Abstract
Recent studies of genetic abnormalities in pediatric low-grade gliomas (LGGs) have focused on activation of the ERK/MAPK pathway by KIAA1549-BRAF gene fusions in the majority of pilocytic astrocytomas (PAs) and by rare mutations in elements of the pathway across histopathologically diverse LGGs. This study reports that MYB, an oncogene not previously implicated in gliomagenesis, is activated in a diverse subset of pediatric LGGs. The study cohort comprised 57 pediatric LGGs and a comparative cohort of 59 pediatric high-grade gliomas (HGGs). The LGG cohort included 34 PAs and 23 diffuse gliomas; fibrillary astrocytomas (n=14), oligodendroglial tumors (n=7), and angiocentric gliomas (n=2). MYB copy number abnormalities were disclosed using Affymetrix 6.0 SNP arrays and confirmed using interphase fluorescence in situ hybridization. Novel MYB amplifications that upregulate MYB RNA and protein expression were demonstrated in 2/14 diffuse astrocytomas. In addition, focal deletion of the terminal region of MYB was seen in 1 of 2 angiocentric gliomas (AGs). Increased expression of MYB was demonstrated by quantitative RT-PCR and immunohistochemistry. MYB upregulation at the protein level was demonstrated in a proportion of diffuse LGGs (60%), pilocytic astrocytomas (41%), and HGGs (19%), but abnormalities at the genomic level were only a feature of diffuse gliomas. Our data suggest that MYB may have a role in a subset of pediatric gliomas, through a variety of mechanisms in addition to MYB amplification and deletion.
Keywords: MYB, glioma, pediatric, amplification
Introduction
Central nervous system (CNS) tumors constitute approximately 25% of neoplastic disease in childhood [17,39], They are the major cause of cancer-related death in children and young adults [16]. Primary CNS neoplasms in adults and children are classified into four grades by histopathological features and broadly subdivided into low-grade (WHO grades I-II) and high-grade (WHO grades III-IV) tumors [30]. High-grade gliomas (HGG) predominate in adults, and low-grade gliomas (LGGs) form the majority of tumors in children. Excluding (low-grade) ependymomas, which are regarded as a distinct disease, pediatric LGGs are a heterogeneous group, comprising tumors thought to be derived from astrocytic or oligodendroglial lineages. They include pilocytic astrocytoma (PA, WHO grade I) and a range of WHO grade II tumors: diffuse astrocytoma (DA), oligodendroglioma (O), oligoastrocytoma (OA) and rarer entities, such as pilomyxoid astrocytoma, pleomorphic xanthoastrocytoma (PXA), and the recently described angiocentric glioma (AG) [30].
Adult and pediatric gliomas share similar histological appearances, but display different genetic and biological characteristics. In adults, mutations affecting the two isocitrate dehydrogenase (IDH) enzymes characterize the majority of WHO grade II-III astrocytomas [2,38,59]. IDH1 mutations have been reported in pediatric DAs, but at a much lower frequency than in adults [11,60]. TP53 mutations are also common in adult diffuse (fibrillary) astrocytomas (WHO grade II), and the majority of these tumors will undergo further transformation to anaplastic astrocytoma or glioblastoma [37]. In contrast, TP53 mutations are rarely seen in pediatric DAs, and less than 10% of these tumors undergo progression to a HGG [7,35,43]. Deletions of chromosome arms 1p and 19q are seen in approximately 80% of adult oligodendrogliomas and are associated with a favorable outcome [23,49,53]. Oligodendrogliomas in childhood are rare; few contain 1p/19q loss, and no survival advantages are associated with 1p/19q deletions in this age group [15,25,42].
Pediatric LGGs are generally regarded as a single group for therapeutic purposes, despite their histopathological heterogeneity. For many types, such as PA, surgical resection is the treatment of choice, and completeness of excision is the main determinant of survival [46]. However, complete resection is often not possible when tumors are situated deep within midline structures at the base of the brain or brain stem, or when tumors are diffusely infiltrative. In these situations, adjuvant chemotherapy and radiotherapy are effective [14], but overall survival beyond fifteen years for unresectable tumors is poor. Pediatric LGGs may cause significant long-term morbidity, despite their indolent clinical course [45]. Such challenges can only be met through an improved understanding of the biology and genetics of pediatric LGG. Increased understanding of the disease will facilitate more effective treatment strategies, particularly those using targeted small molecule drugs.
In this study, a genome-wide analysis of copy number abnormalities (CNAs) was undertaken in pediatric LGGs (WHO grades I and II). Novel CNAs at 6q23 involving gain of MYB were revealed in two DAs and subsequently confirmed as amplifications by interphase fluorescence in situ hybridization (iFISH). MYB was overexpressed, and nuclear accumulation of MYB protein was demonstrated by immunohistochemistry in both cases. Focal deletion of the 3′ region of MYB was also found in 1 of 2 angiocentric gliomas (AGs). Both AGs displayed increased MYB expression and nuclear immunoreactivity for the protein. MYB is an oncogene, which has not previously been implicated in gliomagenesis. Our data are presented in the context of examining MYB status and defining other genetic abnormalities across a large series of pediatric LGGs and in a comparative cohort of pediatric HGGs.
Materials and methods
Tumor cohort
LGGs were surgical specimens from 57 patients (Tables 1a and 1b) and included 14 diffuse (fibrillary) astrocytomas (DAs), 5 oligoastrocytomas (OAs), 2 oligodendrogliomas (Os) and 2 angiocentric gliomas (AGs), all from cerebral cortex, 25 cerebellar PAs, and 9 supratentorial PAs from the cerebral cortex (n=6) and thalamus (n=3). Age at diagnosis ranged from 1-19 years, with a median age of 6 years. There were 29 males and 28 females within the cohort. Tissue samples were snap-frozen at the time of surgical resection and were obtained prior to adjuvant therapy. Matching formalin-fixed, paraffin-embedded (FFPE) samples of these tumors were used for immunohistochemical and molecular cytogenetic studies. A series of archived FFPE pediatric HGGs (n=59) represented a comparative series for immunohistochemical and cytogenetic studies and consisted of 42 glioblastomas (GBs), 11 anaplastic astrocytomas, 3 anaplastic OAs, and 3 (high-grade) astroblastomas. None appeared to have progressed from a low-grade astrocytoma. All patients were negative for NF1, and optic pathway gliomas were not included. A detailed histopathological review of all tumors was undertaken (DWE). DNA and RNA were extracted from frozen tissue using previously described methods [13].
Table 1a.
Clinical data and summary of results for diffuse cerebral LGGs
| Code | Age | Sex | 6q23 CNA by array |
Additional CNA by array |
RAF gene fusion |
Mutation screening |
MYB CNA by FISH |
RT-PCR MYB Exons 8-9 |
MYB IHC |
|---|---|---|---|---|---|---|---|---|---|
| DA1 | 8 | F | Gain | − | − | − | Amplified | 504 | ++ |
| DA2 | 6 | F | Gain | − | − | − | Amplified | 12 | + |
| DA3 | 6 | F | − | − | − | − | − | 156 | ++ |
| DA4 | 6 | F | − | − | − a | − | − | 147 | ++ |
| DA5 | 11 | M | − | − | − | − | − | 3 | ++ |
| DA6 | 4 | M | − | − | − | − | − | − | + |
| DA7 | 5 | M | − | − | − | − | − | − | + |
| DA8 | 9 | M | − | − | − | BRAF p.V600E | − | − | + |
| DA9 | 7 | F | − | − | − | − | − | − | 0 |
| DA10 | 3 | M | − | − | − | − | − | − | 0 |
| DA11 | 9 | F | ND | ND | − | − | − | − | 0 |
| DA12 | 3 | M | − | − | − | − | − | − | ND |
| DA13 | 17 | M | − | Gain of 7b | − | − | Polysomy | − | ND |
| DA14 | 4 | M | − | − | − | − | − | − | ND |
| OA1 | 6 | F | − | − | − | − | − | − | ++ |
| OA2 | 14 | M | − | − | − | − | − | − | 0 |
| OA3 | 14 | M | − | − | − | − | Polysomy | − | 0 |
| OA4 | 2 | F | − | − | − | − | − | − | 0 |
| OA5 | 5 | M | − | Loss of 1p/19q | − | − | − | − | 0 |
| O1 | 3 | M | − | − | − | − | − | 4 | + |
| O2 | 15 | M | − | Loss of 1p/19q | − | − | − | − | 0 |
| AG1 | 3 | M | Loss | − | − | − | − | 2306 | ++ |
| AG2 | 11 | M | − | − | − | − | − | 909 | + |
DA, diffuse astrocytoma; OA, oligoastrocytoma; O, oligodendroglioma; AG, angiocentric glioma. Age, age at diagnosis in years. CNA, copy number abnormality; ND, not done.
