Abstract
Tumor necrosis factor-α (TNFα) was cloned over 2 decades ago and its identification in part led to the discovery of a super family of tumor necrosis factors (TNFs) and their receptors. TNFα signals through two transmembrane receptors, TNFR1 and TNFR2, and regulates a number of critical cell functions including cell proliferation, survival, differentiation, and apoptosis. Macrophages are the major producers of TNFα and interestingly are also highly responsive to TNFα. Aberrant TNFα production and TNF receptor signaling have been associated with the pathogenesis of several diseases, including rheumatoid arthritis, Crohn’s disease, atherosclerosis, psoriasis, sepsis, diabetes, and obesity. TNFα has been shown to play a pivotal role in orchestrating the cytokine cascade in many inflammatory diseases and because of this role as a “master-regulator” of inflammatory cytokine production, it has been proposed as a therapeutic target for a number of diseases. Indeed anti-TNFα drugs are now licensed for treating certain inflammatory diseases including rheumatoid arthritis and inflammatory bowel disease. In this review we discuss the discovery of TNFα and its actions especially in regulating macrophage biology. Given its importance in several human diseases, we also briefly discuss the role of anti-TNFα therapeutics in the treatment of inflammatory diseases.
Keywords: TNFα, disease, inflammation, macrophage, arthritis, inflammatory bowel disease
I. INTRODUCTION
It has been known for over a century that bacterial-derived endotoxins can cause hemorrhagic necrosis of tumors in humans. However, it was not until 1962 that O’Malley et al.1 first demonstrated that this effect of endotoxins is indirect. They showed that serum from animals treated with lipopolysaccharides (LPS) could trigger hemorrhagic necrosis of tumors in animals not exposed to LPS. O’Malley et al. further proposed that the effect of endotoxin is mediated by a “tumor-necrotizing factor.” One decade later, Carswell et al.2 showed that the serum of bacillus Calmette-Guérin–infected mice treated with endotoxin contained a substance, which they named “tumor necrosis factor” (TNF) and further demonstrated that the actions of TNF on tumor necrosis were similar to that of the endotoxin. They demonstrated that the TNF-positive serum was equally effective to that of endotoxin in causing hemorrhagic necrosis of methylcholanthrene A-induced fibrosarcoma (Meth A). In addition, they showed that TNF can cause necrosis of several transplanted tumors similar to that of Meth A.2
One decade after these observations, the cDNA for human and murine TNFα were cloned and expressed in Escherichia coli.3,4 Human TNFα was also purified to homogeneity from the cell culture supernatants of the HL-60 cell line as a protein with a molecular weight of 17,000 by Aggarwal et al.5 Interestingly, in some independent studies Beutler and colleagues identified TNF as a factor that induces LPS-induced wasting or cachexia in mice.6,7 Subsequent studies have demonstrated that TNF is a prototypic member of a large superfamily known as the TNF/TNFR superfamily, which now comprises more than 40 members. Considerable advances have been made in our understanding of the biology and the clinical role of TNFα. In this review, we focus mainly on the role of TNFα in macrophage biology and how these studies are relevant to the pathogenesis of many inflammatory diseases.
II. STRUCTURE AND SYNTHESIS OF TNFα
The TNFα gene is present as a single copy gene on human chromosome 6 (murine chromosome 17).8 The gene consists of four exons and three introns. Interestingly, more than 80% of the mature TNFα sequence is encoded in the fourth exon. Exons I and II mainly contain the leader peptide sequence. Messenger RNA for TNFα is expressed in a wide range of cells, including monocytes and macrophages. TNFα gene expression is regulated at the transcriptional level by several factors, including nuclear factor kappa b (NFκB) and nuclear factor activated T cells (NF-AT). TNFα production is also regulated at the translational level via the UA-rich sequence in the 3′ untranslated region of human TNFα mRNA8,9 Human TNFα is expressed as a 27-kDa (233 amino acid) protein that is then proteolytically cleaved to a 17-kDa (157 amino acid) molecule. The 76-amino-acid presequence in the 27-kDa protein is highly conserved, and seems to serve to anchor the precursor protein to the membrane. This membrane integrated 27-kDa TNFα (mTNFα) undergoes proteolytic cleavage by a metalloprotease TNFα-converting enzyme (TACE), resulting in the 17-kDa soluble TNFα or sTNFα.10,11 The 17-KDa TNFα protomers are composed of two antiparallel β-pleated sheets with antiparallel β-strands, which form a jelly-roll β-structure. It is believed that mTNFα and sTNFα regulate biological responses at autocrine/paracrine and endocrine levels, respectively.12,13
III. TNFα SIGNAL TRANSDUCTION
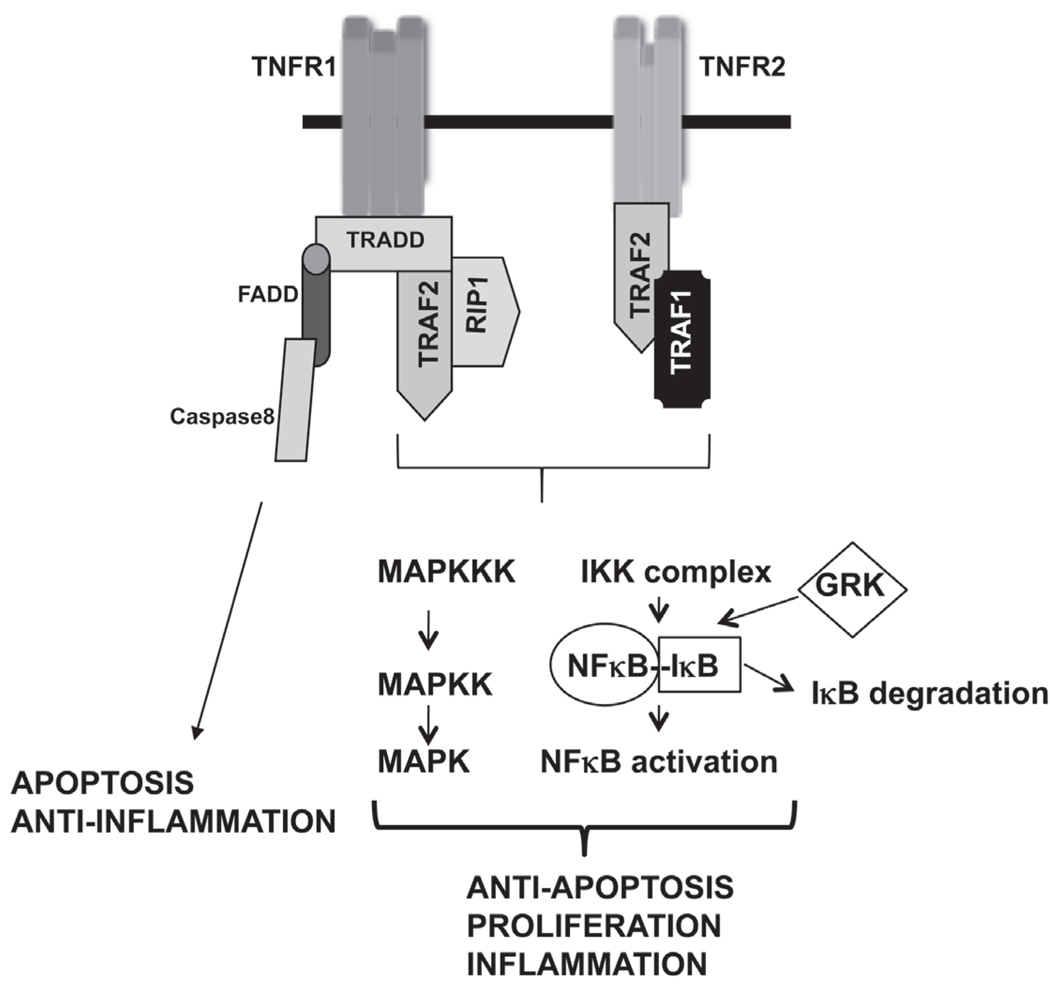
TNFα is a pleiotropic cytokine produced by many different types of cells in the body. However, cells of the monocytic lineage—such as macrophages, astroglia, microglia, Langerhans cells, Kupffer cells, and alveolar macrophages—are the primary synthesizers of TNFα.14,15 TNFα acts through two transmembrane receptors: TNF receptor 1 (TNFR1), also known as p55 or p60, and TNF receptor 2 (TNFR2), also known as p75 or p80 (Fig. 1). TNFR1 and TNFR2 both contain four cysteine-rich repeats in their extracellular domains, forming elongated shapes that interact with the lateral grooves of TNFα trimer formed between two of its three protomers.16 TNFR1 is constitutively expressed in most mammalian tissues, whereas the expression of TNFR2 is highly regulated and is typically expressed in the cells of the immune system. Binding of TNFα onto TNFR1 is considered to be an irreversible mechanism, whereas binding of TNFα onto TNFR2 has both rapid on and off kinetics. Therefore, it has been suggested that TNFR2 might act as a “ligand passer” to TNFR1 in some cells, increasing the local concentration of TNFα at the cell surface through rapid ligand binding and dissociation.17
FIGURE 1.
Signaling from TNFα receptors.
