Abstract
Objective
The two-dimensional multi-echo recombined gradient echo (MERGE) technique automatically acquires and sums multiple gradient echoes at various echo times in cervical spine magnetic resonance (MR) imaging. This technique increases the grey–white matter contrast within the spinal cord and should also improve the depiction of cervical cord lesions. The aim of this study was to qualitatively and quantitatively evaluate MERGE imaging compared with T2-weighted fast spin-echo (T2WFSE) imaging for depicting multiple sclerosis (MS) lesions in the cervical cord.
Methods
Nineteen consecutive patients (10 males and 9 females; age range 22–62 years, mean age 43.6 years) with clinically diagnosed MS were examined with cervical spinal cord MR imaging at 3 T including both MERGE and T2WFSE imaging. Qualitative evaluation for MS lesion conspicuity was performed. The quantitative criterion utilized to compare MERGE imaging with T2WFSE imaging was the lesion-to-background contrast-to-noise ratio (CNR).
Results
MERGE imaging showed 79 lesions and missed 1 that was depicted on T2WFSE imaging. T2WFSE imaging showed 46 lesions and missed 34 that were depicted on MERGE imaging. MERGE imaging was markedly superior to T2WFSE imaging in rendering greater lesion conspicuity. In the quantitative evaluation, the lesion-to-background CNR upon MERGE imaging was significantly higher than that upon T2WFSE imaging (P < 0.001, paired t-test).
Conclusions
MERGE imaging in the cervical spinal cord increases detection and conspicuity of MS lesions. Strong consideration should be given to utilizing axial MERGE images in the diagnosis and follow-up study of cervical cord MS.
Keywords: Neuroimaging, Multi-echo recombined gradient echo, Multiple sclerosis, Cervical spinal cord
Introduction
Detection of hyperintense lesions on T2-weighted magnetic resonance (MR) images is important in the assessment of damage to the spinal cord in patients with multiple sclerosis (MS).1,2 Finding spinal cord lesions can also help in differential diagnosis. Several studies have shown that spinal cord lesions are very uncommon in people with other neurological diseases2–8 and the existence of T2-hyperintensities can increase the confidence in the diagnosis of MS. This is true especially when MR imaging abnormalities of the brain white matter are interpreted as non-specific or equivocal. In addition, hyperintense lesions on T2-weighted images are often associated with sensory or motor symptoms and can be a marker of disease activity.9–11 Therefore, the detection of spinal cord lesions is critical for the evaluation of MS.
Multi-echo recombined gradient echo (MERGE) MR imaging is an imaging technique designed to image the cervical spine. This technique automatically acquires and sums multiple gradient echoes at various echo times. MERGE increases the gray–white matter contrast within the spinal cord and should theoretically increase lesion conspicuity. A similar technique has been proven to be useful for detecting abnormalities of the cervical spinal cord, such as hemorrhage and edema, but no patients with MS were analyzed.12 Another study also concluded that gradient-recalled echo sequences at a 1.5 T system offer better anatomic and pathological delineation of intramedullary disease, but only three patients with definitive diagnoses of MS were included and only two of these cases were imaged in the axial plane.13 The purpose of this study was to evaluate the potential of MERGE imaging for depicting MS lesions in the cervical spinal cord at 3 T, and to compare it qualitatively and quantitatively with a conventional technique, T2-weighted fast spin-echo (T2WFSE) imaging.
Methods
We reviewed the medical records and imaging database from our institution. We selected consecutive patients with clinically diagnosed MS who underwent cervical spinal cord MR imaging, including both MERGE imaging and T2WFSE imaging. We excluded patients who had cervical degenerative changes that could cause spinal cord changes similar to MS lesions. Nineteen patients (10 men and 9 women; age range 22–62 years, median 44 years) met the criteria and were included in this study. Fifteen subjects were classified as having relapsing–remitting MS, two as primary progressive type, and one as a clinically isolated syndrome. Axial MERGE and T2WFSE imaging are part of our standard protocol for scanning the cervical spinal cord in patients with MS. This retrospective study was reviewed by an Institutional Review Board, which approved waiver of informed consent and Health Insurance Portability and Accountability Act (HIPAA) authorization.
