Abstract
Children born very preterm, even when intelligence is broadly normal, often experience selective difficulties in executive function and visual-spatial processing. Development of structural cortical connectivity is known to be altered in this group, and functional magnetic resonance imaging (fMRI) evidence indicates that very preterm children recruit different patterns of functional connectivity between cortical regions during cognition. Synchronization of neural oscillations across brain areas has been proposed as a mechanism for dynamically assigning functional coupling to support perceptual and cognitive processing, but little is known about what role oscillatory synchronization may play in the altered neurocognitive development of very preterm children. To investigate this, we recorded magnetoencephalographic (MEG) activity while 7–8 year old children born very preterm and age matched full-term controls performed a visual short-term memory task. Very preterm children exhibited reduced long-range synchronization in the alpha-band during visual short-term memory retention, indicating that cortical alpha rhythms may play a critical role in altered patterns functional connectivity expressed by this population during cognitive and perceptual processing. Long-range alpha-band synchronization was also correlated with task performance and visual-perceptual ability within the very preterm group, indicating that altered alpha-oscillatory mechanisms mediating transient functional integration between cortical regions may be relevant to selective problems in neurocognitive development in this vulnerable population at school age.
Keywords: magnetoencephalography, neural synchrony, preterm child, alpha oscillation, neural networks, functional connectivity, developmental neuroimaging, short-term memory
Introduction
Understanding the long-term consequences of very preterm birth on brain development is of tremendous importance due to greatly increased survival rates in recent decades, together with the high and increasing prevalence of cognitive, behavioural and motor deficits in this population (Allen, 2008; Roberts et al., 2010) which impose tremendous demands on health care systems, families, and education systems (Behrman and Sith Butler, 2007). Very preterm children, even when intelligence is broadly normal and major neurological impairment is avoided, commonly exhibit selective problems in areas such as visual processing (Grunau et al., 2002; Rickards et al., 2001; Taylor et al., 2004) and executive function (Anderson and Doyle, 2004; Marlow et al., 2007; Mulder et al., 2009). The alterations in functional brain activity underlying these selective difficulties in the cognitive and perceptual development of very preterm children, however, remain poorly understood.
Neuroimaging research has provided considerable evidence of altered structural brain development in very preterm children, and has indicated that many of these alterations are related to cognitive outcome (see Hart et al., 2008; Ment et al., 2009 for reviews). The prevalent pattern of brain injury in this population is white matter damage, and investigation of microstructural development indicates that white matter abnormalities are evident in very preterm children even in the absence of major impairment or brain damage as detected using conventional imaging methods, indicating altered development of structural connectivity within this vulnerable population (see Miller and Ferriero 2009, for review). This heightened susceptibility of white matter to insult in the preterm infant is related to the selective vulnerability of the oligodendrocyte progenitor cells during the neonatal period corresponding to premature birth (Back et al., 2002, 2007; Khwaja and Volpe, 2008; McQuillen and Ferriero, 2004), as well as the importance of this developmental epoch for the establishment of structural connectivity (see Kostović and Jovanov-Milošević, 2006 for review). Altered white matter development has been associated with poor cognitive outcome in children born very preterm (Counsell et al., 2008; Edgin et al., 2008; Hart et al., 2008; Ment et al., 2009), suggesting that the development of structural connectivity is relevant to problems in cognitive and perceptual development underlying academic difficulties experienced by these children at school age.
Altered patterns of functional connectivity between cortical regions during cognition, measured by very low frequency (< 1 Hz) correlations in blood oxygen level dependent (BOLD) signal fluctuations (fcMRI), have also been reported in children and adolescents born very preterm, even in the absence of major intellectual or neurological impairment (Gozzo et al., 2009; Schafer et al., 2009). Altered network dynamics associated with prematurity are already evident during the neonatal period (Smyser et al., 2010). Importantly, different patterns of functional connectivity are observed even when performance does not differ from that of full-term controls (Schafer et al., 2009), indicating that even when individuals born very preterm successfully recruit activation and functional integration within a network of task-relevant brain regions to support cognitive processing, they express different cortical dynamics than are observed in controls. Such results focusing on altered cortical processing during successful cognition in this population suggest that neonatal insult and subsequent recovery may involve functional reorganization which impacts how communication between brain regions is recruited to support cognitive operations. This perspective is reinforced by the observation of different relationships between cognitive performance and activation in specific cortical regions in this population (Schafer et al., 2009), and observations of different patterns of cortical activation during successful visual-perceptual processing in young adults born very prematurely (Narberhaus et al., 2009).
Noninvasive measurement of functional interactions between regions of human cortex and between cortical and subcortical areas can also be indexed by the synchronization of neuromagnetic oscillations (Hari and Salmelin, 1997; Ribary et al., 1991; Varela et al., 2001). This approach offers certain advantages over fcMRI such as a greatly increased temporal resolution, allowing decomposition of connectivity into time-frequency space, and measurement of fast neural oscillations (synchronized dendritic currents) directly underlying information processing and communication in the brain. Accumulating evidence indicates that synchronization of neural oscillations between brain regions is a general mechanism for assigning transient functional connectivity to support cognition, perception, and motor control (Llinás and Ribary, 1993; Llinás et al., 1994; Ribary, 2005; Schnitzler and Gross, 2005; Uhlhaas et al., 2009a; Varela et al., 2001; Ward, 2003). Neurons, or neural populations, which oscillate in synchrony are able to consistently exchange bursts of action potentials during the depolarized phase of the receiving neuron's ongoing membrane potential fluctuations, thereby increasing the efficacy and fidelity of information exchange (Fries, 2005). Perceptual development during childhood is paralleled by the development of event-related long-range synchronization (Uhlhaas et al., 2009b), and the development of cortical oscillations is correlated with the development of cognitive ability (Benasich et al., 2008). Characteristic disturbances in long-range oscillatory synchrony have also been associated with a variety of neurological and psychiatric conditions (see Schnitzler and Gross 2005; Uhlhaas et al., 2009a for reviews), underscoring the relevance of such processes for understanding altered brain function in clinical populations. Long-range oscillatory connectivity has been found to be altered in conditions affecting childhood learning, such as attention deficit/hyperactivity disorder (ADHD) and autism (Mazaheri et al., 2010; Murias et al., 2007; Uhlhaas et al., 2009a), indicating that the study of such mechanisms represents a promising avenue for examining functional connectivity related to issues in the neurocognitive development of special pediatric populations such as very preterm children.
The present study sought to investigate whether long-range neuromagnetic synchronization was altered during successful visual short-term memory (STM) retention in children born very preterm. This cognitive task was selected as it engages visual-spatial and executive processes, faculties for which very preterm children commonly show selective difficulties (Anderson and Doyle, 2004; Grunau et al., 2002; Marlow et al., 2007), and because visual short-term and working memory retention has been reliably associated with long-range synchronization in multiple frequency ranges in adults (Palva et al., 2005; Palva et al., 2010a; Palva et al., 2010b) and children (Doesburg et al., 2010a). We focused on successful cognition (i.e. correct trials) as our goal was to investigate potential reorganization of functional brain dynamics supporting cognition in this vulnerable population. To this end, magnetoencephalographic (MEG) activity was recorded while school-age very preterm children (≤32 weeks) without major intellectual or neurological impairment and age-matched full-term controls performed a visual STM task and phase synchronization between MEG sensors was calculated.
Methods
Subjects
The groups comprised 34 children born very preterm (≤32 weeks; mean age at MEG 7.72 years [SD = 0.29]; mean gestational age at birth 30.1 weeks [SD = 2.2]) and 12 full-term control children (mean age at MEG 7.54 years [SD = 0.15]). The very preterm group consisted of 21 girls and 13 boys; the full term control group comprised 4 girls and 8 boys. The very preterm subjects, as well as 7 of the control subjects, were recruited from an ongoing longitudinal study on the effects of neonatal pain-related stress on the neurocognitive development of very preterm children (e.g. Grunau et al., 2007, 2009). Non-longitudinal full-term control children were recruited from the same community as those enrolled in the ongoing study. For our primary analysis, we selected 12 very preterm children from the group of 34 that were best matched to the control children on the basis of age, sex, verbal IQ (assessed using the Wechsler Intelligence Scale for Children 4th Ed. (Wechsler, 2003, see below) and handedness. This was done in order to investigate altered long-range neuromagnetic synchronization in children born very preterm without the confounding influence of disparate signal-to-noise ratio between groups caused by an unequal number of subjects. In the case of twins, we did not include both twins in the matched group of 12 very preterm children. This yielded a matched group of 12 preterm children with a mean age at MEG recording of 7.79 years (SD = 3.0) and a mean gestational age at birth of 29.5 weeks (SD = 3.0). We also analyzed long-range phase locking in the group of 34 very preterm children in order to establish that reported group differences pertained to a wider sample of preterm children, given the variability of neurocognitive outcomes in this population even when children with major impairments have been excluded (see Allen, 2008), and to investigate correlations between task performance, cognitive ability, and cortical network dynamics in very preterm children. Neonatal characteristics for the very preterm children in both groups are presented in Table 1. Eleven very preterm children were excluded because they had been diagnosed with a major sensory-motor impairment, showed periventricular leukomalacia (PVL) or grade III–IV intraventricular haemorrhage (IVH) on neonatal ultrasound according to Papile’s classification (Papile et al., 1978), received neuroactive medication (e.g. Ritalin) on the test day, or presented artefacts which prevented effective MEG recording (e.g. metal dental work). Subject exclusion on the basis of neurological abnormalities detected using neonatal ultrasound was employed as this is the standard method for screening for structural brain abnormalities in children born very prematurely. Following subject exclusion, 34 very preterm children remained for analysis in the present study, and 12 of those children were matched to the 12 control children for our primary analysis. Eye examinations to detect retinopathy of prematurity were undertaken if the child was born very prematurely with a birth weight of less than 1250 grams, and would begin around the 42nd day of life.