TNFα binds to both TNFR1 and TNFR2 with high affinity. There is, however, some species specificity in terms of the receptor subtype and TNFα binding. It has been shown that human TNFα binds only to the mouse TNFR1.18,19 There are also other unique differences between TNFR1 and TNFR2. For example, the cytoplasmic portion of TNFR1, but not TNFR2, contains a death domain. In addition, TNFR2 can be fully activated only by mTNFα and not by sTNFα.20 Both TNFR1 and TNFR2 can be cleaved from the cell surface by the matrix metalloproteinase family of enzymes in response to an inflammatory signal. Interestingly, these cleaved extracellular domains of the TNF receptors retain the ability to bind TNFα, and thus function as endogenous inhibitors of TNFα.21
Activation from TNFR1 is responsible for a large number of inflammatory responses classically attributed to TNFα. This has Volume 20, Number 2, 2010 been extensively demonstrated by experiments using receptor-specific antibodies,22,23 receptor-specific ligands,24,25 and TNFR1- or TNFR2-deficient mice.14,26,27 TNFα trimer binds to the extracellular domain of TNFR1, releasing the inhibitory protein, silencer of death domains (SODD), from the intracellular domain of TNFR1. The intracellular domain of the oligomerized TNFR1 is then bound by an adaptor protein TNF receptor-associated death domain (TRADD),28 which recruits additional adaptor proteins: receptor interacting protein-1 (RIP-1), a serine/threonine kinase,29 and TNFR-associated factor 2 (TRAF2), an E3 ubiquitin ligase (Fig. 1).30 This complex is then internalized and the TRADD-RIP-1-TRAF2 complex is released from TNFR1. These adapter proteins are then involved in activating key signaling pathways. RIP-1 recruitment of MEKK-3 and transforming growth factor-beta (TGFβ)-activated kinase (TAK1) subsequently activates the IKK (inhibitor of κB kinase) complex. The IKK complex then phosphorylates (primarily by IKKβ) IκBα, as well as other IκB proteins, which then leads to the ubiquitination and degradation of IκBα. This then results in the release of NFκB subunits that are bound to IκBα under unstimulated conditions. The free NFκB subunits translocate into the nucleus and evoke gene transcription.31–36
In addition to RIP1-MEKK3-TAK1–mediated activation of the NFκB pathway, TRAF2 has also been shown to activate NFκB by binding to the IKK complex37 and by recruiting inhibitor of cellular apoptosis proteins (cIAP)-1 and cIAP-2. These caspase inhibitors also possess ubiquitin ligase activity by which they play a role in IκB degradation.31 Interestingly, it has been shown that TNF induction of NFκB activation in macrophages can be mediated by c-Src, a nonreceptor tyrosine kinase.38 Therefore, there are many variations of the signaling mediators for TNFα-induced signaling pathways, depending on the cell type. In this regard, we recently showed that TNFα-induced NFκB activation in a macrophage cell line is dependent on G-protein coupled receptor kinases-2 and -5.39 G-protein coupled receptor kinases were originally discovered for their role in the desensitization of G-protein coupled receptors.40 In a recent study, we found that GRK2 and GRK5 can directly interact with and phosphorylate IκBα and mediate TNFα-induced NFκB activation in Raw264.7 macrophages (Fig. 1). We also found that IKKβ was dispensable in this particular cell line because knock-down of IKKβ did not affect TNFα-induced IκBα phosphorylation, whereas knockdown of GRK2 and GRK5 blocked TNFα-induced IκBα phosphorylation and NFκB activation. It is not clear if this role of GRK2 and 5 is specific for macrophages or for this particular macrophage cell line. Sorriento et al.41 recently reported that GRK5 is a negative regulator of NFκB signaling in endothelial cells, and this role of GRK5 is independent of its kinase activity in an endothelial cell line. Thus, the role of GRKs in NFκB signaling may depend on the cellular context.
Stimulation of TNFR also activates a MAP3K called apoptosis-signaling kinase-1 (ASK-1)42 that associates with TRAF2 in the TRADD-RIP-1-TRAF2 complex, activating MAP2Ks, MEK-4, and MEK-6, which in turn activate c-Jun N-terminal kinases (JNKs) and p38 MAPK.43 JNK and p38 MAPK subsequently activate transcription of many genes via transcription factors such as AP-1. In addition to JNK and p38 MAPKs, TNFR also activates the ERK signaling pathway via activation of TPL2-MEK-ERK pathway.35 Stimulation of the TPL2 pathway involves the IKKβ-mediated phosphorylation of NFκB1 p105, which is stoichiometrically associated with TPL2 under unstimulated conditions. IKKβ phosphorylation of p105 leads to ubiquitination and partial degradation of p105, which then releases TPL2. The free TPL2 then activates the MEK-ERK signaling.44–47 In addition to these signaling pathways, TNFR1 activation is also involved in pro-apoptotic signaling via the Fas-associated death domain. Micheau et al.48 showed that TNFR1-induced pro-apoptotic signaling is mediated by the formation of two distinct signaling complexes. Complex-I is formed rapidly at the plasma membrane and consists of TNFR1, TRADD, RIP, TRAF2, and c-IAP1, and this complex triggers NFκB response without affecting apoptosis. However, a second complex is formed that lacks the TNFR1 but consists of FADD and procaspases-8 and -10. This complex is formed in the cytoplasm and initiates apoptosis if the NFκB signaling does not induce antiapoptotic proteins such as FLIPL.
Although TNFR1 is responsible for most cellular responses to TNFα (including cytotoxicity, cell growth, NFκB activation, and upregulation of adhesion and cytokine genes), TNFR2 signaling has been reported to be important for proliferation of lymphoid cells. In some cell types TNFR2 has also been shown to be important for cytotoxicity as well as NFκB activation.49–54 Using TNFR1 knockout mice, Pfeffer et al.14 demonstrated that TNFR1 plays a crucial role in endotoxemia from lipopolysaccharides or from Staphylococcus aureus enterotoxin B. The same research group, however, showed that TNFR1 knockout mice succumb to infection from Listeria monocytogenes because of severely impaired bacterial clearing. Using a similar model, Rothe et al.26 showed that although the TNFR1 knockout mice are resistant to the lethal effects of low doses of LPS after D-galactosamine sensitization, they remain susceptible to high dose LPS. In another study using TNFR2 knockout mice, Erickson et al.27 showed that the TNFR2 knockout mice show normal T-cell development. However, TNF-induced tissue necrosis is markedly inhibited in these mice, suggesting that TNFR2 plays an important role in TNF-mediated necrotic effects. Using fibroblasts from TNFR1 and TNFR2 knockout mice, Kalb et al.55 demonstrated that TNFR1 and TNFR2 activate signaling pathways with different kinetics. Therefore, depending on the cell type, TNFR1 and TNFR2 may have distinct, as well as overlapping, roles in signal transduction and gene expression.
IV. ROLE OF MACROPHAGES IN INFLAMMATION AND DISEASE
Macrophages are innate immune cells that form the first line of defense against invading pathogens. In addition, these cells also play a crucial role in tissue homeostasis, coordination of adaptive immune responses, inflammation, and repair.57 Elie Metchnikoff first coined the term “macrophage” to describe large mononuclear phagocytic cells.56 Cells related to macrophages have also been found in early life forms. In addition, some protozoans also have certain features similar to that of mammalian macrophages.57 Among the mononuclear phagocytic system macrophages are the major differentiated cell types. Macrophages also exhibit significant structural and functional heterogeneity and are distributed throughout the body.57 They are present in the liver, lungs, lymphoid organs, gastrointestinal tract, central nervous system, serous cavities, bone, synovium, and skin, and thus participate in a variety of physiological and pathophysiological processes.57
Macrophages represent a major defense system against invasion of the host by a range of microorganisms including that of bacteria, viruses, fungi, and protozoa.57 They are involved in the recognition, phagocytosis, and destruction of the organisms. In addition, macrophages are also involved in antigen presentation and secretion of a wide variety of products, including enzymes, enzyme inhibitors, cytokines, chemokines, complement components, coagulation factors, and arachidonic acid intermediates.57 Interestingly, apart from secretion of a repertoire of cytokines/chemokines, macrophages also respond to these products in an autocrine/paracrine manner, thus accentuating the inflammatory response. Because of these various functions of macrophages, these cells have been implicated in a number of disease processes including rheumatoid arthritis, autoimmune and primary immunodeficiency diseases, Alzheimer’s, wound healing processes, and atherosclerosis, as well as in tumor biology. Because of this crucial role in various physiological and pathophysiological processes, mechanisms by which macrophages respond to extracellular stimuli have been examined at great depths. In this regard, several studies have determined the specific role of TNFα signaling mechanisms and their biological consequences with respect to macrophage biology.
V. REGULATION OF MACROPHAGE BIOLOGY BY TNFα
A. Role of TNFα in Inflammation
TNFα is a powerful pro-inflammatory agent that regulates many facets of macrophage function. It is rapidly released after trauma, infection, or exposure to bacterial-derived LPS and has been shown to be one of the most abundant early mediators in inflamed tissue.58 Among its various functions is its pivotal role in orchestrating the production of a pro-inflammatory cytokine cascade. TNFα is thus considered to be a “master regulator” of pro-inflammatory cytokine production.59 In addition to pro-inflammatory cytokines, TNFα also increases lipid signal transduction mediators such as prostaglandins and platelet activating factor.60 Based on these roles, TNFα has been proposed as a central player in inflammatory cell activation and recruitment and is suggested to play a critical role in the development of many chronic inflammatory diseases (Fig. 2).61
FIGURE 2.