MR studies were performed in all patients on a 3.0 T system (HDx Signa, GE Medical Systems, Milwaukee, WI, USA). A dedicated phased array spine coil was used. Axial MERGE images were obtained with time to repetition (TR) of 760–1100 ms, average echo time (TE) of 10.8 ms, field of view (FOV) of 18 × 18, imaging matrix of 320 × 190, the number of excitations (NEX) = 2, acquisition time of 4 minutes 17 seconds to 7 minutes 6 seconds, and a flip angle of 15°. With a bandwidth of 41 kHz the four TEs were obtained at 6.75, 9.45, 12.15, and 14.85 ms. Axial T2WFSE images were obtained with TR of 3000–5300 ms, TE of 120 ms, FOV of 20 × 20, imaging matrix of 448 × 224, NEX = 3, bandwidth of 50 kHz, acquisition time of 3.4–5.2 minutes, and flip angle of 90°. Each sequence had 3.0 mm section thickness and 1.0 mm section gap. Equal numbers of images at the same levels were obtained with each sequence. To minimize cerebrospinal fluid (CSF) pulsation, flow compensation was used for both imaging modalities. Post-contrast T1-weighted imaging was also performed as a standard imaging practice, but not used as a reference in the comparison between MERGE and T2WFSE imaging.
The qualitative analysis was performed by a neuroradiologist and a radiologist experienced at reading cervical spine MR imaging. The radiologists reached a consensus regarding the presence and number of lesions. To minimize the visual misjudgment in detecting lesions, we varied window widths and levels for each sequence to obtain the best depiction of the lesions. The window width is the range of pixel values displayed across an image and the level is the central window width value. Blinding to image type (MERGE or T2WFSE) could not be performed because the radiologists could easily distinguish the imaging sequence. We added 10 normal controls to the qualitative analysis to evaluate whether the MERGE technique may cause false-positive lesions in the spinal cord. All images from both patients and controls were randomly blended for reviewing.
The MERGE images and T2WFSE images were separately evaluated regarding the presence or absence of cervical spinal cord lesions. The number and location of lesions depicted with each image modality were documented. Then both image modalities were re-evaluated side-by-side to compare lesion detection on a lesion-by-lesion basis.
The lesion conspicuity was compared between the two image modalities. The lesions were categorized into three groups: MERGE images were superior to T2WFSE images; MERGE images were equal to T2WFSE images; and MERGE images were inferior to T2WFSE images. Statistical significance was determined with the sign test. A significant difference was accepted if the P value was less than 0.05.
The quantitative criterion used for comparing MERGE imaging with T2WFSE imaging was the lesion-to-background contrast-to-noise ratio (CNR), which was calculated according to the following formula: CNR = (Slesion–Sbackground)/SDnoise, where Slesion is the signal intensity of the lesion, Sbackground is the signal intensity of the spinal cord surrounding the lesion, and SDnoise is the standard deviation of the image noise measured on the image along the phase-encoding direction in an area outside the cervical spine. The areas of the regions of interest drawn in the lesions ranged from 5 to 25 (mean = 11) mm2 depending on lesion size. The statistical significance of the quantitative data was determined with the paired t-test. A significant difference was accepted if the P value was less than 0.05.
Results
Cervical spinal cord lesions were found on MERGE images and/or T2WFSE images in 17 of 19 cases. When separately evaluated, 79 lesions were found on MERGE images and 32 on T2WFSE images. On side-by-side comparison, 14 more lesions were found on T2WFSE images, but no more lesions were found on MERGE images. MERGE images showed 79 lesions and missed 1 that was depicted on T2WFSE imaging. T2WFSE images showed 46 lesions and missed 34 that were depicted on MERGE images (Fig. 1). A total of 45 lesions were found on both MERGE and T2WFSE images. No abnormal spinal cord signal, which could be confused with an MS lesion, was found in the 10 normal controls. Motion artifact obscured evaluation at one level for one of the normal controls.
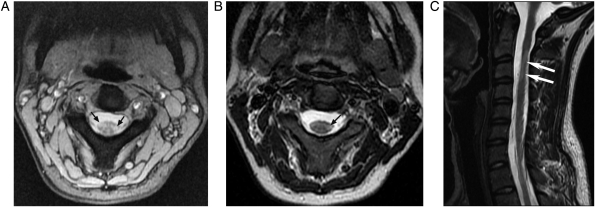
Figure 1.
