Abstract
Background and Purpose
HMGB1 is a nuclear protein and an alarmin that signals cell damage in response to injury. It is believed that following release from injured cells, HMGB1 binds to its receptors to stimulate cross-talk among cells and to drive components of the inflammatory cascade. This study was intended to investigate the role of extracellular HMGB1 in ischemic stroke by examining the response of the zymogen, matrix metalloproteinase-9 (MMP-9) to HMGB1, in vivo and in vitro.
Methods
TLR2, TLR4, RAGE and MMP-9 expression was examined using quantitative RT-PCR in primary cultured neurons, astrocytes as well as in mouse brain following HMGB1 addition. MMP-9 expression/activity was examined using zymography. Middle cerebral artery occlusion was induced for 60 minutes using a filament model.
Results
MMP-9 and TLR4 are highly expressed in neurons, astrocytes and mouse brain. HMGB1 addition to neuronal and glial cell cultures caused MMP-9 upregulation in a dose- and time-dependent manner. Lack of TLR4 function attenuated MMP-9 expression induced by HMGB1 in vitro. Following striatal microinjection of HMGB1, MMP-9 was upregulated, and the response was independent of TNFα. Interestingly, MMP-9 upregulation was reduced in TLR4 missense mutant mice after ischemia compared to wild-type controls as was infarct volume.
Conclusion
Our results suggest that HMGB1 triggers MMP-9 upregulation in neurons and astrocytes predominantly via TLR4 after cerebral ischemia. Hence, targeting HMGB1/TLRs signaling pathway may reduce the acute inflammatory response and reduce tissue damage in cerebral ischemia.
Keywords: cerebral ischemia, matrix metalloproteinase, high-mobility group box 1
Introduction
Stroke is the third leading cause of death in America. Although the pathophysiological processes of stroke have been extensively studied, the mechanisms of early neurovascular dysfunction are not fully understood. After onset of arterial occlusion, ischemic injury develops rapidly in a heterogeneous manner. Many molecular mechanisms have been implicated including excitotoxicity, ionic imbalance, oxidative and nitrosative stress and programmed cell death pathways comprising apoptosis, necroptosis and autophagy1, 2. Inflammation is also broadly triggered with an elevation in cytokines and chemokines3, 4, and various neurovascular proteases including matrix metalloproteinases (MMPs)5-8. Because these cascades of ischemic pathophysiology are so complex, it has not been easy to find therapeutic approaches that are clinically effective.
Within the neurovascular unit, neurons are the most vulnerable cells to hypoxic ischemic injury9. Damaged neurons can be detected in mouse models as early as 30 minutes after middle cerebral artery occlusion (MCAO)10. We previously showed that high-mobility group box 1 (HMGB1), a chromatin protein, is rapidly released from injured cells after ischemia. Extracellular HMGB1 is a prototypical member of the so-called alarmin family of mediators that mediate cross-talk between injured cells and relative healthy cells around damaged tissues11-13. Released HMGB1 binds to its receptors (RAGE, TLR2 and TLR4) and augments inflammation via the upregulation of TNFα, IL-1β and other cytokines14-17. Inhibition of HMGB1 by either siRNA or neutralizing antibodies is neuroprotective18, 19. However, it is likely that HMGB1 may induce more than just inflammatory cytokines per se, since blockade of cytokines is not always beneficial after cerebral ischemia20.
In this study, we hypothesize that HMGB1 may also act by upregulating MMP-9, a potentially deleterious neurovascular protease. A large body of experimental and clinical evidence implicates MMP-9 in cerebral ischemic injury1, 7, 21, 22. Upregulation of MMP-9 causes blood-brain barrier degradation, vasogenic edema and hemorrhage22, 23. If HMGB1 is indeed rapidly released from sites of initial cell death after cerebral ischemia, it would serve as a logical candidate for upregulating MMP-9 and hence expanding the areas of brain damage after stroke. Here, we use a combination of neural cell cultures and in vivo mouse models of focal cerebral ischemia to test this hypothesis. Our data show that HMGB1 upregulates MMP-9 in neurons and astrocytes via the TLR4 receptor, and in missense mutant mice with dysfunctional TLR4 signaling, MMP-9 levels and brain injury are reduced after focal cerebral ischemia.
