, neutral; 7, very enjoyable) | ||||
| Mean ± sem | 4.74 ± 0.22 (n = 76) | 5.04 ± 0.38 (n = 25) | 4.82 ± 0.36 (n = 73) | 4.81 ± 0.22 (n = 28) |
| Pa | 0.487 | 0.975 | ||
| How often do you have an orgasm (climax during sex)? (1, never; 4, about half the time; 7, every time) | ||||
| Mean ± sem | 3.77 ± 0.25 (n = 74) | 4.44 ± 0.31 (n = 25) | 3.85 ± 0.26 (n = 86) | 4.18 ± 0.30 (n = 28) |
| Pa | 0.150 | 0.459 | ||
| How often do you suffer from lack of vaginal lubrication (wetness) during sex? (1, every time; 4, about half the time; 7, never) | ||||
| Mean ± sem | 3.30 ± 0.22 (n = 73) | 2.60 ± 0.32 (n = 25) | 3.39 ± 0.23 (n = 69) | 2.48 ± 0.28 (n = 29) |
| Pa | 0.102 | 0.026 | ||
| How often do you suffer from pain during intercourse? (1, every time; 4, about half the time; 7, never) | ||||
| Mean ± sem | 2.97 ± 0.22 (n = 70) | 2.28 ± 0.36 (n = 25) | 2.94 ± 0.22 (n = 67) | 2.43 ± 0.37 (n = 28) |
| Pa | 0.104 | 0.214 |
P for comparison between the different CAH subgroups.
In their responses to the CAH wellbeing questionnaire, only 22 and 24% of female and male patients, respectively, indicated that they were concerned about the impact of CAH on their long-term health. Interestingly, only 35% of both male and female patients reported concern about their height. By contrast, 61 and 46% of female and male patients, respectively, were concerned about their weight. Only 26 and 18% of male and female patients, respectively, were worried about their fertility potential, whereas 46% of women and 38% of men were unhappy about their sex life.
Discussion
This study establishes that the objective and subjective health status in adult CAH patients in the United Kingdom is poor. This was observed for both sexes and in patients with classic and nonclassic CAH alike. Females with classic CAH were the most affected. All patients, but in particular women with classic CAH, suffered from a high prevalence of obesity (52.2% compared with 26.5% in women of 16–64 yr of age participating in the HSE 2006), hypercholesterolemia, insulin resistance, osteopenia, and osteoporosis, and women with classic CAH also had increased diastolic blood pressure when compared with HSE participants. Glucocorticoid overtreatment is a possible contributor to the observed adverse metabolic profile, with women with classic CAH most frequently receiving long-acting synthetic glucocorticoids, combined treatment with several glucocorticoid preparations, and reverse circadian glucocorticoid administration. The notion of excess glucocorticoid treatment in this cohort was further supported by the frequent observation of suppressed androgens, striae, and a greater reduction in lumbar over femoral BMD, a common finding in glucocorticoid-induced osteoporosis (23). Participants were receiving a variety of different glucocorticoids, with different doses and frequencies and treatment prescribed in a reverse circadian pattern to some participants (see Table 1). As a consequence, the study has limited power to detect differences in outcomes between different treatment groups, and a larger study is required to obtain this important information.
A number of previous studies in small CAH cohorts of young age have reported reduced BMD (24,25,26). A single-center study in 55 adult women with classic CAH showed a high incidence of osteopenia and osteoporosis (27) and also described increased fat mass and increased insulin levels in patients over 30 yr (13). These preliminary reports are corroborated by our findings, although it should be recognized that only a proportion of our patients had BMD measurement at the discretion of the investigator, and therefore, there may be a bias to find an increased incidence of osteoporosis.
The frequent use of long-acting synthetic glucocorticoids and reverse circadian glucocorticoid administration with the highest dose at bedtime, as observed in our cohort, contrasts with current pediatric practice where the consensus suggests the use of the endogenous hormone hydrocortisone (10). The benefit of reverse circadian glucocorticoid administration has never been established, and a recent study reported improved overnight control of androgen excess in CAH by using a hydrocortisone formulation capable of delayed and sustained release (8), which may represent an option for improved glucocorticoid replacement (7). Despite glucocorticoid over-replacement, the control of androgen excess in our cohort was generally poor with the majority of patients having either elevated or suppressed androgens. 17OHP levels were even more variable, and half of our patients had levels higher than 36 nmol/liter after the intake of their last glucocorticoid dose [median time from last intake to blood sampling was 3.75 (interquartile range 2.25–12.5) hours]. This suggests that the currently recommended 17OHP target range, 12–36 nmol/liter before morning dose (2), strongly favors over-replacement and generally questions the suitability of 17OHP as a monitoring tool in CAH with current therapy regimens.