TNFα plays a central role in the perpetuation of inflammation in chronic diseases.
B. Role of TNFα in Macrophage Activation
Toll-like receptors induce the production of TNFα from macrophages that, in addition to other factors, activates macrophages. Exogenous addition of TNFα, however, activates macrophages only after priming with interferon gamma (IFNγ). TNFα and IFNγ exhibit a cross-talk at the level of TNFR1 to induce activation of macrophages. It has been shown that TNFα induces a stronger activation of NFκB in the presence of IFN-γ. IFN-γ signaling causes nuclear localization of STAT1α that precludes it from being recruited at TNFR1, leading to an enhanced TNFα-induced NFκB activation.62 Activated macrophages can migrate to sites of inflammation, where they encounter pathogens and lyse them. This is accomplished by an increased production of toxic oxygen species and via induction of inducible nitric oxide synthase (iNOS) to produce nitric oxide (NO). In a recent study, Magez et al.63 demonstrated that the control of Trypanosoma congolense infection is dependent upon macrophage- and neutrophil-derived soluble TNFα. They also showed that intact TNFR1 signaling via nitric oxide pathway was essential for this event. Using an in vivo approach, Salkowski et al.64 demonstrated that LPS-induced iNOS expression in the liver is, in part, dependent on TNFR signaling in the macrophages.
Studies on TNFα signaling in macrophages have mostly focused on the acute and transient activation of signal transduction pathways and transcription factors such as NFκB. However, a recent study investigated the responses of primary macrophages during a 2-day period after TNFα stimulation.65 The results from this study showed that TNFα induces an autocrine loop that is characterized by a low and sustained production of IFN-β. IFN-β was found to act synergistically with canonical TNFα signal to induce a sustained expression of genes encoding inflammatory molecules and a delayed expression of genes encoding interferon-response molecules. These molecules are then thought to prime macrophages for enhanced responses to subsequent challenge with microbial products or cytokines. This feed-forward loop plays an important role in sustaining inflammation.65
C. Role of TNFα in Resolution of Inflammation
In addition to its role in the initiation and perpetuation of inflammation, TNFα has also been shown to be important in the resolution of inflammation. In a study by Michlweska et al.,66 the authors examined the role of TNFα on LPS-mediated efferocytosis of neutrophils by human monocyte-derived macrophages. They demonstrated that LPS-mediated inhibition of efferocytosis of neutrophils by macrophages is mediated via TNFα. Because efferocytosis plays a crucial role in clearing neutrophils, this role of TNFα is especially important in the resolution of inflammation.
D. Role of TNFα in Proliferation, Apoptosis, and Differentiation of Macrophages
Studies have shown that the long-term survival of macrophages is dependent on autocrine signaling by TNFα.67 Because TNFα mediates many of the pathological effects of LPS-TLR4 in conditions such as septic shock, it is suggested that prolonged macrophage survival mediated by TNFα plays an important role in sepsis.68 In an effort to determine the role of TNFR in Fas-induced apoptosis, Takada et al.69 generated macrophage cell lines derived from wild-type mice as well as from TNFR1, TNFR2, and TNFR1/2 genetically deleted mice. The authors demonstrated that both TNFR1 and TNFR2 were required for Fas-induced macrophage apoptosis because both TNFR1 and TNFR2 deleted cells were resistant to anti-Fas-induced apoptosis. A similar cross-talk mechanism was also shown for another member of the TNF family, namely RANKL (receptor activator for NF-κB ligand). Takada and Aggarwal70 showed that RANKL signaling in macrophages is modulated by TNF receptors. In contrast to its reported roles in macrophage apoptosis, Guilbert et al.71 showed that TNFα increases the proliferation of growth-competent macrophages in the presence of macrophage colony-stimulating factor-1 (M-CSF). TNFα has also been shown to enhance the production of macrophages in vitro from primitive mouse hematopoietic progenitor cells.72
In addition to these roles, Witsell and Schook73 demonstrated that TNFα has macrophage differentiation capabilities. TNFα gene transcripts are expressed during differentiation of bone marrow-derived macrophages. To test the importance of this, these authors used an antisense approach and blocked TNFα gene expression during differentiation. They found that in the absence of TNFα (in the differentiating macrophages), the cells followed a proliferative program instead of going through the differentiation program. These results suggest that “autocrine” TNFα effects are important in promoting the macrophage differentiation. Taken together, these results suggest that similar to other cell types, TNFα plays an important role in the proliferation, apoptosis, and differentiation of macrophages.
E. Role of TNFα in Atherosclerosis
There is now strong evidence for the role of macrophage-derived TNFα in the development of atherosclerosis.74–77 Because macrophage scavenger receptor plays an important role in foam cell formation, Hsu et al. examined the effect of TNFα on macrophage scavenger receptor expression. They found that TNFα downregulates macrophage scavenger receptor gene expression and protein via transcriptional and post-transcriptional processes.78 In another study, TNFα-induced MAPK pathway was shown to be important in the transcriptional regulation of macrophage scavenger receptor and increase in the receptor expression. However, long-term treatment with TNFα led to downregulation of the scavenger receptor and foam cell formation potentially via a post-transcriptional mechanism.79 Because macrophage scavenger receptor and foam cell formation play critical roles in the pathogenesis of atherosclerosis, these studies further implicate an important role of macrophage TNFα in atherosclerosis. In inflammatory models, TNFα has also been shown to be important in the upregulation of adhesion molecules that are critical in extravasation of monocytes. Studies have shown that this role of TNFα is mediated via the NFκB pathway.80
F. Role of TNFα in Osteoclastogenesis
Osteoclasts derived from the monocyte/macrophage lineage are an important cell type that regulates bone formation and remodeling under normal conditions, as well as in pathologic bone loss seen in diseases such as rheumatoid arthritis. Members of the TNF superfamily, especially TNFα and RANKL, are intricately involved in osteoclast differentiation and function as well as in bone destruction through osteoclast activation.81 In this regard, RANK-knockout mice show severe osteopetrosis with total occlusion of the bone marrow space within the endosteal bone. These mice also lack osteoclasts but have normal osteoclast progenitors.82 There is strong evidence that TNFα can induce formation of osteoclasts via a mechanism that is independent of RANKL signaling.83 In addition, TNFα has been shown to promote bone resorption in vitro and in vivo and induces secretion of RANKL in osteoblastic cells.84–89 TNFα has also been shown to be an important mediator of LPS-induced osteoclastogenesis.90 Furthermore, TNFα alone or in combination with interleukin 1 (IL-1) increases osteoclast numbers seen at sites of bone resorption.91 TNFα also increases the numbers of circulating osteoclast precursors by promoting their proliferation and differentiation in the bone marrow.92 In addition, TNFα, in the presence of M-CSF has also been reported to directly act on osteoclast precursors and induce osteoclast differentiation independent of RANKL.93 Activation of p38 MAP kinase plays an important role in TNF-induced osteoclast differentiation.94 Moreover, TNFα has been shown to promote the survival of differentiated osteoclasts, which generally have a short life span. This function of TNFα seems to be dependent on the phosphatidylinositol 3-kinase, Akt, and MEK/ERK signaling pathways.95 TNFα-induced osteoclast formation has also been shown to be dependent on TRAF2.96
In contrast, Lam et al.97 have shown that although TNFα alone fails to induce differentiation of murine osteoclast precursors, priming with RANKL (with a concentration that is insufficient to induce osteoclastogenesis by itself) dramatically enhances TNFα-induced osteoclast differentiation. Moreover, TNFα, in association with RANKL, activates these cells to resorb bone, causing joint erosions.98 Taken together, these results suggest that TNFα (alone or together with RANKL) does play an important physiological role in the development and differentiation of the musculoskeletal system. In light of the fact that TNFα levels are increased in inflammatory musculoskeletal diseases, TNFα has been proposed to mediate local bone destruction in these diseases.99
The physiological functions of TNFα in macrophages are numerous; thus, any aberration in its production or signaling in part plays a crucial role in the pathogenesis of many inflammatory diseases. Therefore, it is not surprising that TNFα and its receptors have been targeted for therapeutic development for the treatment of inflammatory diseases.