DA4 was previously reported to contain a KIAA1549-BRAF fusion [13], but this was not confirmed on repeated analysis for this study.
whole chromosome gain. Mutation screening included BRAF exon 15, KRAS exons 2 and 3, IDH1 exon 4 and IDH2 exons 4-5. Reverse-transcription PCR using primers within MYB exons 8-9, expressed as a normalized ratio. Negative results showed ratios equal to or less than a (normal) control. IHC, immunohistochemistry: ++, strong nuclear immunoreactivity in nearly all tumor cells; +, scattered tumor cells with weak to moderately strong nuclear immunoreactivity; 0, no immunoreactivity in tumor cells.
Table 1b.
Clinical data and summary of results for pilocytic astrocytomas
| Code | Site | Age in years |
Sex | 7q34 CNA by array |
RAF gene fusion | Mutation screening |
MYB CNA by FISH |
MYB IHC |
|---|---|---|---|---|---|---|---|---|
| PA1 | Cb | 3 | M | − | KIAA1549-BRAF Ex 15-Ex 9 | − | − | + |
| PA2 | Cb | 5 | F | Gain | KIAA1549-BRAF Ex 15-Ex 9 | − | − | + |
| PA3 | Cb | 13 | M | Gain | KIAA1549-BRAF Ex 15-Ex 9 | − | − | + |
| PA4 | Cb | 10 | M | Gain | KIAA1549-BRAF Ex 16-Ex 9 | − | − | + |
| PA5 | Cb | 12 | F | Gain | KIAA1549-BRAF Ex 16-Ex 9 | − | − | + |
| PA6 | Cb | 7 | M | Gain | KIAA1549-BRAF Ex 16-Ex 9 | − | − | + |
| PA7 | Cb | 15 | F | Gain | KIAA1549-BRAF Ex 16-Ex 9 | − | − | + |
| PA8 | Cb | 19 | M | Gain | KIAA1549-BRAF Ex 19-Ex 9 | − | − | + |
| PA9 | Cb | 2 | F | − | − | KRAS p.G12A | − | + |
| PA10 | Cb | 3 | F | − | − | − | − | + |
| PA11 | Cb | 6 | M | − | − | − | − | + |
| PA12 | Cb | 18 | F | Gain | KIAA1549-BRAF Ex 15-Ex 9 | − | Polysomy | 0 |
| PA13 | Cb | 7 | M | Gain | KIAA1549-BRAF Ex 15-Ex 9 | − | − | 0 |
| PA14 | Cb | 7 | M | Gain | KIAA1549-BRAF Ex 16-Ex 9 | − | − | 0 |
| PA15 | Cb | 2 | F | Gain | KIAA1549-BRAF Ex 16-Ex 9 | − | − | 0 |
| PA16 | Cb | 9 | M | Gain | KIAA1549-BRAF Ex 16-Ex 9 | − | − | 0 |
| PA17 | Cb | 10 | M | Gain | KIAA1549-BRAF Ex 16-Ex 9 | − | − | 0 |
| PA18 | Cb | 2 | F | Gain | KIAA1549-BRAF Ex 16-Ex 9 | − | − | 0 |
| PA19 | Cb | 4 | F | Gain | KIAA1549-BRAF Ex 16-Ex 9 | − | − | 0 |
| PA20 | Cb | 1 | F | Gain | KIAA1549-BRAF Ex 16-Ex 9 | − | − | 0 |
| PA21 | Cb | 4 | F | Gain | KIAA1549-BRAF Ex 16-Ex 11 | − | ND | ND |
| PA22 | Cb | 3 | F | − | KIAA1549-BRAF Ex 16-Ex 11 | − | ND | ND |
| PA23 | Cb | 14 | F | − | KIAA1549-BRAF Ex 16-Ex 11 | − | − | ND |
| PA24 | Cb | 15 | F | Gain | KIAA1549-BRAF Ex 18-Ex 10 | − | − | 0 |
| PA25 | Cb | 13 | F | − a | SRGAP3-RAF1 Ex 11-Ex 8 | − | − | 0 |
| PA26 | Ce | 2 | M | Gain | KIAA1549-BRAF Ex 15-Ex 9 | − | − | + |
| PA27 | Ce | 14 | M | − | KIAA1549-BRAF Ex 15-Ex 9 | − | − | 0 |
| PA28 | Ce | 5 | F | − | KIAA1549-BRAF Ex 15-Ex 9 | − | − | 0 |
| PA29 | Ce | 4 | M | Gain | KIAA1549-BRAF Ex 16-Ex 9 | − | − | 0 |
| PA30 | Ce | 18 | F | Gain of 7b | − | − | − | 0 |
| PA31 | Ce | 10 | F | − | − | − | − | 0 |
| PA32 | Th | 5 | F | ND | KIAA1549-BRAF Ex 15-Ex 9 | − | ND | ND |
| PA33 | Th | 6 | M | Gain | KIAA1549-BRAF Ex 16-Ex 9 | − | − | 0 |
| PA34 | Th | 13 | F | ND | − | BRAF p.V600E | − | ND |
PA, pilocytic astrocytoma; Cb, cerebellum; Ce, cerebral cortex; Th, thalamus. CNA, copy number abnormality.
PA25 contained 3p25 copy number gain by array analysis;
whole chromosome gain. ND, not done. Mutation screening included BRAF exon 15, KRAS exons 2 and 3, IDH1 exon 4 and IDH2 exons 4-5. IHC, immunohistochemistry: ++, strong nuclear immunoreactivity in nearly all tumor cells; +, scattered tumor cells with weak to moderately strong nuclear immunoreactivity; 0, no immunoreactivity in tumor cells.
The principles of the study and access to tumors and clinical data were in accordance with Institutional Review Board and MREC regulations: XPD07-107/IRB and Tissue Resource Request No. 07-007 (St Jude Children’s Research Hospital); REC No. 2002/112 (University of Newcastle) and ICMS/PR/09/77 (University of London).
Affymetrix 6.0 SNP array analysis
Tumor DNA from 54 LGGs was analyzed for CNAs using the Affymetrix Genome-Wide Human 6.0 SNP array according to standard Affymetrix protocols. Processed data were normalized and subjected to circular binary segmentation using previously described methods [13]. Genomic Identification of Significant Targets in Cancer (GISTIC) was used to identify significant CNAs [4]. Data were visualized using the Integrative Genomics Viewer (IGV; 1.4.2), http://www.broadinstitute.org/igv.