In contrast to the suggestion of glucocorticoid overtreatment, many of our patients appeared insufficiently treated with mineralocorticoids, as indicated by raised plasma renin in more than half of all patients and even in one third of the patients receiving mineralocorticoid replacement. Aldosterone levels during the neonatal period and childhood differ substantially from those during adulthood (2,3). Therefore, reassessment of volume status and mineralocorticoid replacement requirements during early adulthood is an important management step that should not be overlooked (4,5,9).
Quality of life in our patients was significantly compromised, to a similar if not greater extent than previously observed in adrenal insufficiency (28). Of note, our disease-specific questionnaire revealed that the significantly reduced final height had a lesser self-perceived impact on the adult patients than obesity or compromised sex life.
Similar to some smaller previous studies (29,30,31,32), we found a low fertility rate in the overall cohort but a greater fertility rate in those wanting to be fertile. Reasons will vary but may include the consequences of genital surgery (30) and the increased incidence of homosexual orientation (11,33). In our female patients, pain during intercourse was a major perceived problem, supporting previous findings in smaller cohorts (30,34).
Over the last decade, it has become increasingly clear that TARTs, i.e. nodular hyperplasia arising from cells that have many characteristics of adrenocortical cells and migrated with the testis during fetal development (35), represent a significant problem in male CAH patients because they can severely compromise fertility (12,36,37,38,39). This is supported by the findings in our study that identified TARTs in the majority of male patients studied with ultrasound, albeit that only a small number were investigated. TARTs usually indicate poor disease control and often are reversible after adjustment of glucocorticoid treatment. It is of concern that four of our patients underwent gonadectomy on the assumption of a seminoma, with histology revealing TARTs, clearly indicating the need for increased awareness of all clinicians involved in the management of male CAH patients.
A major finding of our study was the low capture rate, despite extensive recruitment by major United Kingdom endocrine centers. Even when accounting for the recruitment rate of 54% and overlooking the much higher number of to-be-expected nonclassic CAH patients, we are left with the fact that less than 10% of the expected adult United Kingdom CAH patients are under endocrine specialist care. Where are the other patients? It is likely that most patients born more than 60 yr ago are now deceased because they were born before the availability of life-saving glucocorticoid treatment. Also, some of the younger patients may not have survived, with 60% of our patients presenting in the neonatal period being diagnosed only after life-threatening adrenal crisis, consequent to the lack of neonatal CAH screening in the United Kingdom. This will affect in particular male patients who do not present with ambiguous genitalia and thus fail to trigger early diagnosis, which may contribute to the lower capture of male patients in our study. For the majority of the missing younger patients, we assume that many will either receive no medical attention or are under the care of the family physician or gynecologist. We should also be cautious that we may have a selection bias in that healthier patients may not seek medical care.
In conclusion, this study demonstrates that health outcomes in adults with CAH are generally poor in the CAH patients receiving care at specialist centers studied and that currently only a small minority of patients in the United Kingdom receive specialist care. Achieving improved care by raising awareness of all physicians and enhancing transition of care between pediatric and adult endocrinologists can be expected to improve outcomes; higher numbers will also translate into increased expertise of the involved specialists. We recommend that all adult patients with CAH should be offered care in endocrine centers with appropriate expertise, involving a multidisciplinary team, including endocrinologists, gynecologists, geneticists, psychologists, urologists, and specialist nurses. We would also stress the importance of transitional care between pediatric and adult endocrinologists. The prevalent use of long-acting synthetic glucocorticoids and in particular reverse circadian glucocorticoid administration in adults with CAH is not supported by sufficient evidence, and improving options for glucocorticoid treatment in CAH is an important item on the research agenda.
Supplementary Material
Acknowledgments
We are grateful to John Brazier (University of Sheffield, Sheffield, UK) and Andreas Hinz (University of Leipzig, Leipzig, Germany) for granting access to SF-36 and HADS reference cohorts, respectively, and to Jill Harrison (University of Edinburgh, Edinburgh, UK) for conducting insulin assays.