(A) Axial MERGE, (B) axial T2WFSE, and (C) sagittal T2WFSE images of the cervical cord in a 27-year-old woman with relapsing–remitting MS. Two lesions at the C2–C3 level are depicted in (A) (arrows). Only one lesion at the C2–C3 level is visible in (B) (arrow). The lesion on the left side is depicted at the C2–C3 level in (C) (arrows). The lesion on the right side is not visible in sagittal T2WFSE images (not shown).
Among the 14 lesions that were initially missed on the T2WFSE images, 4 lesions were just slightly hyperintense (Fig. 1) and 2 lesions were obscured by CSF flow artifact. In the remaining eight lesions, both slight hyperintensity and CSF flow artifact were the reasons for false-negative results when T2WFSE images were evaluated alone. No flow artifact was seen on the MERGE images in the 10 lesions affected by CSF flow artifact on the T2WFSE images (Fig. 2). The lesion missed on MERGE imaging but depicted on T2WFSE imaging was obscured on MERGE imaging by patient movement artifact.
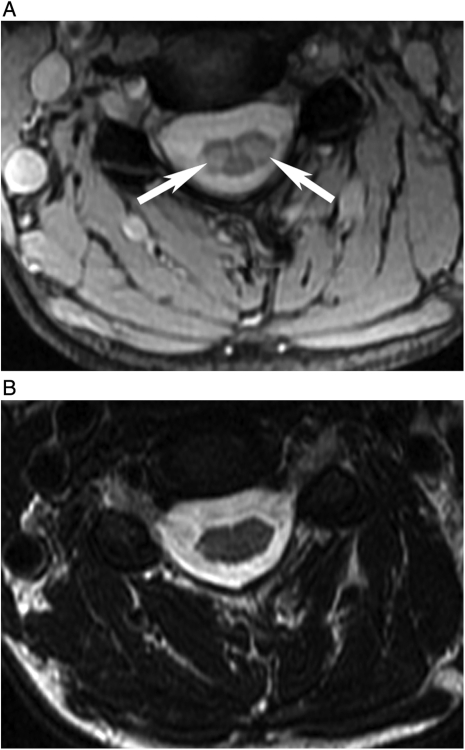
Figure 2.
(A) MERGE and (B) T2WFSE images of the cervical cord at the C4–C5 level in a 44-year-old woman with relapsing–remitting MS. Two lesions are depicted in (A) (arrows). CSF flow artifact is seen in (B) and the lesions are not visible.
For the qualitative comparison of lesion conspicuity, MERGE images were superior to the T2WFSE images in 41 lesions (Fig. 3) and MERGE images were identical to T2WFSE images in 4 lesions (P < 0.001). No lesion was more conspicuous on T2WFSE images than on MERGE images.
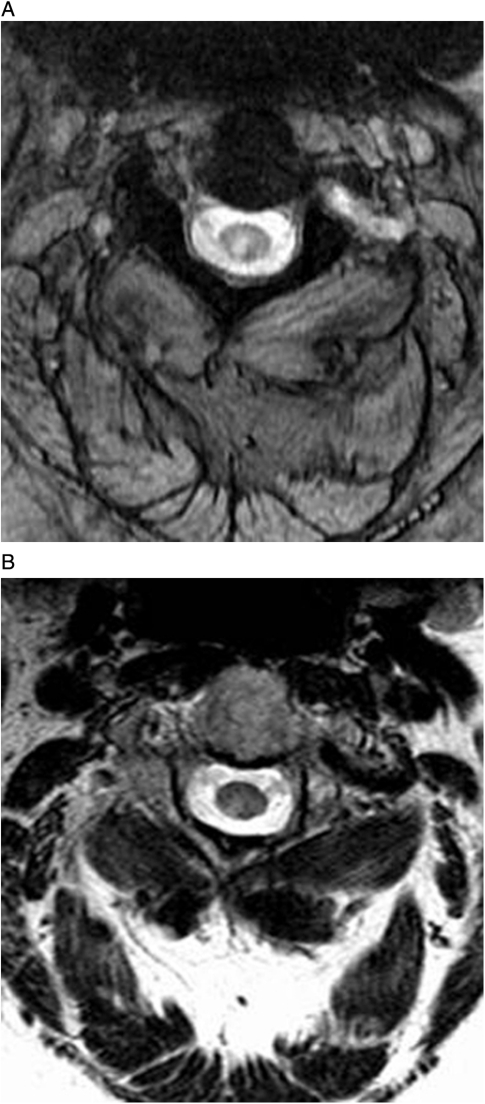
Figure 3.