VI. THERAPEUTIC AGENTS TARGETING TNFα IN INFLAMMATORY DISEASE
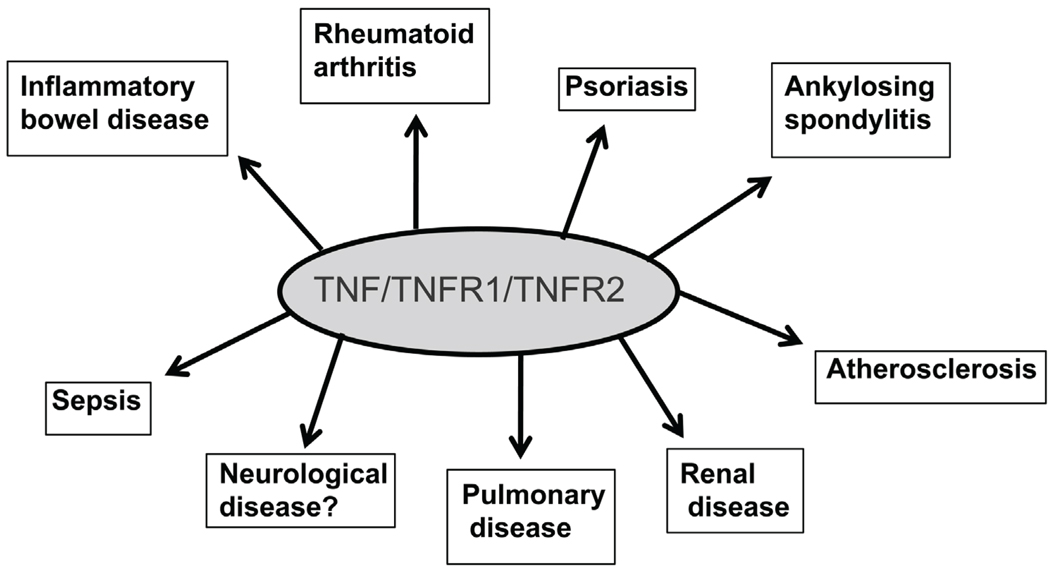
Even though TNFα is important for normal homeostatic mechanisms including host defense, dysregulated production of TNFα has been found in several inflammatory diseases. In addition, TNFα-activated macrophages are the principal components of the immunopathology of many autoimmune diseases.100 Thus, macrophage-derived TNFα is now implicated in a number of diseases, including rheumatoid arthritis, inflammatory bowel disease, psoriatic arthritis, ankylosing spondylitis, juvenile chronic arthritis, atherosclerosis, and sepsis (Fig. 3).101–104 After a number of laboratory-based studies and human clinical trials, five drugs (TNFα blocking agents) are currently licensed for treating some of these diseases, including rheumatoid arthritis and Crohn’s disease. These five drugs are as follows: 1) etanercept is a recombinant human soluble fusion protein of TNFR2 coupled to the Fc portion of IgG105; 2) infliximab is an anti-TNF human-murine chimeric IgG1 monoclonal antibody106; 3) adalimumab is a human anti-human TNFα antibody that was produced by phage display107; (4) certolizumab pegol is a PEGylated TNFα antibody108; and 5) golimumab is a human anti-TNFα IgG1κ monoclonal antibody that can be administered by the patient.109
FIGURE 3.
TNFα and its receptors play a major role in a number of inflammatory diseases. Anti-TNFα agents are clinically used to treat some of these diseases.
A. TNFα Blockers in Rheumatoid Arthritis
TNFα is considered to be the key inflammatory cytokine in rheumatoid arthritis and is found in high levels in patients with the disease. Its dysregulated secretion by macrophages is involved in both inducing and maintaining synovitis. It has been shown that TNFα activates p38 and ERK MAPKs in the synovial macrophages.110 TNFα also affects the bone marrow, causing anemia in patients with rheumatoid arthritis.111 Based on extensive studies on the role of TNFα in rheumatoid arthritis, TNFα was targeted for the treatment of this disease. Since their first license for clinical use in 1998, TNFα antagonists have been used with good therapeutic benefits. Several studies suggest that anti-TNFα therapeutics may work by affecting signaling pathways resulting in cell cycle arrest, apoptosis, and suppression of cytokine production. For instance, etanercept and infliximab have been shown to induce apoptosis in monocytes and macrophages both in synovial fluid and peripheral blood in vivo.112 Furthermore, treatment with TNFα blockers reduces the number of infiltrating synovial granulocytes and macrophages as well as reduces the expression of chemokines IL-8 and monocyte chemotactic protein-1.113
B. TNFα Blockers in Inflammatory Bowel Disease, Ankylosing Spondylitis, and Psoriasis
Similar to its role in rheumatoid arthritis, TNFα has also been shown to be crucial in the pathogenesis of inflammatory bowel disease.114–116 Clinical studies demonstrate that infliximab is effective in the treatment of patients with Crohn’s disease and ulcerative colitis.117,118 Infliximab is also effective in mucosal healing in patients who are refractory to conventional treatment using corticosteroids and/or immunosuppressive agents.119,120 In patients with ankylosing spondylitis, both etanercept and infliximab have been shown to be effective in inducing and maintaining remission.121–129 Because psoriasis is an inflammatory skin disorder, patients with psoriasis also develop inflammatory arthritis.130,131 Clinical studies have shown that anti-TNFα agents are effective in treating the dermatological, as well as the articular, components of psoriasis.
C. TNFα in Other Diseases
TNFα has been shown to play an important role in the pathogenesis of neurological diseases. Microglial cells (from monocytic lineage) in the central nervous system are one of the major producers of TNFα and participate in a number of pathophysiological conditions in the brain.132 With regard to disease pathogenesis, TNFα in the brain has been shown to have both harmful and beneficial effects, thus making it a difficult therapeutic target in neurological diseases.133–137 In one study in humans, neutralization of TNFα did not benefit patients with relapsing-remitting multiple sclerosis and, in fact, was shown to increase the disease process.138 Therefore, further extensive studies on the “basic science” aspects of TNFα in the central nervous system are essential before it can be targeted for treating neurological diseases.
There is accumulating evidence that patients with rheumatoid arthritis and other chronic inflammatory diseases have increased incidence of cardiovascular disease. TNFα has been implicated in this process, and clinical trials have shed some evidence that the TNFα blocking in rheumatoid arthritis patients ameliorates the cardiovascular risk.139 TNFα has also been implicated in diseases of other systems such as respiratory and renal disease and there is some evidence that the TNFα blocking agents may be effective in some of these conditions.140,141 Further research in both basic science as well as clinical research is essential before exploring the therapeutic potential of anti-TNFα agents in other diseases such as cancer, diabetes, obesity, and so forth.
VII. PERSPECTIVES AND CONCLUSIONS
Although actions of TNFα have been known for more than a century, TNFα was identified only in 1975 and cloned a decade later. The last 2 decades of active research on TNFα have not only unraveled a TNF/TNFR superfamily, but have also led to seminal advances in our understanding of the role of macrophage-derived TNFα in various inflammatory disease processes. Importantly, the discovery of TNFα and its current clinical use represent a classic example of how laboratory-based research can be translated into clinical medicine for treating inflammatory disease. Current biotherapies in rheumatoid arthritis target TNFα by repetitively administering recombinant proteins to block TNFα. However, it is necessary to target specific cells and tissues and to avoid the frequent administration of recombinant proteins. Gene therapy and targeting TNFα at the RNA level seem to be promising approaches. These techniques can be used for the specific suppression of immune system targets and also for replacing the frequent administration. In one such study, murine macrophages transfected with siRNA against TNFα showed marked inhibition of TNFα secretion. In addition, TNFα siRNA delivered in vivo using liposomes caused a 50% to 70% decrease in articular and systemic TNFα secretion, suggesting some promise in these methods for future therapeutics.142 Further research on the most efficient ways to block TNFα selectively is needed.
Of further importance is the knowledge that we have obtained from clinical studies with regard to the mechanisms of TNFα actions, which helps return clinical medicine back to laboratory-based research to answer some critical and unexpected clinical findings. In this regard, it should be noted that more that one third of patients suffering from any disease against which anti-TNFα drugs have been approved do not benefit clinically from anti-TNFα treatment. Future studies are thus needed to investigate if these differences are due to different mechanisms of disease pathogenesis in different patients or due to the presence of other regulators in the pathogenesis of disease. This can be achieved by laboratory-based research in which we can further investigate the molecular mechanisms of these disease processes as well as the mechanisms of “normal” TNFα signaling. In understanding the normal regulators of the signaling process, especially in macrophages, we can then begin to understand if aberrant regulation of these “regulators” contributes to the heterogeneity in disease process. This will not only help us to understand the overall mechanisms of TNFα signaling in normal and inflammatory diseases, but it will also lead to identification of potential therapeutic targets that may be superior to currently available therapeutics. As noted above, TNFα is equally essential in maintaining a normal homeostasis even though its aberrant production and signaling can lead to inflammatory disease process. Therefore, selective targeting of the “pathogenic” signaling pathway may render better outcomes than are currently available.
ACKNOWLEDGMENTS
Research in Dr. Parameswaran’s laboratory is funded by grants from the National Institutes of Health (HL095637, AR055726, and AR056680).