Materials and Methods
Middle cerebral artery occlusion model
C57 Black/6j, C3H/heouj and C3H/Hej male mice (20 to 25 g, 10-12 weeks old) were purchased from The Jackson Laboratory (Bar Harbor, Maine). All experiments were performed following institutionally approved protocols in accordance with The National Institutes of Health Guide for the Care and Use of Laboratory Animals under institutional guidelines. The filament MCAO model was used, as previously described10, 24. Animals were anesthetized with isoflurane in 70% nitrous oxide and 30% oxygen. A silicone-coated 8-0 monofilament was used to occlude the middle cerebral artery for 1 hour. After 1 h MCAO, the filament was withdrawn for reperfusion. Temporal cerebral blood flow was monitored by laser-Doppler (Perimed, Stockholm, Sweden) to confirm occlusion and reperfusion. Rectal temperature was maintained 36.5°C-37.5°C. The mice that died due to seizure within 1 hour after occlusion and termination of anesthesia were excluded. Infarct volume was measured 24 hours after MCAO by 2,3,5-triphenyltetrazolium chloride (TTC) staining.
Cell cultures
Primary neuronal cultures were isolated from E-16 mouse embryo and cultured in NeuroBasal medium with 2% B27 (Invitrogen) on polyethyleminime-coated plates25. Neurons were grown for 10-14 days before use. Primary mixed glial cultures were prepared from postnatal day 1 pups and cultured in DMEM with 10% FBS. Low passage glial cells (3-4) were used for our experiments.
Quantitative real time RT-PCR
RNA was isolated from either cultured cells or brain tissues and treated with DNase I to remove genomic DNA. cDNA was generated by using SuperScript III kit (Invitrogen). Samples were run in triplicates, with negative controls (no cDNA). Primers and Taqman FAM labeled probes were mixed with AmpliTaq Fast Universal PCR Master Mix (Applied Biosystems) and then diluted with ddH2O up to 25μl. Gene specific products were normalized to an endogenous control of 18S rRNA.
Immunostaining
Immunostaining was performed as previously described26. Briefly, fresh frozen coronal brain sections were fixed with 4% phosphate-buffered paraformaldehyde. The following antibodies and dilution conditions were used: polyclonal MMP-9 antibody (1:200; Abcam), neurons were stained with NeuN (1:1000; Pharmingen) and astrocytes were stained with glial fibrillary acidic protein (GFAP) (1:1000; Sigma). Fluorescent-stained sections were analyzed by confocal microscopy.
Statistics
Messenger RNA expression levels were quantified by real-time RT-PCR. mRNA amounts were then averaged by group/treatment and compared by ANOVA followed by Dunnet post hoc analysis. p ≤ 0.05 was considered statistically significant.
Results
HMGB1 upregulates MMP-9 in neurons and astrocytes
Exposure of primary neurons and astrocytes to increasing concentrations of HMGB1 caused an upregulation of MMP-9 mRNA as measured by real time RT-PCR (Fig. 1A and 1B). This transcriptional response was followed by release of MMP-9 into the extracellular space as shown by zymography on conditioned media (Fig. 1C). The response in astrocytes was higher than in neurons. MMP-2 was not detected in the conditioned media and not detected after HMGB1 addition.
1.