Dual-color interphase fluorescence in situ hybridization (iFISH)
Dual-color iFISH was performed on 5μm FFPE tissue array sections using standard methods [13]. Probes were derived from BAC clones (BACPAC Resources, Oakland, CA), labeled with either AlexaFluor-488 or Rhodamine fluorochromes and validated on normal control metaphase spreads to confirm chromosomal location. BAC clone RP11-104D9 was used to target MYB at 6q23.3 in combination with a control probe to 6p (RP11-945O22 and RP11-186N7). Clones RP11-702H8 and RP11-1025G11 were used to probe the co-amplified regions of gain at 6q23.3 in DA1 and DA2, respectively. Clones RP11-366H19 and RP11-170P19 were used to probe the regions of possible copy number loss in DA1 and DA2. RP1-233K16, RP1-120G22 and RP1-202O8 were used to probe 1p36.3, in combination with control probes RP11-54H19 and RP11-336K24 to 1q22. Clones RP11-927F22 and RP11-105H7 were used to probe 19q13.32, in combination with control probes CTD-2538G9 and CTD-2528A14 to 19p13.11.
Immunohistochemistry
Immunohistochemistry was undertaken on FFPE material using a monoclonal antibody to the N-terminal region of MYB (Abcam EP769Y; Cambridge, MA). Staining was performed on the BondMax automated staining system using standard methodology (Leica Microsystems, Bannockburn, IL). Slides were deparaffinized, pretreated with ER2 (EDTA pH8) and incubated with antibody (1:50) dilution for 15 minutes at room temperature. Detection was performed using the Refine Polymer from Leica Microsystems and slides were counterstained with hematoxylin.
DNA qPCR
The human genomic MYB gene sequence was retrieved from Ensembl (Transcript ID: ENST00000341911) and used as a template for designing all real-time PCR oligonucleotide primers (Table 2). Individual real-time PCR reactions (20μl) contained 1 × SYBR Green I master buffer, different concentrations of sense and antisense primers (Table 2) and 50ng of genomic DNA. Real-time PCR was performed in a LightCycler480® (Roche), using the default thermocycler program for all genes: 5 minutes of pre-incubation at 95°C followed by 45 cycles for 10 seconds at 95°C, 15 seconds at 60°C and 30 seconds at 72°C. At the end of each reaction, melting curves were acquired and analyzed. For real time PCR data analysis, an advanced relative quantification method was adapted to estimate copy numbers in MYB using Lightcycler 480® software (Roche). Human genomic DNA extracted from blood (Roche) contained 2 normal copies of MYB and was used as the copy number calibrator. Two genes (GPR15 and ZNF80) contained constant copy number in all LGG samples and were used as reference genes to normalize the quantitative data. Results for each tumor sample were expressed as the N-fold copy number of MYB relative to the calibrator. Assays were repeated if amplification curves did not reflect exponential kinetic parameters or if the N-fold copy number was lower than 0.7 or higher than 1.3. When N-fold was between these values (0.7 < N-fold < 1.3), tumor samples were judged to contain 2 normal copies of the target gene.
Table 2.
Primers for DNA qPCR
| Gene | Primer | Sequence | Conc (nM) | Amplicon (bp) |
|---|---|---|---|---|
| MYB exon 8 | Forward Reverse |
CCTGAGAAGGAAAAGCGAAT GAATGGATATATGGAGCCTTGC |
300 300 |
194 |
| MYB exon 16 | Forward Reverse |
CTTTCTCCATCAGCCTTGTAGCAG GGAAGATGTCATCTGCTCCTCCAT |
500 500 |
76 |
| ZNF80 | Forward Reverse |
CTGTGACCTGCAGCTCATCCT TAAGTTCTCTGACGTTGACTGATGTG |
300 300 |
120 |
| GPR15 | Forward Reverse |
GGTCCCTGGTGGCCTTAATT TTGCTGGTAATGGGCACACA |
300 300 |
101 |
Quantitative reverse-transcription PCR (RT-PCR)
First strand cDNA was synthesized using Superscript II reverse transcriptase and random hexamers according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Primers were designed from published MYB cDNA sequences using Primer 6 software (Table 3). RT-PCR amplification mixtures (25μl) contained 25ng template cDNA, 1 × SYBR Green I Master mix buffer (Roche, Indianapolis, IN) and 300 nM forward and reverse primers. Reactions were run on a Lightcycler 480® Real-Time PCR machine (Roche, Indianapolis, IN). The cycling conditions comprised 10 minutes at 95°C and 45 cycles at 95°C for 10 seconds, 60°C for 15 seconds and 72°C for 20 seconds. MYB gene expression was analyzed by an Advanced Relative Quantification method using Lightcycler 480® software (Roche, Indianapolis, IN). In brief, target quantities were normalized to GAPDH and B2M (references) and calibrated using qPCR Human Reference cDNA (Clontech, Mountain View, CA) prepared from a mixture of total RNAs collected from adult normal human tissues. All quantities were expressed as N-fold related to the calibrator.
Table 3.
Primers for RT PCR
| Gene | Primer |
|---|---|
| MYB exon 8 forward | TGCTCCTAATGTCAACCGAGA |
| MYB exon 9 reverse | GCGCTTTCTTCAGGTAGGG |
| GAPDH forward | CTCTGCTCCTCCTGTTCGAC |
| GAPDH reverse | GCCCAATACGACCAAATCC |
| B2M forward | GGGATCGAGACATGTAAGCAG |
| B2M reverse | CAAGCAAGCAGAATTTGGAA |
5′ and 3′ rapid amplification of cDNA ends (RACE)
5′ and 3′ RACE was performed using the SMARTer RACE cDNA amplification kit (Clontech, Mountain View, CA), following the manufacturer’s instructions. Reactions were run on a Lightcycler 480® Real-Time PCR machine (Roche, Indianapolis, IN).
Gene sequence analysis
The mutational hotspots of BRAF (exon 15) and KRAS (exons 2 and 3) were sequenced using previously published primers [13]. The mutational hotspots for IDH1 (exon 4) and IDH2 (exons 4-5) were also sequenced using the following primers; IDH1 exon 4 5′-GAGCTCTATATGCCATCACTGC-3′ (sense) and 5′-CAAGTTGGAAATTTCTGGGC-3′ (antisense), IDH2 exons 4-5 5′-ATTCTGGTTGAAAGATGGCG-3′ (sense) and 5′-CAGAAGAAAGGAAAGCCACG-3′ (antisense). Direct sequencing of PCR products was performed using BigDye version 3.1 chemistry and a 3730XL DNA analyzer (Applied Biosystems, Foster City, CA). Results were screened using MacVector version 8.3 sequence analysis software (Cary, NC).
Statistical Analysis
Comparisons of MYB immunoreactivity between different tumor groups were performed using Pearson’s Chi2 test. The Wilcoxon rank sum test was used to compare the cumulative genomic burden between MYB-amplified and non-amplified samples.