Table 1.
Characteristics of very preterm children in the group of 12 matched to the full-term controls and the larger group of 34 subjects (which includes the matched group of 12) used in the correlation and confirmatory synchronization analyses.
| Preterm n=12 |
Preterm n=34 |
|
|---|---|---|
| Gestational age (wks) | 29.5 (3.0) | 30.1 (2.2) |
| Birthweight (g) | 1407 (534) | 1418 (433) |
| Days on Mechanical Ventilation | 10.6 (17.6) | 6.2 (12.3) |
| Small for Gestational Age (n, %) | 1 (8.3%) | 2 (5.9%) |
| Singleton (n, %) | 9 (75%) | 17 (50%) |
| IVH Grade I – II | 2 (16.7%) | 4 (11.8%) |
| Retinopathy of Prematurity | 1 (8.3%) | 3 (8.8%) |
| Verbal Comprehension Index (WISC-IV) | 103.0 (10.3) | 102.1 (12.3) |
| Perceptual Reasoning Index (WISC-IV) | 96.5 (12.3) | 102.9 (14.2) |
| Full-scale IQ (WISC-IV) | 95.9 (9.9) | 100.9 (12.4) |
| Mother's Education (yrs) | 15.7 (2.7) | 15.6 (2.5) |
| Mother Completed High School | 12 (100%) | 34 (100%) |
| Boys/Girls | 8/4 | 13/21 |
MEG Recording and Experimental Task
MEG was recorded using a 151 channel MEG system (CTF systems; Coquitlam, Canada). Subjects were in the supine position during recording, and data were digitized at 1200 Hz and stored offline for analysis. Fiducial coils were attached at left and right preauricular points and at the nasion, and each was energized at a characteristic narrow-band frequency above the frequency range of analyzed neural activity. Subjects were monitored by a research assistant within the magnetically shielded chamber throughout recording. Stimuli were presented on a screen positioned 40 cm above the child’s eyes. Prior to MEG recording eyesight was tested using the Snellen eye chart. When necessary, vision was corrected to normal using a nonmagnetic medigoggle system.
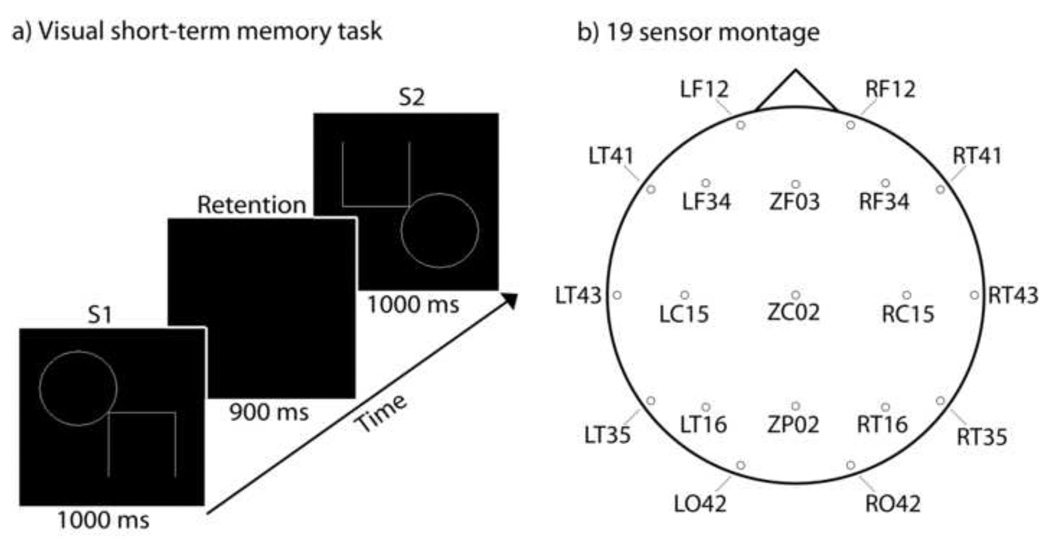
On each trial of the visual STM task (Figure 1A) the child viewed an initial stimulus (S1) for 1000 ms, followed by a 900 ms retention interval during which they were instructed to remember the initial stimulus. A second stimulus (S2) was then presented for 1000 ms. When stimuli appeared they were surrounded by a bounding box (10 cm; 14.25° visual angle.) This bounding box remained on-screen during the STM retention interval. A 1700 – 2000 ms interval followed each trial. The child was instructed to press one of two buttons on a response box to indicate whether the second stimulus was the same or different from the first stimulus. Stimuli were considered different if they (a) were different sizes, (b) had changed orientation, or (c) had part of the image added or removed. All subjects were trained on the task and on mapping responses to the correct buttons prior to recording. Stimuli were adapted from the Beery-Buktenica Developmental Test of Visual-Motor Integration (5th Edition, © 2004), with permission from the publisher. Three blocks of 60 trials were presented to each subject; a short break period was given between each block. Subjects were instructed to respond as quickly and accurately as possible. One-tailed t-tests were used to assess group differences in performance, as poorer performance was expected for the preterm group given their selective difficulties in executive function and visual processing (Anderson and Doyle, 2004; Grunau et al., 2002; Marlow et al., 2007), both of which play a critical role in visual STM.
Figure 1.
a) The stimulus display and its time course on a single trial. b) Locations and channel names of sensors used in the 19 channel montage.
Developmental Testing
The Wechsler Intelligence Scale for Children 4th Ed. (WISC-IV; Wechsler, 2003) was administered by a psychometrician to assess cognitive ability of each child; as well, visual perception, motor coordination, and visual-motor integration were assessed with the Beery-Buktenica Developmental Test of Visual-Motor Integration 5th Edition, © 2004 (Beery VMI). Psychometric data was used for the purposes of subject matching and to investigate potential relationships between MEG activity and cognitive, perceptual, and motor function.
MEG analysis
Our analysis of long-range phase synchronization focused on the STM retention interval (between the first and second stimuli) as long-range MEG synchronization in multiple frequency ranges has been reliably reported during visual short-term and working memory maintenance in both adults (Palva et al., 2005; Palva et al., 2010a; Palva et al., 2010b) and school age children (Doesburg et al., 2010a), making it ideal for the study of potential alterations in inter-regional phase locking in children born very preterm. As our aim was to investigate possible alteration and reorganization of functional connectivity in children born very preterm, we concentrated our analysis on instances of successful cognition, i.e. trials on which the subjects responded correctly. Group differences in such an analysis most directly reflect functional reorganization, as they indicate that even when children very preterm children successfully recruit neural processes to support cognitive processing, different patterns are observed. Moreover, successful and unsuccessful instances of visual perception and visual memory function are associated with different patterns of long-range phase synchronization (i.e. Melloni et al. 2007; Supp et al., 2007; Rodriguez et al., 1999). As the visual STM task was selected because of its dependence on executive and visual-perceptual processes, areas in which children born very preterm show selective difficulty (Anderson and Doyle, 2004; Grunau et al., 2002; Marlow et al., 2007), group differences in accuracy on the task were expected. As such, in an analysis including all trials it would not be possible to distinguish if any observed group differences were due to reorganization of function in preterm children or instead attributable simply to differences in brain responses related to successful versus unsuccessful cognitive processing. Selection of correct trials only for analysis yielded an average of 134.2 (18.2) trials for each subject in the group of 12 full-term controls for a total of 1610 trials, and an average of 117.7 (27.1) trails for each of the very preterm children in the matched group of 12 for a total of 1412 trials. Very preterm children in the larger group of 34 had an average of 117.5 (25.8) per subject for a total of 3996 correct trials.