(A) MERGE and (B) T2WFSE images of the cervical cord at the C2–C3 level in a 47-year-old woman with secondary progressive MS. The lesion is depicted in both images, but more conspicuously in (A).
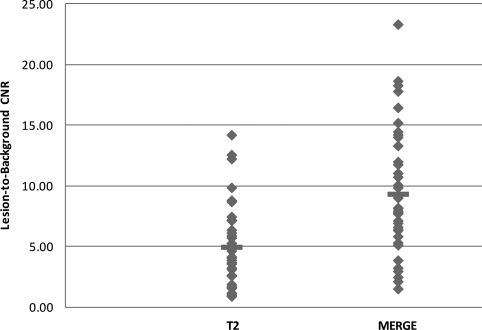
Among the 45 lesions found on both MERGE and T2WFSE images, the lesion-to-background CNR was calculated for 38 lesions. Four lesions occupied nearly the whole spinal cord on the axial section and the spinal cord surrounding the lesions was too small to have the signal intensity measured. In the remaining three lesions, signal intensity could not be accurately measured due to either a strong CSF flow artifact on T2WFSE images (two lesions) or a gradient artifact on MERGE images (one lesion). The lesion-to-background CNR on the MERGE images ranged from 1.47 to 23.27 (mean ± SD, 9.3 ± 5.3). The lesion-to-background CNR on the T2WFSE images ranged from 0.86 to 14.16 (mean ± SD, 4.9 ± 3.3). There was a significant difference between the two, with P < 0.001 (Fig. 4).
Figure 4.
Graph shows a comparison of the lesion-to-background CNR between MERGE (1.47–23.27) and T2WFSE (0.86–14.16) images. The difference between the means is statistically significant (P < 0.001). The horizontal lines indicate the mean CNR in each group.
Discussion
The spinal cord is frequently involved in MS and imaging the spinal cord is often an essential element in the diagnosis of MS.14 T2-weighted imaging can detect cord lesions in as many as 90% of MS patients.3,15–18 However, low contrast of lesions to the surrounding spinal cord may result in small lesions being missed. Rocca et al. found that 10 of 83 lesions were false negative on FSE sequences because of having only slight hyperintensity.19 In our study, 12 slightly hyperintense lesions were missed when T2WFSE images were evaluated without referring to MERGE images, but were seen on the MERGE images. No lesions that would be mistaken for MS spinal cord lesions were identified on the normal control MERGE images. These findings indicate the superiority of the MERGE sequence.
Among the 45 lesions found on both the MERGE and T2WFSE images, 41 lesions were more conspicuous on the MERGE images. In contrast, no lesion was found to be more conspicuous on T2WFSE images than on MERGE images. Quantitatively, the lesion-to-background CNR was significantly higher on the MERGE images than on the T2WFSE images.
The spinal cord is a small and mobile structure.20 Detection of spinal cord lesions using MR imaging represents a problem related to the challenge of spatial resolution and suboptimal contrast. The MERGE technique is a gradient-echo method with multiple bipolar gradient-echo formations that combines the signal from the individual echoes. The early echoes provide increased signal-to-noise ratio, while later echoes boost contrast.21 The TEs utilized on the T2WFSE sequence are longer than on the MERGE sequence. The longer TEs on the T2WFSE sequence contribute to greater phase encoding artifact (image distortion) from CSF flow effects. By contrast, the shorter TEs on the MERGE sequence limit phase errors, reducing the image distortion from CSF flow.13 These factors likely contribute toward MERGE imaging increasing the conspicuity of lesions to the surrounding spinal cord, and this is extraordinarily important for detecting MS lesions in the spinal cord.