CaHASE investigators (in alphabetical order) include Prof. W. Arlt (Birmingham), Dr. U. Ayyagari (Oxford), Dr. S. Ball (Newcastle), Prof. J. S. Bevan (Aberdeen), Dr. S. A. Booth (Aberdeen), Dr. U. Bradley (Belfast), Sister L. Breen (St. Thomas’, London), Dr. P. V. Carroll (St. Thomas’, London), Dr. M. Clements IWatford), T, Chambers (Manchester), Dr. T. R. Cole (Birmingham), Prof. J. M. C. Connell (Dundee/Glasgow), Dr. G. Conway (University College Hospitals, London), Dr. M. Daly (Exeter), Prof. J. R. Davis (Manchester), Sister A. Doane (Sheffield), Dr. E. J. Doherty (St. Thomas’, London), Dr. T. S. Han (University College Hospitals, London), Prof. I. A. Hughes (Cambridge), Dr. S. Hunter (Belfast), Sister V. Ibbotson (Sheffield), Dr. N. Krone (Birmingham), Sister J. MacDonald (Oxford), Dr. K. Mullen (Belfast), Dr. S. Peacey (Bradford), Dr. C. Perry (Glasgow), Dr. D. W. Ray (Manchester), Dr. D. A. Rees (Cardiff), Prof. R. J. M. Ross (Sheffield), Prof. M. Scanlon (Cardiff), Dr. H. Simpson (Cambridge), Prof. P. M. Stewart (Birmingham), Sister S. E. Stewart (Birmingham), Dr. R. H. Stimson (Edinburgh), Dr. J. P. Vora (Liverpool), Dr. D. Wake (Edinburgh), Sister E. Walker (Watford), Prof. B. R. Walker (Edinburgh), Prof. J. A. H. Wass (Oxford), Sister P. Whittingham (Liverpool), Dr. D. S. Willis (Society for Endocrinology), Sister D. Wright (Bradford), and Prof. F. C. Wu (Manchester).
Footnotes
CaHASE gratefully acknowledges support from The Clinical Endocrinology Trust (United Kingdom Registered Charity Number 288679) and the Society for Endocrinology. W. A. receives grant support from the Medical Research Council UK (Program Grant G0900567) and the European Community (FP7 Collaborative Research Project EuroDSD). N. K. is a Wellcome Trust Clinician Scientist Fellow (GR079865MA).
Clinical trial registration number at www.ClinicalTrials.gov is NCT00749593.
Disclosure Summary: R. J. R. is a founding director and equity holder in Diurnal Ltd. that is developing new hydrocortisone preparations for patients with CAH. All other authors have nothing to disclose.
First Published Online August 18, 2010
Abbreviations: BMI, Body mass index; CAH, congenital adrenal hyperplasia; HADS, Hospital Anxiety and Depression Scale; HSE, Health Surveys for England; IIEF-5, five-item version of the International Index of Erectile Function; 17OHP, 17-hydroxyprogesterone; SF-36, 36-item short-form health survey; TART, testicular adrenal rest tumor.
References
- Arlt W, Allolio B 2003 Adrenal insufficiency. Lancet 361:1881–1893 [DOI] [PubMed] [Google Scholar]
- Merke DP, Bornstein SR 2005 Congenital adrenal hyperplasia. Lancet 365:2125–2136 [DOI] [PubMed] [Google Scholar]
- Speiser PW, White PC 2003 Congenital adrenal hyperplasia. N Engl J Med 349:776–788 [DOI] [PubMed] [Google Scholar]
- Arlt W, Krone N 2007 Adult consequences of congenital adrenal hyperplasia. Horm Res 68(Suppl 5):158–164 [DOI] [PubMed] [Google Scholar]
- Merke DP 2008 Approach to the adult with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 93:653–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debono M, Ross RJ, Newell-Price J 2009 Inadequacies of glucocorticoid replacement and improvements by physiological circadian therapy. Eur J Endocrinol 160:719–729 [DOI] [PubMed] [Google Scholar]
- Debono M, Ghobadi C, Rostami-Hodjegan A, Huatan H, Campbell MJ, Newell-Price J, Darzy K, Merke DP, Arlt W, Ross RJ 2009 Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab 94:1548–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Vanryzin C, Sinaii N, Kim MS, Nieman LK, Ravindran S, Calis KA, Arlt W, Ross RJ, Merke DP 2010 A pharmacokinetic and pharmacodynamic study of delayed- and extended-release hydrocortisone (Chronocort) versus conventional hydrocortisone (Cortef) in the treatment of congenital adrenal hyperplasia. Clin Endocrinol (Oxf) 72:441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie CM, Crouch NS, Rumsby G, Creighton SM, Liao LM, Conway GS 2006 Congenital adrenal hyperplasia in adults: a review of medical, surgical and psychological issues. Clin Endocrinol (Oxf) 64:2–11 [DOI] [PubMed] [Google Scholar]