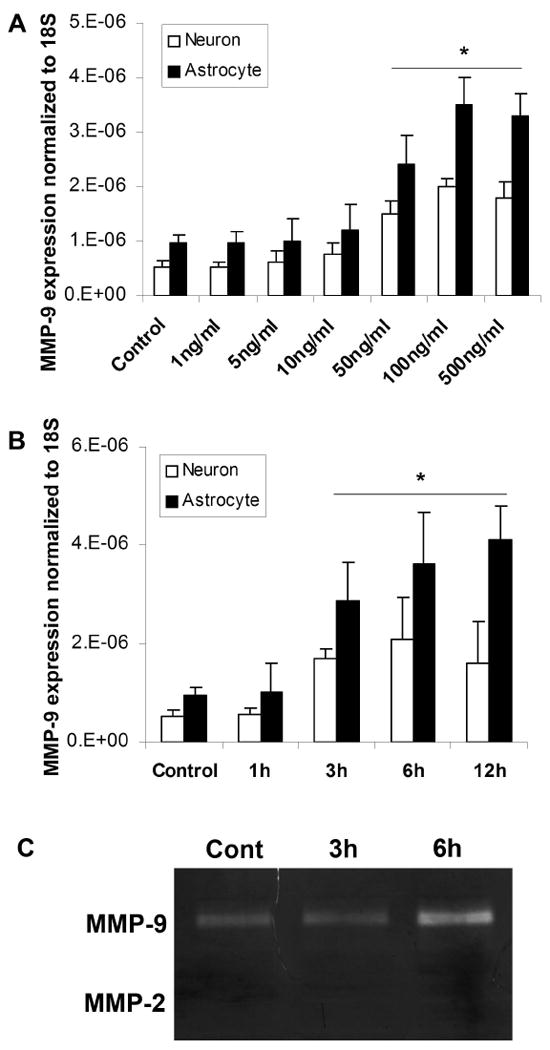
HMGB1 induces MMP-9 expression in neurons and astrocytes. Primary cultured neurons and astrocytes were treated with different doses of HMGB1 (A; 6h incubation) for various incubation periods (B; 100ng/ml of HMGB1). MMP-9 expression in the cells increases in a time and dose-dependent manner. C: Gel zymography confirms MMP-9 upregulation in the conditioned media from astrocytes. No MMP-2 is detected in the media. The data are the means±SD. *: p<0.05.
HMGB1 receptors are present in cultured neurons and astrocytes
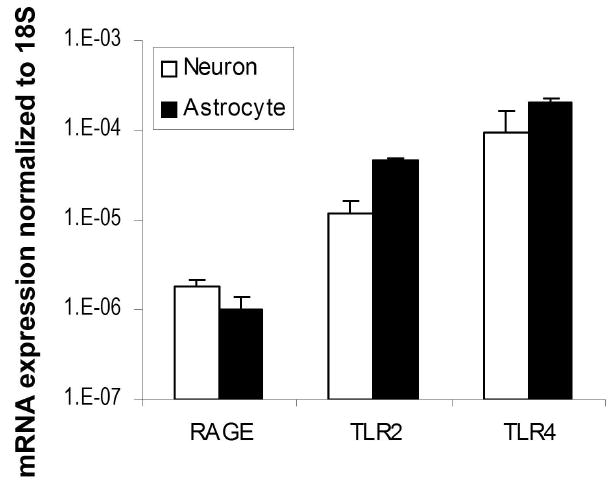
Three putative receptors for HMGB1 have been proposed - RAGE, TLR2 and TLR4. Using real-time RT-PCR, we quantitatively examined the expression levels of these 3 receptors in primary mouse cortical neurons and astrocytes. Our data confirmed that the mRNAs for the receptors were expressed in both cell types. RAGE levels were the lowest whereas TLR4 expression levels were the highest (Fig. 2).
2.
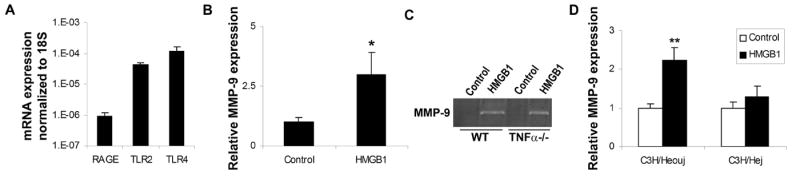
Expression of TLR4, TLR2 and RAGE, HMGB1 putative receptors in cultured neurons and astrocytes. Total RNA was isolated from primary cultured neurons and astrocytes. TLR4, TLR2 and RAGE mRNAs were analyzed by quantitative real time PCR. The expression levels were normalized to 18S expression. The data are shown as the mean±SD. Y-axis is a log scale.