To standardize head location within and between subjects dipolar source solutions were computed for each fiducial coil 30 times per second, producing a continuous record of head location throughout MEG recording. The MEG data were then aligned to a common position by performing an inverse solution, data rotation, and forward solution 30 times each second (Wilson et al., 2007). The high frequency activity emitted by the fiducial coils during recording was then removed from the MEG record using notch filters and the data were downsampled to 300 Hz. Epochs were extracted surrounding each correct trial of the STM task. Ocular and nonocular artefacts were then removed using a principal components analysis (PCA) based procedure (see Herdman and Cheyne, 2009). This approach was chosen over that of rejecting artefact contaminated epochs as child data imposes limitations on the number of trials available for analysis.
A montage of 19 MEG sensors distributed roughly evenly over the head was selected for the analysis of long-range phase locking (Figure 1B). This approach was chosen, rather than that of analyzing synchronization between all available sensor pairs, (i) because synchronization between neighbouring sensors is more likely to be spurious (both sensors recording the lead field of a single oscillating source), and (ii) in order to reduce computational load associated with pair wise comparisons. Data were then digitally filtered at 1 Hz intervals from 4 – 60 Hz (passband = f ± 0.05f, where f represents the filter frequency). We then calculated the analytic signal
of the filtered waveform for each epoch, f(t), where f̃ (t) is the Hilbert transform of f(t) and , to obtain the instantaneous phase, ϕ(t), and amplitude, A(t), at each sample point. Phase locking values (PLVs) were computed from the differences of the instantaneous phases for 12 analyzed sensor pairs for each analyzed frequency, for example, sensors j and k, at each point in time, t, across the N available epochs (Lachaux et al., 1999):
PLV is a real number between 0 (no phase locking, random phase difference) and 1 (maximum phase locking, constant phase difference). In order to identify changes in long-range synchronization relevant to task demands PLVs were standardized relative to a 500 ms baseline interval immediately preceding the onset of S1. Standardization was accomplished by subtracting the mean baseline PLV from the PLV for each data point and dividing by the standard deviation of the baseline PLV. The resulting PLV scores indicate standardized changes from the average baseline PLV. To assess group differences in long range phase locking, we calculated averaged PLVs both for a given sensor pair within each group. PLVs from the control group were then subtracted from the PLVs for the very preterm group, creating a time-series of difference-PLVs (DPLVs) between the compared groups. DPLVs were then standardized at each time-point (for a given sensor pair and frequency) by subtracting the mean of the baseline DPLV and dividing the standard deviation of the baseline DPLV (see Doesburg et al., 2009).
To assess the statistical reliability of changes from baseline in long-range synchronization for individual sensor pairs, in order to reveal the topography of event-related changes in long-range synchrony, we employed the surrogate statistical method (see Lachaux et al., 1999). To this end we shuffled the epochs and computed PLVs for the shuffled data for each frequency and time point combination for each pair of sources. The resulting PLVs were then standardized relative to the (shuffled) pre-S1 baseline. This process was repeated 2000 times to create a surrogate PLV distribution. The percentile rankings of PLV values obtained from real (unshuffled) data from the normalized PLV values within the surrogate distributions were used to assess the statistical significance of observed changes in synchronization (i.e. for a two-tailed test with an alpha level = 0.05 significant synchronization entails real PLV above 1950 surrogates; desynchronization entails real PLV below all but 50 surrogates). In order to assess the statistical significance of group differences for individual sensor pairs we used surrogate methods for comparison of event-related phase locking (see Doesburg et al., 2009). We shuffled the epochs for both of the to-be-compared group’s data and computed PLVs for the shuffled data for each frequency and data point combination for each analyzed sensor pair. The resulting PLVs were used to create DPLVs as was done for the unshuffled data. The resulting DPLVs were then standardized relative to the (shuffled) pre-S1 baseline interval. This process was repeated 2000 times to create a surrogate distribution for each set of the DPLVs. The percentile rankings of the unscrambled DPLVs within the surrogate distributions created from shuffled data were used to assess the statistical significance of group differences in synchronization/desynchronization for each sensor pair.
In order to assess the statistical significance of global patterns of long-range synchronization during STM retention using a method which did not require large numbers of comparisons we employed t-tests at peak frequencies of group differences. Group differences in global long-range alpha band synchronization, for example, were assessed by averaging 10 Hz PLV across each of 171 sensor pairs and each time point within the STM retention interval for each subject. This produced a single value for global alpha-band PLV for each subject, and t-tests were used to test the significance of group differences. We used two-tailed t-tests in these MEG analyses, and unequal variances were assumed if the variances between groups differed by more than 0.1 standard deviation. All statistical tests were performed using MATLAB and group differences and correlations were considered significant when p < 0.05. Analyses of global amplitude were similarly accomplished by averaging instantaneous amplitude values across all sensors in the 19 channel montage and all time points during retention for a given frequency.
Results
Task Performance
Full-term controls performed more accurately on the visual STM task than the 12 matched preterm children (p < 0.05) and the larger group of 34 very preterm children (p < 0.02). Accuracy was 74.5% (SD = 10.1) for the full-term children, 65.4% (SD = 15.0) for the matched group of 12 very preterm children, and 65.4% for the larger group of 34 very preterm children (SD = 14.1).
Long-range phase synchronization
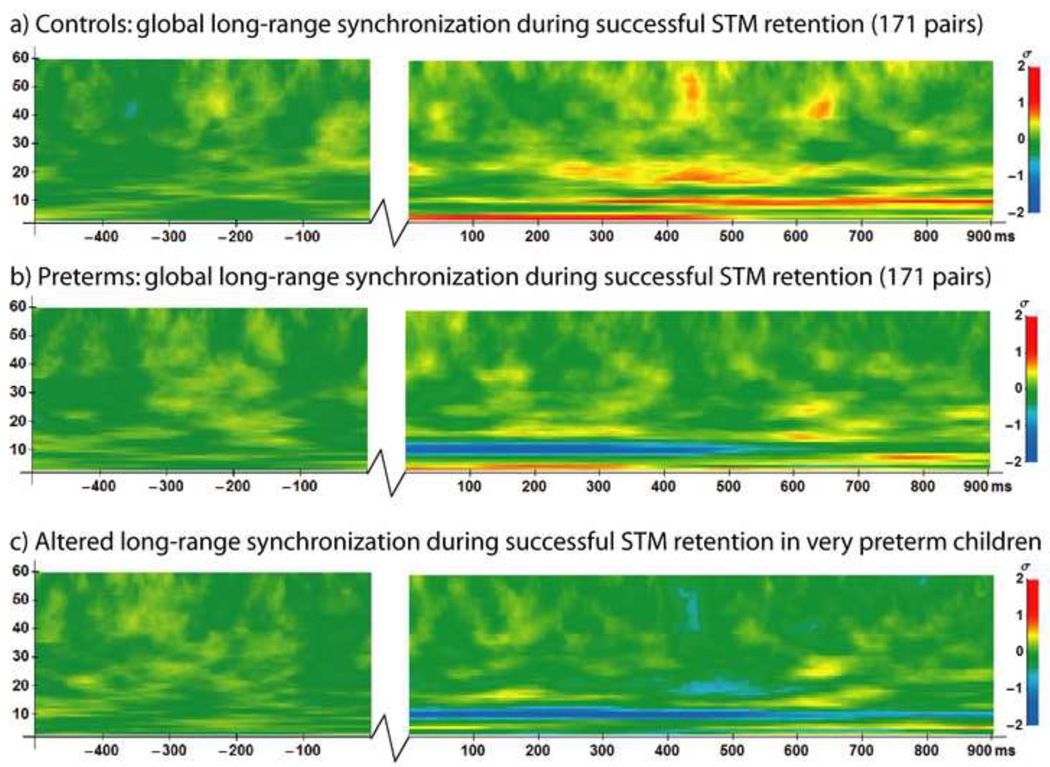
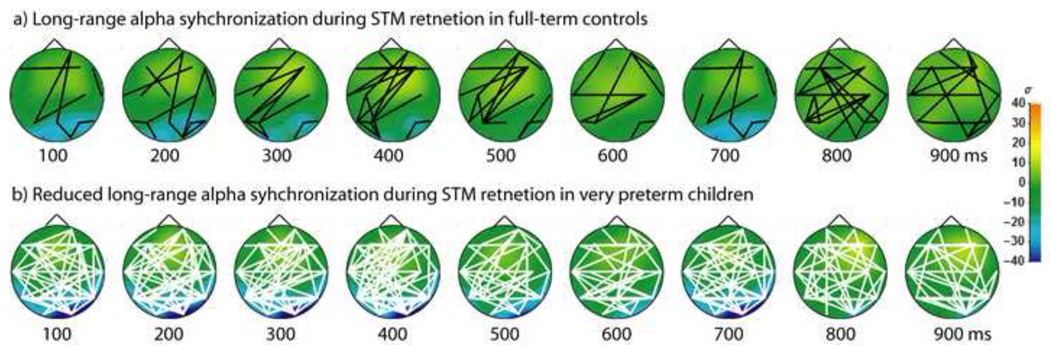
During successful visual short-term memory retention, fullterm controls expressed sustained synchronization in the theta (4 – 7 Hz), alpha (8 – 14 Hz) and beta frequency ranges (15 – 30 Hz), together with periodic gamma-band (30 – 60 Hz) synchronization (Figure 2A). These results are consistent with our previous work establishing a normative pattern of long-range synchronization during short-term memory retention in children age 6 – 10 years using the experimental paradigm and PLV analysis methods employed in the present study (Doesburg et al., 2010a). Very preterm children, conversely, expressed different patterns of long-range synchronization than full-term controls (Figure 2B). Differences between full-term controls and the matched preterm group were concentrated within the alpha frequency range and centred at 10 Hz, as preterm children exhibited long-range desynchronization, rather than synchronization, of alpha oscillations (Figure 2C). Long-range phase synchronization in very preterm children in frequency ranges outside the alpha-band, however, more closely resembled the pattern observed in the controls. Global alpha-band amplitude was reduced for both full-term controls and very preterm children and did not differ significantly between groups. Topographic analysis of long-range alpha-band synchronization in full-term controls indicated a widespread pattern which included considerable frontoposterior connectivity (Figure 3A), as established previously (Doesburg et al., 2010a). Analysis of the topography of group differences revealed a widespread pattern of alpha-band desynchronization in very preterm children, relative to controls (Figure 3B). This alpha-band desynchronization in very preterm children, relative to controls, expressed an interhemispheric concentration.