- Joint LWPES/ESPE CAH Working Group 2002 Consensus statement on 21-hydroxylase deficiency from the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. J Clin Endocrinol Metab 87:4048–4053 [DOI] [PubMed] [Google Scholar]
- Bachelot A, Plu-Bureau G, Thibaud E, Laborde K, Pinto G, Samara D, Nihoul-Fékété C, Kuttenn F, Polak M, Touraine P 2007 Long-term outcome of patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res 67:268–276 [DOI] [PubMed] [Google Scholar]
- Cabrera MS, Vogiatzi MG, New MI 2001 Long term outcome in adult males with classic congenital adrenal hyperplasia. J Clin Endocrinol Metab 86:3070–3078 [DOI] [PubMed] [Google Scholar]
- Falhammar H, Filipsson H, Holmdahl G, Janson PO, Nordenskjöld A, Hagenfeldt K, Thorén M 2007 Metabolic profile and body composition in adult women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 92:110–116 [DOI] [PubMed] [Google Scholar]
- Jääskeläinen, Voutilainen R 2000 Long-term outcome of classical 21-hydroxylase deficiency: diagnosis, complications and quality of life. Acta Paediatr 89:183–187 [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- Garratt A, Schmidt L, Mackintosh A, Fitzpatrick R 2002 Quality of life measurement: bibliographic study of patient assessed health outcome measures. BMJ 324:1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware Jr JE, Sherbourne CD 1992 The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30:473–483 [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP 1983 The hospital anxiety and depression scale. Acta Psychiatr Scand 67:361–370 [DOI] [PubMed] [Google Scholar]
- Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM 1999 Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 11:319–326 [DOI] [PubMed] [Google Scholar]
- McCoy NL 2000 The McCoy Female Sexuality Questionnaire. Qual Life Res 9:739–745 [Google Scholar]
- National Centre for Social Research, Department of Epidemiology and Public Health at the Royal Free and University College Medical School; Commissioned by Department of Health 2004 Health Survey for England 2003. London: Stationery Office [Google Scholar]
- Health and Social Care Information Centre 2008 Health Survey for England 2006. Vol 1: CVD and risk factors adults. Joint Health Surveys Unit. In: Craig R, Mindell J, eds. London: Information Centre [Google Scholar]
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP 2007 Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 18:1319–1328 [DOI] [PubMed] [Google Scholar]
- Cameron FJ, Kaymakci B, Byrt EA, Ebeling PR, Warne GL, Wark JD 1995 Bone mineral density and body composition in congenital adrenal hyperplasia. J Clin Endocrinol Metab 80:2238–2243 [DOI] [PubMed] [Google Scholar]
- Chakhtoura Z, Bachelot A, Samara-Boustani D, Ruiz JC, Donadille B, Dulon J, Christin-Maître S, Bouvattier C, Raux-Demay MC, Bouchard P, Carel JC, Leger J, Kuttenn F, Polak M, Touraine P; Centre des Maladies Endocriennes Rares de la Croissance and Association Surrénales 2008 Impact of total cumulative glucocorticoid dose on bone mineral density in patients with 21-hydroxylase deficiency. Eur J Endocrinol 158:879–887 [DOI] [PubMed] [Google Scholar]
- Sciannamblo M, Russo G, Cuccato D, Chiumello G, Mora S 2006 Reduced bone mineral density and increased bone metabolism rate in young adult patients with 21-hydroxylase deficiency. J Clin Endocrinol Metab 91:4453–4458 [DOI] [PubMed] [Google Scholar]