HMGB1-induced MMP-9 occurs via TLR4 signaling
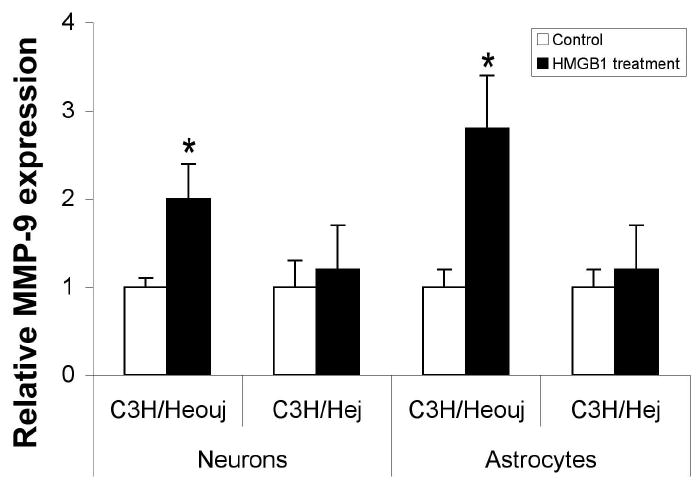
To further examine the mechanism of HMGB1-induced MMP-9 upregulation, we investigated whether TLR4 was involved. Astrocytes and neurons were isolated from the C3H/Hej mutant mouse, which expresses a loss-of-function missense mutation in third exon of tlr4 gene27, 28. For comparison, cells were isolated from C3H/Heouj mice (wild type control). Both wild-type and TLR4 mutant neurons and astrocytes were exposed to 100ng/ml recombinant HMGB1 for 6 hrs and MMP-9 expression was examined by RT-PCR. HMGB1 induced a clear increase in MMP-9 in wild type astrocytes and neurons but not in TLR4 mutant cells (Fig. 3), suggesting that TLR4 signaling is required for HMGB1-induced MMP-9 upregulation.
3.
HMGB1-induced MMP-9 upregulation is attenuated in TLR4 mutant neurons and astrocytes. Neurons and astrocytes were isolated from either C3H/Heouj (wild-type) or C3H/Hej (mutant) mice and treated with 100ng/ml HMGB1 for 6 hours. MMP-9 expression was analyzed by real time PCR. The data show means±SD relative to control. *: <0.01.
HMGB1 induces MMP-9 upregulation in brain
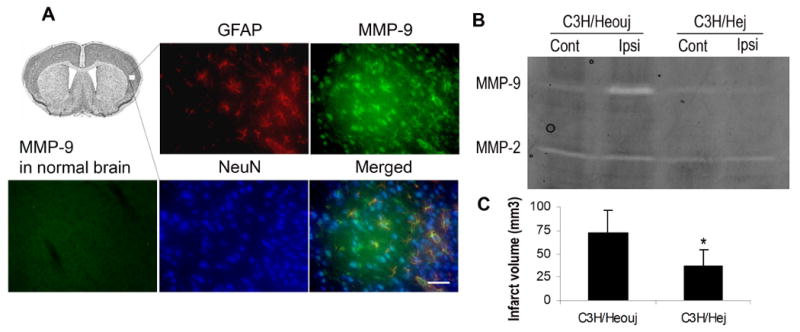
RT-PCR demonstrated that all 3 HMGB1 receptors (RAGE, TLR2 and TLR4) were detectable in mouse brain homogenates similar in quantity and relative expression to each other as in the culture data (Fig. 4A). Once again, levels of the TLR4 expression were much higher than TLR2 and RAGE expression levels. Stereotactic injection of HMGB1 directly into normal striatum caused a significant upregulation in MMP-9 in striatal homogenates (Fig. 4B). This response appeared to be independent of TNFα Fig. 4C) as evidenced by a similar MMP-9 response following injections of HMGB1 in TNFα knockout and wild-type mice (Fig. 4C). HMGB1 also significantly caused MMP-9 upregulation in wild type C3H strain comparable to the response in the C57B/6 strain. Interestingly, upregulation of MMP-9 induced by HMGB1 is attenuated in TLR4 mutant mice (Fig. 4D). The data are consistent with the results from in vitro experiments.
4.