Figure 2.
Time-frequency plots of global long-range synchronization during the 500 ms baseline period preceding the onset of the first stimulus (−500 to 0 ms) and during the 900 ms STM retention interval between the first and second stimuli (0 to 900 ms) on correct trials. Jagged lines indicate temporal discontinuity (time period between baseline and retention interval). a) Normative pattern of long-range synchronization during successful visual STM retention in 12 full-term controls. b) Long-range synchronization on correct trials in the matched group of 12 very preterm children. c) Group differences in global long-range phase synchronization, where blue represents reduced synchronization in 12 matched preterm children relative to controls during successful visual STM retention, and yellow/red represents increased synchronization in preterm children relative to controls. Legends at right.
Figure 3.
a) Topography of long-range alpha-band (10 Hz) synchronization in controls during successful visual STM retention. Black lines indicate statistically significant synchronization between sensors (p < 0.05). b) Topography of altered long-range alpha-band (10 Hz) desynchronization, relative to controls, during successful visual STM retention in children born very preterm. White lines indicate statistically significant reductions in long-range synchronization in 12 matched very preterm children, relative to controls (p < 0.001). Colours represent amplitude; legend at right.
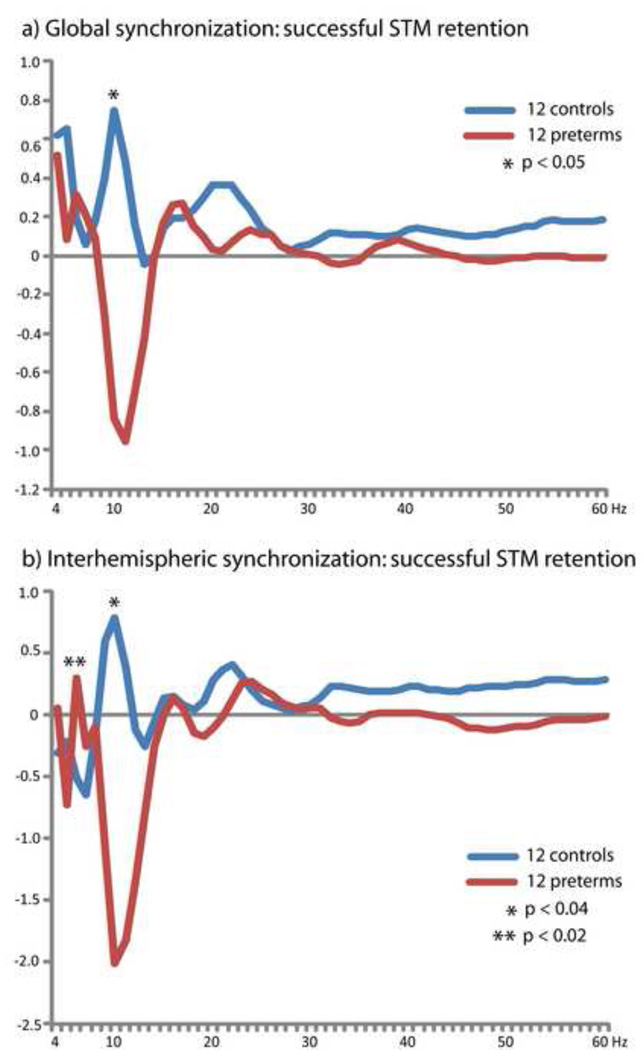
To reveal the spectral density of global long-range phase locking during successful visual STM retention for full-term controls and our matched preterm group, we averaged PLVs at each analyzed frequency over each time point within the STM retention interval and over all 171 analyzed sensor pairs. This revealed a pronounced and statistically significant (p < 0.05) group difference in the alpha-band (10 Hz), wherein very preterm children exhibited task-dependent desynchronization while the full-term controls expressed synchronization (Figure 4A). A second peak was also observed in the very preterm group in the high theta frequency range which was distinct from the low-theta peak observed in both preterms and controls, although this difference was not statistically significant. As the topographic mapping of group differences in long-range alpha synchronization revealed an interhemispheric concentration of desynchronization in very preterm children, we also calculated the spectral density of interhemispheric phase locking for each group. This was accomplished by averaging PLVs over each time point within the STM retention interval and over each of 64 sensor pairs that had one sensor over each cerebral hemisphere (Figure 4B). This revealed reduced alpha-band (10 Hz) synchronization in the matched very preterm group (p < 0.04), as was seen in the analysis of global phase locking, together with the emergence a distinct additional peak in the high theta-band (6 Hz) for the very preterm children which was not observed in the controls (p < 0.02).
Figure 4.
Spectral density of long-range phase locking for groups 12 controls and 12 matched very preterm children obtained by averaging PLVs across all time points during the STM retention period for each frequency. a) Global spectrum of long-range synchronization obtained be averaging over all 171 analyzed sensor pairs. b) Spectral density of interhemispheric synchronization obtained by averaging over all sensor pairs which included a sensor over each hemisphere (64 pairs). Note the reduced alpha-band (8 – 14 Hz) synchronization, and the emergence of an additional peak in the theta-band (4 – 7 Hz), in very preterm children.
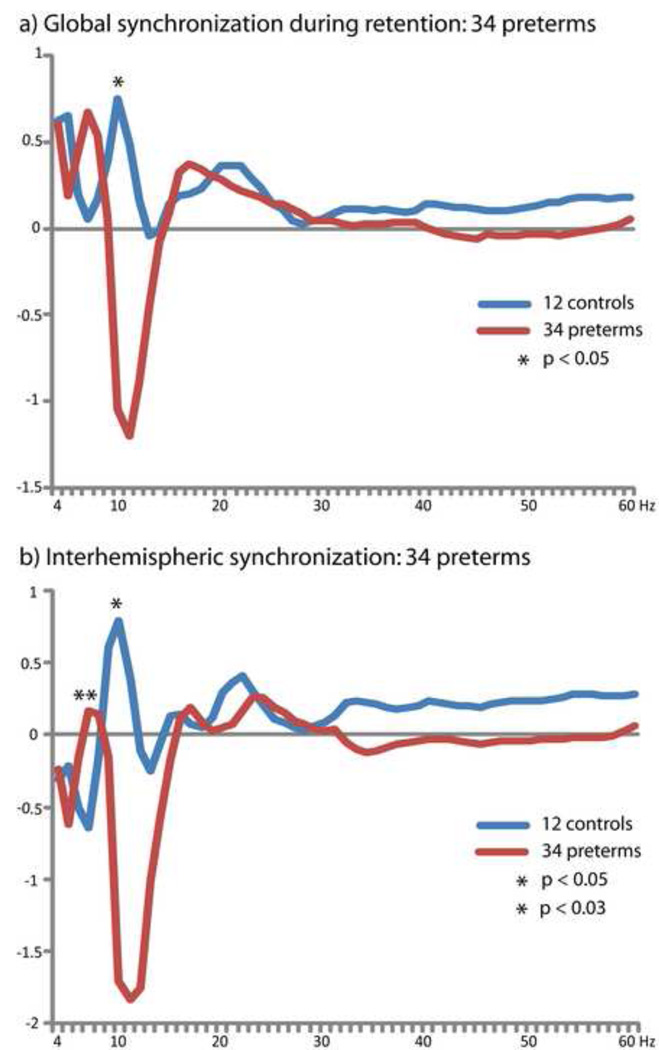
To confirm that observed group differences were also present in a larger group of very preterm children, given the variability of outcome in this population (Allen, 2008), and to investigate correlations between altered oscillatory cortical dynamics and cognitive function given the statistical demands of correlation analysis on sample size (Cohen, 1992), we repeated our analysis of global and interhemispheric long-range synchronization using all 34 very preterm subjects. Analysis of global long-range phase synchronization confirmed group differences (p < 0.05) in the alpha-band (10 Hz), as well as demonstrating that this larger group of very preterm children also showed long-range desynchronization in the alpha-band (Figure 5A). As was seen in the group of 12 very preterm children, an additional peak was observed in the high theta-band (n.s.). Interhemispheric synchronization in the alpha-band (10 Hz) was also reduced in the group of 34 children born very preterm (p < 0.05; Figure 5B), and these children also expressed an additional peak in interhemispheric phase locking in the high theta-band (6 Hz) which was not present in the full-term children (p < 0.03).