- Falhammar H, Filipsson H, Holmdahl G, Janson PO, Nordenskjöld A, Hagenfeldt K, Thorén M 2007 Fractures and bone mineral density in adult women with 21-hydroxylase deficiency. J Clin Endocrinol Metab 92:4643–4649 [DOI] [PubMed] [Google Scholar]
- Hahner S, Loeffler M, Fassnacht M, Weismann D, Koschker AC, Quinkler M, Decker O, Arlt W, Allolio B 2007 Impaired subjective health status in 256 patients with adrenal insufficiency on standard therapy based on cross-sectional analysis. J Clin Endocrinol Metab 92:3912–3922 [DOI] [PubMed] [Google Scholar]
- Casteras A, De SP, Rumsby G, Conway GS 2009 Reassessing fecundity in women with classical congenital adrenal hyperplasia (CAH): normal pregnancy rate but reduced fertility rate. Clin Endocrinol (Oxf) 70:833–837 [DOI] [PubMed] [Google Scholar]
- Gastaud F, Bouvattier C, Duranteau L, Brauner R, Thibaud E, Kutten F, Bougnères P 2007 2007 Impaired sexual and reproductive outcomes in women with classical forms of congenital adrenal hyperplasia. J Clin Endocrinol Metab 92:1391–1396 [DOI] [PubMed] [Google Scholar]
- Hagenfeldt K, Janson PO, Holmdahl G, Falhammar H, Filipsson H, Frisén L, Thorén M, Nordenskjöld A 2008 Fertility and pregnancy outcome in women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Hum Reprod 23:1607–1613 [DOI] [PubMed] [Google Scholar]
- Jääskeläinen J, Hippeläinen M, Kiekara O, Voutilainen R 2000 Child rate, pregnancy outcome and ovarian function in females with classical 21-hydroxylase deficiency. Acta Obstet Gynecol Scand 79:687–692 [PubMed] [Google Scholar]
- Frisén L, Nordenström A, Falhammar H, Filipsson H, Holmdahl G, Janson PO, Thorén M, Hagenfeldt K, Möller A, Nordenskjöld A 2009 Gender role behavior, sexuality, and psychosocial adaptation in women with congenital adrenal hyperplasia due to CYP21A2 deficiency. J Clin Endocrinol Metab 94:3432–3439 [DOI] [PubMed] [Google Scholar]
- Nordenskjöld A, Holmdahl G, Frisén L, Falhammar H, Filipsson H, Thorén M, Janson PO, Hagenfeldt K 2008 Type of mutation and surgical procedure affect long-term quality of life for women with congenital adrenal hyperplasia. J Clin Endocrinol Metab 93:380–386 [DOI] [PubMed] [Google Scholar]
- Claahsen-van der Grinten HL, Otten BJ, Sweep FC, Span PN, Ross HA, Meuleman EJ, Hermus AR 2007 Testicular tumors in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency show functional features of adrenocortical tissue. J Clin Endocrinol Metab 92:3674–3680 [DOI] [PubMed] [Google Scholar]
- Claahsen-van der Grinten HL, Otten BJ, Takahashi S, Meuleman EJ, Hulsbergen-van de Kaa C, Sweep FC, Hermus AR 2007 Testicular adrenal rest tumors in adult males with congenital adrenal hyperplasia: evaluation of pituitary-gonadal function before and after successful testis-sparing surgery in eight patients. J Clin Endocrinol Metab 92:612–615 [DOI] [PubMed] [Google Scholar]
- Martinez-Aguayo A, Rocha A, Rojas N, García C, Parra R, Lagos M, Valdivia L, Poggi H, Cattani A; Chilean Collaborative Testicular Adrenal Rest Tumor Study Group 2007 Testicular adrenal rest tumors and Leydig and Sertoli cell function in boys with classical congenital adrenal hyperplasia. J Clin Endocrinol Metab 92:4583–4589 [DOI] [PubMed] [Google Scholar]
- Reisch N, Flade L, Scherr M, Rottenkolber M, Pedrosa Gil F, Bidlingmaier M, Wolff H, Schwarz HP, Quinkler M, Beuschlein F, Reincke M 2009 High prevalence of reduced fecundity in men with congenital adrenal hyperplasia. J Clin Endocrinol Metab 94:1665–1670 [DOI] [PubMed] [Google Scholar]
- Stikkelbroeck NM, Otten BJ, Pasic A, Jager GJ, Sweep CG, Noordam K, Hermus AR 2001 High prevalence of testicular adrenal rest tumors, impaired spermatogenesis, and Leydig cell failure in adolescent and adult males with congenital adrenal hyperplasia. J Clin Endocrinol Metab 86:5721–5728 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.