Abstract
Previously, Anderson, Ramo, Cummins, and Brown (2010) described six distinct patterns of alcohol and other drug (AOD) use during the decade following adolescents’ treatment for alcohol and other substance use disorders (A/SUD). This time period represents a phase of significant neurodevelopment and the influence of substance use on the brain is a concern. In the present study we examined patterns of neuropsychological function over these 10 years in relation to the AOD trajectories identified for youth as they transition into their twenties. Participants were part of a longitudinal research project following adolescents with and without A/SUD who received neuropsychological examinations at baseline and up to 7 times thereafter spanning 10 years (N=213; 46% female at baseline). Neuropsychological trajectories were significantly related to substance involvement patterns over time on measures of verbal learning and memory (ps=.011 to <.0001), visuospatial memory (p=.0002), and verbal attention/working memory (p=.020), with heavier use patterns generally followed by poorer cognition. Heavy use of alcohol alone was independently associated with poorer verbal memory over time. Further, substance withdrawal symptoms during each follow-up time point were related to poorer verbal learning and memory scores (ps<.05), while substance abuse/dependence diagnostic criteria were not related to neuropsychological performance levels. These findings suggest that AOD use during adolescence and young adulthood may primarily influence performance that relies on later maturing brain structures, although further research is needed. Higher levels of AOD withdrawal symptoms may signify greater neuropsychological impairment, reflecting potential neurotoxic effects of AOD use.
Keywords: Adolescence, alcohol abuse, drug abuse, neuropsychological assessment, longitudinal
Alcohol and other drug (AOD) involvement often begins in adolescence, and some teens develop alcohol use disorders that may require professional treatment (Clark, Buckstein, & Cornelius, 2002; Rohde, Lewinsohn, & Seeley, 1996). Previously, Anderson, Ramo, Cummins, and Brown (2010) described six distinct patterns of AOD use during the decade following adolescent treatment for alcohol and other substance use disorders (A/SUD): (1) Abstainers/Infrequent Users, (2) Late Adolescent Resurgence, (3) Emerging Adulthood Resurgence, (4) Frequent Drinkers, (5) Frequent Drinkers/Drug Dependent, and (6) Chronic. This 10-year time period represents a phase of transition into young adulthood (Aseltine & Gore, 2005; Brown et al., 2008; Chassin, Flora, & King, 2004; Kypri, McCarthy, Coe, & Brown, 2004). Anderson et al. (2010) found that long-term AOD use appears to negatively influence educational attainment, occupational and socioeconomic status, and marriage/cohabitation relationships during young adulthood.
In addition to the direct impact of AOD use on social and occupational functioning, heavy use may also indirectly affect these outcomes via neurocognitive damage. AOD use tends to peak during a time of ongoing brain development, with normative neuromaturation processes continuing throughout adolescence and into early adulthood (Gogtay et al., 2004; Gogtay & Thompson, 2010). The prefrontal cortex (primarily involved in executive functions and inhibiting impulses) and lateral temporal lobes (responsible for integrating memory, audiovisual information, and object recognition) appear to be the last cortical structures to mature (Gogtay et al., 2004; Jernigan & Gamst, 2005). Neuromaturation in certain brain areas (e.g., the corpus callosum) has been associated with improved neuropsychological abilities in healthy adolescents (Fryer et al., 2008), thus linking brain development to improved cognition.
The potential influence of substance use on the brain during neurodevelopment is concerning. In older adults, heavy alcohol use appears to impact several brain regions including the hippocampus (Jernigan et al., 1991; Sullivan, Marsh, Mathalon, Lim, & Pfefferbaum, 1995), which is responsible for learning and remembering new information (Eichenbaum, 1999; Foster et al., 1999; Squire, 1992). Rodent studies suggest that the hippocampus and frontal lobes are vulnerable to heavy alcohol (White & Swartzwelder, 2004) and cannabinoid intake (Hampson & Deadwyler, 2000), and that the juvenile brain is particularly sensitive to ethanol binge-induced brain damage relative to the adult brain (Crews, Braun, Hoplight, Switzer, & Knapp, 2000). Studies of heavy alcohol-use by human teens have reported smaller hippocampal volumes relative to controls (De Bellis et al., 2000; Medina, Schweinsburg, Cohen-Zion, Nagel, & Tapert, 2007; Nagel, Schweinsburg, Phan, & Tapert, 2005). Other studies have suggested smaller prefrontal white matter volumes in adolescents with AUDs compared to controls (De Bellis et al., 2005; Medina et al., 2008), and adolescent marijuana use has been associated with abnormal cerebellar volumes (Medina, Nagel, & Tapert, 2010).
These and other differences in structural brain integrity may relate to brain functioning. Adolescent heavy drinkers have demonstrated different brain activation patterns during spatial working memory (Tapert et al., 2004) and verbal encoding (Schweinsburg, McQueeny, Nagel, Eyler, & Tapert, 2010) on functional magnetic resonance imaging tasks relative to non-using controls, especially in fronto-cerebellar, occipital, and hippocampal regions. Neuropsychological studies of adolescents with A/SUD have also reported deficits in verbal and non-verbal memory (Brown, Tapert, Granholm, & Delis, 2000), language (Moss, Kirisci, Gordon, & Tarter, 1994), executive functioning (Giancola, Mezzich, & Tarter, 1998; Moss et al., 1994), processing speed (Medina, Hanson et al., 2007), IQ, and attention (Tarter, Mezzich, Hsieh, & Parks, 1995). Previously, our group found that in addition to short-term neurocognitive deficits (Brown et al., 2000), youth treated for A/SUD during adolescence developed deficits in visuospatial functioning, attention, and visuospatial construction over the decade following treatment (Hanson, Medina, Padula, Tapert, & Brown, in press; Tapert & Brown, 1999; Tapert, Granholm, Leedy, & Brown, 2002). These previous reports compared baseline functioning during treatment (3 weeks of abstinence) to performance at the various endpoints (4, 8, and 10 years). However, substance involvement varies markedly from mid-adolescence to mid-twenties, and the substance use trajectories identified in Anderson et al. (2010) provide a unique means to examine the concomitant changes in cognition in relation to the fluctuations in substance involvement during this developmental period.
Given the salient increases in frequency and intensity of alcohol use during late adolescence and young adulthood (Grant & Dawson, 1997), and recent findings that some youth with SUD appear to only use alcohol in adulthood (Anderson et al., 2010), it is important to consider whether continued use of alcohol alone during this developmental period may be detrimental to neurocognitive functioning. Historically among youth with polysubstance involvement, neurocognitive functioning has been examined in relation to combined use of alcohol and all other drugs. However, the impact of use of multiple substances appears to vary. In general, use of multiple substances is associated with poorer neurocognitive performance. However, in one study, adults who abused alcohol (but not other substances) demonstrated poorer cognitive efficiency than those who abused both alcohol and marijuana (Nixon, Paul, & Phillips, 1998). Similarly, healthy non-abusing teens showed a decrease in verbal memory with the onset of drinking and accumulation of alcohol withdrawal symptoms, yet those who used both marijuana and alcohol appeared unaffected by withdrawal episodes (Mahmood, Jacobus, Bava, Scarlett, & Tapert, 2010). This is consistent with a potential “protective effect” of joint alcohol and marijuana use on hippocampal volumes (Medina, Schweinsburg et al., 2007) and white matter integrity (Jacobus et al., 2009) identified in recent imaging studies. These results suggest that the effects of alcohol alone may differ from the concomitant use of alcohol and other drugs on the brain and cognition.
As noted above, withdrawal experience cumulatively provokes cognitive impairment. In mice, repeated alcohol withdrawal episodes appear to negatively affect spatial learning (Freund, 1970). Alcohol-related seizures in human adult alcoholics were associated with smaller temporal lobe white matter volumes (Sullivan, Marsh, Mathalon, Lim, & Pfefferbaum, 1996), and a greater number of alcohol withdrawal episodes was linked with poorer verbal and visual memory (Glenn, Parsons, Sinha, & Stevens, 1988). Among adolescents, more alcohol and drug withdrawal symptoms have been associated with poorer visuospatial function, executive function, attention, and verbal learning and memory (Brown et al., 2000; Hanson et al., in press; Tapert & Brown, 1999; Tapert et al., 2002). Withdrawal and hangover symptoms have also significantly predicted: 1) brain activation during a spatial working memory task in adolescents with AUD (Tapert et al., 2004), 2) decreased white matter integrity among adolescent binge drinkers with no AUD history (McQueeny et al., 2009), and 3) deteriorating attentional skills in boys who initiate heavy drinking (Squeglia, Spadoni, Infante, Myers, & Tapert, 2009). Together, these relationships suggest that withdrawal symptoms may reflect distinct negative effects on brain function and appear to particularly impact memory.
The primary goal of this investigation was to identify long-term (ten year) patterns of neuropsychological functioning in relation to the dominant trajectories of AOD use for youth with alcohol and drug use disorder histories during this decade of study. Healthy controls with no A/SUD history were also included for comparison. Based on previous findings, we had three primary hypotheses:
We first hypothesized that greater exposure to substances and more persistence of AOD use would be linked to poorer memory, visuospatial skills, attention, and executive functioning during the critical period of development from adolescence to young adulthood. In particular, we expected that cognitive skills would be most impacted during periods of heaviest use but also that adverse cumulative effects would be evident among youth and young adults with the most persistent AOD involvement trajectories. Likewise, we presumed that clinical youth who abstained from substance use over the 10-year follow-up period (Abstainers/Infrequent Users) would perform similarly to community controls without histories of problematic AOD use.
Second, we hypothesized that neuropsychological deficits (especially memory) would be associated with heavy use of alcohol (the primary substance used during this period) even when other drugs are not commonly used.
Third, we postulated that poorer neurocognitive functioning over the decade that is associated with AOD involvement is more a function of the physiological impact of the substances (as measured by withdrawal symptoms) than dependence symptoms per se (which reflect functional life problems associated with substance use).
Methods
Participants
Participants (N = 213; ages 13–18 years, M = 15.7, SD = 1.5; 46% female) were part of a longitudinal research project following adolescents with (clinical youth: n = 151, or 71% of this sample) and without (community controls: n = 62, or 29% of this sample) alcohol and other substance use disorders (Brown, Myers, Mott, & Vik, 1994; Brown, Vik, & Creamer, 1989; Hanson et al., in press). The current study examines performance on neuropsychological assessments administered at baseline and semi-annually thereafter up to 10 years (i.e., at 6 months and 1, 2, 4, 6, 8, and 10 years after baseline).