HMGB1 upregulates MMP-9 in mouse brain. A: expressions of RAGE, TLR2 and TLR4 in normal C57B/6 mice examined by real time PCR. The expression level is normalized to 18S expression. Y-axis is a log scale. B: Recombinant HMGB1 (100ng) or saline (control) was injected into C57B/6 mouse brains and MMP-9 mRNA was examined 6 hours after injection by real time PCR. The real time PCR data are the means±SD. *: p<0.01. C: recombinant HMGB1 (100ng) or saline control was injected into TNFα wild-type (WT) or knockout mouse brains and MMP-9 mRNA expression was assayed 6 hours after injection by gel zymography. No significant difference was observed between wild-type and knockout mice. D: 100ng of HMGB1 or saline (control) were injected into C3H mouse brains and mRNA of MMP-9 was analyzed by real time PCR. **: p<0.01.
MMP-9 and infarction volumes are reduced in TLR4 mutant mice
To test the hypothesis that HMGB1 induces MMP-9 via TLR4 in ischemic brain injury, we subjected TLR4 mutant mice and their wild type controls to 1 hour transient middle cerebral artery occlusion. MMP-9 levels were increased in neurons and astrocytes within ischemic tissue areas in wild type (Fig. 5A), which is consistent with the region of HMGB1 release 10. MMP-9 expression/activation was significantly lower in the ischemic cortex of TLR4 mutant mice compared to the wild-type controls at 2 hours after reperfusion (Fig. 5B). Correspondingly, 24 h infarction volumes were also significantly smaller in TLR4 mutant mice compared with wild-type mice (Fig. 5C).
5.
MMP-9 expression and infarct volume after MCAO. A: The wild-type mice were subjected to 1h MCAO and sacrificed 6 hours after reperfusion. MMP-9 expression in cerebral cortex was examined by immunostaining. Upper left image shows representative region of MMP-9 examined in neurons (NeuN) and astrocytes (GFAP). No significant MMP-9 expression was detected in normal brains (lower left image). Scale bar: 50μm. B: MMP-9 expression examined by zymography was attenuated in cerebral cortex of TLR4 mutants (C3H/Hej) compared to wild-type mice (C3H/Heouj) after MCAO. C: Infarct volume was reduced in C3H/Hej mice examined by TTC staining 24h after MCAO. Data are means±SD. *: p<0.05.
Discussion
HMGB1 belongs to a family of molecules called alarmins11. Members of this family participate in cell-cell signaling to herald and respond to conditions of tissue stress. Passive release of HMGB1 from dying cells is a sign of necrotic damage. This is a broad-based phenomenon; HMGB1 has been shown to be released from neuronal cells10, 18, hepatocytes29, and cadiomyocytes30. In addition to passive release, actively-regulated secretion of HMGB1 may also occur, but primarily in immune cells15.
Extracellular HMGB1 is known to induce complex cascades of signaling via binding to its receptors, including RAGE, TLR2 and TLR4. The downstream effects are also wide-ranging. HMGB1 can trigger inflammation15, cardiac regeneration31, and neurite outgrowth32. Many of these cascades are mediated by well-conserved pathways such as MAP kinase, NF-κB and AP-1 transcriptional responses33. In the context of stroke, these pathways may be especially relevant since they are known to upregulate MMP-934, 35. HMGB1 release occurs as early as 30 minutes after ischemia10, MMP-9 elevations are described to take place after 6-12 hours. The temporal profile implicates these molecules may be related. In the present study, we showed that HMGB1 upregulated MMP-9 in cultured neurons and astrocytes, as well as in mouse brain. This phenomenon was primarily mediated through the TLR4 receptor, which was highly expressed in neuronal and astrocyte cultures as well as mouse brain. MMP-9 upregulation via HMGB1 expression was attenuated in TLR4 missense mutant neural cells compared to wild-type control cells after HMGB1 treatment. Finally, MMP-9 expression in cerebral cortex of TLR4 mutant mice was lower than wild-type controls after focal cerebral ischemia. Taken together, our findings suggest that after stroke onset, HMGB1 released from rapidly dying cells may bind onto constitutively expressed TLR4 receptors in adjacent brain, thus upregulating MMP-9 and expanding neurovascular damage and ischemic brain injury.