Results
MYB copy number abnormalities in LGGs
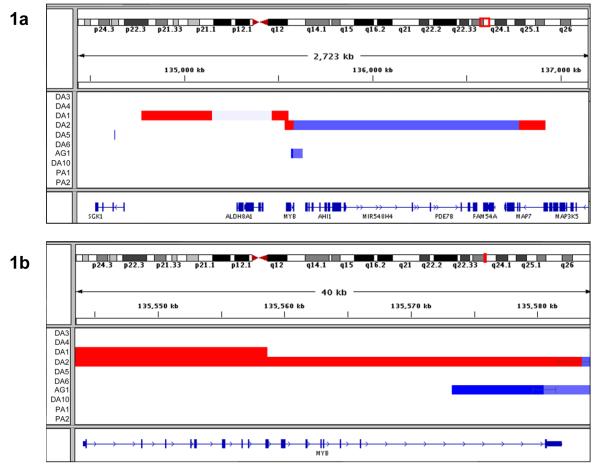
Analysis of 54 LGGs using the Affymetrix 6.0 SNP array revealed discrete copy number gains at chromosome band 6q23 in 2 (14%) of 14 DAs (Fig. 1a, Table 1a). DA1 showed a 90Kb region of copy number gain at 6q23. This included MYB exons 1-8 and terminated within MYB exon 9 (Fig. 1b). DA2 contained a 43.6Kb region of gain encompassing the majority of the MYB locus, ending within the 3′ untranslated region (UTR) of MYB exon 16. Distinct regions centromeric or telomeric from MYB were also co-amplified in each case. DA1 showed a large 370Kb region of copy number gain at 6q23 centromeric to MYB, including the non-coding gene AJ606331. The two gained regions in DA1 were separated by approximately 309Kb of possible copy number loss. DA2 showed a 136Kb region of gain telomeric to MYB, including the first exon of MAP7 and the terminal exon of MAP3K5. In this tumor, the two amplified regions were separated by approximately 1200Kb of apparent copy number loss. Regions of copy number gain within MYB were subsequently confirmed by DNA qPCR. DNA copy number levels, expressed as a normalized ratio, were elevated at MYB exon 8 in DA1 and DA2 (equal to 9 and 8, respectively). The DNA copy number ratio at MYB exon 16 was also elevated (equal to 7) in DA2, and was normal in DA1, matching the copy number aberrations identified by SNP array analysis. Sixteen representative LGGs were investigated in this way, and no other samples showed DNA copy number gains within MYB.
Fig. 1.
DNA CNAs at chromosome band 6q23 identified by Affymetrix SNP 6.0 analysis; gains are shown in red, losses in blue. (a) Two diffuse astrocytomas (DA1, DA2) showed gains within MYB and each had an additional region of amplification centromeric or telomeric from MYB, respectively. One further tumor (AG1) showed a focal deletion of the 3′ terminal region of MYB. (b) Detailed mapping of the regions of copy number change within MYB showed that gained regions terminated within exon 9 in DA1 and within the UTR of exon 16 in DA2. Exon 16 alone was lost in AG1. (The start and end of segmented regions of gain or loss are shown by black bars, horizontal black lines are used to connect between segments and do not denote a confidence interval.)
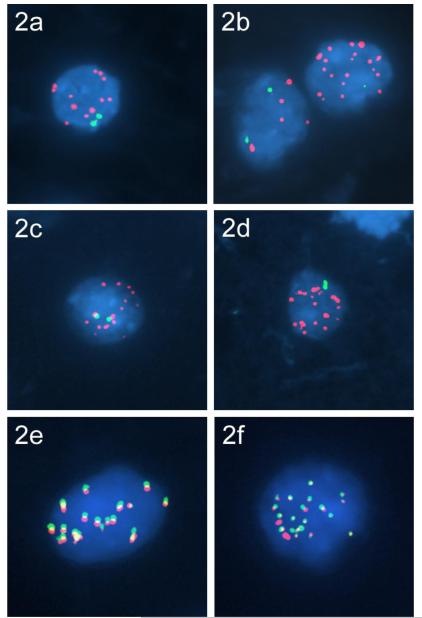
Dual-color interphase FISH (iFISH) confirmed amplification of the MYB locus at 6q23 in both DAs with copy number gains. Multiple copies of the MYB signal were seen in the form of double minutes in more than 80% of tumor cell nuclei, in the presence of two homologues of chromosome 6p (Fig. 2a-b). Fifty-two further LGGs were probed in this way, but did not demonstrate MYB amplification (Tables 1a and 1b). Three LGGs (DA13, OA3, PA12) showed polysomy 6, with 3-8 copies per cell.
Fig. 2.
Focal amplifications at 6q23 identified by iFISH. Multiple copies of MYB (red) were seen as double minutes within DA1 (a) and DA2 (b), in combination with control signals to 6p (green). Multiple copies of the amplified region at 6q23 that included AJ606331 are seen as double minutes (red) within DA1 (c), in combination with control signals to 6p (green). Multiple copies of the amplified region at 6q23 that included MAP7 and MAP3K5 are seen as double minutes (red) within DA2, in combination with control signals to 6p (green) (d). Co-localization of MYB and the additional amplified regions within double minutes were confirmed using probes to target MYB (red) and AJ606331 (green) in DA1 (e) and MYB (red) and MAP7 and MAP3K5 (green) in DA2 (f).
Further iFISH was performed to investigate the co-amplified regions that included AJ606331 or MAP7/MAP3K5 in DA1 and DA2, respectively. Multiple copies of these co-amplified regions were found as double minutes, and these were confirmed to co-localize with MYB (Fig. 2c-f). Regions of apparent copy number loss identified by array analysis were also probed using iFISH. Hemizygous deletion was confirmed between the amplified region of MYB and MAP7/MAP3K5 in DA2; however, no deletion was found in the region between AJ606331 and MYB in DA1, where two copies of the target probe were present (data not shown).
Affymetrix SNP array analysis also revealed a discrete region of DNA copy number loss at 6q23 in one angiocentric glioma (AG1) (Figs. 1a, b). AG1 showed a region of loss of approximately 59Kb, including MYB exon 16. The coding exons for the 5′ promoter and central transactivation region remained intact in this case. This region of copy number loss was subsequently confirmed by DNA qPCR. A normalized ratio of 0.5 was shown at MYB exon 16, with normal DNA copy number at MYB exon 8, matching the copy number change shown by SNP array analysis. No other LGG showed DNA copy number loss within MYB by DNA qPCR.
MYB expression by RT-PCR and immunohistochemistry in LGGs
RT-PCR was undertaken to measure MYB expression levels in all 57 LGGs, using primers situated within MYB exons 8-9. The central transactivating domain in MYB is encoded by exons 6-8 (amino acids 223-313). Increased MYB expression was seen in 8 LGGs. These comprised 5 DAs, 2 AGs, and 1 oligodendroglioma, and included all cases with MYB copy number aberrations (Table 1a). No PAs showed increased expression of MYB by RT-PCR.
MYB immunoreactivity was investigated in 49/57 LGGs (Tables 1a, 1b). Two patterns were demonstrated; strong nuclear immunoreactivity was a feature of nearly all tumor cells in 6 LGGs, all diffuse gliomas; 4 of 11 DAs, including one MYB-amplified case (DA1), 1 of 5 oligoastrocytomas, and 1 of 2 angiocentric gliomas (Fig. 3). Weak to moderately strong immunoreactivity was found in scattered tumor cells in 18 LGGs. These comprised 4 of 11 DAs, including the second MYB-amplified case (DA2), 1 of 2 oligodendrogliomas, 12 of 29 PAs, and the second angiocentric glioma. No immunoreactivity was found in the remaining 25 LGGs. The frequency of MYB immunoreactivity was higher among DAs (8/11; 73%) when compared to PAs (12/29; 41%). Five diffuse gliomas with increased MYB RNA and protein expression showed no corresponding copy number changes within MYB by DNA qPCR, array or FISH analysis (Table 1a). These data indicate that MYB upregulation may be modulated by further mechanisms, in addition to DNA CNAs.
Fig. 3.
MYB immunoreactivity (IR) in low-grade gliomas. Two broad categories of MYB IR were demonstrated; (i) strong nuclear IR of nearly all tumor cells as demonstrated by this diffusely infiltrating astrocytoma, in which normal glia and blood vessels are immunonegative (a, b), and weak to moderately strong nuclear IR of scattered tumor cells in this cerebellar pilocytic astrocytoma (c, d), and this anaplastic astrocytoma (e,f).