Clinical participants were individuals admitted to inpatient adolescent substance abuse treatment centers in San Diego County between 1986–1990 and were demographically representative of the greater San Diego County school population. At project intake, the clinical sample of youth met diagnostic criteria for alcohol abuse or dependence and typically met criteria for at least one other SUD (94%). To reduce the potential confound of co-morbid psychiatric disorders, clinical participants were excluded for concurrent DSM-III-R Axis I psychopathology (American Psychiatric Association, 1987) at project intake aside from conduct disorder (CD; 94% with CD symptoms at study intake, M # of symptoms = 5.9; SD = 2.5) (Brown, D’Amico, McCarthy, & Tapert, 2001). Sixteen subjects (9.6%) of the original clinical sample (n = 167) were not included in these analyses because of death, incarceration, or missing data (e.g., moved out of the area).
The comparison sample was recruited through advertisements in the same communities where clinical participants were located. Community youth were selected to be similar to clinical youth on age (±1 year), years of education (±1 year), gender, socioeconomic status (SES; both groups were middle class, on average; ns), ethnic background (58% of controls vs. 55% of clinical sample were Caucasian; ns), and prevalence of family history of AUD and SUD (53% of controls vs. 64% of clinical youth; ns). Although the groups were within one year in age and education, the clinical youth were slightly older (M=15.9 vs. 15.0 years; p<.001) and, consequently, had more education by half of a year (M=9.4 vs. 8.9 years; p<.10). The community youth also tended to have more girls (56% female) than the clinical youth (41% female; p=.05). The purpose of including community controls was to examine how clinical youth compared to individuals who did not develop AOD problems. Therefore, community adolescents were excluded if they had a history of AUD, SUD, or alcohol or drug use problems at project intake or developed such problems over the course of study (n = 45). Follow-up rates across the assessments (6 months and 1, 2, 4, 6, 8, and 10 years) ranged from 82–97% with no significant differences between clinical and community samples.
Youth were excluded at project intake if they (1) did not have a parent or guardian to corroborate biographical, health, and substance involvement information; (2) lived >50 miles from the research facility; (3) had an Axis I psychiatric disorder other than conduct disorder predating the onset of regular substance use; (4) did not speak English fluently; (5) had a history of head trauma with loss of consciousness >2 minutes; (6) had any medical or physical condition or used medication that could compromise neuropsychological (NP) performance (e.g., epilepsy, migraine); or (7) had prenatal exposure to alcohol (mother drank 3 or more drinks on an occasion or >3 times per month during pregnancy). Follow-up assessments were conducted in person or by phone (>50 miles from research site) with no NP assessment for those living further than 50 miles from the research site. Informed assent and consent, approved by the University of California, San Diego Institutional Review Board and clinical agencies, was independently obtained from each participant and parent.
Measures
Structured clinical interview
Youth and collateral reporters were interviewed separately using a 90-minute confidential structured clinical interview (Brown et al., 1994; Brown et al., 1989) to gather demographic and substance involvement information, as well as social, academic, and occupational functioning, family history of A/SUD, maternal drinking during pregnancy, physical and emotional health problems, and SES. At each follow up time point, a modified interview assessed the participant and a collateral reporter (e.g., parent, domestic partner, roommate, as appropriate) to update this information and collect new data on substance use, treatment, current living situations, changes in significant relationship and parenthood status, and current financial/employment status. SES was determined using the Hollingshead Index (Hollingshead, 1965) based on the education, occupation, and income of parents at intake and of youth after age 21 (higher scores reflect lower SES).
Customary Drinking and Drug Use Record (CDDR)
The CDDR (Brown et al., 2001; Brown et al., 1998) gathered self-report information on the use of alcohol (beer, wine, liquor) and drugs (marijuana, amphetamines, barbiturates, hallucinogens, cocaine, inhalants, opiates, and prescription medications or other drugs not previously specified). The Lifetime Version of the CDDR (administered at intake) assessed lifetime AOD use and the Current Version (administered at all time points) assessed average 30-day use in the last 3 months and use since the previous assessment (e.g., 2 years). For earlier time points, DSM-III-R classifications of dependence were recoded using DSM-IV criteria (American Psychiatric Association, 1987, 1994). The CDDR incorporates items from the Cahalan drinking classification procedure (Cahalan, 1970), Drug Indulgence Index (Lu, 1974), and Alcohol Dependence Scale (Horn, Skinner, Wanberg, & Foster, 1984). Good internal consistency, test–retest reliability, and inter-rater reliability, as well as convergent and discriminant validity, have been demonstrated in adolescent and young adult samples using these time periods (Brown et al., 1998; Stewart & Brown, 1995).
Neuropsychological test battery
The NP battery at project intake (1986–1990) included the following measures: Wechsler Intelligence Scale for Children–Revised (WISC–R) (Wechsler, 1974) Vocabulary, Similarities, Block Design, Arithmetic, and Digit Span (digits forward, digits backward) subtests; Wechsler Memory Scale (WMS) Visual Reproduction subtest (Wechsler, 1945); California Verbal Learning Test (CVLT) (Delis, Kramer, Kaplan, & Ober, 1987); and Trail Making Test (Reitan & Wolfson, 1993). At Year 2, the WISC-R subtests were changed to the Wechsler Adult Intelligence Scale–Revised (WAIS–R) (Wechsler, 1981) to coincide with the age of the participants, and at Year 10, the CVLT was replaced by the newer CVLT-II (Delis, Kramer, Kaplan, & Ober, 2000). At Years 8 and 10, WMS Visual Reproduction was exchanged for the Rey-Osterrieth Complex Figure test (ROCF) (Osterrieth, 1944) copy and 30-minute delayed recall, using the Taylor system for scoring accuracy (Lezak, 1995). This replacement was made in order to update the battery and provide a more complex measure of visuospatial organization and memory. For tests with alternate forms, these were administered to reduce the influence of practice effects. The duration of NP testing was approximately 2 hours at each assessment time point.
Procedures
Recruitment and assessment procedures for this longitudinal study have been previously published (Brown et al., 2001; Brown et al., 1994; Brown et al., 1989). Clinical and community youths were administered the structured interview and NP test battery by a trained psychometrist at project intake and at each follow-up (6 months and 1, 2, 4, 6, 8, and 10 years after intake). Clinical youth completed the initial assessment following a 3-week detoxification period. Community youth and follow-up assessments were conducted at the research facility or the participant’s residence.
After study intake, participants were contacted by phone, mail, and e-mail to schedule follow-up assessments. Substance involvement information was obtained on nearly 100% the cohort through in-person (nearly 70% at the 10 year follow-up) or phone interviews (out of driving range) to maximize follow-up participation (Anderson et al., 2010; Hanson et al., in press). Youths and collateral reporters were assessed privately by alternate trained interviewers to maximize self-disclosure and confidentiality and to provide independent corroboration of data. We used a standardized approach to composite youth and collateral reported information. Specifically, youth reports of use or problems were counted even when not identified in collateral reports, and heavier use / more consequences were included if verified by objective information (e.g., toxicology screens, public information) (see Brown et al., 1989, 1994 for details). To confirm self- and collateral reports, approximately 10–15% of youth were randomly selected to complete urine toxicology screens and Breathalyzers at each follow-up assessment. The results indicated 97% consistency between self-report and biological verification with 1.5% of participants reporting use of a substance that was not identified on the tox screen (marijuana in all cases) and 1.4% reporting no use when a substance was identified on the tox screen. Biological verification results were used to recode the substance use data.
Substance Use Trajectory Classes
The major longitudinal patterns of AOD use were previously determined by latent class growth analysis (LCGA) using multiple AOD variables (Anderson et al., 2010). LCGA is a special case of growth mixture modeling (Jung & Wickrama, 2008; Nagin, 1999) and can be used to describe complex patterns of longitudinal development by mixtures of trajectories that are fit to latent classes. Under a set of strong assumptions the latent classes can be interpreted as representing actual groupings in the population.
In the LCGA used here, alcohol and other substances were modeled as two distinct variables in order to describe differential fluctuations in use over the 10-year period to result in a dual trajectory model (Brame, Nagin, & Tremblay, 2001). Both variables were modeled across time with a quadratic function. At each follow-up, alcohol use frequency was measured by totaling the average monthly alcohol use episodes summed across beer, wine, and hard liquor in the 3 months prior to assessment (0–90). The average monthly drug use variable was the composite use of 8 drug classes in the past 3 months (0–240; marijuana, amphetamines, barbiturates, hallucinogens, cocaine, inhalants, opiates, and prescription medications or other drugs not previously specified). Because of the statistical properties of these response variables, they were treated as count variables and assumed to be Poisson distributed in the model. Multiple substance use measures were used to validate the substance use trajectory classes (e.g., binge drinking, maximum drinks per episode, drug type, polysubstance use, dependence symptoms).
A model with 6 latent classes was adopted in Anderson et al. (2010). The classes adopted were established by comparing models with 3–9 classes. These models make strong assumptions regarding the number of underlying classes and require model selection routines to be used to choose the class structure. These models were initially screened with adjusted Bayesian information criterion (BIC), entropy, and posterior probabilities (PP). Both entropy (.998) and PP (.96-.99) were very high in the 6 class model. Final selection was determined by interpretability and discriminant validity (Anderson et al., 2010; Jung & Wickrama, 2008). We also elected to include the demographically similar community controls in the current analysis for two reasons: 1) to allow for continuity with our previous analyses of this sample (Brown et al., 2000; Hanson et al., in press; Tapert & Brown, 1999; Tapert et al., 2002), and 2) to provide some reflection on how the clinical youth may have performed had they refrained from heavy AOD use.
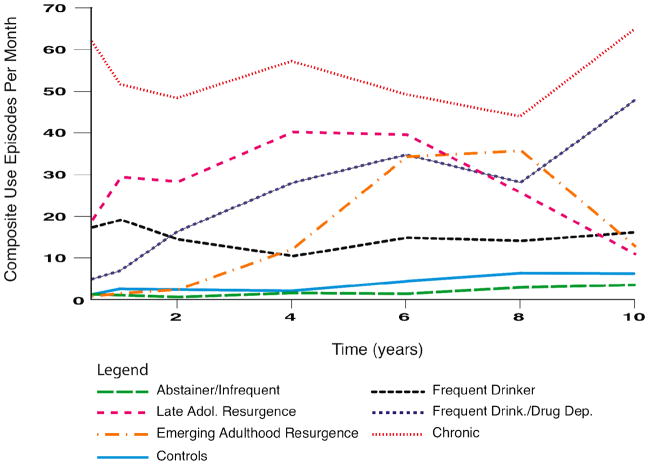
The six identified AOD use trajectory classes are described as follows (see Figure 1 for composite substance use episodes per month of the six clinical sample classes and community controls):
Figure 1.
Composite alcohol and other drug use episodes per month among the alcohol and other drug use trajectory classes and community controls across the 10-year follow-up period. At each follow-up, the average monthly drug and alcohol use episodes in the 3 months prior to the assessment was summed across each type of substance (e.g., episodes of beer + wine + hard liquor [possible range = 0–90], as well as episodes of marijuana + any of the 7 other drug use classes [possible range = 0–240]). Because multiple types of alcohol or drugs could be used in a given day, frequencies may exceed 30.