REFERENCES
- 1.O'Malley WE, Achinstein B, Shear MJ. Action of bacterial polysaccharide on tumors. II. Damage of sarcoma 37 by serum of mice treated with Serratia Marcescens polysaccharide, and induced tolerance. J Natl Cancer Inst. 1962;29:1169–1175. [Google Scholar]
- 2.Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pennica D, Nedwin GE, Hayflick JS, Seeburg PH, Derynck R, Palladino MA, Kohr WJ, Aggarwal BB, Goeddel DV. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 312(5996):724–729. doi: 10.1038/312724a0. 1984 Dec 20–1985 Jan 2. [DOI] [PubMed] [Google Scholar]
- 4.Pennica D, Hayflick JS, Bringman TS, Palladino MA, Goeddel DV. Cloning and expression in Escherichia coli of the cDNA for murine tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6060–6064. doi: 10.1073/pnas.82.18.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal BB, Kohr WJ, Hass PE, Moffat B, Spencer SA, Henzel WJ, Bringman TS, Nedwin GE, Goeddel DV, Harkins RN. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985 Feb 25;260(4):2345–2354. [PubMed] [Google Scholar]
- 6.Beutler B, Mahoney J, Le Trang N, Pekala P, Cerami A. Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264.7 cells. J Exp Med. 1985 May 1;161(5):984–995. doi: 10.1084/jem.161.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahoney JR, Jr, Beutler BA, Le Trang N, Vine W, Ikeda Y, Kawakami M, Cerami A. Lipopolysaccharide-treated RAW 264.7 cells produce a mediator that inhibits lipoprotein lipase in 3T3-L1 cells. J Immunol. 1985 Mar;134(3):1673–1675. [PubMed] [Google Scholar]
- 8.Spriggs DR, Deutsch S, Kufe DW. Genomic structure, induction, and production of TNF-alpha. Immunol Ser. 1992;56:3–34. [PubMed] [Google Scholar]
- 9.Vilcek J, Lee TH. Tumor necrosis factor. New insights into the molecular mechanisms of its multiple actions. J Biol Chem. 1991 Apr 25;266(12):7313–7316. [PubMed] [Google Scholar]
- 10.Kriegler M, Perez C, DeFay K, Albert I, Lu SD. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988 Apr 8;53(1):45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- 11.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997 Feb 20;385(6618):729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 12.Perez C, Albert I, DeFay K, Zachariades N, Gooding L, Kriegler M. A nonsecretable cell surface mutant of tumor necrosis factor (TNF) kills by cell-to-cell contact. Cell. 1990 Oct 19;63(2):251–258. doi: 10.1016/0092-8674(90)90158-b. [DOI] [PubMed] [Google Scholar]
- 13.Grell M. Tumor necrosis factor (TNF) receptors in cellular signaling of soluble and membrane-expressed TNF. J Inflamm. 1995;47(1–2):8–17. [PubMed] [Google Scholar]
- 14.Pfeffer K, Matsuyama T, Kundig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Krönke M, Mak TW. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993 May 7;73(3):457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 15.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995 Jun;2(6):561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 16.Banner DW, D'Arcy A, Janes W, Gentz R, Schoenfeld HJ, Broger C, Loetscher H, Lesslauer W. Crystal structure of the soluble human 55 kd TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell. 1993 May 7;73(3):431–445. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- 17.Tartaglia LA, Pennica D, Goeddel DV. Ligand passing: the 75-kDa tumor necrosis factor (TNF) receptor recruits TNF for signaling by the 55-kDa TNF receptor. J Biol Chem. 1993 Sep 5;268(25):18542–18548. [PubMed] [Google Scholar]
- 18.Lewis M, Tartaglia LA, Lee A, Bennett GL, Rice GC, Wong GH, Chen EY, Goeddel DV. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2830–2834. doi: 10.1073/pnas.88.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tartaglia LA, Goeddel DV. Two TNF receptors. Immunol Today. 1992 May;13(5):151–153. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- 20.Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G, Pfizenmaier K, Scheurich P. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995 Dec 1;83(5):793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 21.Van Zee KJ, Kohno T, Fischer E, Rock CS, Moldawer LL, Lowry SF. Tumor necrosis factor soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor alpha in vitro and in vivo. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4845–4849. doi: 10.1073/pnas.89.11.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shalaby MR, Sundan A, Loetscher H, Brockhaus M, Lesslauer W, Espevik T. Binding and regulation of cellular functions by monoclonal antibodies against human tumor necrosis factor receptors. J Exp Med. 1990 Nov 1;172(5):1517–1520. doi: 10.1084/jem.172.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheehan KC, Pinckard JK, Arthur CD, Dehner LP, Goeddel DV, Schreiber RD. Monoclonal antibodies specific for murine p55 and p75 tumor necrosis factor receptors: identification of a novel in vivo role for p75. J Exp Med. 1995 Feb 1;181(2):607–617. doi: 10.1084/jem.181.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Ostade X, Vandenabeele P, Everaerdt B, Loetscher H, Gentz R, Brockhaus M, Lesslauer W, Tavernier J, Brouckaert P, Fiers W. Human TNF mutants with selective activity on the p55 receptor. Nature. 1993 Jan 21;361(6409):266–269. doi: 10.1038/361266a0. [DOI] [PubMed] [Google Scholar]
- 25.Barbara JA, Smith WB, Gamble JR, Van Ostade X, Vandenabeele P, Tavernier J, Fiers W, Vadas MA, Lopez AF. Dissociation of TNF-alpha cytotoxic and proinflammatory activities by p55 receptor- and p75 receptor-selective TNF-alpha mutants. Embo J. 1994 Feb 15;13(4):843–850. doi: 10.1002/j.1460-2075.1994.tb06327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothe J, Lesslauer W, Lötscher H, Lang Y, Koebel P, Köntgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993 Aug 26;364(6440):798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 27.Erickson SL, de Sauvage FJ, Kikly K, Carver-Moore K, Pitts-Meek S, Gillett N, Sheehan KC, Schreiber RD, Goeddel DV, Moore MW. Decreased sensitivity to tumour-necrosis factor but normal T-cell development in TNF receptor- 2-deficient mice. Nature. 1994 Dec 8;372(6506):560–563. doi: 10.1038/372560a0. [DOI] [PubMed] [Google Scholar]
- 28.Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell. 1995 May 19;81(4):495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 29.Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996 Apr;4(4):387–396. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi M, Rothe M, Goeddel DV. Anatomy of TRAF2. Distinct domains for nuclear factor-kappaB activation and association with tumor necrosis factor signaling proteins. J Biol Chem. 1996 Aug 16;271(33):19935–19942. doi: 10.1074/jbc.271.33.19935. [DOI] [PubMed] [Google Scholar]
- 31.Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005 Aug;7(8):758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004 Sep 15;18(18):2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 33.Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harbor Perspect Biol. 2009 Nov;1(5):a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mercurio F, Manning AM. Multiple signals converging on NF-kappaB. Curr Opin Cell Biol. 1999 Apr;11(2):226–232. doi: 10.1016/s0955-0674(99)80030-1. [DOI] [PubMed] [Google Scholar]
- 35.Sun SC, Ley SC. New insights into NF-kappaB regulation and function. Trends Immunol. 2008 Oct;29(10):469–478. doi: 10.1016/j.it.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 37.Devin A, Lin Y, Yamaoka S, Li Z, Karin M, Liu Z. The alpha and beta subunits of IkappaB kinase (IKK) mediate TRAF2-dependent IKK recruitment to tumor necrosis factor (TNF) receptor 1 in response to TNF. Mol Cell Biol. 2001 Jun;21(12):3986–3994. doi: 10.1128/MCB.21.12.3986-3994.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abu-Amer Y, Ross FP, McHugh KP, Livolsi A, Peyron JF, Teitelbaum SL. Tumor necrosis factor-alpha activation of nuclear transcription factor-kappaB in marrow macrophages is mediated by c-Src tyrosine phosphorylation of Ikappa Balpha. J Biol Chem. 1998 Nov 6;273(45):29417–29423. doi: 10.1074/jbc.273.45.29417. [DOI] [PubMed] [Google Scholar]
- 39.Patial S, Luo J, Porter KJ, Benovic JL, Parameswaran N. G-protein-coupled-receptor kinases mediate TNFalpha-induced NF-kappaB signalling via direct interaction with and phosphorylation of IkappaBalpha. Biochem J. 2010 Jan 1;425(1):169–178. doi: 10.1042/BJ20090908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ribas C, Penela P, Murga C, Salcedo A, Garcia-Hoz C, Jurado-Pueyo M, Aymerich I, Mayor F., Jr The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta. 2007 Apr;1768(4):913–922. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 41.Sorriento D, Ciccarelli M, Santulli G, Campanile A, Altobelli GG, Cimini V, Galasso G, Astone D, Piscione F, Pastore L, Trimarco B, Iaccarino G. The G-protein-coupled receptor kinase 5 inhibits NFkappaB transcriptional activity by inducing nuclear accumulation of IkappaB alpha. Proc Natl Acad Sci U S A. 2008 Nov 18;105(46):17818–17823. doi: 10.1073/pnas.0804446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishitoh H, Saitoh M, Mochida Y, Takeda K, Nakano H, Rothe M, Miyazono K, Ichijo H. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol Cell. 1998 Sep;2(3):389–395. doi: 10.1016/s1097-2765(00)80283-x. [DOI] [PubMed] [Google Scholar]