Figure 5.
Altered long-range phase locking during successful visual STM retention confirmed using a larger group of 34 very preterm children for both a) global synchronization (171 sensor pairs) and, b) interhemispheric synchronization (64 sensor pairs).
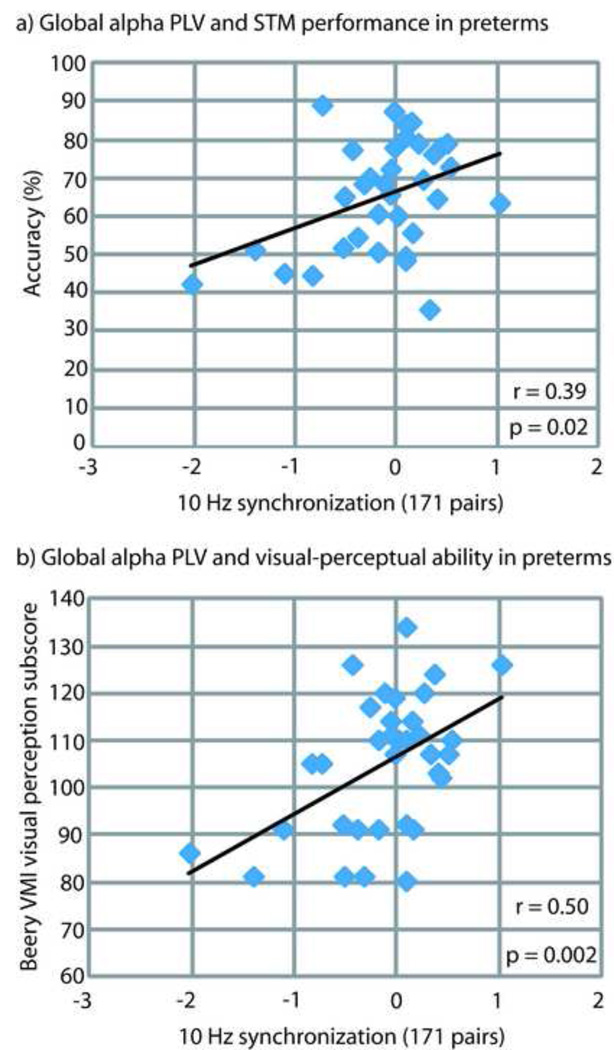
Global long-range alpha-band synchronization was correlated with accuracy on the visual STM task within the group of 34 very preterm children (r = 0.39; p = 0.022; Figure 6A). To investigate the relationship between altered long-range alpha-band synchronization and cognitive function within this group, we calculated the correlation of global long-range alpha-band synchronization with the WISC-IV full scale IQ, and the Beery visual-motor integration, motor coordination, and visual perception scores. Altered long-range alpha-band synchronization during visual STM retention was not correlated with full-scale IQ, but was positively correlated with the visual perception subscale of the Beery (r = 0.50; p = 0.002; Figure 6B). Table 2 details all correlation data for altered long-range alpha-band synchronization and cognitive ability. The full-term controls did not display the same relationships between global long-range alpha-band (10 Hz) synchronization and accuracy on the visual STM task (r = −0.24; p = 0.46) or the visual perception subscore of the Beery (r = 0.005; p = 0.81). Very preterm children and full term controls exhibited roughly similar variability across subjects, expressed in standard deviations, in global long-range alpha-band PLV and the visual perception subscale of the Beery (12 controls = 15.09; 12 preterms = 11.14; 34 preterms = 14.64). Global-long range alpha-band synchronization within the group of 34 very preterm children was not correlated with gestational age (r = −0.2; p = 0.27) or maternal years of education (r = −0.07; p = 0.67), and did not differ between very preterm boys and girls (p = 0.83).
Figure 6.
Correlation of global long-range alpha-band synchronization (171 pairs) in 34 very preterm children with a) accuracy on the visual STM task, and b) visual-perceptual ability as measured by the Beery-Buktenica Developmental Test of Visual-Motor Integration.
Table 2.
Correlations between global long-range alpha-band phase synchronization during successful visual short-term memory retention (171 pairs; 10 Hz) and cognitive ability in 34 children born very preterm.
| Correlation Coefficient |
p Value | |
|---|---|---|
| WISC-IV Full-Scale IQ | 0.12 | 0.49 |
| Beery Visual Perception Subscale | 0.50 | 0.002* |
| Beery Visual-Motor Integration Subscale | 0.28 | 0.11 |
| Beery Motor Coordination Subscale | 0.13 | 0.48 |
Discussion
We present the first evidence of altered long-range neuromagnetic synchronization in very preterm children, indicative of altered functional connectivity during visual STM retention. We demonstrate that alterations in long-range synchronization are concentrated within the alpha frequency range, suggesting that alpha oscillations may play a critical role in altered cortical network function in children born very preterm. These results show that differences in long-range alpha synchronization during cognition are evident in very preterm children with broadly normal intelligence and without major sensory or motor impairment. This indicates that children born very preterm, even when their intellectual ability is considered within the normal range, express markedly different cortical network dynamics to support cognitive processing. Research using fcMRI has indicated that children and adolescents born very prematurely recruit different patterns of cortical connectivity to support cognitive processing (Gozzo et al., 2009; Schafer et al., 2009). These findings suggest functional reorganization during the cortical development of very preterm children, and/or the use of compensatory strategies. This outlook is supported by MEG findings that a larger number of dipoles are required to account for brain activity within several cortical areas in adolescents born very preterm during the performance of a cognitive task (Frye et al., 2010). This indicates that very preterm adolescents recruit more diffuse brain areas during cognition. The findings of the present study expand our emerging understanding of cortical network function in very preterm children by demonstrating that oscillatory mechanisms mediating functional connectivity during cognition exhibit frequency-specific deviation from patterns of phase locking observed in children born full-term and healthy. As we focused our analysis of group differences on correct trials, our results demonstrate that even when very preterm children successfully recruit functional network connectivity to support visual STM retention, they do so using a different set of oscillatory networks and mechanisms than are observed in full-term children and adults. Analysis of correct trials also ensured that observed group differences were due to altered cortical processing during STM retention, rather than differences between successful and unsuccessful processing, as very preterm children performed less accurately than full-term controls. Alterations in long-range oscillatory synchronization during successful cognition in children born very preterm may be related to altered development of structural connectivity due to selective vulnerabilities in the developing brain during the neonatal period corresponding to premature birth, white matter injury (see Miller and Ferriero, 2009), and/or functional reorganization and compensation related to recovery from adverse effects on brain development experienced by this vulnerable population during the perinatal period (i.e. Schafer et al., 2009).
In the present study, we reproduce and extend findings from our previous work (Doesburg et al., 2010a) indicating that STM retention in healthy children at age 6 to 10 years born full-term entails long-range synchronization in a number of frequency ranges including the theta, alpha and beta-bands. This result is consistent with research on adult humans which has demonstrated increased inter-regional synchronization during visual STM and working memory retention using electroencephalography (EEG) (Sarnthein et al., 1998; Payne and Kounios, 2009; Sauseng et al., 2005), MEG (Palva et al., 2005; Palva et al., 2010a; Palva et al., 2010b; Doesburg et al., 2010a) and implanted electrodes (Tallon-Baudry et al., 2001). Such synchronization has been interpreted as functional connectivity within a distributed cell assembly recruited to maintain the memory trace. Very preterm children, relative to controls, exhibited reduced long-range synchronization during STM retention in the alpha frequency range. Strikingly, this was manifest as task-dependent long-range alpha-band desynchronization in children born very preterm, whereas task-dependent synchronization was observed in full-term controls. Very preterm children, however, expressed a pattern of long-range synchronization more similar to that observed in controls in other frequency ranges. The significance of alpha synchronization to the development perceptual and cognitive function is evidenced by observation of age-related changes in event-related long-range alpha-band synchronization throughout childhood and adolescence during visual perception (Uhlhaas et al., 2009b), as well as age-related changes in alpha oscillatory responses during cognition throughout childhood (Krause et al., 2001; Yordanova and Kolev, 1996, 1997). The functional role of alpha oscillations in visual STM retention is demonstrated by findings that individual differences in visual STM span can be predicted from the parameters of alpha oscillations (Maltseva and Maslobev, 1997) and long-range alpha-band synchronization (Palva et al., 2010a).