Class 1: Abstainers/Infrequent Users (n = 42)
This class demonstrated abstinence or the lowest frequency of AOD use over time. Aside from residual dependence symptoms during the initial 2 years after treatment, participants in this class did not meet DSM AUD or SUD criteria. However, those not abstinent used in a limited fashion over time. For example, at year 8, approximately 40% in this class used alcohol at least once in the prior two years (<5 times/mo.) and about 10% reported recent marijuana use (<1 use/mo.).
Class 2: Late Adolescent Resurgence (n = 27)
This class demonstrated an increase in substance use after baseline, particularly during late adolescence, with a subsequent decrease in early adulthood. Relative to Class 3 (Emerging Adulthood Resurgence), substance use and the prevalence of drug (but not alcohol) use disorders peaked earlier (rising from year 2 through year 4) and recovered earlier (slow, steady decline until around year 10). Through year 6, approximately 80–95% of this class used alcohol (average of 20 times/mo.), marijuana (10–15 episodes/mo.), and other drugs (about 5 episodes/mo.; primarily amphetamines, cocaine, and hallucinogens), and 40–50% used these substances at year 10 (≤10 episodes/mo.).
Class 3: Emerging Adulthood Resurgence (n = 22)
This pattern involved low frequency AOD use during the two years after treatment with a sharp increase in drug use and SUD prevalence beginning at year 4 (SUD rates = 5% at 2 years, 20% at 4 years, 45% at 6 years). Substance use peaked through years 6 and 8 with 70–90% using alcohol (10–12 episodes/mo.), marijuana (10–15 episodes/mo.), or other drugs (10 episodes/mo.). While this group drank alcohol less frequently than the Late Adolescent Resurgence class, they used other drugs more heavily (amphetamines, in particular). Substance use then decreased through year 10 with only 40–60% using alcohol, marijuana, and/or other drugs (<15 episodes/mo.).
Class 4: Frequent Drinkers (n = 25)
This trajectory class was characterized by sustained high frequency drinking over time and relatively low rates of other drug use. At year 2, drug use declined sharply (40% using around 5 episodes/mo., typically marijuana), and no one in this class met drug dependence criteria from years 4 through 10 (<1 drug use episode/mo.). These youth drank alcohol at rates similar to the prior two classes (10–15 episodes/mo. in years 4 to 10) and had similar rates of alcohol dependence relative to other classes.
Class 5: Frequent Drinkers/Drug Dependent (n = 26)
Although the initial rates of AOD use following treatment were relatively low, AOD use frequencies and SUD rates rose sharply across time (25% at year 1 to 45% at year 6) and exceeded the previous 3 classes by year 10. Approximately 80% of this class was using marijuana from year 4 (10 episodes/mo.) through year 10 (20 episodes/mo.), while alcohol and other drug use rose from about 80% at year 4 (about 10 alcohol and 5 other drug episodes/mo.) to nearly 100% of the sample using these substances at year 10 (about 20 alcohol and 10 other drug episodes/mo., primarily amphetamines and hallucinogens).
Class 6: Chronic (n = 9)
This pattern of AOD involvement had the highest frequency of AOD use and SUD rates at nearly all time points (e.g., 86% at 6 months; 50% at year 4) but the lowest probability of class membership. A range of 80–100% of this class used alcohol throughout the 10 years (20 episodes/mo. at 6 months to 40 episodes/mo. at year 10). Further, 80–100% of this class used marijuana and other drugs through year 6 (15–20 marijuana episodes/mo.; 5–10 other drug episodes/mo., especially amphetamines, cocaine, and hallucinogens), and about 65% used these substances from years 8 through 10, though at slightly lower frequencies. Due to a small sample size at years 8 and 10, the Chronic users were excluded from analyses at those time points.
Community Controls (n = 62)
This comparison class was free from A/SUD symptoms at project intake and throughout the decade of study. Controls generally had low rates of alcohol and marijuana use and little to no other drug use across time. Approximately 30% of controls used some alcohol from baseline to year 4 (1–2 episodes/mo., on average), and 50–75% used alcohol from years 6 through 10 (2.5–5 times/mo.) coinciding with reaching the legal drinking age. Marijuana use rates fluctuated throughout the follow-up period from less than 2% (at 6 months and 2 years) to 12–13% (years 6 and 8) with an average frequency of ≤1 time/mo.
Demographics
Mean ages within each trajectory class at baseline ranged from 15.0 (SD = 1.7; Controls) to 16.4 (SD = 1.1; Frequent Drinkers; F(6,206) = 3.88, p < .01). In general, heavier substance use classes had fewer females than males (range = 22.2% to 33.3% female), while the Frequent Drinkers, Abstainers/Infrequent Users, and Controls were more balanced in terms of gender (range = 52% to 57% female; Fisher’s exact = 0.025). The overall sample was 55% Caucasian (range = 44% [Chronic] to 58% [Controls]; ns). At baseline, the substance use trajectory classes did not significantly differ in regards to parental SES levels (M Hollingshead score = 26.9 for Emerging Adulthood Resurgence [upper middle class] to 38.8 for Chronic [middle class]). As expected, Controls had fewer conduct disorder symptoms at baseline (M = 1.6; SD = 0.3) compared to the clinical youth, with clinical youth ranging from a mean of 3.3 (SD = 0.3; Abstainers/Infrequent Users) to 4.7 (SD = 0.4; Emerging Adulthood Resurgence) non-substance-related CD symptoms (F(6,206) = 11.72, p < .001). Finally, although the classes did not differ significantly in their family history of substance use disorders, the Frequent Drinkers (68%), Late Adolescent (85%), and Emerging Adulthood Resurgence classes (64%) had higher rates of family histories of SUD than the other classes and Controls (range = 53 to 58%).
Although there were no significant differences in follow-up rates for neuropsychological testing (requiring face- to- face assessments) between trajectory classes, follow-up rates were highest at year 4 (M = 95%) and, as expected, declined over the subsequent follow-up years (year 6: M = 73%; year 8: M = 60%; year 10: M = 46%). The primary reason for not completing NP testing at each time point was moving more than 50 miles from the testing facility. Overall, the median number of days since the last alcohol or other drug use prior to each neuropsychological testing session was 4 days (IQR = 28). However, most classes had a mean of at least 30 days since last use at each follow-up assessment (except for Chronic users), and recency of alcohol use for Controls and Abstainers/Infrequent Users was comparable. Over the 10-year period, alcohol was used by the largest proportion of the sample, and marijuana was the most commonly used illicit drug, followed by stimulants and hallucinogens.
Statistical Analysis
NP scores were calculated using standardized procedures at each time point. Neuropsychological trajectories for the substance use classes were described and compared using Linear Mixed Effects (LME) models (Frees, 2004; West, Welch, & Galecki, 2007). Each NP variable was modeled separately and included fixed effects for time (in years), time squared (to allow for curvilinear trajectories), the seven AOD trajectory classes, and their time interactions, and a random effect for participant. Models were estimated using maximum likelihood (ML), which produced similar estimates to models using restricted maximum likelihood (REML). The overall models were evaluated with likelihood ratio tests (Rabe-Hesketh & Skrondal, 2005). The statistical significance of including the trajectory classes in each model was also tested with a likelihood ratio test (LR Test). Where LR Tests establish statistical significance of an individual factor (e.g. trajectory classes), post-hoc comparisons among individual classes were conducted based on pairwise Wald tests. As the ROCF was only administered at two time points, results were modeled with first order time effects and their intake WMS Visual Reproduction scores as a covariate.
The Abstainers/Infrequent Users were used as the reference group in these analyses. Comparison to this AOD use trajectory class allowed us to evaluate how the other substance use classes might have performed if they had maintained abstinence after substance use treatment. Further, inclusion of the Community Controls provided an additional evaluation of how individuals may have performed if they had never used alcohol or other drugs heavily. Finally, comparison of the various AOD trajectory classes allowed us to examine whether certain substance use patterns were associated with specific neurocognitive consequences. The Chronic trajectory class was included to provide useful clarification of the neurocognitive functioning of those with the most severe and persistent substance involvement but was only analyzed up to the 6-year follow-up due to a small sample size at the 8- and 10-year time points.
Because the inclusion of any covariate in the primary analysis created an overly complex model, hypotheses about the relationship between substance withdrawal and dependence and neuropsychological performance were separately evaluated with LME models. NP measures were modeled with time, time squared, number of symptoms, and their interactions.
Results
Neuropsychological Function in Clinical versus Control Participants
As with previous reports on this sample (Brown et al., 2000; Hanson et al., in press; Tapert & Brown, 1999; Tapert et al., 2002), we first examined the combined clinical participants compared to the community controls on neuropsychological measures during the ten years of the study (see Table 1 for a brief summary). Although the groups were not significantly different at intake when the clinical sample had three weeks of abstinence, clinical participants performed worse than community controls on measures of verbal attention/working memory, and verbal learning and memory at multiple time points over the subsequent 10-year period. The overall neurocognitive differences between the clinical and community youth provided further rationale to examine how the various substance use trajectories influenced the pattern of performance in each neuropsychological domain.
Table 1.
Means (and standard deviations) on selected neuropsychological measures for controls and combined clinical participants over 10 years.
| Measure | Sample | Time (years) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Intake | 0.5 | 1 | 2 | 4 | 6 | 8 | 10 | ||
| CVLTa | |||||||||
| List A Trial 1 | Clinical | 7.5 (1.9) | 7.9 (2.1) | 8.7 (2.3) | 8.5 (2.8) | 8.2* (2.5) | 7.8* (2.3) | 8.1 (2.3) | 6.6 (1.9) |
| Control | 7.3 (1.7) | 7.9 (2.2) | 9.4 (2.5) | 9.4 (2.2) | 9.1* (2.2) | 8.7* (2.1) | 8.9 (2.3) | 6.7 (1.8) | |
| SDFR | Clinical | 11.7 (4.4) | 12.0 (4.8) | 12.7 (4.5) | 12.7 (5.9) | 12.4* (6.4) | 12.3 (4.4) | 12.0* (4.9) | 11.1 (8.0) |
| Control | 12.1 (4.4) | 12.0 (6.5) | 12.7 (4.3) | 13.1 (4.3) | 13.5* (3.6) | 13.3 (3.8) | 13.4* (3.1) | 12.6 (6.2) | |
| LDFR | Clinical | 12.1 (2.7) | 12.4 (2.7) | 13.1 (2.5) | 12.8 (3.2) | 12.8 (3.0) | 12.5* (3.1) | 12.4* (2.9) | 11.9 (2.8) |
| Control | 12.1 (2.8) | 12.4 (3.1) | 13.3 (2.6) | 13.4 (2.5) | 13.7 (2.4) | 13.9* (2.4) | 13.4* (2.9) | 13.3 (2.2) | |
| Rec. Disc. (%) | Clinical | 95.9 (4.4) | 95.8 (4.8) | 96.4 (4.5) | 95.6 (5.9) | 95.7 (6.4) | 95.6* (4.4) | 95.6* (4.9) | 94.1 (8.0) |
| Control | 95.9 (4.4) | 95.5 (6.5) | 97.0 (4.3) | 97.2 (4.3) | 97.4 (3.6) | 97.5* (3.8) | 97.8* (3.1) | 96.2 (6.2) | |
| Arithmeticb | Clinical | 10.4 (3.2) | 10.5* (2.7) | 10.5** (2.9) | 10.0** (2.6) | 9.1** (2.4) | 9.2* (2.4) | 9.6 (2.4) | 9.8 (2.7) |
| Control | 10.7 (3.1) | 11.5* (2.5) | 12.7** (3.0) | 11.6** (2.9) | 11.1** (2.8) | 10.2* (2.7) | 10.1 (2.6) | 10.4 (3.0) | |
| ROCF 30 min.c | Clinical | 16.3 (5.4) | 15.6 (5.1) | ||||||
| Control | 18.1 (5.3) | 16.0 (5.9) | |||||||
California Verbal Learning Test (CVLT) raw scores (List A Trial 1; SDFR=short delay free recall; LDFR=long delay free recall) and percent correct (recognition discriminability); CVLT-II administered at years 8 and 10.