- 43.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997 Jan 3;275(5296):90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 44.Rousseau S, Papoutsopoulou M, Symons A, Cook D, Lucocq JM, Prescott AR, O'Garra A, Ley SC, Cohen P. TPL2-mediated activation of ERK1 and ERK2 regulates the processing of pre-TNF alpha in LPS-stimulated macrophages. J Cell Sci. 2008 Jan 15;121(Pt 2):149–154. doi: 10.1242/jcs.018671. [DOI] [PubMed] [Google Scholar]
- 45.Belich MP, Salmeron A, Johnston LH, Ley SC. TPL-2 kinase regulates the proteolysis of the NF-kappaB-inhibitory protein NF-kappaB1 p105. Nature. 1999 Jan 28;397(6717):363–368. doi: 10.1038/16946. [DOI] [PubMed] [Google Scholar]
- 46.Das S, Cho J, Lambertz I, Kelliher MA, Eliopoulos AG, Du K, Tsichlis PN. Tpl2/cot signals activate ERK, JNK, and NF-kappaB in a cell-type and stimulus-specific manner. J Biol Chem. 2005 Jun 24;280(25):23748–23757. doi: 10.1074/jbc.M412837200. [DOI] [PubMed] [Google Scholar]
- 47.Eliopoulos AG, Wang CC, Dumitru CD, Tsichlis PN. Tpl2 transduces CD40 and TNF signals that activate ERK and regulates IgE induction by CD40. EMBO J. 2003 Aug 1;22(15):3855–3864. doi: 10.1093/emboj/cdg386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003 Jul 25;114(2):181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 49.Tartaglia LA, Weber RF, Figari IS, Reynolds C, Palladino MA, Jr, Goeddel DV. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9292–9296. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tartaglia LA, Goeddel DV, Reynolds C, Figari IS, Weber RF, Fendly BM, Palladino MA., Jr Stimulation of human T-cell proliferation by specific activation of the 75-kDa tumor necrosis factor receptor. J Immunol. 1993 Nov 1;151(9):4637–4641. [PubMed] [Google Scholar]
- 51.Gehr G, Gentz R, Brockhaus M, Loetscher H, Lesslauer W. Both tumor necrosis factor receptor types mediate proliferative signals in human mononuclear cell activation. J Immunol. 1992 Aug 1;149(3):911–917. [PubMed] [Google Scholar]
- 52.Hohmann HP, Brockhaus M, Baeuerle PA, Remy R, Kolbeck R, van Loon AP. Expression of the types A and B tumor necrosis factor (TNF) receptors is independently regulated, and both receptors mediate activation of the transcription factor NF-kappa B. TNF alpha is not needed for induction of a biological effect via TNF receptors. J Biol Chem. 1990 Dec 25;265(36):22409–22417. [PubMed] [Google Scholar]
- 53.Laegreid A, Medvedev A, Nonstad U, Bombara MP, Ranges G, Sundan A, Espevik T. Tumor necrosis factor receptor p75 mediates cell-specific activation of nuclear factor kappa B and induction of human cytomegalovirus enhancer. J Biol Chem. 1994 Mar 11;269(10):7785–7791. [PubMed] [Google Scholar]
- 54.Heller RA, Song K, Fan N, Chang DJ. The p70 tumor necrosis factor receptor mediates cytotoxicity. Cell. 1992 Jul 10;70(1):47–56. doi: 10.1016/0092-8674(92)90532-h. [DOI] [PubMed] [Google Scholar]
- 55.Kalb A, Bluethmann H, Moore MW, Lesslauer W. Tumor necrosis factor receptors (Tnfr) in mouse fibroblasts deficient in Tnfr1 or Tnfr2 are signaling competent and activate the mitogen-activated protein kinase pathway with differential kinetics. J Biol Chem. 1996 Nov 8;271(45):28097–28104. doi: 10.1074/jbc.271.45.28097. [DOI] [PubMed] [Google Scholar]
- 56.Karnovsky ML. Metchnikoff in Messina: a century of studies on phagocytosis. N Engl J Med. 1981 May 7;304(19):1178–1180. doi: 10.1056/NEJM198105073041922. [DOI] [PubMed] [Google Scholar]
- 57.Ross JA, Auger MJ. The biology of the macrophage. In: Burke B, Lewis CE, editors. The macrophage. 2nd ed. New York (NY): Oxford University Press Inc; 2002. [Google Scholar]
- 58.Feldmann M, Brennan FM, Elliott M, Katsikis P, Maini RN. TNF alpha as a therapeutic target in rheumatoid arthritis. Circ Shock. 1994 Aug;43(4):179–184. [PubMed] [Google Scholar]
- 59.Maini RN, Elliott MJ, Brennan FM, Feldmann M. Beneficial effects of tumour necrosis factor-alpha (TNF-alpha) blockade in rheumatoid arthritis (RA) Clin Exp Immunol. 1995 Aug;101(2):207–212. doi: 10.1111/j.1365-2249.1995.tb08340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 61.Clark IA. How TNF was recognized as a key mechanism of disease. Cytokine Growth Factor Rev. 2007 Jun–Aug;18(3–4):335–343. doi: 10.1016/j.cytogfr.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 62.Wesemann DR, Benveniste EN. STAT-1 alpha and IFN-gamma as modulators of TNF-alpha signaling in macrophages: regulation and functional implications of the TNF receptor 1:STAT-1 alpha complex. J Immunol. 2003 Nov 15;171(10):5313–5319. doi: 10.4049/jimmunol.171.10.5313. [DOI] [PubMed] [Google Scholar]
- 63.Magez S, Radwanska M, Drennan M, Fick L, Baral TN, Allie N, Jacobs M, Nedospasov S, Brombacher F, Ryffel B, De Baetselier P. Tumor necrosis factor (TNF) receptor-1 (TNFp55) signal transduction and macrophage-derived soluble TNF are crucial for nitric oxide-mediated Trypanosoma congolense parasite killing. J Infect Dis. 2007 Sep 15;196(6):954–962. doi: 10.1086/520815. [DOI] [PubMed] [Google Scholar]
- 64.Salkowski CA, Detore G, McNally R, van Rooijen N, Vogel SN. Regulation of inducible nitric oxide synthase messenger RNA expression and nitric oxide production by lipopolysaccharide in vivo: the roles of macrophages, endogenous IFN-gamma, and TNF receptor-1-mediated signaling. J Immunol. 1997 Jan 15;158(2):905–912. [PubMed] [Google Scholar]
- 65.Yarilina A, Park-Min KH, Antoniv T, Hu X, Ivashkiv LB. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon-response genes. Nat Immunol. 2008 Apr;9(4):378–387. doi: 10.1038/ni1576. [DOI] [PubMed] [Google Scholar]
- 66.Michlewska S, Dransfield I, Megson IL, Rossi AG. Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: key role for TNF-alpha. FASEB J. 2009 Mar;23(3):844–854. doi: 10.1096/fj.08-121228. [DOI] [PubMed] [Google Scholar]
- 67.Lombardo E, Alvarez-Barrientos A, Maroto B, Bosca L, Knaus UG. TLR4-mediated survival of macrophages is MyD88 dependent and requires TNF-alpha autocrine signalling. J Immunol. 2007 Mar 15;178(6):3731–3739. doi: 10.4049/jimmunol.178.6.3731. [DOI] [PubMed] [Google Scholar]
- 68.Conte D, Holcik M, Lefebvre CA, Lacasse E, Picketts DJ, Wright KE, Korneluk RG. Inhibitor of apoptosis protein cIAP2 is essential for lipopolysaccharide-induced macrophage survival. Mol Cell Biol. 2006 Jan;26(2):699–708. doi: 10.1128/MCB.26.2.699-708.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takada Y, Sung B, Sethi G, Chaturvedi MM, Aggarwal BB. Evidence that genetic deletion of the TNF receptor p60 or p80 inhibits Fas mediated apoptosis in macrophages. Biochem Pharmacol. 2007 Oct 1;74(7):1057–1064. doi: 10.1016/j.bcp.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takada Y, Aggarwal BB. Evidence that genetic deletion of the TNF receptor p60 or p80 in macrophages modulates RANKL-induced signaling. Blood. 2004 Dec 15;104(13):4113–4121. doi: 10.1182/blood-2004-04-1607. [DOI] [PubMed] [Google Scholar]
- 71.Guilbert LJ, Winkler-Lowen B, Smith A, Branch DR, Garcia-Lloret M. Analysis of the synergistic stimulation of mouse macrophage proliferation by macrophage colony-stimulating factor (CSF-1) and tumor necrosis factor alpha (TNF-alpha) J Leukoc Biol. 1993 Jul;54(1):65–72. doi: 10.1002/jlb.54.1.65. [DOI] [PubMed] [Google Scholar]
- 72.Fahlman C, Jacobsen FW, Veiby OP, McNiece IK, Blomhoff HK, Jacobsen SE. Tumor necrosis factor-alpha (TNF-alpha) potently enhances in vitro macrophage production from primitive murine hematopoietic progenitor cells in combination with stem cell factor and interleukin-7: novel stimulatory role of p55 TNF receptors. Blood. 1994 Sep 1;84(5):1528–1533. [PubMed] [Google Scholar]
- 73.Witsell AL, Schook LB. Tumor necrosis factor alpha is an autocrine growth regulator during macrophage differentiation. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4754–4758. doi: 10.1073/pnas.89.10.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tipping PG, Hancock WW. Production of tumor necrosis factor and interleukin-1 by macrophages from human atheromatous plaques. Am J Pathol. 1993 Jun;142(6):1721–1728. [PMC free article] [PubMed] [Google Scholar]