To date, three putative HMGB1 receptors have been reported – RAGE, TLR2 and TLR4. Baseline RAGE expression is low in brain and is inducible hours after injury36, 37. Our data showed that TLR2 and TLR4 were constitutively expressed in brain tissue and primary cultured neurons and astrocytes, consistent with other reports37-39. In our cell and animal models, TLR4 expression levels were highest compared to the other receptors. HMGB1-induced MMP-9 responses were mostly suppressed after blockade of TLR4 signaling. These data suggest that HMGB1 can act immediately around damaged areas upon release, and TLR4 may play a predominant role in the very early phases after stroke onset. Nevertheless, it remains possible that interactions with the other receptors may be important as ischemic injury evolves. Further studies will be required to assess these questions.
Because HMGB1 can participate in so many overlapping pathways, dissecting the precise signaling pathways involved is not easy. Our findings suggest that TLR4 activation is required for MMP-9 induction and neurovascular injury. But of course, HMGB1 can also induce many other cytokines such as TNFα and IL-1β, which may promote MMP-9 upregulation indirectly. In our study, we used TNFα knockout mice to control for one such alternate pathway. Stereotactic injection of recombinant HMGB1 robustly elevated MMP-9 expression in TNFα knockout mice, suggesting that at least in mouse models, the induction of MMP-9 by HMGB1 can be TNFα-independent. However, the interactions between HMGB1, MMP-9 and other cytokines will have to be carefully elucidated in future studies.
Along with the downregulation of MMP-9, a significant reduction of infarct volume was observed in TLR4 mutant mice after focal cerebral ischemia. There is also a clear time-dependent response. Increase of MMP-9 expression may worsen blood-brain barrier leakage and potentiate edema and inflammation. This finding suggests that release of HMGB1 from damaged neural cells can signal neighboring cells to increase blood vessel permeability and recruits immune cells thus worsening the progression of brain damage. But there is a caveat. If TLR4 mutant mice are protected against stroke by other undefined mechanisms, then any reduction in MMP-9 might be an indirect effect as well. In our model system, this may be unlikely. The reduction in ischemic MMP-9 levels within the TLR4 mutant brains occur very early, within 3 hrs after arterial occlusion. At these early time points, brain infarction has not fully progressed yet so that any decrease in MMP-9 levels would not be explained by smaller volumes of brain injury. Others have proposed that TLR4 is involved in cell death after stroke29, 40, and dysfunction of TLR4 leads to smaller infarction and lower MMP-9 expression40. Our study is consistent with these previous ideas, and further provides novel evidence showing that extracellular HMGB1 induces MMP-9 upregulation via a TNFα independent mechanism by activating TLR4. Since other MMPs such as MMP-3 also contribute to ischemia-induced cell death, whether upregulation of other MMPs through HMGB1-TLR4 pathway remains to be further addressed.
In conclusion, our study shows that extracellular HMGB1 signaling can serve as a mediator of cross-talk between cells upon release after stroke and brain injury. The ability of HMGB1 to activate TLR4 signaling and upregulate MMP-9 provides a novel mechanism by which neurovascular injury is amplified after initial ischemic injury in the brain. Targeting the HMGB1/TLR4 signaling pathway may be a novel therapeutic approach for stroke.
Acknowledgments
This work was supported by NIH R21NS056214 (MAM), 5R01NS010828-33 (MAM), R01NS48422 (EHL), R01NS56458 (EHL), R37NS37074 (EHL) and Massachusetts General Hospital Neuroscience Center Core Facility (NIH P30-NS045776). Jianhua Qiu was partly supported by AHA N0335154 and Massachusetts General Hospital interim support fund. John Sims was supported by NIH K08 NS049241. We thank Masaki Nishimura, Yumei Wang and Igor Bagayev for their excellent assistance.
Contributor Information
Jianhua Qiu, Department of Neurology and Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Jian Xu, Department of Neurology and Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA; Department of Neurosurgery, Huashan Hospital, Shanghai, China.
Yi Zheng, Department of Neurology and Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Ying Wei, Department of Neurology and Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Xiaoxia Zhu, Department of Neurology and Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA; Department of Rheumatology, Huashan Hospital, Shanghai, China.