WISC-R (baseline, 6 month, and 1 year follow-ups) and WAIS-R (years 2–10) Arithmetic age-corrected scaled scores.
Rey Osterrieth Complex Figure test (ROCF) 30-minute delayed recall (administered at years 8 and 10).
p<.05;
p<.01
AOD Trajectory Classes and Neuropsychological Functioning
Hypothesis 1: Patterns of neurocognitive functioning will be related to the progression and fluctuations in alcohol and drug involvement during the 10 years of study
As expected, the AOD use trajectory classes did not differ in their language (WISC-R/WAIS-R Vocabulary and Similarities) or psychomotor speed (Trails A time-to-completion) throughout the 10-year follow-up period. However, neuropsychological performance was significantly related to substance use trajectories over time on measures of verbal learning and memory, visuospatial memory, and verbal attention/working memory, as described below.
Verbal Learning & Memory
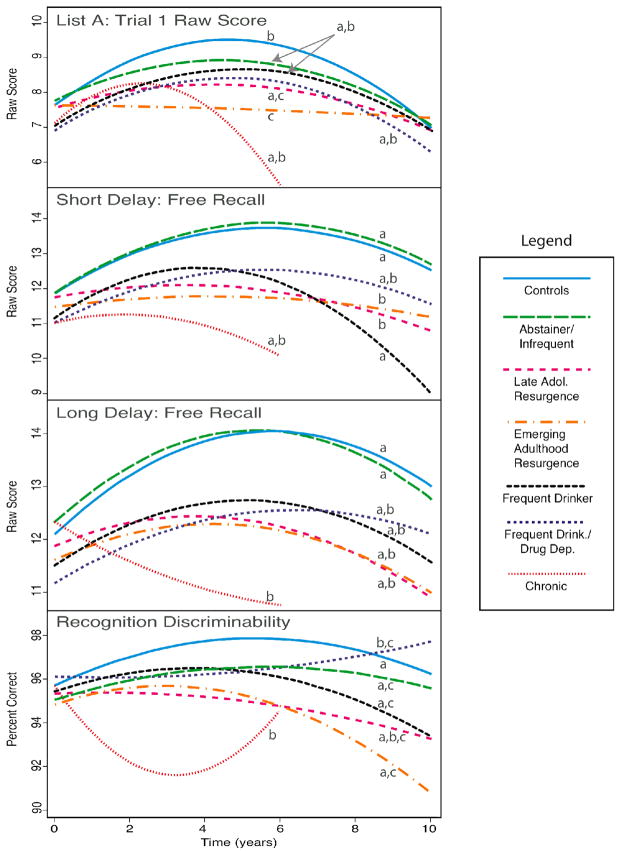
The AOD trajectories were significant predictors of CVLT List A Trial 1 raw scores (LR χ2 (18) = 36.70, p = .006) demonstrating differences in initial verbal learning over time based on substance use patterns (see Table 2). Controls and most AOD trajectory classes initially improved in verbal learning after baseline, peaking around the 4 to 6-year follow-ups (recalling about 1 more word than at baseline) and slightly declining through year 10 (see Figure 2). However, the two classes that had a resurgence of AOD involvement in late adolescence or early adulthood showed divergent patterns of verbal learning over the 10 years. Specifically, the Emerging Adulthood Resurgence class failed to show the normative increase in performance and remained relatively flat on this measure over time. The Late Adolescence Resurgence class showed a smaller peak in learning over time compared to Controls. Notably, the Chronic users showed an earlier peak in verbal learning at the 2-year follow-up but subsequently declined markedly through year 6, differing significantly from the Emerging Adulthood Resurgence class (Chronic users were not included at the 8- and 10-year time points due to sample size).
Table 2.
Summary of longitudinal analysis of changes in verbal learning and memory, visual memory, and attention over the 10-year follow-up period based on substance use trajectory classes. Predictor values are unstandardized B coefficients (and standard errors).
| Model | List A: Trial 1 | CVLTa | Recognition Discriminability | WISC/WAISb | ROCFc | |
|---|---|---|---|---|---|---|
| Short Delay Free Recall | Long Delay Free Recall | Arithmetic | 30-minute Delayed Recall | |||
| Log Likelihood | −2176.05 | −2271.96 | −2188.48 | −3022.36 | −2283.55 | −373.48 |
| Wald chi2 | 127.13 | 134.04 | 123.22 | 46.03 | 67.81 | 51.04 |
| Model P value | <.0001 | <.0001 | <.0001 | 0.0008 | <.0001 | <0.0001 |
| Predictors | ||||||
| Time | .53(.13)** | . 71(.13)* | .65(.12)** | .55(.29) | −.29(.13)* | −.59(.05) |
| Time2 | −.06(.01)** | .0004(.00001)** | .0004(.00001)* | −.05(.03) | .03(.01) | − |
| Intake Visual Reproductiond | - | - | - | - | - | .54(.18)** |
| Late Adol. Resurg. | −.18(.53) | −.12(.69) | −.45(.68) | .29(1.21) | −.36(.66) | −3.01(2.18) |
| Emerge. Adult. Resurg. | −.12(.55) | −.40(.71) | −.72(.71) | −.21(1.25) | −.66(.68) | −3.21(1.20) |
| Frequent Drinkers | −.68(.55) | −.71(.70) | −.82(.70) | .39(1.25) | −.36(.68) | −8.40(1.90)** |
| Frequent Drinkers/Drug Dep. | −.85(.55) | −.85(.70) | −1.15(.69) | 1.07(1.24) | −.37(.68) | −4.21(2.32) |
| Chronic | −.62(.83) | −.85(1.06) | −.01(1.05) | .64(1.88) | −.74(1.00) | − |
| Controls | −.13(.42) | −.04(.54) | −.24(.54) | .66(.94) | .86(.52) | −1.57(1.49) |
| Late Adol. Resurg. x Time | −.21(.23) | −.50(.24)* | −.35(.22) | −.48(.52) | −.03(.24) | −.2.28(1.13)* |
| Emerge. Adult. Resurg. x Time | −.54(.23)* | −.56(24)* | −.32(.22) | .03(.53) | −.04(.23) | .26(.1.10) |
| Frequent Drinkers x Time | .12(.22) | .03(.23) | −.16(.21) | .03(.51) | −.07(.23) | 2.4(.88)** |
| Heavy Drink/Drug Dep. x Time | .14(.22) | −.16(.23) | −.21(.20) | −.62(.49) | −.24(.23) | 2.63(1.14)* |
| Chronic x Time | .44(.56) | −.45(.59) | −1.06(.53)* | −3.07(1.29)* | −.20(.39) | - |
| Controls x Time | .28(.17) | −.03(.18) | −.03(.16) | .26(.39) | .22(.18) | −.33(.71) |
| Late Adol. Resurg.x Time2 | .02(.02) | .03(.03) | .02(.02) | .02(.06) | −.01(.03) | - |
| Emerge. Adult. Resurg. x Time2 | .06(.03)* | .05(.03) | .02(.02) | −.05(.06) | .01(.02) | - |
| Frequent Drinkers x Time2 | −.01(.02) | −.03(.02) | .01(.02) | −.03(.05) | .01(.02) | - |
| Heavy Drink/Drug Dep. x Time2 | −.01(.02) | −.01(.02) | −.03(.02) | −.07(.05) | .02(.02) | - |
| Chronic x Time2 | −.15(.09) | −.01(.10) | −.09(.09) | .44(.22)* | .01(.05) | - |
| Controls x Time2 | −.03(.02) | −.00(.02) | −.00(.02) | −.03(.04) | −.03(.02) | - |
| Constant | 7.75(.32)** | 11.87(.41)** | 12.33(.41)** | 95.04(.72)** | 10.6(.40)** | 14.87(2.05)** |
| Random Effects | ||||||
| Participant | 1.51(.10) | 2.23(.13) | 2.30(.13) | 3.40(.23) | 2.11(.12) | 3.83 (.45) |
Note. Abstainers/Infrequent Users were used as the reference group in these analyses.
CVLT = California Verbal Learning Test; CVLT-II administered at years 8 and 10.
WISC-R (baseline, 6 month, and 1 year follow-ups) and WAIS-R (years 2–10).
ROCF = Rey Osterrieth Complex Figure test (administered at years 8 and 10). Time2 coefficients are not present because only two time points were analyzed. The Chronic use class was not included due to a small sample size at later time points.
ROCF analysis controlled for Wechsler Memory Scale Visual Reproduction immediate and delayed recall scores at intake.
p<.05;
p<.01
Figure 2.
Verbal learning and memory (California Verbal Learning Test) performance across the 10-year follow-up period. Lines with different letters (a, b, c) significantly differ from each other at p<.05.
Note. CVLT List A Trial 1, Short Delay Free Recall, and Long Delay Free Recall raw scores were based on a possible total 16 words. Recognition Descriminability depicts the percent correct on the recognition trial.
The substance use trajectory classes predicted CVLT Short Delay Free Recall (SDFR) performance over time (LR χ2(18) = 56.93, p < .0001) showing a relationship between substance use patterns and short-term verbal recall (see Table 2). The classes performed similarly at baseline, and Controls and Abstainers/Infrequent Users improved in SDFR after baseline, peaking around year 6. However, the two classes with the most acute resurgence of AOD use (Late Adolescence Resurgence and Emerging Adulthood Resurgence) remained relatively flat over time, recalling 1–2 fewer words than Controls and Abstainers/Infrequent Users from years 2 through 10 (see Figure 2). While the Frequent Drinkers improved initially, they showed a steeper decline in SDFR through year 10, recalling about 2 fewer words than the Late Adolescence and Emerging Adulthood Resurgence classes. The Chronic users generally showed a downward trend, although this did not reach statistical significance.