- 75.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999 Jan 14;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 76.Schreyer SA, Peschon JJ, LeBoeuf RC. Accelerated atherosclerosis in mice lacking tumor necrosis factor receptor p55. J Biol Chem. 1996 Oct 18;271(42):26174–26178. doi: 10.1074/jbc.271.42.26174. [DOI] [PubMed] [Google Scholar]
- 77.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 78.Hsu HY, Nicholson AC, Hajjar DP. Inhibition of macrophage scavenger receptor activity by tumor necrosis factor-alpha is transcriptionally and post-transcriptionally regulated. J Biol Chem. 1996 Mar 29;271(13):7767–7773. doi: 10.1074/jbc.271.13.7767. [DOI] [PubMed] [Google Scholar]
- 79.Hsu HY, Twu YC. Tumor necrosis factor-alpha -mediated protein kinases in regulation of scavenger receptor and foam cell formation on macrophage. J Biol Chem. 2000 Dec 29;275(52):41035–41048. doi: 10.1074/jbc.M003464200. [DOI] [PubMed] [Google Scholar]
- 80.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995 Jul;9(10):899–909. [PubMed] [Google Scholar]
- 81.Feng X. Regulatory roles and molecular signaling of TNF family members in osteoclasts. Gene. 2005 Apr 25;350(1):1–13. doi: 10.1016/j.gene.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 82.Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999 Sep 15;13(18):2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem. 2000 Feb 18;275(7):4858–4864. doi: 10.1074/jbc.275.7.4858. [DOI] [PubMed] [Google Scholar]
- 84.Hofbauer LC, Lacey DL, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Interleukin-1beta and tumor necrosis factor-alpha, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone. 1999 Sep;25(3):255–259. doi: 10.1016/s8756-3282(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 85.Bertolini DR, Nedwin GE, Bringman TS, Smith DD, Mundy GR. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986 Feb 6–12;319(6053):516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- 86.Kitazawa R, Kimble RB, Vannice JL, Kung VT, Pacifici R. Interleukin-1 receptor antagonist and tumor necrosis factor binding protein decrease osteoclast formation and bone resorption in ovariectomized mice. J Clin Invest. 1994 Dec;94(6):2397–2406. doi: 10.1172/JCI117606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thomson BM, Mundy GR, Chambers TJ. Tumor necrosis factors alpha and beta induce osteoblastic cells to stimulate osteoclastic bone resorption. J Immunol. 1987 Feb 1;138(3):775–779. [PubMed] [Google Scholar]
- 88.Lerner UH, Ohlin A. Tumor necrosis factors alpha and beta can stimulate bone resorption in cultured mouse calvariae by a prostaglandin-independent mechanism. J Bone Miner Res. 1993 Feb;8(2):147–155. doi: 10.1002/jbmr.5650080205. [DOI] [PubMed] [Google Scholar]
- 89.van der Pluijm G, Most W, van der Wee-Pals L, de Groot H, Papapoulos S, Lowik C. Two distinct effects of recombinant human tumor necrosis factor-alpha on osteoclast development and subsequent resorption of mineralized matrix. Endocrinology. 1991 Sep;129(3):1596–1604. doi: 10.1210/endo-129-3-1596. [DOI] [PubMed] [Google Scholar]
- 90.Abu-Amer Y, Ross FP, Edwards J, Teitelbaum SL. Lipo-polysaccharide-stimulated osteoclastogenesis is mediated by tumor necrosis factor via its P55 receptor. J Clin Invest. 1997 Sep 15;100(6):1557–1565. doi: 10.1172/JCI119679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Konig A, Muhlbauer RC, Fleisch H. Tumor necrosis factor alpha and interleukin-1 stimulate bone resorption in vivo as measured by urinary [3H]tetracycline excretion from prelabeled mice. J Bone Miner Res. 1988 Dec;3(6):621–627. doi: 10.1002/jbmr.5650030607. [DOI] [PubMed] [Google Scholar]
- 92.Yao Z, Li P, Zhang Q, Schwarz EM, Keng P, Arbini A, Boyce BF, Xing L. Tumor necrosis factor-alpha increases circulating osteoclast precursor numbers by promoting their proliferation and differentiation in the bone marrow through up-regulation of c-Fms expression. J Biol Chem. 2006 Apr 28;281(17):11846–11855. doi: 10.1074/jbc.M512624200. [DOI] [PubMed] [Google Scholar]
- 93.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, Suda T. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000 Jan 17;191(2):275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matsumoto M, Sudo T, Maruyama M, Osada H, Tsujimoto M. Activation of p38 mitogen-activated protein kinase is crucial in osteoclastogenesis induced by tumor necrosis factor. FEBS Lett. 2000 Dec 1;486(1):23–28. doi: 10.1016/s0014-5793(00)02231-6. [DOI] [PubMed] [Google Scholar]
- 95.Lee SE, Chung WJ, Kwak HB, Chung CH, Kwack KB, Lee ZH, Kim HH. Tumor necrosis factor-alpha supports the survival of osteoclasts through the activation of Akt and ERK. J Biol Chem. 2001 Dec 28;276(52):49343–49349. doi: 10.1074/jbc.M103642200. [DOI] [PubMed] [Google Scholar]
- 96.Kanazawa K, Kudo A. TRAF2 is essential for TNF-alpha-induced osteoclastogenesis. J Bone Miner Res. 2005 May;20(5):840–847. doi: 10.1359/JBMR.041225. [DOI] [PubMed] [Google Scholar]
- 97.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000 Dec;106(12):1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goldring SR, Gravallese EM. Pathogenesis of bone lesions in rheumatoid arthritis. Curr Rheumatol Rep. 2002 Jun;4(3):226–231. doi: 10.1007/s11926-002-0069-y. [DOI] [PubMed] [Google Scholar]
- 99.Isomaki P, Punnonen J. Pro- and anti-inflammatory cytokines in rheumatoid arthritis. Ann Med. 1997 Dec;29(6):499–507. doi: 10.3109/07853899709007474. [DOI] [PubMed] [Google Scholar]
- 100.Flavell RA. The relationship of inflammation and initiation of autoimmune disease: role of TNF super family members. Curr Top Microbiol Immunol. 2002;266:1–9. doi: 10.1007/978-3-662-04700-2_1. [DOI] [PubMed] [Google Scholar]
- 101.Feldmann M, Brennan FM, Elliott MJ, Williams RO, Maini RN. TNF alpha is an effective therapeutic target for rheumatoid arthritis. Ann N Y Acad Sci. 1995 Sep 7;766:272–278. doi: 10.1111/j.1749-6632.1995.tb26675.x. [DOI] [PubMed] [Google Scholar]
- 102.Kollias G, Douni E, Kassiotis G, Kontoyiannis D. On the role of tumor necrosis factor and receptors in models of multiorgan failure, rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease. Immunol Rev. 1999 Jun;169:175–194. doi: 10.1111/j.1600-065x.1999.tb01315.x. [DOI] [PubMed] [Google Scholar]
- 103.Beutler B, Cerami A. The biology of cachectin/TNF—a primary mediator of the host response. Annual review of immunology. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- 104.Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc Res. 2008 Aug 1;79(3):360–376. doi: 10.1093/cvr/cvn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, Jackson CG, Lange M, Burge DJ. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999 Jan 28;340(4):253–259. doi: 10.1056/NEJM199901283400401. [DOI] [PubMed] [Google Scholar]
- 106.Maini RN, Breedveld FC, Kalden JR, Smolen JS, Davis D, Macfarlane JD, Antoni C, Leeb B, Elliott MJ, Woody JN, Schaible TF, Feldmann M. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998 Sep;41(9):1552–1563. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 107.den Broeder AA, Joosten LA, Saxne T, Heinegard D, Fenner H, Miltenburg AM, Frasa WL, van Tits LJ, Buurman WA, van Riel PL, van de Putte LB, Barrera P. Long term anti-tumour necrosis factor alpha monotherapy in rheumatoid arthritis: effect on radiological course and prognostic value of markers of cartilage turnover and endothelial activation. Ann Rheum Dis. 2002 Apr;61(4):311–318. doi: 10.1136/ard.61.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Choy EH, Hazleman B, Smith M, Moss K, Lisi L, Scott DG, Patel J, Sopwith M, Isenberg DA. Efficacy of a novel PEGylated humanized anti-TNF fragment (CDP870) in patients with rheumatoid arthritis: a phase II double-blinded, randomized, dose-escalating trial. Rheumatology (Oxford) 2002 Oct;41(10):1133–1137. doi: 10.1093/rheumatology/41.10.1133. [DOI] [PubMed] [Google Scholar]
- 109.Zhou H, Jang H, Fleischmann RM, Bouman-Thio E, Xu Z, Marini JC, Pendley C, Jiao Q, Shankar G, Marciniak SJ, Cohen SB, Rahman MU, Baker D, Mascelli MA, Davis HM, Everitt DE. Pharmacokinetics and safety of golimumab, a fully human anti-TNF-alpha monoclonal antibody, in subjects with rheumatoid arthritis. J Clin Pharmacol. 2007 Mar;47(3):383–396. doi: 10.1177/0091270006298188. [DOI] [PubMed] [Google Scholar]
- 110.Gortz B, Hayer S, Tuerck B, Zwerina J, Smolen JS, Schett G. Tumour necrosis factor activates the mitogen-activated protein kinases p38alpha and ERK in the synovial membrane in vivo. Arthritis Res Ther. 2005;7(5):R1140–R1147. doi: 10.1186/ar1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Voulgari PV, Kolios G, Papadopoulos GK, Katsaraki A, Seferiadis K, Drosos AA. Role of cytokines in the pathogenesis of anemia of chronic disease in rheumatoid arthritis. Clin Immunol. 1999 Aug;92(2):153–160. doi: 10.1006/clim.1999.4736. [DOI] [PubMed] [Google Scholar]