Analysis of MYB abnormalities in high-grade gliomas
Pediatric HGGs (n=54) were evaluated by iFISH, and none demonstrated MYB amplification. Many tumors (n=21; 38%) had a balanced (2:2) profile, but a high proportion of assessable tumors (23/55; 42%) showed polysomy (3N or 4N) of chromosome 6. Relative loss of MYB against a background of hyperploidy was detected in 6 tumors, and monosomy of chromosome 6 was detected in 4 HGGs. The remaining tumor, a giant cell GB, contained between one and four extra copies of MYB in its cells.
MYB immunoreactivity was assessed in 59 HGGs. A minority (19%) contained scattered tumor cells with weak to moderately strong nuclear immunoreactivity (Fig. 3). None contained widespread strong immunoreactivity, as seen in some low-grade diffuse gliomas. Anaplastic astrocytomas (5/11) and GBs (5/42) were equally represented among immunopositive tumors, and there was one immunopositive astroblastoma. However, the frequency of MYB immunoreactivity among anaplastic astrocytomas (45%) was significantly higher than among GBs (12%; P=0.011).
No gene fusions involving MYB
Potential gene fusions were sought between the co-amplified genomic regions in DA1 and DA2 using several methods. DA1 had limited material available for study. PCR for rapid amplification of cDNA ends (RACE) was performed in 5′ and 3′ directions for both samples. No novel sequences were identified to suggest that gene fusions with MYB had occurred. The 5′ region was intact in both DAs; however, the sequence from DA1 was found to terminate within MYB exon 9 as indicated by array analysis. Oligonucleotide primers were designed within the candidate fusion partner genes MYB, MAP7 and MAP3K5, and PCR was then performed using cDNA from DA2. No PCR products could be amplified. Finally, genomic DNA from DA2 was investigated for the presence of DNA insertions flanking the 5′ and 3′ ends of MYB using standard methods [19]. No additional genetic material was detected.
No chromosomal instability in MYB-amplified DAs
Chromosomal instability was investigated as a possible cause for the genetic abnormalities in the two DAs with MYB amplification. The number of genetic lesions (sum of gains and losses) and the cumulative genetic burden (sum of cumulative gains and losses in Kb) were calculated. Segmentation data were filtered to exclude known germline copy number variants, using ≥ 8 markers within a segment. Data were compared between the two DAs with MYB amplification and 38 representative LGGs, to identify whether more genetic rearrangements were present in the two DAs, indicating chromosomal instability. No differences were found in the number of genetic lesions (P=0.73) or in the cumulative genetic burden (P=0.43), respectively.
Further genetic aberrations in LGGs
Twenty-one LGGs contained a discrete copy number gain of 1.9Mb at chromosome band 7q34 by Affymetrix 6.0 SNP array analysis; all were PAs (Table 1b). The cohort was screened for the presence of KIAA1549-BRAF fusions as previously described [13]. Twenty-seven of 34 PAs (79%) were subsequently confirmed to contain KIAA1549-BRAF fusions and five different KIAA1549-BRAF fusion variants were identified. Seven PAs did not contain KIAA1549-BRAF fusions. Of these, one cerebellar PA contained an SRGAP3-RAF1 fusion. One oligodendroglioma (O2) and one oligoastrocytoma (OA5) contained hemizygous deletions of chromosomes 1p and 19q by array analysis and iFISH. Fifty-five LGGs were probed in this way, and no other cases contained 1p/19q deletions.
Mutation screening
Fifty-seven pediatric LGGs were screened for activating mutations at known mutation hotspots in BRAF (exon 15), KRAS (exons 2 and 3) and for somatic mutations known to perturb enzymatic function in IDH1 (exon 4) and IDH2 (exons 4 and 5). Two samples were found to contain the BRAF p.V600E activating mutation. One was a cerebral DA which also showed homozygous CDKN2A loss by copy number analysis and iFISH. The other was a thalamic PA. This sample had not been screened by SNP array, but showed polysomy at the CDKN2A locus by iFISH. A KRAS p.G12A activating mutation was identified in one fusion-negative cerebellar PA. No mutations were identified within KRAS exon 3, IDH1 exon 4 or IDH2 exons 4-5 within the cohort.
Discussion
Pediatric LGGs are dominated by cerebellar PAs (WHO grade I), which are generally cured by surgery [1,46]. However, diffusely infiltrating gliomas (WHO grade II) and PAs that seed the neuraxis or occur at sites where resection is impossible cause significant morbidity and mortality and represent a considerable therapeutic challenge. This challenge will only be met by an increased understanding of the biology of these tumors. Recent recognition that pediatric PAs demonstrate activation of the ERK/MAPK pathway, many as a result of a KIAA1549-BRAF gene fusion, has helped to direct functional studies and drug discovery for this disease [13,20,21,52,55]. Grade II pediatric gliomas remain enigmatic entities. Extrapolation from data on adult grade II LGGs to childhood disease clearly has limitations; while the histological features of adult and pediatric LGGs show considerable overlap, their clinical and molecular characteristics do not. The rate of anaplastic progression in pediatric grade II LGGs is much lower than in their adult counterparts, and the frequency of specific molecular genetic abnormalities, such as TP53 or IDH1 mutations in diffuse astrocytomas [2,35,43,51] and 1p/19q loss in oligodendrogliomas [42,47], is much lower in pediatric tumors. The present study of 57 well characterized pediatric LGGs reports novel data on MYB, a gene not previously implicated in gliomagenesis. Amplifications of MYB, apparently driving upregulation of the gene product, occurred at low frequency in DAs, but not in PAs.
The oncogene MYB encodes a nuclear DNA-binding leucine zipper transcription factor, which can act as both a transcriptional activator and repressor [3,48]. MYB is the cellular homologue of v-Myb, which is found in the avian retroviruses E26 and AMV and induces leukemic transformation in birds [5,29]. MYB is one of a family of closely related transcriptional regulators that modulate cell maturation and proliferation and determine lineage fate. Others include MYBL1 (AMYB) and MYBL2 (BMYB). All three proteins contain a highly conserved DNA binding site (the MYB binding site, MBS), which binds to the sequence t/cAACt/gG [6]. Three key domains are found within MYB; the N-terminal DNA binding domain, where three MBSs are situated as tandem repeats, the centrally located transactivating domain, and the C-terminal negative regulatory domain [36]. MYB has a short half-life of approximately 30 minutes in vivo, and undergoes various post-translational modifications, including ubiquitylation, phosphorylation, acetylation and sumoylation, which modify levels of DNA-binding or transcriptional activation. In general, MYB induces gene activation, although repression has been documented in a few situations. Over 80 genes have been confirmed as MYB targets, including MYB itself, but these vary considerably between different cell types [28,48]. Most MYB studies have been conducted on the hematopoietic system, and little is known about the actions of MYB within the CNS. MYB expression has recently been shown to be negatively regulated by microRNAs, including miR-15a, miR-16-1, and miR-150. These bind within the 3′UTR and inhibit mRNA translation of MYB [8,58,61].
MYB is critically important for hematopoiesis and is highly expressed within immature multi-potent stem cells. Levels are successively down-regulated as differentiation proceeds along myeloid and lymphoid lineages [3,56]. Complete loss of Myb is lethal in mouse embryos, which appear to develop normally until day 14, but then die as myeloid and erythroid development is severely disrupted [34]. Further murine studies have demonstrated functions for cMyb in colonic stem cells and in regions of postnatal neurogenesis [48,54]. Normal mice express high levels of cMyb mRNA at E15 within brain, colon and liver. Levels fall in brain and liver by P7, and are almost undetectable in adult mice [50]. Relative to cortex, high levels of cMyb mRNA and protein are found in the subventricular zone (SVZ) of adult mice [31], and cMyb immunoreactivity has been identified in the majority of SVZ cell types.