The AOD trajectory classes also predicted CVLT Long Delay Free Recall (LDFR) performance (LR χ2(18) = 42.38, p = .001) demonstrating an association between delayed verbal recall and substance use over time (see Table 2). Similar to SDFR, the classes generally showed a slight improvement in LDFR following the baseline assessment, peaking around years 4 to 6 (recalling 1–2 more words than at baseline) and declining slightly thereafter (see Figure 2). The Chronic users showed the most pronounced difference in LDFR relative to Controls and Abstainers/Infrequent Users. Rather than showing the normative increase after baseline, Chronic users declined steadily, recalling 1–2 fewer words than at baseline by year 6.
AOD trajectory classes performed similarly in CVLT Recognition Discriminability (i.e., percent correct) at baseline, with about 95 to 96% accuracy, but substance use patterns significantly predicted recognition discriminability over the subsequent ten years (LR χ2(18) = 34.58, p = .011; see Table 2). Most classes either increased or decreased gradually over time (see Figure 2). However, the Chronic users differed in their trajectory compared to Controls, Abstainers/Infrequent Users, Emerging Adulthood Resurgence, and Frequent Drinkers. The Chronic users decreased significantly from baseline to the 4-year follow-up (92% accuracy) and then improved in recognition performance at year 6 (94% accuracy). The Frequent Drinkers/Drug Dependent class also differed in their performance relative to Controls, showing a significantly slower increase over time. Finally, the substance use trajectory classes did not predict performance on the other CVLT variables examined, including List A Trials 1 to 5 total and intrusion errors.
Visuospatial Function
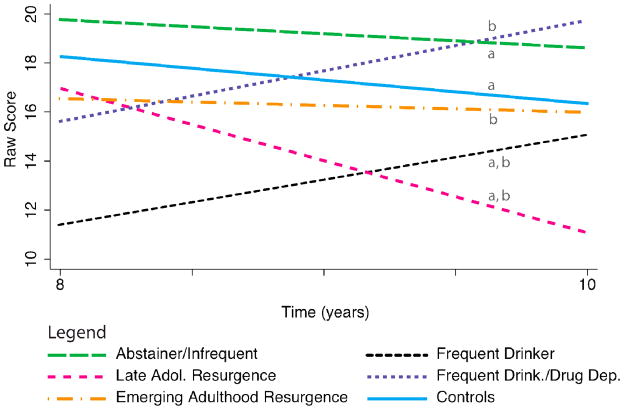
We examined performance on the Rey-Osterrieth Complex Figure (ROCF) copy and 30-minute delayed recall at the 8 and 10-year follow-ups when this test was administered, controlling for WMS Visual Reproduction Immediate and Delayed Recall performances, respectively, at baseline. AOD trajectory classes did not differ on their copy of the ROCF, but substance use patterns were significant predictors of delayed visual recall performance (LR χ2(10) = 33.85, p = .0002; see Table 2). The Controls, Abstainers/Infrequent Users, and Emerging Adulthood Resurgence classes remained relatively stable across time (see Figure 3). In contrast to the stability of those who abstained or used substances infrequently, the Late Adolescence Resurgence class showed a significant decline in delayed visual recall over the 8 to 10-year period, decreasing by about 6 raw score points (out of a possible total of 36 points). The Frequent Drinkers performed worse than most other classes at year 8 and showed a divergent pattern across time, improving by 3–4 points. The Frequent Drinkers/Drug Dependent class also improved from year 8 to year 10, although the trajectory differed from Frequent Drinkers. The Chronic users were not included in this analysis at 8 and 10 year time points. The AOD trajectory classes did not show statistically different visuospatial construction (Block Design) performance over the 10-year follow-up period.
Figure 3.
Visuospatial memory performance (Rey Osterrieth Complex Figure 30-minute delayed recall) from the 8- to 10-year follow-up time points. Lines with different letters (a, b, c, d) significantly differ from each other at p<.05.
Note. ROCF raw score is based on a possible total of 36 points.
Attention, Working Memory, and Executive Function
WISC-R/WAIS-R Arithmetic is a mental arithmetic test that requires verbal, attention, working memory, and reasoning skills (Tulsky et al., 2003). Performance on this measure varied over time in relation to AOD use trajectories (LR χ2(18) = 32.29, p = .020) suggesting that verbal attention/working memory is influenced by substance use patterns (see Table 2). At baseline, the Emerging Adulthood Resurgence trajectory class performed significantly worse than Controls (see Figure 4). Controls subsequently decreased slightly by about one scaled score point from baseline to the 10-year follow-up. In comparison, the Frequent Drinkers/Drug Dependent and Emerging Adulthood Resurgence classes showed a statistically different pattern of performance, demonstrating a steeper decline followed by a small improvement in verbal working memory, performing similar to controls by the 10-year follow-up. The Abstainers/Infrequent Users and Frequent Drinkers also showed a similar pattern to these two classes. The Late Adolescence Resurgence and Chronic users deteriorated more steadily over time, generally performing worse than other classes, although this did not reach significance. Finally, we did not find statistically significant differences between AOD use trajectory classes over time on other measures of attention, working memory, or executive function (WISC-R/WAIS-R Digit Span or Trails B time-to-completion).
Figure 4.
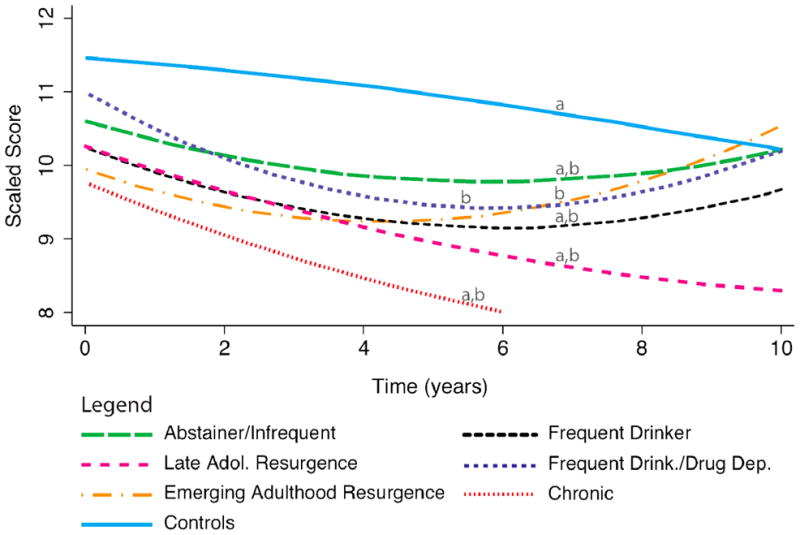
Verbal attention/working memory (WISC-R/WAIS-R Arithmetic) performance across the 10-year follow-up period. Lines with different letters (a, b) significantly differ from each other at p<.05.
Note. WISC-R/WAIS-R Arithmetic scaled scores have a mean of 10 and a standard deviation of 3.
Summary
To summarize, we found that substance use patterns over the 10-year period following AOD treatment were significantly associated with the longitudinal patterns of neuropsychological performance on measures of verbal learning and memory, visuospatial memory, and verbal attention/working memory. In general the Controls and Abstainers/Infrequent Users had the most successful performance over the follow-up period. A sharp increase in substance use during the late teens (Late Adolescence Resurgence) or early adulthood (Emerging Adulthood Resurgence) appeared to have a negative impact on verbal learning and memory and verbal attention/working memory, and a peak in heavy AOD use during late adolescence was also associated with visuospatial memory declines. The Frequent Drinkers/Drug Dependent class showed a divergent pattern in verbal working memory relative to Controls, although they improved in later years. Chronic AOD use throughout the follow-up period was associated with the most impaired performance in verbal learning, recall, and recognition relative to other AOD use patterns. Because heavy use of alcohol (without significant other drug use) was of special interest, the findings regarding the Frequent Drinkers are discussed in more detail below.
Persistent Heavy Alcohol Use and Neuropsychological Functioning
Hypothesis 2: Greater cognitive deficits will be associated with high dose alcohol consumption over time (Frequent Drinkers) compared to concomitant use of alcohol and other drugs (other AOD trajectory classes)
We found that heavy use of alcohol alone was related to verbal and visuospatial memory performance over time. Specifically, the Frequent Drinkers showed a normative increase and subsequent decrease in CVLT List A Trial 1 learning, while the Emerging Adulthood Resurgence class remained relatively unchanged over time (see Table 2; Figure 2). The Frequent Drinkers also differed in their CVLT Short Delay Free Recall trajectory from the Late Adolescence Resurgence and Emerging Adulthood Resurgence classes. Although the Frequent Drinkers showed the same initial increase in verbal short-term memory as most other classes, they showed a steeper decline from years 6 through 10, recalling about 2 fewer words than the two Resurgence classes at year 10. On CVLT Recognition Discriminability, the Frequent Drinkers showed a different pattern than the Chronic class, although the Chronic users differed from most other substance use classes on this measure. In regards to visuospatial memory, the Frequent Drinkers performed worse than the Controls, Abstainers/Infrequent Users, and both Resurgence classes at year 8 on ROCF 30-minute delayed recall (see Table 2). Although Frequent Drinkers showed a divergent pattern from most other classes from the 8 to 10-year follow-up in visuospatial memory, they improved to a similar performance level as Controls (see Figure 3). Frequent Drinkers did not differ from other AOD classes on other aspects of verbal memory (CVLT Total of Trials 1 to 5, Long Delay Free Recall, intrusion errors) or on other neuropsychological measures (WISC-R/WAIS-R Vocabulary, Similarities, Digit Span, Arithmetic, and Block Design; ROCF copy; Trail Making Test).
Relationship of Neuropsychological Function with Substance Withdrawal and Dependence
Hypothesis 3: Poorer neuropsychological functioning over the decade will be more strongly associated with the physiological impact of heavy substance use (i.e., withdrawal symptoms) rather than dependence symptoms
We found that the number of substance withdrawal symptoms over the previous three months at each follow-up time point significantly predicted verbal learning and memory performance (ps < .03). As shown in Table 3, having more withdrawal symptoms negatively affected memory performance for CVLT List A Trial 1 (p < .01), Short Delay Free Recall (p = .05), Long Delay Free Recall (p < .05), and recognition discriminability (p < .01) over time. Additionally, a withdrawal symptoms by time squared interaction was found with CVLT recognition discriminability (p < .01). By contrast, substance dependence symptoms did not show a significant relationship with NP performance during the 10-year follow-up period.
Table 3.