Inspection of group differences in the spectral density of global-long range synchronization reveals that children born very preterm express a second peak in the high theta range (6 Hz), which is distinct from the low-theta peak observed in both preterm and full-term children. In the PLV spectrum of interhemispheric long-range synchronization, a topography in which group differences in long-range alpha synchronization were concentrated, this group difference was more pronounced and statistically significant. The presence of an additional peak within the slower theta frequency range suggests that alpha oscillatory mechanisms mediating functional connectivity during visual STM retention are not entirely absent in very preterm children, but rather are expressed at a slower frequency, indicating reorganized and/or compensatory cortical network dynamics. Reduced interhemispheric synchronization in very preterm children may relate to anatomical brain development as structural alterations of corpus collosum have been reported in children born preterm and are related to functional outcome in this group (see Hart et al., 2008 for review), and resting interhemispheric alpha-band EEG coherence has been related to corpus collosum structure in ageing adults with and without mild cognitive impairment (Teipel et al., 2009). It has also been shown that extremely low birth weight (ELBW) infants measured at term equivalent age express reduced interhemispheric EEG coherence in the 1 – 12 Hz frequency range during sleep (Grieve et al., 2008), and that young adults who were born ELBW expressed greater short-distance, and reduced long-distance, alpha-band resting EEG coherence over the right hemisphere (Miskovic et al., 2009). Such results suggest that alterations in long-range alpha-band synchrony in very preterm children may emerge very early in life and persist into adulthood. A limitation of the present study is that MRI was not implemented to screen or evaluate white matter structure in our cohort of very preterm children, but rather relied on neonatal ultrasound for detection of structural brain abnormalities. Accordingly, it is possible that subtle white matter abnormalities detectable using conventional MRI may have been present in some subjects. Future research will be required to explore the potential relationship between white matter damage, microstructural white matter abnormalities, and altered inter-regional synchronization among cortical and sub-cortical brain areas in very preterm children.
Investigations of resting spectral power lend precedent to the notion that alpha oscillatory mechanisms may be slowed toward the theta frequency range in very preterm children, as it is well known that brain development is characterized by a progressive shift from lower to higher frequencies, a trend that begins in the perinatal period (Okamura et al., 2006) and continues throughout childhood (Clarke et al., 2001) and into early adulthood (Fehr et al., 2002). Children born very preterm express relatively more low-frequency activity during the neonatal period (Okamura et al., 2006), and this tendency persists into school age as evidenced by a greater ratio of low-to-high frequency resting EEG activity (Miskovic et al., 2009) and a slowing of the alpha peak towards the theta frequency range in spontaneous MEG recordings (see Doesburg et al., 2010b). Slowing of alpha oscillations toward the theta-band has been attributed to disordered interaction between cortex and thalamus (Llinás et al., 1999; Hughes and Crunelli, 2005), and very preterm children show altered development of thalamocortical systems (i.e. Anjari et al., 2007; Giménez et al., 2006; Srinivasan et al., 2007). The relationship between altered electromagnetic oscillations and structural brain development in very preterm children represents a promising direction for future study, as recent research indicates that parameters of the alpha rhythms are related to white matter architecture (Valdés-Hernández et al., 2010).
Analysis of long-range synchronization within the group of 34 very preterm children confirmed that observed alterations in long-range synchronization were also present in a larger group of very preterm children, and also provided sufficient sample size to investigate the potential relationship of altered long-range alpha-band synchronization with performance on the visual STM task and measures of cognitive ability. Within the group of 34 very preterm children global alpha-band synchronization was correlated with accuracy on the visual STM task and with a visual perception task that did not have memory demands, but importantly was not a function of full-scale IQ. This indicates that altered long-range alpha synchronization may be a mechanism underlying selective difficulties in very preterm children, as this population is known to commonly express problems in visual perceptual function, even when intelligence is broadly normal and in the absence of neurological, sensory or motor impairment (see Grunau et al., 2002). Alternatively stated, the degree to which very preterm children express altered long-range alpha-band synchronization during successful visual STM maintenance is correlated with the extent of reduced visual-perceptual function. As global alpha synchronization was not correlated with gestational age in very preterm children, and because alpha desynchronization was observed in preterm children near the upper limit of gestational ages included in the present study, the altered cortical dynamics reported presently appear to affect children born across the entire range of gestational ages investigated (≤32 weeks). Critically, children born full-term and healthy did not exhibit the same pattern of correlations between long-range alpha synchronization, accuracy on the STM task, and visual-perceptual function, indicating that altered long-range alpha synchronization in very preterm children is relevant to specific issues surrounding neurocognitive development within this vulnerable population, rather than variation within the population of school age children more generally. Although the relatively small sample size of full-term controls in the present study was not sufficient for a proper statistical evaluation of the correlation between alpha-band synchronization and visual-perceptual ability, it was sufficient to establish the direction of correlation (see Cohen, 1992). Observation that this correlation was in the opposite direction than was observed for the very preterm children indicates that the relationship between long-range synchronization and cognitive function was not similar across full-term and preterm groups. These differential relationships are unlikely to have arisen because of differences in variance across the groups, as variability on these measures were roughly similar in both populations, and neither group trended toward greater variability for all relevant measures (alpha PLV, STM accuracy, and Beery visual perception subscore). Global alpha amplitude did not exhibit a similar, or statistically significant, relationship to STM performance or visual-perceptual function, indicating that alterations of alpha-mediated mechanisms underlying functional connectivity, rather than local activation, were of particular relevance to perceptual function in school age children born very preterm. Confirmation of observed group differences in long-range synchronization comparing 12 controls and 34 very preterm children also indicates that these effects could not have arisen due to signal-to-noise ratio imbalances in our primary analysis (i.e. more correct trials for the full term controls), as greater signal-to-noise ratio would be present for the very preterm group in this secondary analysis.
As with any study investigating synchronization between sensors or electrodes arrayed over the surface of the head, the potential influence of volume conducted neural synchrony is an important consideration. There are several reasons, however, that the effects reported in the present study are not attributable to the influence of volume conduction. As in Doesburg et al., (2010a), we found that global long-range alpha-band synchronization was increased in full-term children, whereas global alpha-band amplitude was decreased, demonstrating that these PLV and instantaneous amplitude measures were sensitive to very distinct aspects of brain activity and indicating that the observed normative pattern of long-range alpha synchronization during STM retention could not arise through volume conduction. The view that PLV values represent neural processes distinct from local amplitude changes is further buttressed by the finding that although global long-range alpha-band synchronization showed opposite directionality between the groups and differed significantly, both preterm and full-term children showed global alpha amplitude reductions during STM retention and did not differ statistically on this measure. Moreover, long-range alpha phase locking showed correlations with accuracy on the visual STM task and with visual perceptual function within the very preterm group which were not observed for global alpha amplitude. Furthermore, increased long-range alpha synchronization was observed in controls between posterior sensors showing reduced alpha amplitude, and relative desynchronization in very preterm children was observed between frontal sensors expressing relative increases alpha amplitude. Due to the distributed lead fields of the axial gradiometers, however, a limitation of the present study is that it is not possible to infer the putative engagement of specific cortical regions in large-scale network connectivity. Future research combining source localization and long-range synchronization analysis methods will be required to precisely map altered connectivity and communication between specific brain regions underlying selective cognitive difficulties in children born very preterm.
Conclusion
We present the first evidence of altered long-range neuromagnetic synchronization in children born very preterm, indicative of altered functional connectivity during visual STM retention. Atypical long-range synchronization was concentrated in the alpha frequency range, with possible reorganization of cortical network dynamics involving the lower theta frequency range, and correlated with visual-perceptual function in very preterm children. The results of the present study suggest that altered patterns of functional connectivity mediated by cortical alpha oscillations may underlie selective difficulties in visual-perceptual ability common within this vulnerable population at school age.