Various human neoplasms harbor genomic rearrangements and CNAs within MYB. Balanced reciprocal t(6;7)(q23;q34) translocations have been found in a few patients with T-ALL [9]. These create a derivative chromosome 6 with a novel juxtaposition involving MYB and the regulatory sequence for the T-cell receptor-beta gene. MYB duplications characterize disease in an additional group of T-ALL patients. Focal copy number gains were found to encompass the whole MYB locus, and tandem duplication was demonstrated using FISH [9,26]. MYB amplifications have also been identified in several solid tumors. High-level amplifications occurred in 29% of hereditary BRCA1 breast cancers, which compares with 2% in sporadic breast cancers [24]. Furthermore, over-expression of MYB mRNA was seen by Northern blot analysis in 2 of 5 BRCA1 tumors with no MYB amplification, suggesting the presence of an alternate mechanism of up-regulation. MYB DNA copy number gains have been demonstrated in approximately 10% of pancreatic carcinomas and FISH using the P2 pancreatic cancer cell line confirmed amplification of MYB as homogeneously staining regions [57]. Studies of CNS tumors using array-based comparative genomic hybridization (CGH) have identified MYB copy number gain in one supratentorial primitive neuroectodermal tumor, a finding validated by semi-quantitative multiplex PCR [32], and in 5 of 10 medulloblastomas, although these results were not confirmed by another method [22].
A novel t(6;9) translocation between MYB and the nuclear transcription factor NFIB has recently been identified in adenoid cystic carcinomas (ACCs) of the breast and salivary glands [33,40]. The terminal exon and 3′UTR of MYB are lost in the translocation; this region contains the binding sites for several microRNAs (miR-15a, miR-16-1, miR-150) that negatively regulate MYB transcriptional activity. Upregulation of MYB (RNA and protein) was seen in ACCs, and levels of MYB target genes were also elevated in comparison to normal salivary gland tissue. The initial report identified fusions in all examined ACCs, while a recent study of salivary gland tumors found fusions in 28% primary and 35% metastatic ACCs [33,40]. MYB overexpression appears to be a key alteration in ACC development; increased expression was seen in 85% fusion-positive ACCs. It was also found in 60% of fusion-negative ACCs, although the mechanism for this is currently unknown.
The present series of LGGs included 23 diffuse gliomas; DAs, oligodendrogliomas, oligoastrocytomas, and angiocentric gliomas, and 34 PAs from three anatomic sites. Three diffuse LGGs showed novel MYB abnormalities at the genomic level, but MYB CNAs were not demonstrated in PAs or in a series of pediatric HGGs. Our data demonstrated two possible genomic mechanisms for MYB upregulation; MYB amplification in two DAs and loss of the 3′ region of MYB in a single angiocentric glioma were both associated with increased expression at RNA and protein levels. Both examples of MYB amplification contained the promoter region and transactivating domain, and translated products would be predicted to retain their transcription factor function. The nature of genomic loss in the angiocentric glioma is similar to the situation seen within MYB-NFIB fusions in ACCs, where MYB upregulation occurs through loss of micro-RNA binding regions within the 3′-UTR of MYB. Discrete copy number loss at 6q24-25 by metaphase comparative genomic hybridization (CGH) has previously been reported in 1 of 8 angiocentric gliomas [44], and this may represent the same region of loss demonstrated here.
Array data for both DAs with MYB amplification indicated additional regions of amplification on 6q23. iFISH confirmed the presence of double minutes at these loci and showed that both amplicons were not continuous with the MYB amplicon. The amplicon situated centromeric to MYB in DA1 contains the non-coding gene AJ606331. This is postulated to encode a short RNA, and expression has been documented in human testis and placenta [10]. The amplicon telomeric to MYB in DA2 included the first exon of MAP7 and the terminal exon of the mitogen-activated protein kinase MAP3K5 Most. of MAP3K5 exon 30 encodes the 3′UTR, this contains many stop codons, so would not be expected to produce a protein, even if it were transcribed. The significance of these disparate additional amplifications remains to be determined. The close proximity of the amplified regions led us to search for possible gene fusions between MYB and MAP7 / MAP3K5. No fusion could be found between these potential candidates, but our data do not entirely rule out the existence of such a fusion, particularly if a complex rearrangement is present. Chromosomal instability was considered as a possible cause for the genetic rearrangements in the two DAs with MYB amplification, but no significant differences were found when comparing the number of genetic gains and losses between these cases and a representative group of LGGs with no CNAs.
MYB upregulation was not confined to the diffuse gliomas with MYB amplification or deletion; immunohistochemistry disclosed increased nuclear expression of MYB in 41% of PAs and 19% of HGGs. These are lower frequencies than that for diffuse LGGs (60%), in which MYB immunoreactivity was also more pronounced; all tumors with strong nuclear immunoreactivity in nearly all tumor cells (++ cases) were found among diffuse gliomas. In some diffuse LGGs without MYB CNAs, MYB expression at the RNA level was increased, and in others and in all MYB-immunopositive PAs MYB expression was not elevated, suggesting multiple mechanisms account for MYB upregulation across pediatric gliomas. With one exception from the cerebral cortex, all MYB-immunopositive PAs were cerebellar, and the MYB-immunopositive oligodendroglial tumors did not show 1p/19q loss. Otherwise, LGGs with MYB upregulation did not demonstrate any distinctive clinical or genetic features.
This series of LGGs includes 10 cerebral DAs and 8 cerebellar PAs from our previous study of KIAA1549-BRAF gene fusions and MAPK pathway activation in LGGs. In total, we have now presented genetic data on 89 pediatric LGGs, including 24 diffuse gliomas – 15 cerebral DAs, 7 oligodendrogliomas or oligoastrocytomas, and 2 angiocentric gliomas, 58 PAs – 48 from the cerebellum and 10 from other sites, 6 pilomyxoid gliomas – 4 supratentorial and 2 spinal, and one PXA. In accord with other reports in the literature, this dataset highlights the following clinicopathological / genetic associations; tumors with KIAA1549-BRAF or SRGAP3-RAF1 fusion genes are all PAs, most commonly from the cerebellum or pilomyxoid gliomas (one hypothalamic pilomyxoid astrocytoma and one spinal tumor) [13,18,20,21,27,52], tumors with BRAFp.V600E activating mutations are histologically varied (DA, PA, or PXA) and sometimes harbor concomitant deletion of CDKN2A [12,41,51], IDH1/2 mutations are not a feature of pediatric diffuse gliomas [11,60], and 1p/19q loss occurs in oligodendroglial tumors (oligodendroglioma or oligoastrocytoma) at a much lower frequency in children than in adults [25,42,47].
This study provides data on MYB abnormalities in a large series of pediatric LGGs that have been comprehensively analyzed for a range of genetic abnormalities already implicated in LGG development. Novel MYB amplifications were disclosed at a relatively low frequency (14%) in DAs, but they supplement very few genetic aberrations so far reported for these enigmatic WHO grade II tumors. Further, our immunohistochemical data indicate that upregulation of MYB can occur through mechanisms other than gene amplification or deletion of the 3′UTR, and that it can be a feature of other types of LGG and even some HGGs, suggesting a broader role for MYB in gliomagenesis.
Acknowledgements
This work was supported by the Pediatric Low-Grade Astrocytoma (PLGA) Foundation, the American Lebanese Syrian Associated Charities (ALSAC), the Samantha Dickson Brain Tumour Trust, and Cancer Research UK.
Contributor Information
Ruth G. Tatevossian, Department of Pathology, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105 USA
Bo Tang, Department of Pathology, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105 USA.
James Dalton, Department of Pathology, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105 USA.
Tim Forshew, Queen Mary University of London, Centre for Neuroscience and Trauma, Blizard Institute of Cell and Molecular Science, Barts and the London School of Medicine and Dentistry, London E1 2AT UK.