Summary of covariate analysis examining relationship of combined alcohol and other drug withdrawal and dependence symptoms with neuropsychological performance over 10 years. Values are unstandardized B coefficients (and standard errors).
| WISC/WAISa | List A: Trial 1 | CVLTb | Recognition Discriminability | ROCFc | ||
|---|---|---|---|---|---|---|
| Arithmetic | Short Delay Free Recall | Long Delay Free Recall | 30-minute Delayed Recall | |||
| Substance Withdrawal (past 3 mo.) | ||||||
| Log Likelihood | −2277.15 | −2166.59 | −2254.96 | −2186.06 | −3002.72 | −380.58 |
| Wald Chi2 | 33.59 | 96.65 | 80.12 | 77.01 | 23.11 | 10.89 |
| Model P value | <0.0001 | <0.0001 | <0.0001 | 0.0000 | 0.0003 | 0.0278 |
| Time (months) | −.026(.01)** | .046(.006)** | .045(.006)** | .046(.006)** | .050(.013)** | −.036(.029) |
| Time2 | .0002(.00005)** | .0004(.00005)** | .0004(.00005)** | .0004(.0001)** | −.0004(.0001)** | − |
| Substance Withdrawal | −.023(.014) | −.007(.014) | −.007(.001) | −.007(.014) | .042(.031) | −.16(.24) |
| Substance Withdrawal x Time | −.001(.001) | −.003(.001)* | −.0002(.0001)* | −.003(.001)* | −.011(.003)** | .032(.028) |
| Substance Withdrawal x Time2 | .0001(.0001) | .0001(.0001) | .0001(.001) | .0001(.0001) | .0001(.00003)** | − |
| Constant | 10.84(.20)** | 7.50(.16)** | 11.65(.21)** | 7.57(.165)** | 95.29(.38)** | 12.07(1.90)** |
| WMS Visual Reproduction c | - | - | - | - | - | .54(.19)** |
| Random Effects: Participant | 2.18 | 1.53 | 2.27 | 1.51 | 3.48 | 3.93 |
| Substance Dependence (past 3 mo.) | ||||||
| Log Likelihood | −1901.27 | −1815.29 | −1885.64 | −1832.32 | −2528.91 | −380.20 |
| Wald Chi2 | 52.24 | 60.66 | 61.45 | 42.60 | 16.96 | 10.65 |
| Model P value | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0046 | 0.0308 |
| Time | −.030(.008)** | .040(.007)** | .048(.007)** | .039(.007)** | .056(.017)** | −.034(.031) |
| Time2 | .0002(.0001)** | −.0004(.0001)** | −.0004(.0001)** | .0003(.0001)** | −.0005(.0001)** | − |
| Substance Dependence | .035(.047) | −.021(.045) | −.016(.047) | −.021(.043) | .001(.104) | −.223(.325) |
| Substance Dependence x Time | −.004(.002) | −.003(.002) | −.002(.002) | −.002(.002) | −.009(.005) | .006(.018) |
| Substance Dependence x Time2 | .00003(.00002) | .00003(.00002) | .000001(.00002) | .00001(.00002) | .0001(.00005) | - |
| Constant | 11.006(.230)** | 7.787(.204)** | 11.609(.240)** | 12.008(.234)** | 95.245(.467)** | 12.275(1.924)** |
| WMS Visual Reproduction c | - | - | - | - | - | .554(.192)** |
| Random Effects: Participant | 2.125 | 1.673 | 2.351 | 2.397 | 3.661 | 3.881 |
WISC-R (baseline, 6 month, and 1 year follow-ups) and WAIS-R (years 2–10).
CVLT = California Verbal Learning Test; CVLT-II administered at years 8 and 10.
Rey Osterrieth Complex Figure test (ROCF; administered at years 8 and 10) analysis controlled for Wechsler Memory Scale (WMS) Visual Reproduction performance at intake.
p≤.05;
p<.01
Discussion
This study sought to determine how specific patterns of alcohol and drug use following adolescent treatment are related to the progression of neuropsychological functioning over 10 years from mid-adolescence to the mid-twenties. This period represents a time of significant neurodevelopment (Gogtay et al., 2004) that may be influenced by substance use (Brown et al., 2008). The results showed that substance use patterns over the 10 years following A/SUD treatment were significantly related to verbal learning and memory, verbal attention/working memory, and visuospatial memory, with heavier use generally resulting in poorer cognition. Heavy use of alcohol alone over the follow-up period (with little or no other substance use) was associated with poorer verbal short-term memory compared to re-initiating heavy other drug use in late adolescence or early adulthood. Finally, recent substance withdrawal (but not dependence) symptoms were related to poorer verbal learning and recognition memory performance.
The current findings suggest that for youth with a history of alcohol and other substance use disorders, subsequent use of either alcohol or other drugs during adolescence and young adulthood may negatively impact verbal and visuospatial memory and verbal attention/working memory. The most chronic and severe class of AOD users demonstrated the poorest verbal memory over time, particularly on delayed verbal recall and recognition. Further, individuals whose AOD use substantially increased either during late adolescence or early adulthood had poorer verbal learning and memory over time relative to individuals with minimal or no AOD use during the decade of study. Although the substance use trajectory classes significantly predicted first trial learning on the CVLT, performance on the overall learning measure (total of trials 1–5) was not predicted by the classes. This finding suggests that the class differences in short and long delay free recall were not primarily a function of differential learning among the classes, but rather reflect difficulty in recalling or recognizing information. Interestingly, individuals whose AOD use increased during late adolescence showed a visuospatial memory decline during a period when their use was also decreasing, suggesting that visuospatial memory may be differentially sensitive to continued substance use during this time period.
Finally, individuals who were both frequent drinkers and drug dependent performed worse than community controls over time on a measure of verbal attention/working memory, although their pattern of performance was similar to several other AOD use classes. Interestingly, the substance use trajectory classes did not predict performance on another measure of verbal attention and working memory (WISC-R/WAIS-R Digit Span). One possible explanation for this discrepancy is the higher association of academic achievement in mathematics with WAIS-R Arithmetic versus Digit Span (r=.46 versus .35, respectively, with WRAT-3 Arithmetic; as cited in Reynolds, & Kamphaus, 2003). Anderson et al. (2010) reported that the AOD trajectory classes significantly predicted educational attainment, and therefore, educational differences in mathematics could have had some influence on their WISC-R/WAIS-R Arithmetic performance. Still, other measures that are more strongly associated with academic achievement (e.g., Vocabulary, Similarities; Heaton, Ryan, & Grant, 2009) did not appear to be affected by educational differences in this sample.
The association of verbal memory, visuospatial function, and attention decrements with heavier or more symptomatic substance use among young adults who underwent A/SUD treatment during adolescence supports our previous reports (Brown et al., 2000; Hanson et al., in press; Tapert & Brown, 1999; Tapert et al., 2002). Together, these findings suggest that individuals who generally abstain from AOD use following adolescent A/SUD treatment perform at similar cognitive levels as community youth without any alcohol or drug problems. However, continued heavy, chronic AOD use or a significant increase in use during late adolescence or early adulthood may adversely impact cognition even after AOD intake is reduced. For example, while the Late Adolescent Resurgence class decreased the frequency of alcohol and drug use at the 8 and 10 year follow-ups (approximate ages 24 and 26, respectively), these reductions did not result in subsequent improvements in learning and memory. This may indicate that damage resulting from sustained AOD use during certain stages of neurocognitive development persists beyond periods of heavy use.
As stated, we found evidence that use of alcohol alone (with minimal or no other drug use) may differentially affect cognition compared to combined alcohol and other drug use. Frequent drinkers initially demonstrated similar verbal short-term memory to individuals with other substance use patterns, but by the 10-year follow-up, frequent drinkers recalled fewer items than other classes after a short delay. Frequent drinkers also showed worse delayed visuospatial memory than other classes at an 8-year follow-up, but despite steady alcohol use, they performed similarly to controls two years later. Although these results are preliminary in nature, several studies have found that heavy alcohol use is associated with hippocampal abnormalities in animals (White & Swartzwelder, 2004), human adults (Jernigan et al., 1991; Sullivan et al., 1995), and adolescents (De Bellis et al., 2000; Medina, Schweinsburg et al., 2007; Nagel et al., 2005). Previous studies have also suggested that use of alcohol alone may be more detrimental than combined use of alcohol plus marijuana (Jacobus et al., 2009; Mahmood et al., 2010; Medina, Schweinsburg et al., 2007; Nixon et al., 1998). While the excitotoxic effects of alcohol on the brain have been demonstrated (Crews, 2008; Crews, Waage, Wilkie, & Lauder, 1999), further evidence suggests that the antioxidant properties of marijuana may have a paradoxical “protective effect” from alcohol’s neurotoxic consequences (Hamelink, Hampson, Wink, Eiden, & Eskay, 2005; Marsicano et al., 2003). Since marijuana was the most common drug of abuse other than alcohol in this sample, a protective effect may account for the poorer verbal memory performance of the heavy alcohol users as compared to the other substance use classes. However, this is purely speculative, and additional research is needed to determine the effects of heavy alcohol use versus combined alcohol and other drug use on cognition.
Finally, we found that withdrawal symptoms at each follow-up time point were related to poorer verbal learning, recall, and recognition, while substance dependence symptoms were not related to neurocognitive performance. These findings concur with several previous studies reporting that increasing alcohol or drug withdrawal symptoms were associated with poorer cognition (Brown et al., 2000; Freund, 1970; Glenn et al., 1988; Hanson et al., in press; Tapert et al., 2002), as well as the functional and structural integrity of the brain (McQueeny et al., 2009; Sullivan et al., 1996; Tapert et al., 2004). Together, these studies support Parsons & Stevens (1986) hypothesis that cognitive impairment increases with cumulative ethanol withdrawal episodes, and our findings extend this phenomenon to youth. While the possible mechanisms of brain damage from substance use and withdrawal are still being explored, there are suggestions from experimental animal studies. For example, rapid cell death and reduced neuro- and gliogenesis appear to occur within the hippocampus both during exposure to high levels of alcohol (i.e., binge drinking) and acute alcohol withdrawal (Crews et al., 2004). This damage may be caused by oxidative stress and inflammation from heavy alcohol use, as well as changes in glutamate exitotoxicity during withdrawal. The susceptibility of the hippocampus to alcohol neurotoxicity may subsequently result in mnemonic deficits. Fortunately, neuronal integrity may rebound to normal levels after prolonged abstention from alcohol (Crews et al., 2004), as was suggested by the normal neuropsychological functioning within the abstaining or infrequently using class in the present study.