Eng H Lo, Department of Neurology and Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Michael A Moskowitz, Department of Neurology and Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
John R Sims, Department of Neurology and Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
References
- 1.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 2.Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55:310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: New opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: Effects of gene knockout and enzyme inhibition with bb-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39:279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg GA, Estrada EY, Mobashery S. Effect of synthetic matrix metalloproteinase inhibitors on lipopolysaccharide-induced blood-brain barrier opening in rodents: Differences in response based on strains and solvents. Brain Res. 2007;1133:186–192. doi: 10.1016/j.brainres.2006.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- 10.Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, Savitz SI, Salomone S, Moskowitz MA. Early release of hmgb-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab. 2008;28:927–938. doi: 10.1038/sj.jcbfm.9600582. [DOI] [PubMed] [Google Scholar]
- 11.Haraldsen G, Balogh J, Pollheimer J, Sponheim J, Kuchler AM. Interleukin-33 - cytokine of dual function or novel alarmin? Trends Immunol. 2009;30:227–233. doi: 10.1016/j.it.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Yang QW, Wang JZ, Li JC, Zhou Y, Zhong Q, Lu FL, Xiang J. High-mobility group protein box-1 and its relevance to cerebral ischemia. J Cereb Blood Flow Metab. 30:243–254. doi: 10.1038/jcbfm.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faraco G, Fossati S, Bianchi ME, Patrone M, Pedrazzi M, Sparatore B, Moroni F, Chiarugi A. High mobility group box 1 protein is released by neural cells upon different stresses and worsens ischemic neurodegeneration in vitro and in vivo. J Neurochem. 2007;103:590–603. doi: 10.1111/j.1471-4159.2007.04788.x. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Wang H, Mason JM, Levine J, Yu M, Ulloa L, Czura CJ, Tracey KJ, Yang H. Recombinant hmgb1 with cytokine-stimulating activity. J Immunol Methods. 2004;289:211–223. doi: 10.1016/j.jim.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Lotze MT, Tracey KJ. High-mobility group box 1 protein (hmgb1): Nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 16.Tian L, Rauvala H, Gahmberg CG. Neuronal regulation of immune responses in the central nervous system. Trends Immunol. 2009;30:91–99. doi: 10.1016/j.it.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 17.O’Connor KA, Hansen MK, Rachal Pugh C, Deak MM, Biedenkapp JC, Milligan ED, Johnson JD, Wang H, Maier SF, Tracey KJ, Watkins LR. Further characterization of high mobility group box 1 (hmgb1) as a proinflammatory cytokine: Central nervous system effects. Cytokine. 2003;24:254–265. doi: 10.1016/j.cyto.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW, Lee MH, Han PL, Park JS, Lee JK. Hmgb1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci. 2006;26:6413–6421. doi: 10.1523/JNEUROSCI.3815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu K, Mori S, Takahashi HK, Tomono Y, Wake H, Kanke T, Sato Y, Hiraga N, Adachi N, Yoshino T, Nishibori M. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. Faseb J. 2007 doi: 10.1096/fj.07-8770com. [DOI] [PubMed] [Google Scholar]
- 20.Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nat Med. 2002;8:1363–1368. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- 21.Lo EH. T time in the brain. Nat Med. 2009;15:844–846. doi: 10.1038/nm0809-844. [DOI] [PubMed] [Google Scholar]