Andrew R. Lawson, Queen Mary University of London, Centre for Neuroscience and Trauma, Blizard Institute of Cell and Molecular Science, Barts and the London School of Medicine and Dentistry, London E1 2AT UK
Jing Ma, Hartwell Center for Bioinformatics and Biotechnology, St. Jude Children’s Research Hospital, Memphis, TN 38105 USA.
Geoff Neale, Hartwell Center for Bioinformatics and Biotechnology, St. Jude Children’s Research Hospital, Memphis, TN 38105 USA.
Sheila A. Shurtleff, Department of Pathology, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105 USA
Simon Bailey, Sir James Spence Institute of Child Health, Royal Victoria Infirmary, Newcastle upon Tyne, UK.
Amar Gajjar, Department of Oncology, St Jude Children’s Research Hospital, Memphis, TN 38105, USA.
Suzanne J. Baker, Department of Developmental Neurobiology, St Jude Children’s Research Hospital, Memphis, TN 38105, USA
Denise Sheer, Queen Mary University of London, Centre for Neuroscience and Trauma, Blizard Institute of Cell and Molecular Science, Barts and the London School of Medicine and Dentistry, London E1 2AT UK.
David W. Ellison, Department of Pathology, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105 USA
References
- 1.Arora RS, Alston RD, Eden TO, et al. Age-incidence patterns of primary CNS tumors in children, adolescents, and adults in England. Neuro Oncol. 2009;11:403–413. doi: 10.1215/15228517-2008-097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balss J, Meyer J, Mueller W, et al. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116:597–602. doi: 10.1007/s00401-008-0455-2. [DOI] [PubMed] [Google Scholar]
- 3.Bender TP, Kremer CS, Kraus M, Buch T, Rajewsky K. Critical functions for c-Myb at three checkpoints during thymocyte development. Nat Immunol. 2004;5:721–729. doi: 10.1038/ni1085. [DOI] [PubMed] [Google Scholar]
- 4.Beroukhim R, Getz G, Nghiemphu L, et al. Assessing the significance of chromosomal aberrations in cancer: methodology and application to glioma. Proc Natl Acad Sci U S A. 2007;104:20007–20012. doi: 10.1073/pnas.0710052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beug H, von Kirchbach A, Doderlein G, Conscience JF, Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979;18:375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- 6.Biedenkapp H, Borgmeyer U, Sippel AE, Klempnauer KH. Viral myb oncogene encodes a sequence-specific DNA-binding activity. Nature. 1988;335:835–837. doi: 10.1038/335835a0. [DOI] [PubMed] [Google Scholar]
- 7.Broniscer A, Baker SJ, West AN, et al. Clinical and molecular characteristics of malignant transformation of low-grade glioma in children. J Clin Oncol. 2007;25:682–689. doi: 10.1200/JCO.2006.06.8213. [DOI] [PubMed] [Google Scholar]
- 8.Chung EY, Dews M, Cozma D, et al. c-Myb oncoprotein is an essential target of the dleu2 tumor suppressor microRNA cluster. Cancer Biol Ther. 2008;7:1758–1764. doi: 10.4161/cbt.7.11.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clappier E, Cuccuini W, Kalota A, et al. The C-MYB locus is involved in chromosomal translocation and genomic duplications in human T-cell acute leukemia (T-ALL), the translocation defining a new T-ALL subtype in very young children. Blood. 2007;110:1251–1261. doi: 10.1182/blood-2006-12-064683. [DOI] [PubMed] [Google Scholar]
- 10.Close J, Game L, Clark B, et al. Genome annotation of a 1.5 Mb region of human chromosome 6q23 encompassing a quantitative trait locus for fetal hemoglobin expression in adults. BMC Genomics. 2004;5:33. doi: 10.1186/1471-2164-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Carli E, Wang X, Puget S. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:2248. doi: 10.1056/NEJMc090593. author reply 2249. [DOI] [PubMed] [Google Scholar]
- 12.Dougherty MJ, Santi M, Brose MS, et al. Activating mutations in BRAF characterize a spectrum of pediatric low-grade gliomas. Neuro Oncol. 2010 doi: 10.1093/neuonc/noq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forshew T, Tatevossian RG, Lawson AR, et al. Activation of the ERK/MAPK pathway: a signature genetic defect in posterior fossa pilocytic astrocytomas. J Pathol. 2009;218:172–181. doi: 10.1002/path.2558. [DOI] [PubMed] [Google Scholar]
- 14.Fouladi M, Hunt DL, Pollack IF, et al. Outcome of children with centrally reviewed low-grade gliomas treated with chemotherapy with or without radiotherapy on Children’s Cancer Group high-grade glioma study CCG-945. Cancer. 2003;98:1243–1252. doi: 10.1002/cncr.11637. [DOI] [PubMed] [Google Scholar]
- 15.Fuller CE, Perry A. Molecular diagnostics in central nervous system tumors. Adv Anat Pathol. 2005;12:180–194. doi: 10.1097/01.pap.0000175117.47918.f7. [DOI] [PubMed] [Google Scholar]
- 16.Geraci M, Birch JM, Alston RD, Moran A, Eden TO. Cancer mortality in 13 to 29-year-olds in England and Wales, 1981-2005. Br J Cancer. 2007;97:1588–1594. doi: 10.1038/sj.bjc.6604080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80:2321–2332. doi: 10.1002/(sici)1097-0142(19971215)80:12<2321::aid-cncr14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Horbinski C, Hamilton RL, Nikiforov Y, Pollack IF. Association of molecular alterations, including BRAF, with biology and outcome in pilocytic astrocytomas. Acta Neuropathol. 2010;119:641–649. doi: 10.1007/s00401-009-0634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang AM, Rehm EJ, Rubin GM. Recovery of DNA sequences flanking P-element insertions in Drosophila: inverse PCR and plasmid rescue. Cold Spring Harb Protoc. 2009;2009 doi: 10.1101/pdb.prot5199. pdb prot5199. [DOI] [PubMed] [Google Scholar]
- 20.Jones DT, Kocialkowski S, Liu L, et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68:8673–8677. doi: 10.1158/0008-5472.CAN-08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones DT, Kocialkowski S, Liu L, et al. Oncogenic RAF1 rearrangement and a novel BRAF mutation as alternatives to KIAA1549:BRAF fusion in activating the MAPK pathway in pilocytic astrocytoma. Oncogene. 2009;28:2119–2123. doi: 10.1038/onc.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kagawa N, Maruno M, Suzuki T, et al. Detection of genetic and chromosomal aberrations in medulloblastomas and primitive neuroectodermal tumors with DNA microarrays. Brain Tumor Pathol. 2006;23:41–47. doi: 10.1007/s10014-006-0201-1. [DOI] [PubMed] [Google Scholar]
- 23.Kanner AA, Staugaitis SM, Castilla EA, et al. The impact of genotype on outcome in oligodendroglioma: validation of the loss of chromosome arm 1p as an important factor in clinical decision making. J Neurosurg. 2006;104:542–550. doi: 10.3171/jns.2006.104.4.542. [DOI] [PubMed] [Google Scholar]
- 24.Kauraniemi P, Hedenfalk I, Persson K, et al. MYB oncogene amplification in hereditary BRCA1 breast cancer. Cancer Res. 2000;60:5323–5328. [PubMed] [Google Scholar]
- 25.Kreiger PA, Okada Y, Simon S, et al. Losses of chromosomes 1p and 19q are rare in pediatric oligodendrogliomas. Acta Neuropathol. 2005;109:387–392. doi: 10.1007/s00401-004-0976-2. [DOI] [PubMed] [Google Scholar]
- 26.Lahortiga I, De Keersmaecker K, Van Vlierberghe P, et al. Duplication of the MYB oncogene in T cell acute lymphoblastic leukemia. Nat Genet. 2007;39:593–595. doi: 10.1038/ng2025. [DOI] [PubMed] [Google Scholar]