Clinical Implications
Decrements in learning, memory, and attention may contribute to poorer academic achievement (Brown et al., 2000; Lynskey & Hall, 2000; Moss et al., 1994) and occupational functioning among these alcohol and drug involved young adults, resulting in lower high school and college graduation rates, lower occupational attainment, and lower SES (Anderson et al., 2010). While the social consequences of heavy substance use (e.g., increased relational conflict, reduced time spent tending to responsibilities, increased prevalence of legal problems) are likely involved, cognitive decrements may also contribute to poorer outcomes via reduced decision-making abilities and poorer performance in developmentally important arenas. Lowered cognition in conjunction with a deficient repertoire of coping skills has been shown to have an adverse impact on sustained abstention (e.g., Tapert, Brown, Myers, & Granholm, 1999). However, if cognitively compromised youth are provided with concrete coping skills to deal with high-risk situations, they may more successfully avoid relapse, thus preventing further damage and allowing for neurocognitive recovery. Further, cognitive decrements may be subtle and emerge slowly, and substance users may remain unaware of specific cognitive changes, especially if they have been present for several years. For this reason, youth may benefit from systematic personal neuropsychological testing feedback (Brown, et al., 2000; Ramo, Myers, & Brown, 2007). Finally, youth with an alcohol or drug dependence history who, after treatment, use alcohol heavily without use of other drugs may appear to be progressing from a psychosocial perspective and remain unnoticed by treatment professionals (Anderson et al., 2010), yet they may not be developing their full neurocognitive potential. The current study showed that heavy alcohol users performed similarly to or worse than classes who abused both alcohol and other drugs. It appears that continued heavy alcohol use (as well as other drug use) has measurable adverse neurocognitive impacts which are not currently considered in the diagnostic criteria for abuse or dependence.
Limitations
In order to accurately interpret these findings, it is important to consider the possibility that recent substance use may have impacted performance such that classes with a higher use frequency performed worse due to short-term effects of recent use rather than as a result of incurring more extensive, long-term neurocognitive damage. Unfortunately, we were not able to examine the differential effects of withdrawal within each class. Further, while recent substance use may be considered a limitation, another perspective is that it increases generalization of these findings to substance users who are similar to any of the regular users included here. That is, if they continue using, they may continue to have negative cognitive effects. Second, we did not examine the effects of specific substances on cognition. Because multiple substances are commonly used together or in succession during this age period (Brown, et al., 2000), our approach was to examine how different patterns of alcohol and other drug use impacted cognition over time. Thus, it is difficult to attribute any deficit to a particular drug especially in the context of polysubstance use. Third, it is possible that participants may have over- or underreported their substance use. To minimize this possibility, we chose a measure with good test-retest reliability, interviewed significant others to corroborate participant report, and requested that a random sample of participants complete Breathalyzer and urinalysis at each time point. These corroborated reports and biological specimens demonstrated good agreement with participants’ self-report in this and other samples (Brown et al., 1998). Fourth, the small sample size of the Chronic use class limited our ability to detect statistical differences between classes, especially at the 8- and 10-year follow-ups. Although other substance use classes were larger, an overall increased sample size would provide additional power to detect subtle differences or interactions over time.
We excluded for Axis I psychiatric disorders at baseline in order to focus more specifically on the effects of substance use on cognition, since various psychiatric disorders have been associated with cognitive deficits (e.g., Gallagher, Reid, & Ferrier, 2009; Hammar & Ardal, 2009). Although we did not exclude for psychiatric disorders at the follow-up timepoints, this may limit the generalizability of our findings to substance abusing populations without comorbid psychiatric disorders. However, clinical youth were not excluded for the presence of conduct disorder symptoms at baseline, given the high rates of comorbidity in this population (e.g., Farmer, Seeley, Kosty, & Lewinsohn, 2009). While this may help generalize these findings to other substance abusing youth and young adults, the presence of conduct disorder or antisocial personality disorder symptomotology could account for some of the cognitive deficits seen here. Although the substance abusing youth did not differ significantly from each other in the number of CD symptoms present at study intake, this is certainly an issue to examine in future research. In addition, the complexity of the analyses completed in this study and issues of statistical power limited our ability to fully examine other potential interacting factors, such as age, gender, ethnicity, SES, education, and family history of A/SUD. Finally, many of the neuropsychological tests in this battery tap into multiple cognitive abilities (e.g., WISC-R/WAIS-R Arithmetic), some of which are intercorrelated (e.g., scores on the CVLT), and conversely a single test (e.g., Trails B) does not fully assess a cognitive domain such as executive function. The descriptions of the neuropsychological domains are purely organizational and were not based on a factor analysis. Despite the potential limitations, we believe the current findings are important and relatively rare in examining the fluctuations in cognition in relation to various substance use patterns over the course of a decade.
Conclusions and Future Directions
In conclusion, this study found that verbal learning and memory, visuospatial memory, and verbal attention/working memory decrements were significantly related to patterns of AOD use unfolding from middle adolescence to the middle twenties for those youth who received A/SUD treatment during adolescence. Further, heavy use of alcohol alone was associated with verbal short-term memory decline. Finally, greater AOD withdrawal symptoms may signify poorer verbal learning and recognition and may reflect the neurotoxic effects of AOD use on the brain. Fortunately, individuals who discontinue AOD use during this time period appear to largely recover their cognitive functions, suggesting possible neurological repair. These findings imply that alcohol and other drug use during adolescence and young adulthood may primarily impact functions subserved by the lateral temporal lobes and prefrontal cortex, which are among the last cortical structures to reach maturity (Gogtay et al., 2004; Jernigan & Gamst, 2005). Previous studies also implicate other brain areas, including parietal regions (Tapert et al., 2004).
More research is needed to determine the mechanisms underlying the impact of individual substances and combinations of drugs (e.g., alcohol alone versus alcohol plus marijuana) on brain integrity. Future studies may determine how substance use during neurodevelopment affects the brain on micro- and macro-structural levels. Evaluation of substance users after varying lengths of abstinence may help determine the duration of any injury or cognitive decrements after substance use is discontinued. Future research may also examine how co-morbid Axis I psychiatric disorders (e.g., depression, anxiety, attention disorders) may influence and interact with cognition in the context of long-term substance use. Particular attention should be given to co-morbid conduct disorder and antisocial personality disorder in substance using populations, since these externalizing disorders have been associated with cognitive deficits (e.g., Finn et al., 2009). The influence of other demographic and developmental factors on cognition over time in substance users is also important to explore (e.g., gender; SES; ethnicity; family history of A/SUD; prenatal exposure to nicotine, alcohol, drugs, or other environmental toxins). Finally, this battery represents a sampling of various cognitive abilities, and future studies may expand upon the neuropsychological domains examined.
Acknowledgments
This research was supported by the following National Institutes of Health grants and fellowships: R37 AA007033-23, 5 R21 AA017321-02, and 1 R01 DA021905-01A1 (PI: Brown). The authors would like to acknowledge the contributions of treatment sites and research staff making this extended research possible. In addition, we would like to thank Mark Nakamura for his contributions to the statistical analysis.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/adb
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: American Psychiatric Association; 1987. rev ed. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Anderson KG, Ramo DE, Cummins K, Brown SA. Alcohol and drug involvement after adolescent treatment and functioning during emerging adulthood. Drug and Alcohol Dependence. 2010;107:171–181. doi: 10.1016/j.drugalcdep.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aseltine RH, Jr, Gore S. Work, postsecondary education, and psychosocial functioning following the transition from high school. Journal of Adolescent Research. 2005;20(6):615–639. [Google Scholar]
- Brame B, Nagin DS, Tremblay RE. Developmental trajectories of physical aggression from school entry to late adolescence. Journal of Child Psychology and Psychiatry. 2001;42:503–512. [PubMed] [Google Scholar]
- Brown SA, D’Amico EJ, McCarthy DM, Tapert SF. Four year outcomes from adolescent alcohol and drug treatment. Journal of Studies on Alcohol. 2001;62(3):381–388. doi: 10.15288/jsa.2001.62.381. [DOI] [PubMed] [Google Scholar]
- Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, et al. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics. 2008;121(Suppl 4):S290–310. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59(4):427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Mott MA, Vik PW. Correlates of success following treatment for adolescent substance abuse. Applied & Preventive Psychology. 1994;3:61–73. [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: Effects of protracted alcohol use. Alcoholism: Clinical and Experimental Research. 2000;24:164–171. [PubMed] [Google Scholar]
- Brown SA, Vik PW, Creamer VA. Characteristics of relapse following adolescent substance abuse treatment. Addictive Behaviors. 1989;14:291–300. doi: 10.1016/0306-4603(89)90060-9. [DOI] [PubMed] [Google Scholar]
- Cahalan D. Problem drinkers. San Francisco: Jossey-Bass; 1970. [Google Scholar]
- Chassin L, Flora DB, King K. Trajectories of alcohol and drug use and dependence from adolescence to adulthood: The effects of familial alcoholism and personality. Journal of Abnormal Psychology. 2004;113:483–498. doi: 10.1037/0021-843X.113.4.483. [DOI] [PubMed] [Google Scholar]
- Clark DB, Buckstein O, Cornelius J. Alcohol use disorders in adolescents: Epidemiology, diagnosis, psychosocial interventions, and pharmacological treatment. Pediatric Drugs. 2002;4(8):493–502. doi: 10.2165/00128072-200204080-00002. [DOI] [PubMed] [Google Scholar]
- Crews FT. Effects of alcohol abuse on brain neurochemistry. In: Brick J, editor. Handbook of the medical consequences of alcohol and drug abuse. 2. New York, NY: The Haworth Press/Taylor and Francis Group; 2008. pp. 123–176. The Haworth Press series in neuropharmacology. [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcoholism: Clinical and Experimental Research. 2000;24(11):1712–1723. [PubMed] [Google Scholar]
- Crews FT, Collins MA, Dlugos C, Littleton J, Wilkins L, Neafsey EJ, et al. Alcohol-induced neurodegeneration: When, where and why? Alcoholism: Clinical and Experimental Research. 2004;28(2):350–364. doi: 10.1097/01.alc.0000113416.65546.01. [DOI] [PubMed] [Google Scholar]