- 22.Leonardo CC, Pennypacker KR. Neuroinflammation and mmps: Potential therapeutic targets in neonatal hypoxic-ischemic injury. J Neuroinflammation. 2009;6:13. doi: 10.1186/1742-2094-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke. 2008;39:3372–3377. doi: 10.1161/STROKEAHA.108.514026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Endres M, Laufs U, Huang Z, Nakamura T, Huang P, Moskowitz MA, Liao JK. Stroke protection by 3-hydroxy-3-methylglutaryl (hmg)-coa reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1998;95:8880–8885. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu J, Whalen MJ, Lowenstein P, Fiskum G, Fahy B, Darwish R, Aarabi B, Yuan J, Moskowitz MA. Upregulation of the fas receptor death-inducing signaling complex after traumatic brain injury in mice and humans. J Neurosci. 2002;22:3504–3511. doi: 10.1523/JNEUROSCI.22-09-03504.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu J, Takagi Y, Harada J, Rodrigues N, Moskowitz MA, Scadden DT, Cheng T. Regenerative response in ischemic brain restricted by p21cip1/waf1. J Exp Med. 2004;199:937–945. doi: 10.1084/jem.20031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson J, Riblet R, Taylor BA. The response of recombinant inbred strains of mice to bacterial lipopolysaccharides. J Immunol. 1977;118:2088–2093. [PubMed] [Google Scholar]
- 28.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective lps signaling in c3h/hej and c57bl/10sccr mice: Mutations in tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 29.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor hmgb1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Germani A, Limana F, Capogrossi MC. Pivotal advances: High-mobility group box 1 protein--a cytokine with a role in cardiac repair. J Leukoc Biol. 2007;81:41–45. doi: 10.1189/jlb.0306165. [DOI] [PubMed] [Google Scholar]
- 31.Limana F, Germani A, Zacheo A, Kajstura J, Di Carlo A, Borsellino G, Leoni O, Palumbo R, Battistini L, Rastaldo R, Muller S, Pompilio G, Anversa P, Bianchi ME, Capogrossi MC. Exogenous high-mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac c-kit+ cell proliferation and differentiation. Circ Res. 2005;97:e73–83. doi: 10.1161/01.RES.0000186276.06104.04. [DOI] [PubMed] [Google Scholar]
- 32.Merenmies J, Pihlaskari R, Laitinen J, Wartiovaara J, Rauvala H. 30-kda heparin-binding protein of brain (amphoterin) involved in neurite outgrowth. Amino acid sequence and localization in the filopodia of the advancing plasma membrane. J Biol Chem. 1991;266:16722–16729. [PubMed] [Google Scholar]
- 33.Takeda K, Akira S. Tlr signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Kolev K, Skopal J, Simon L, Csonka E, Machovich R, Nagy Z. Matrix metalloproteinase-9 expression in post-hypoxic human brain capillary endothelial cells: H2o2 as a trigger and nf-kappab as a signal transducer. Thromb Haemost. 2003;90:528–537. doi: 10.1160/TH03-02-0070. [DOI] [PubMed] [Google Scholar]
- 35.Gum R, Lengyel E, Juarez J, Chen JH, Sato H, Seiki M, Boyd D. Stimulation of 92-kda gelatinase b promoter activity by ras is mitogen-activated protein kinase kinase 1-independent and requires multiple transcription factor binding sites including closely spaced pea3/ets and ap-1 sequences. J Biol Chem. 1996;271:10672–10680. doi: 10.1074/jbc.271.18.10672. [DOI] [PubMed] [Google Scholar]
- 36.Freixes M, Rodriguez A, Dalfo E, Ferrer I. Oxidation, glycoxidation, lipoxidation, nitration, and responses to oxidative stress in the cerebral cortex in creutzfeldt-jakob disease. Neurobiol Aging. 2006;27:1807–1815. doi: 10.1016/j.neurobiolaging.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Pichiule P, Chavez JC, Schmidt AM, Vannucci SJ. Hypoxia-inducible factor-1 mediates neuronal expression of the receptor for advanced glycation end products following hypoxia/ischemia. J Biol Chem. 2007;282:36330–36340. doi: 10.1074/jbc.M706407200. [DOI] [PubMed] [Google Scholar]
- 38.Ziegler G, Prinz V, Albrecht MW, Harhausen D, Khojasteh U, Nacken W, Endres M, Dirnagl U, Nietfeld W, Trendelenburg G. Mrp-8 and -14 mediate cns injury in focal cerebral ischemia. Biochim Biophys Acta. 2009;1792:1198–1204. doi: 10.1016/j.bbadis.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Yoon HJ, Jeon SB, Kim IH, Park EJ. Regulation of tlr2 expression by prostaglandins in brain glia. J Immunol. 2008;180:8400–8409. doi: 10.4049/jimmunol.180.12.8400. [DOI] [PubMed] [Google Scholar]
- 40.Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]