Acknowledgements
We would like to thank Dr. Ivan Cepeda and Gisela Gosse for coordinating the study, Katia Jitlina, Amanda Degenhardt and Julie Unterman for their help in data collection, and John Gaspar and Wenqi Sun for their help with data analysis. We thank Teresa Cheung, Dr. Hal Weinberg, Dr. Mario Liotti and Dr. Daniel Weeks of Simon Fraser University for their help in designing the study. We also thank Dr. Keiichi Kitajo, RIKEN Brain Science Institute, and Dr. Kentaro Yamanaka, University of Tokyo, for help in developing the phase synchrony analysis programs. This work was supported by grant RO1 HD039783 from the Kennedy Shriver Institute of Child Health and Human Development of the National Institutes of Health (NICHD/NIH) to R.E.G., who received salary support from the Child and Family Research Institute (CFRI). S.M.D. was supported by a Pain in Child Health (PICH) Training Award and a Neuroscience Training Award from the Canadian Institutes for Health Research (CIHR), and is currently supported by a Postdoctoral Fellowship award from the Michael Smith Foundation for Health Research (MSFHR) and the CFRI. S.P.M. is supported by a Clinician Scientist award from CIHR and a Michael Smith Scholar award from MSFHR. U.R. holds a Chair Leadership Chair in Cognitive Neuroscience in Early Childhood Health and Development supported by the BC Leading Edge Endowment Fund (BC LEEF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen MC. Neurodevelopmental outcomes of preterm infants. Curr Opin Neurobiol. 2008;21:123–128. doi: 10.1097/WCO.0b013e3282f88bb4. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Doyle LW. Executive functioning in school-age children who were born very preterm of with extremely low birth weight in the 1990s. Pediatrics. 2004;114(1):50–57. doi: 10.1542/peds.114.1.50. [DOI] [PubMed] [Google Scholar]
- Anjari M, Srinivasan L, Allsop JM, Hajnal JV, et al. Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. NeuroImage. 2007;35:1021–1027. doi: 10.1016/j.neuroimage.2007.01.035. [DOI] [PubMed] [Google Scholar]
- Back SA, Han BH, Luo NL, Chricton CA, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;15(22):455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Riddle A, McClure MM. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke. 2007;38(2):724–730. doi: 10.1161/01.STR.0000254729.27386.05. [DOI] [PubMed] [Google Scholar]
- Beery KE, Buktenica NA, Beery NA. Beery-Buktenica Developmental Test of Visual-Motor Integration. 5th Ed. Psychological Corporation; 2004. [Google Scholar]
- Behrman RE, Stith Butler A, editors. Institute of Medicine Committee on Understanding Premature Birth and Assuring Healthy Outcomes Board on Health Sciences Outcomes: Premature Birth: Causes, Consequences and Prevention. Washington DC: The National Academies Press; 2007. [Google Scholar]
- Benasich AA, Gou Z, Choudhury N, Harris KD. Early cognitive and language skills are linked to resting frontal gamma power across the first three years. Behav Brain Res. 2008;195(2):215–222. doi: 10.1016/j.bbr.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Age and sex effects in the EEG: development of the normal child. Clin Neurophysiol. 2001;112:806–814. doi: 10.1016/s1388-2457(01)00488-6. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psycol Bull. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Counsell SJ, Edwards AD, Chew ATM, Anjari M, et al. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008;131:3201–3208. doi: 10.1093/brain/awn268. [DOI] [PubMed] [Google Scholar]
- Doesburg SM, Green JJ, McDonald JJ, Ward LM. From local inhibition to long-range integration: a functional dissociation of alpha-band synchronization across cortical scales in visuospatial attention. Brain Res. 2009;1303:97–110. doi: 10.1016/j.brainres.2009.09.069. [DOI] [PubMed] [Google Scholar]
- Doesburg SM, Herdman AT, Ribary U, Cheung T, et al. Long-range synchronization and local desynchronization of alpha oscillations during visual short-term memory retention in children. Exp Brain Res. 2010a;201(4):719–727. doi: 10.1007/s00221-009-2086-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doesburg S, Ribary U, Herdman A, Moiseev A, Cheung T, Miller S, Weinberg H, Grunau R. Magnetoencephalography reveals thalamocortical dysrhythmia in children born very preterm. Conference Abstract: Biomag 2010 - 17th International Conference on Biomagnetism.2010b. [Google Scholar]
- Edgin JO, Inder TE, Anderson PJ, Hood KM, et al. Executive functioning in preschool children born very preterm: relationship with early white matter pathology. J Int Neuropsychol Soc. 2008;14:90–101. doi: 10.1017/S1355617708080053. [DOI] [PubMed] [Google Scholar]
- Fehr T, Bott C, Haeberle A, Rockstroth B. MEG power spectrum and age: differences between adolescents and adults. 13th International Meeting on Biomagnetism; VDE Verlag. 2002. http:biomag2002.uni-jena.de/ [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cog Sci. 2005;9(10):474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Frye RE, Malmberg B, McLean J, 3rd, Swank P, et al. Increased left prefrontal activation during an auditory language task in adolescents born preterm at high risk. Brain Res. 2010;1336:89–97. doi: 10.1016/j.brainres.2010.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giménez M, Junqué A, Narberhaus A, Botet F. Correlations of thalamic reductions with verbal fluency impairment in those born prematurely. Neuroreport. 2006;17(5):463–466. doi: 10.1097/01.wnr.0000209008.93846.24. [DOI] [PubMed] [Google Scholar]
- Gozzo Y, Vohr B, Lacadie C, Hampson M, et al. Alterations in neural connectivity in preterm children at school age. Neuroimage. 2009;48:458–463. doi: 10.1016/j.neuroimage.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve PG, Isler JR, Izraelit A, Peterson BS, et al. EEG functional connectivity in term age extremely low birth weight infants. Clin Neurophysiol. 2008;119:2712–2720. doi: 10.1016/j.clinph.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RE, Haley DW, Whitfield MF, Weinberg J, Yu W, Thiessen P. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J Pediatr. 2007;150(2):151–156. doi: 10.1016/j.jpeds.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RE, Whitfield MF, Davis C. Pattern of learning disabilities in children with extremely low birth weight and broadly average intelligence. Arch Pediatr Adolesc Med. 2002;156(6):615–620. doi: 10.1001/archpedi.156.6.615. [DOI] [PubMed] [Google Scholar]
- Grunau RE, Whitfield MF, Petrie-Thomas J, Synnes AR, Cepeda IL, et al. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain. 2009;143(1–2):138–146. doi: 10.1016/j.pain.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R, Salmelin R. Human cortical oscillations: a neuromagnetic view through the skull. Trends Neurosci. 1997;20(1):44–49. doi: 10.1016/S0166-2236(96)10065-5. [DOI] [PubMed] [Google Scholar]
- Hart AR, Whitby EW, Griffiths PD, Smith MF. Magnetic resonance imaging and developmental outcome following preterm birth: review of current evidence. Dev Med Child Neurol. 2008;50:655–663. doi: 10.1111/j.1469-8749.2008.03050.x. [DOI] [PubMed] [Google Scholar]
- Herdman AT, Cheyne D. A practical guide to MEG and beamforming. In: Handy TC, editor. Brain signal analysis: advances in neuroelectric and neuromagnetic methods. Cambridge: MIT Press; 2009. pp. 99–140. [Google Scholar]
- Hughes SW, Crunelli V. Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neuroscientist. 2005;11(4):357–372. doi: 10.1177/1073858405277450. [DOI] [PubMed] [Google Scholar]
- Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Fetal Neonatal Ed. 2008;93:F153–F161. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostović I, Jovanov-Milošević N. Development of cerebral connections during the first 20–45 weeks’ gestation. Sem Fetal Neonat Med. 2006;11:415–422. doi: 10.1016/j.siny.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Krause CM, Salminen PA, Sillanmaki L, Holopainen IE. Event related desynchronization and synchronization during a memory task in children. Clin Neurophysiol. 2001;111:2071–2078. doi: 10.1016/s1388-2457(01)00684-8. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Ribary U. Coherent 40-Hz oscillation characterizes dream state in humans. Proc Natl Acad Sci USA. 1993;90:2078–2081. doi: 10.1073/pnas.90.5.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci USA. 1999;96(26):15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR, Ribary U, Joliot M, Wang XJ. Content and context in temporal thalamocortical binding. In: Buzsaki G, Llinas R, Singer W, Berthoz A, Christen Y, editors. Temporal Coding in the Brain. Heidelberg: Springer-Verlag; 1994. pp. 251–272. [Google Scholar]
- Maltseva IV, Maslobev YP. Alpha rhythms parameters and short-term memory span. Int J Psychophysiol. 1997;26(1–3):396–380. doi: 10.1016/s0167-8760(97)00776-9. [DOI] [PubMed] [Google Scholar]