- 112.Catrina AI, Trollmo C, af Klint E, Engstrom M, Lampa J, Hermansson Y, Klareskog L, Ulfgren AK. Evidence that anti-tumor necrosis factor therapy with both etanercept and infliximab induces apoptosis in macrophages, but not lymphocytes, in rheumatoid arthritis joints: extended report. Arthritis Rheum. 2005 Jan;52(1):61–72. doi: 10.1002/art.20764. [DOI] [PubMed] [Google Scholar]
- 113.Taylor PC, Peters AM, Paleolog E, Chapman PT, Elliott MJ, McCloskey R, Feldmann M, Maini RN. Reduction of chemokine levels and leukocyte traffic to joints by tumor necrosis factor alpha blockade in patients with rheumatoid arthritis. Arthritis Rheum. 2000 Jan;43(1):38–47. doi: 10.1002/1529-0131(200001)43:1<38::AID-ANR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 114.Breese EJ, Michie CA, Nicholls SW, Murch SH, Williams CB, Domizio P, Walker-Smith JA, MacDonald TT. Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology. 1994 Jun;106(6):1455–1466. doi: 10.1016/0016-5085(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 115.Murch SH, Braegger CP, Walker-Smith JA, MacDonald TT. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993 Dec;34(12):1705–1709. doi: 10.1136/gut.34.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999 Mar;10(3):387–398. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 117.Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, Rutgeerts P ACCENT I Study Group. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002 May 4;359(9317):1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 118.Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, Kamm MA, Korzenik JR, Lashner BA, Onken JE, Rachmilewitz D, Rutgeerts P, Wild G, Wolf DC, Marsters PA, Travers SB, Blank MA, van Deventer SJ. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004 Feb 26;350(9):876–885. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 119.Oussalah A, Danese S, Peyrin-Biroulet L. Efficacy of TNF antagonists beyond one year in adult and pediatric inflammatory bowel diseases: a systematic review. Curr Drug Targets. 2010 Feb;11(2):156–175. doi: 10.2174/138945010790309939. [DOI] [PubMed] [Google Scholar]
- 120.Willert RP, Lawrance IC. Use of infliximab in the prevention and delay of colectomy in severe steroid dependant and refractory ulcerative colitis. World J Gastroenterol. 2008 Apr 28;14(16):2544–2549. doi: 10.3748/wjg.14.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rudwaleit M, Baeten D. Ankylosing spondylitis and bowel disease. Best Pract Res Clin Rheumatol. 2006 Jun;20(3):451–471. doi: 10.1016/j.berh.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 122.Braun J, Bollow M, Neure L, Seipelt E, Seyrekbasan F, Herbst H, Eggens U, Distler A, Sieper J. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum. 1995 Apr;38(4):499–505. doi: 10.1002/art.1780380407. [DOI] [PubMed] [Google Scholar]
- 123.Francois RJ, Neure L, Sieper J, Braun J. Immunohistological examination of open sacroiliac biopsies of patients with ankylosing spondylitis: detection of tumour necrosis factor alpha in two patients with early disease and transforming growth factor beta in three more advanced cases. Ann Rheum Dis. 2006 Jun;65(6):713–720. doi: 10.1136/ard.2005.037465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gorman JD, Sack KE, Davis JC., Jr Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med. 2002 May 2;346(18):1349–1356. doi: 10.1056/NEJMoa012664. [DOI] [PubMed] [Google Scholar]
- 125.Baraliakos X, Listing J, Rudwaleit M, Brandt J, Sieper J, Braun J. Radiographic progression in patients with ankylosing spondylitis after 2 years of treatment with the tumour necrosis factor alpha antibody infliximab. Ann Rheum Dis. 2005 Oct;64(10):1462–1466. doi: 10.1136/ard.2004.033472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Davis JC, van der Heijde DM, Braun J, Dougados M, Cush J, Clegg D, Inman RD, Kivitz A, Zhou L, Solinger A, Tsuji W. Sustained durability and tolerability of etanercept in ankylosing spondylitis for 96 weeks. Ann Rheum Dis. 2005 Nov;64(11):1557–1562. doi: 10.1136/ard.2004.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Inman RD, Clegg DO, Davis JC, Whitmore JB, Solinger A. Etanercept in adult patients with early onset ankylosing spondylitis. J Rheumatol. 2006 Aug;33(8):1634–1636. [PubMed] [Google Scholar]
- 128.Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W, Gromnica-Ihle E, Kellner H, Krause A, Schneider M, Sörensen H, Zeidler H, Thriene W, Sieper J. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet. 2002 Apr 6;359(9313):1187–1193. doi: 10.1016/s0140-6736(02)08215-6. [DOI] [PubMed] [Google Scholar]
- 129.Baraliakos X, Listing J, Rudwaleit M, Brandt J, Alten R, Burmester G, Gromnica-Ihle E, Haibel H, Schewe S, Schneider M, Sörensen H, Zeidler H, Visvanathan S, Sieper J, Braun J. Safety and efficacy of readministration of infliximab after longterm continuous therapy and withdrawal in patients with ankylosing spondylitis. J Rheumatol. 2007 Mar;34(3):510–515. [PubMed] [Google Scholar]
- 130.Kristensen M, Chu CQ, Eedy DJ, Feldmann M, Brennan FM, Breathnach SM. Localization of tumour necrosis factor-alpha (TNF-alpha) and its receptors in normal and psoriatic skin: epidermal cells express the 55-kD but not the 75-kD TNF receptor. Clin Exp Immunol. 1993 Nov;94(2):354–362. doi: 10.1111/j.1365-2249.1993.tb03457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hueber AJ, McInnes IB. Immune regulation in psoriasis and psoriatic arthritis—recent developments. Immunol Lett. 2007 Dec 15;114(2):59–65. doi: 10.1016/j.imlet.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 132.Feuerstein GZ, Liu T, Barone FC. Cytokines, inflammation, and brain injury: role of tumor necrosis factor-alpha. Cerebrovasc Brain Metab Rev. 1994 Winter;6(4):341–360. [PubMed] [Google Scholar]
- 133.Shohami E, Ginis I, Hallenbeck JM. Dual role of tumor necrosis factor alpha in brain injury. Cytokine Growth Factor Rev. 1999 Jun;10(2):119–130. doi: 10.1016/s1359-6101(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 134.Nawashiro H, Martin D, Hallenbeck JM. Neuroprotective effects of TNF binding protein in focal cerebral ischemia. Brain Res. 1997 Dec 19;778(2):265–271. doi: 10.1016/s0006-8993(97)00981-5. [DOI] [PubMed] [Google Scholar]
- 135.Liu J, Marino MW, Wong G, Grail D, Dunn A, Bettadapura J, Slavin AJ, Old L, Bernard CC. TNF is a potent anti-inflammatory cytokine in autoimmune-mediated demyelination. Nat Med. 1998 Jan;4(1):78–83. doi: 10.1038/nm0198-078. [DOI] [PubMed] [Google Scholar]
- 136.Kassiotis G, Kollias G. Uncoupling the proinflammatory from the immunosuppressive properties of tumor necrosis factor (TNF) at the p55 TNF receptor level: implications for pathogenesis and therapy of autoimmune demyelination. J Exp Med. 2001 Feb 19;193(4):427–434. doi: 10.1084/jem.193.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001 Nov;4(11):1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- 138.TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Neurology. 1999 Aug 11;53(3):457–465. [PubMed] [Google Scholar]
- 139.Wolfe F, Michaud K. Heart failure in rheumatoid arthritis: rates, predictors, and the effect of anti-tumor necrosis factor therapy. Am J Med. 2004 Mar 1;116(5):305–311. doi: 10.1016/j.amjmed.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 140.Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE, Green RH, Bradding P, Brightling CE, Wardlaw AJ, Pavord ID. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med. 2006 Feb 16;354(7):697–708. doi: 10.1056/NEJMoa050580. [DOI] [PubMed] [Google Scholar]
- 141.Lamprecht P, Voswinkel J, Lilienthal T, Nolle B, Heller M, Gross WL, Gause A. Effectiveness of TNF-alpha blockade with infliximab in refractory Wegener's granulomatosis. Rheumatology (Oxford) 2002 Nov;41(11):1303–1307. doi: 10.1093/rheumatology/41.11.1303. [DOI] [PubMed] [Google Scholar]
- 142.Khoury M, Louis-Plence P, Escriou V, Noel D, Largeau C, Cantos C, Scherman D, Jorgensen C, Apparailly F. Efficient new cationic liposome formulation for systemic delivery of small interfering RNA silencing tumor necrosis factor alpha in experimental arthritis. Arthritis Rheum. 2006 Jun;54(6):1867–1877. doi: 10.1002/art.21876. [DOI] [PubMed] [Google Scholar]
- 143.Sacre SM, Drexler SK, Andreakos E, Feldmann M, Brennan FM, Foxwell BM. Could toll-like receptors provide a missing link in chronic inflammation in rheumatoid arthritis? Lessons from a study on human rheumatoid tissue. Ann Rheum Dis. 2007 Nov;66 Suppl 3:iii81–iii86. doi: 10.1136/ard.2007.079012. [DOI] [PMC free article] [PubMed] [Google Scholar]