- Crews FT, Waage HG, Wilkie MB, Lauder JM. Ethanol pretreatment enhances NMDA excitotoxicity in biogenic amine neurons: Protection by brain derived neurotrophic factor. Alcoholism: Cinical and Experimental Research. 1999;23(11):1834–1842. [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, et al. Hippocampal volume in adolescent-onset alcohol use disorders. American Journal of Psychiatry. 2000;157(5):737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus and cerebellar volumes in adolescents and young adults with adolescent onset alcohol use disorders and co-morbid mental disorders. Alcoholism: Clinical and Experimental Research. 2005;29(9):1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test Manual, Research Edition. San Antonio: Psychological Corporation; 1987. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. Manual for the California Verbal Learning Test. 2. San Antonio, TX: Psychological Corp; 2000. (CVLT-II) [Google Scholar]
- Eichenbaum H. The hippocampus and mechanisms of declarative memory. Behavioural Brain Research. 1999;103:123–133. doi: 10.1016/s0166-4328(99)00044-3. [DOI] [PubMed] [Google Scholar]
- Farmer RF, Seeley JR, Kosty DB, Lewinsohn PM. Refinements in the hierarchical structure of externalizing psychiatric disorders: Patterns of lifetime liability from mid-adolescence through early adulthood. Journal of Abnormal Psychology. 2009;118:699–710. doi: 10.1037/a0017205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PR, Rickert ME, Miller MA, Lucas J, Bogg T, Bobova L, et al. Cognitive ability in alcohol dependence: Examining the role of covarying externalizing psychopathology. Journal of Abnormal Psychology. 2009;118:100–116. doi: 10.1037/a0014656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JK, Meikle A, Goodson G, Mayes AR, Howard M, Sunram SI, et al. The hippocampus and delayed recall: Bigger is not necessarily better? Memory. 1999;7:715–732. doi: 10.1080/096582199387823. [DOI] [PubMed] [Google Scholar]
- Frees EW. Longitudinal and Panel Data: Analysis and Applications in the Social Sciences. Cambridge, UK: Cambridge University Press; 2004. [Google Scholar]
- Freund G. Alcohol, barbiturate, and bromide withdrawal syndrome in mice. In: Mello NK, Mendelson JH, editors. Recent advances in studies of alcoholism; An interdisciplinary symposium. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1970. DHEW publication; no. (HSM) 71–9045. [Google Scholar]
- Fryer SL, Frank LR, Spadoni AD, Theilmann RJ, Nagel BJ, Schweinsburg AD, et al. Microstructural integrity of the corpus callosum linked with neuropsychological performance in adolescents. Brain and Cognition. 2008;67:225–233. doi: 10.1016/j.bandc.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher P, Reid KS, Ferrier IN. Neuropsychological functioning in health and mood disorder: Modulation by glucocorticoids and their receptors. Psychoneuroendocrinology. 2009;34S:S196–S207. doi: 10.1016/j.psyneuen.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Mezzich AC, Tarter RE. Disruptive, delinquent and aggressive behavior in female adolescents with a psychoactive substance use disorder: Relation to executive cognitive functioning. Journal of Studies on Alcohol. 1998;59:560–567. doi: 10.15288/jsa.1998.59.560. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Glenn SW, Parsons OA, Sinha R, Stevens L. The effects of repeated withdrawals from alcohol on the memory of male and female alcoholics. Alcohol and Alcoholism. 1988;23:337–342. doi: 10.1093/oxfordjournals.alcalc.a044826. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Thompson PM. Mapping gray matter development: Implications for typical development and vulnerability to psychopathology. Brain and Cognition. 2010;72(1):6–15. doi: 10.1016/j.bandc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of Substance Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Hamelink C, Hampson A, Wink DA, Eiden LE, Eskay RL. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. The Journal of Pharmacology and Experimental Therapeutics. 2005;314(2):780–788. doi: 10.1124/jpet.105.085779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar A, Ardal G. Cognitive functioning in major depression - a summary. Frontiers in Human Neuroscience. 2009;3 doi: 10.3389/neuro.09.026.2009. Article 26, 7 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Cannabinoids reveal the necessity of hippocampal neural encoding for short-term memory in rats. The Journal of Neuroscience. 2000;20(23):8932–8942. doi: 10.1523/JNEUROSCI.20-23-08932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Padula CB, Tapert SF, Brown SA. How does adolescent alcohol and drug use affect neuropsychological functioning in young adulthood?: 10-year outcomes. Journal of Child & Adolescent Substance Abuse. doi: 10.1080/1067828X.2011.555272. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Ryan L, Grant I. In: Neuropsychological Assessment of Neuropsychiatric and Neuromedical Disorders. 3. Grant I, Adams KM, editors. New York: Oxford University Press; 2009. pp. 127–155. [Google Scholar]
- Hollingshead AB. Two-factor index of social position. New Haven, CT: Yale University Press; 1965. [Google Scholar]
- Horn JI, Skinner HA, Wanberg K, Foster FM. Alcohol Dependence Scale (ADS) Toronto: Addiction Research Foundation; 1984. [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, et al. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicology and Teratology. 2009;31:349–355. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Butters N, DiTraglia G, Schafer K, Smith T, Irwin M, et al. Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcoholism: Clinical and Experimental Research. 1991;15:418–427. doi: 10.1111/j.1530-0277.1991.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC. Changes in volume with age: Consistency and interpretation of observed effects. Neurobiology of Aging. 2005;26(9):1271–1274. doi: 10.1016/j.neurobiolaging.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Jung T, Wickrama KAS. An introduction to latent class growth analysis and growth mixture modeling. Social and Personality Psychology Compass. 2008;2/1:302–317. [Google Scholar]
- Kypri K, McCarthy DM, Coe MT, Brown SA. Transition to independent living and substance involvement of treated and high risk youth. Journal of Child and Adolescent Substance Abuse. 2004;13(3):85–100. [Google Scholar]
- Lezak MD. Neuropsychological Assessment. 3. Oxford University Press; New York: 1995. [Google Scholar]
- Lu KH. The indexing and analysis of drug indulgence. International Journal of the Addictions. 1974;9:785–804. doi: 10.3109/10826087409022175. [DOI] [PubMed] [Google Scholar]
- Lynskey M, Hall W. The effects of adolescent cannabis use on educational attainment: A review. Addiction. 2000;95:1621–1630. doi: 10.1046/j.1360-0443.2000.951116213.x. [DOI] [PubMed] [Google Scholar]
- Mahmood O, Jacobus J, Bava S, Scarlett A, Tapert SF. Learning and memory performances in adolescent users of alcohol and marijuana: Interactive effects. Journal of Studies on Alcohol and Drugs. 2010;71:885–894. doi: 10.15288/jsad.2010.71.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, et al. Altered white matter integrity in adolescent binge drinkers. Alcoholism: Cinical and Experimental Research. 2009;33(7):1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after a month of abstinence. Journal of the International Neuropsychological Society. 2007;13:807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: Unique gender effects. Alcoholism: Clinical and Experimental Research. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Tapert SF. Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry Research: Neuroimaging. 2010;182:152–159. doi: 10.1016/j.pscychresns.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicology and Teratology. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HB, Kirisci L, Gordon HW, Tarter RE. A neuropsychologic profile of adolescent alcoholics. Alcoholism: Clinical and Experimental Research. 1994;18:159–163. doi: 10.1111/j.1530-0277.1994.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Research. 2005;139(3):181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagin D. Analyzing developmental trajectories: Asemiparametric, group-based approach. Psychological Methods. 1999;4:139–157. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- Nixon SJ, Paul R, Phillips M. Cognitive efficiency in alcoholics and polysubstance abusers. Alcoholism: Clinical & Experimental Research. 1998;22:1414–1420. doi: 10.1111/j.1530-0277.1998.tb03929.x. [DOI] [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d’une figure complexe. Archives of Psychology. 1944;30:206–356. [Google Scholar]
- Parsons OA, Stevens L. Previous alcohol intake and residual cognitive deficits in detoxified alcoholics and animals. Alcohol & Alcoholism. 1986;21:137–157. [PubMed] [Google Scholar]
- Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling using Stata. College Station, TX: Stata Press; 2005. [Google Scholar]
- Ramo DE, Myers MG, Brown SA. Relapse Prevention for Adolescent Substance Abuse: Overview and Case Examples. In: Witkiewitz K, Marlatt GA, editors. Therapist’s Guide to Evidence-Based Relapse Prevention. New York: Elsevier; 2007. pp. 293–311. [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. Tucson, AZ: Neuropsychology Press; 1993. [Google Scholar]
- Reynolds CR, Kamphaus RW, editors. Handbook of Psychological and Educational Assessment of Children: Intelligence, Aptitude, and Achievement. 2. New York: The Guilford Press; 2003. [Google Scholar]
- Rohde P, Lewinsohn PM, Seeley JR. Psychiatric comorbidity with problematic alcohol use in high school students. Journal of the American Academy of Child & Adolescent Psychiatry. 1996;35:101–109. doi: 10.1097/00004583-199601000-00018. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, McQueeny T, Nagel BJ, Eyler LT, Tapert SF. A preliminary study of fMRI response during verbal encoding among adolescent binge drinkers. Alcohol. 2010;44:111–117. doi: 10.1016/j.alcohol.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. The Journal of Neuroscience. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychology of Addictive Behaviors. 2009;23(4):715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Declarative and nondeclarative memory: Multiple brain systems supporting learning and memory. Journal of Cognitive Neuroscience. 1992;4:222–243. doi: 10.1162/jocn.1992.4.3.232. [DOI] [PubMed] [Google Scholar]
- Stewart DG, Brown SA. Withdrawal and dependency symptoms among adolescent alcohol and drug abusers. Addiction. 1995;90:627–635. doi: 10.1046/j.1360-0443.1995.9056274.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcoholism: Cinical and Experimental Research. 1995;19(1):110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Relationship between alcohol withdrawal seizures and temporal lobe white matter volume deficits. Alcoholism: Clinical & Experimental Research. 1996;20:348–354. doi: 10.1111/j.1530-0277.1996.tb01651.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Neuropsychological correlates of adolescent substance abuse: Four year outcomes. Journal of the International Neuropsychological Society. 1999;5(6):481–493. doi: 10.1017/s1355617799566010. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown SA, Myers MG, Granholm E. The role of neurocognitive abilities in coping with adolescent relapse to alcohol and drug use. Journal of Studies on Alcohol. 1999;60(4):500–508. doi: 10.15288/jsa.1999.60.500. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: Neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society. 2002;8(7):873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, et al. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2004;28(10):1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Mezzich AC, Hsieh YC, Parks SM. Cognitive capacity in female adolescent substance abusers. Drug and Alcohol Dependence. 1995;39(1):15–21. doi: 10.1016/0376-8716(95)01129-m. [DOI] [PubMed] [Google Scholar]
- Tulsky DS, Saklofske DH, Chelune GJ, Heaton RK, Ivnik RJ, Bornstein R, et al., editors. Clinical Interpretation of the WAIS-III and WMS-III. San Diego, CA: Academic Press; 2003. p. 81. [Google Scholar]
- Wechsler D. Wechsler memory scale. New York: Psychological Corporation; 1945. [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children-Revised. San Antonio, TX: The Psychological Corporation; 1974. [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale -Revised. San Antonio, TX: Psychological Corporation; 1981. [Google Scholar]
- West BT, Welch KB, Galecki AT. Linear Mixed Models, A Practical Guide Using Statistical Software. Boca Raton, FL: Chapman & Hall/CRC; 2007. [Google Scholar]
- White AM, Swartzwelder HS. Hippocampal function during adolescence: A unique target of ethanol effects. Annals of the New York Academy of Sciences. 2004;1021:206–220. doi: 10.1196/annals.1308.026. [DOI] [PubMed] [Google Scholar]