- Marlow N, Hennessy EM, Bracewell MA, Wolke D. Motor and executive function at 6 years of age after extremely preterm birth. Pediatrics. 2007;120:793–804. doi: 10.1542/peds.2007-0440. [DOI] [PubMed] [Google Scholar]
- Mazaheri A, Coffey-Corina S, Mangun GR, Bekker EM. Functional disconnection of frontal cortex and visual cortex in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2009.11.022. [DOI] [PubMed] [Google Scholar]
- McQuillen PS, Ferriero DM. Selective vulnerability in the developing central nervous system. Pediatr Neurol. 2004;30:227–235. doi: 10.1016/j.pediatrneurol.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Melloni L, Molina C, Pena M, Torres D, Singer W, et al. Synchronization of neural activity across cortical areas correlates with conscious perception. J Neurosci. 2007;27(11):2858–2865. doi: 10.1523/JNEUROSCI.4623-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ment LR, Hirtz D, Hüppi PS. Imaging of biomarkers of outcome in the developing preterm brain. Lancet Neurol. 2009;8:1042–1055. doi: 10.1016/S1474-4422(09)70257-1. [DOI] [PubMed] [Google Scholar]
- Miller SP, Ferriero DM. From selective vulnerability to connectivity: insights from newborn brain imaging. Trends Neurosci. 2009 doi: 10.1016/j.tins.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskovic V, Schmidt LA, Boyle M, Saigal S. Regional electroencephalogram (EEG) spectral power and hemispheric coherence in young adults born at extremely low birth weight. Clin Neurophysiol. 2009;120:231–238. doi: 10.1016/j.clinph.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Mulder H, Pichford NJ, Haggar MS, Marlow N. Development of executive function and attention in preterm children: a systematic review. Dev Neuropsych. 2009;34(4):393–421. doi: 10.1080/87565640902964524. [DOI] [PubMed] [Google Scholar]
- Murias M, Webb SJ, Greenson J, Dawson G. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biol Psychiatry. 2007;62(3):270–273. doi: 10.1016/j.biopsych.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narberhaus A, Lawrence E, Allin MP, Walshe M, McGuire P, et al. Neural substrates of visual paired associates in young adults with a history of very preterm birth: alterations in fronto-parieto-occipital networks and caudate nucleus. NeuroImage. 2009;47(4):1884–1893. doi: 10.1016/j.neuroimage.2009.04.036. [DOI] [PubMed] [Google Scholar]
- Okumura A, Kubota T, Tsuji T, Kato T, et al. Amplitude spectral analysis of theta/alpha/beta waves in preterm infants. Ped Neurol. 2006;34(1):30–34. doi: 10.1016/j.pediatrneurol.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Palva JM, Monto S, Kulashekhar S, Palva S. Neuronal synchrony reveals working memory networks and predicts individual memory capacity. Proc Natl Acad Sci U S A. 2010a;107(16):7580–7585. doi: 10.1073/pnas.0913113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S, Monto S, Palva JM. Graph properties of synchronized cortical networks during visual working memory maintenance. Neuroimage. 2010b;49(4):3257–3268. doi: 10.1016/j.neuroimage.2009.11.031. [DOI] [PubMed] [Google Scholar]
- Palva JM, Palva S, Kaila K. Phase synchrony among neuronal oscillations in the human cortex. J Neurosci. 2005;25(15):3962–3972. doi: 10.1523/JNEUROSCI.4250-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1500 grams. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- Payne L, Kounios J. Coherent oscillatory networks supporting short-term memory retention. Brain Res. 2009;1247:126–132. doi: 10.1016/j.brainres.2008.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribary U, Ioannides AA, Singh KD, Hasson R, Bolton JPR, Lado F, Mogilner A, Llinas R. Magnetic Field Tomography (MFT) of coherent thalamo-cortical 40-Hz oscillations in humans. Proc Natl Acad Sci USA. 1991;88:11037–11041. doi: 10.1073/pnas.88.24.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribary U. Dynamics of thalamo-cortical network oscillations and human perception. Progr Brain Res. 2005;150:127–142. doi: 10.1016/S0079-6123(05)50010-4. [DOI] [PubMed] [Google Scholar]
- Rickards AL, Kelly EA, Doyle LW, Callanan C. Cognition, academic progress, behavior and self-concept at 14 years of very low birth weight children. J Dev Behav Pediatrics. 2001;22(1):11–18. doi: 10.1097/00004703-200102000-00002. [DOI] [PubMed] [Google Scholar]
- Roberts G, Anderson PJ, De Luca C, Doyle LW. Changes in neurodevelopmental outcome at age eight in geographic cohorts of children born at 22–27 weeks gestational age during the 1990s. Arch Dis Child Fetal Neonatal Ed. 2010;95(2):90–94. doi: 10.1136/adc.2009.165480. [DOI] [PubMed] [Google Scholar]
- Rodriguez E, George N, Lachaux JP, Martinerie J, et al. Perception’s shadow: long distance synchronization of human brain activity. Nature. 1999;397:430–433. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- Sarnthein J, Petsche H, Rappelsberger P, et al. Synchronization between prefrontal and posterior association cortex during human working memory. Proc Natl Acad Sci USA. 1998;95:7092–7096. doi: 10.1073/pnas.95.12.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Doppelmayr S, et al. EEG alpha synchronization and functional coupling during top-down processing in a working memory task. Hum Brain Mapp. 2005;26:148–155. doi: 10.1002/hbm.20150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer RJ, Lacadie C, Vohr B, Kesler SR, et al. Alterations in functional connectivity for language in prematurely born adolescents. Brain. 2009;132:661–670. doi: 10.1093/brain/awn353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler A, Gross J. Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci. 2005;6(4):285–296. doi: 10.1038/nrn1650. [DOI] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, et al. Longitudinal analysis of neural network development in preterm infants. Cer Cortex. 2010 doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan L, Dutta R, Counsell SJ, et al. Quantification of deep gray matter in preterm infants at term-equivalent age using manual volumetry of 3-tesla magnetic resonance images. Pediatrics. 2007;119:759–765. doi: 10.1542/peds.2006-2508. [DOI] [PubMed] [Google Scholar]
- Supp GG, Schögl A, Trujillo-Barreto N, Müller MM, Gruber T. Directed cortical information flow during human object recognition: analyzing induced EEG gamma-band responses in brain’s source space. PLoS One. 2007;2(1):e684. doi: 10.1371/journal.pone.0000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Fischer C. Oscillatory synchrony between human extrastriate areas during visual short-term memory maintenance. J Neurosci. 2001;21(RC177):1–5. doi: 10.1523/JNEUROSCI.21-20-j0008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HG, Minich NM, Klein N, Hack M. Longitudinal outcomes of very low birth weight: neuropsychological findings. J Int Neuropsychol Soc. 2004;10(2):149–163. doi: 10.1017/S1355617704102038. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Pogarell O, Meidl T, Dietrich O, et al. Regional networks underlying interhemispheric connectivity: an EEG and DTI study in healthy ageing and amnesic mild cognitive impairment. Hum Brain Mapp. 2009;30:2098–2119. doi: 10.1002/hbm.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Pipa G, Lima B, Melloni L, Neuenschwander S, et al. Neural synchrony in cortical networks: history, concept and current status. Frontiers Int Neurosci. 2009a;3(17):1–19. doi: 10.3389/neuro.07.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Rodriguez E, Rotarska-Jagiela A, Singer W. Neural synchrony and the development of cortical networks. Trends Cogn Sci. 2010;14(2):72–80. doi: 10.1016/j.tics.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Singer W, Heanshel C, et al. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc Natl Acad Sci USA. 2009b;106(24):9866–9871. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés-Hernández PA, Ojeda-Gonzalez A, Martinez-Montes E, Lage-Castellanos A, et al. White matter architecture rather than cortical surface area correlated with the EEG alpha rhythm. NeuroImage. 2010;49:2328–2339. doi: 10.1016/j.neuroimage.2009.10.030. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2(4):229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Ward LM. Synchronous neural oscillations and cognitive processes. Trends Cogn Sci. 2003;7(12):553–559. doi: 10.1016/j.tics.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scales for Children: (WISC-IV) 4th ed. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- Wilson H, Moiseev A, Podin S, Quraan M. Continuous head localization and data correction in MEG. International Congress Series. 2007;1300:623–626. [Google Scholar]
- Yordanova JY, Kolev VN. Developmental changes in the alpha response system. Electroencephalogr Clin Neurophysiol. 1996;99(6):527–538. doi: 10.1016/s0013-4694(96)95562-5. [DOI] [PubMed] [Google Scholar]
- Yordanova JY, Kolev VN. Alpha response system in children: changes with age. Int J Psychophysiol. 1997;26(1–3):411–430. doi: 10.1016/s0167-8760(97)00779-4. [DOI] [PubMed] [Google Scholar]