- 27.Lawson AR, Tatevossian RG, Phipps KP, et al. RAF gene fusions are specific to pilocytic astrocytoma in a broad paediatric brain tumour cohort. Acta Neuropathol. 2010;120:271–273. doi: 10.1007/s00401-010-0693-y. [DOI] [PubMed] [Google Scholar]
- 28.Lei W, Rushton JJ, Davis LM, Liu F, Ness SA. Positive and negative determinants of target gene specificity in myb transcription factors. J Biol Chem. 2004;279:29519–29527. doi: 10.1074/jbc.M403133200. [DOI] [PubMed] [Google Scholar]
- 29.Lipsick JS, Wang DM. Transformation by v-Myb. Oncogene. 1999;18:3047–3055. doi: 10.1038/sj.onc.1202745. [DOI] [PubMed] [Google Scholar]
- 30.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malaterre J, Mantamadiotis T, Dworkin S, et al. c-Myb is required for neural progenitor cell proliferation and maintenance of the neural stem cell niche in adult brain. Stem Cells. 2008;26:173–181. doi: 10.1634/stemcells.2007-0293. [DOI] [PubMed] [Google Scholar]
- 32.McCabe MG, Ichimura K, Liu L, et al. High-resolution array-based comparative genomic hybridization of medulloblastomas and supratentorial primitive neuroectodermal tumors. J Neuropathol Exp Neurol. 2006;65:549–561. doi: 10.1097/00005072-200606000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitani Y, Li J, Rao PH, et al. Comprehensive Analysis of the MYB-NFIB Gene Fusion in Salivary Adenoid Cystic Carcinoma: Incidence, Variability and Clinicopathological Significance. Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-10-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mucenski ML, McLain K, Kier AB, et al. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991;65:677–689. doi: 10.1016/0092-8674(91)90099-k. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura M, Shimada K, Ishida E, et al. Molecular pathogenesis of pediatric astrocytic tumors. Neuro Oncol. 2007;9:113–123. doi: 10.1215/15228517-2006-036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh IH, Reddy EP. The myb gene family in cell growth, differentiation and apoptosis. Oncogene. 1999;18:3017–3033. doi: 10.1038/sj.onc.1202839. [DOI] [PubMed] [Google Scholar]
- 37.Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445–1453. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peris-Bonet R, Martinez-Garcia C, Lacour B, et al. Childhood central nervous system tumours--incidence and survival in Europe (1978-1997): report from Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:2064–2080. doi: 10.1016/j.ejca.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Persson M, Andren Y, Mark J, et al. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci U S A. 2009;106:18740–18744. doi: 10.1073/pnas.0909114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfister S, Janzarik WG, Remke M, et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest. 2008;118:1739–1749. doi: 10.1172/JCI33656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pollack IF, Finkelstein SD, Burnham J, et al. Association between chromosome 1p and 19q loss and outcome in pediatric malignant gliomas: results from the CCG-945 cohort. Pediatr Neurosurg. 2003;39:114–121. doi: 10.1159/000071647. [DOI] [PubMed] [Google Scholar]
- 43.Pollack IF, Finkelstein SD, Burnham J, et al. Age and TP53 mutation frequency in childhood malignant gliomas: results in a multi-institutional cohort. Cancer Res. 2001;61:7404–7407. [PubMed] [Google Scholar]
- 44.Preusser M, Hoischen A, Novak K, et al. Angiocentric glioma: report of clinico-pathologic and genetic findings in 8 cases. Am J Surg Pathol. 2007;31:1709–1718. doi: 10.1097/PAS.0b013e31804a7ebb. [DOI] [PubMed] [Google Scholar]
- 45.Qaddoumi I, Sultan I, Broniscer A. Pediatric low-grade gliomas and the need for new options for therapy: Why and how? Cancer Biol Ther. 2009;8:4–10. doi: 10.4161/cbt.8.1.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qaddoumi I, Sultan I, Gajjar A. Outcome and prognostic features in pediatric gliomas: a review of 6212 cases from the Surveillance, Epidemiology, and End Results database. Cancer. 2009;115:5761–5770. doi: 10.1002/cncr.24663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raghavan R, Balani J, Perry A, et al. Pediatric oligodendrogliomas: a study of molecular alterations on 1p and 19q using fluorescence in situ hybridization. J Neuropathol Exp Neurol. 2003;62:530–537. doi: 10.1093/jnen/62.5.530. [DOI] [PubMed] [Google Scholar]
- 48.Ramsay RG, Gonda TJ. MYB function in normal and cancer cells. Nat Rev Cancer. 2008;8:523–534. doi: 10.1038/nrc2439. [DOI] [PubMed] [Google Scholar]
- 49.Reifenberger G, Louis DN. Oligodendroglioma: toward molecular definitions in diagnostic neuro-oncology. J Neuropathol Exp Neurol. 2003;62:111–126. doi: 10.1093/jnen/62.2.111. [DOI] [PubMed] [Google Scholar]
- 50.Rosenthal MA, Thompson MA, Ellis S, Whitehead RH, Ramsay RG. Colonic expression of c-myb is initiated in utero and continues throughout adult life. Cell Growth Differ. 1996;7:961–967. [PubMed] [Google Scholar]
- 51.Schiffman JD, Hodgson JG, VandenBerg SR, et al. Oncogenic BRAF mutation with CDKN2A inactivation is characteristic of a subset of pediatric malignant astrocytomas. Cancer Res. 2010;70:512–519. doi: 10.1158/0008-5472.CAN-09-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sievert AJ, Jackson EM, Gai X, et al. Duplication of 7q34 in pediatric low-grade astrocytomas detected by high-density single-nucleotide polymorphism-based genotype arrays results in a novel BRAF fusion gene. Brain Pathol. 2009;19:449–458. doi: 10.1111/j.1750-3639.2008.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith JS, Perry A, Borell TJ, et al. Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J Clin Oncol. 2000;18:636–645. doi: 10.1200/JCO.2000.18.3.636. [DOI] [PubMed] [Google Scholar]
- 54.Stenman G, Andersson MK, Andren Y. New tricks from an old oncogene: gene fusion and copy number alterations of MYB in human cancer. Cell Cycle. 2010;9:2986–2995. doi: 10.4161/cc.9.15.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tatevossian RG, Lawson AR, Forshew T, et al. MAPK pathway activation and the origins of pediatric low-grade astrocytomas. J Cell Physiol. 2010;222:509–514. doi: 10.1002/jcp.21978. [DOI] [PubMed] [Google Scholar]
- 56.Thomas MD, Kremer CS, Ravichandran KS, Rajewsky K, Bender TP. c-Myb is critical for B cell development and maintenance of follicular B cells. Immunity. 2005;23:275–286. doi: 10.1016/j.immuni.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 57.Wallrapp C, Muller-Pillasch F, Solinas-Toldo S, et al. Characterization of a high copy number amplification at 6q24 in pancreatic cancer identifies c-myb as a candidate oncogene. Cancer Res. 1997;57:3135–3139. [PubMed] [Google Scholar]
- 58.Xiao C, Calado DP, Galler G, et al. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell. 2007;131:146–159. doi: 10.1016/j.cell.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 59.Yan H, Bigner DD, Velculescu V, Parsons DW. Mutant metabolic enzymes are at the origin of gliomas. Cancer Res. 2009;69:9157–9159. doi: 10.1158/0008-5472.CAN-09-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao H, Kalota A, Jin S, Gewirtz AM. The c-myb proto-oncogene and microRNA-15a comprise an active autoregulatory feedback loop in human hematopoietic cells. Blood. 2009;113:505–516. doi: 10.1182/blood-2008-01-136218. [DOI] [PMC free article] [PubMed] [Google Scholar]