CSF flow artifact on the FSE sequence causes subtle changes around and in the spinal cord that causes lesions to be missed.19,22,23 When the T2WFSE images were evaluated without referring to the MERGE images, 10 lesions were missed owing (or partly owing) to the effect of the CSF flow artifact. In contrast, all of these 10 lesions were depicted on the MERGE images in which there was no obvious CSF flow artifact. A plausible reason for the reduced CSF artifacts in MERGE is its shorter echo times.13,24
Our results demonstrate that MERGE imaging is superior to T2WFSE imaging for the detection of MS lesions in the cervical spine. On comparison of the MERGE images with the T2WFSE images, more lesions were found on the T2WFSE images. However, more lesions were not found on the MERGE images in the case when T2WFSE images were used as a reference. In addition, the MERGE images only missed one lesion that was shown on the T2WFSE images. In contrast, T2WFSE images missed 34 that were depicted on MERGE images. Like the FSE sequence, the MERGE sequence has a short acquisition time and the whole length of the cervical spinal cord can be scanned within a reasonable time (usually 5–6 minutes). The findings suggest that for the detection of MS lesions, MERGE should replace the T2WFSE, but further studies from different facilities would help confirm the clinical role of MERGE in evaluating MS.
A limitation of this study is that for the MERGE sequence the TRs were automatically selected by the MR sequence based on the number of slices needed for imaging the cervical spinal cord. Therefore, there was a variable range of TRs in the MERGE sequence. The TR of the T2WFSE sequence was varied by the technologist as per standard practice to optimize coverage without markedly changing the imaging time. Another limitation is the sample size of this study. The number of patients and controls were not large. In addition, MR imaging was performed only at 3 T, and the potential of MERGE imaging for depicting MS lesions at 1.5 T is unclear.
In this retrospective study, we were not able to compare MERGE with the fast short-inversion-time inversion recovery (STIR) sequence that was not included in our standard imaging routine. A study has indicated that fast STIR sequence reveals more cervical cord MS lesions than the FSE sequence.19 Moreover, magnetization transfer and diffusion tensor imaging have been applied for assessing spinal cord damage in MS.19,25 Further study is needed to compare the MERGE sequence with these imaging techniques.
Conclusion
Our results for patients with MS have shown that MERGE images are significantly superior to T2WFSE images in detecting cervical spinal cord lesions and rendering greater lesion conspicuity. We believe that MERGE images should be included in the routine MR examination for the assessment of spinal cord damage in patients with MS at 3 T.
References
- 1.Grossman RI, Barkhof F, Filippi M. Assessment of spinal cord damage in MS using MRI. J Neurol Sci 2000;172Suppl 1:S36–9 [DOI] [PubMed] [Google Scholar]
- 2.Simon JH. The contribution of spinal cord MRI to the diagnosis and differential diagnosis of multiple sclerosis. J Neurol Sci 2000;172Suppl 1:S32–5 Review [DOI] [PubMed] [Google Scholar]
- 3.Honig LS, Sheremata WA. Magnetic resonance imaging of spinal cord lesions in multiple sclerosis. J Neurol Neurosurg Psychiatry 1989;52(4):459–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorpe JW, Kidd D, Moseley IF, et al. Spinal MRI in patients with suspected multiple sclerosis and negative brain MRI. Brain 1996;119(Pt 3):709–14 [DOI] [PubMed] [Google Scholar]
- 5.Munoz DG, Hastak SM, Harper B, Lee D, Hachinski VC. Pathologic correlates of increased signals of the centrum ovale on magnetic resonance imaging. Arch Neurol 1993;50(5):492–7 [DOI] [PubMed] [Google Scholar]
- 6.Kirkpatrick JB, Hayman LA. White-matter lesions in MR imaging of clinically healthy brains of elderly subjects: possible pathologic basis. Radiology 1987;162(2):509–11 [DOI] [PubMed] [Google Scholar]
- 7.Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 1993;43(9):1683–9 [DOI] [PubMed] [Google Scholar]
- 8.Bot JC, Barkhof F, Lycklama à Nijeholt G, et al. Differentiation of multiple sclerosis from other inflammatory disorders and cerebrovascular disease: value of spinal MR imaging. Radiology 2002;223(1):46–56 [DOI] [PubMed] [Google Scholar]
- 9.Wiebe S, Lee DH, Karlik SJ, et al. Serial cranial and spinal cord magnetic resonance imaging in multiple sclerosis. Ann Neurol 1992;32(5):643–50 [DOI] [PubMed] [Google Scholar]
- 10.Turano G, Jones SJ, Miller DH, Du Boulay GH, Kakigi R, McDonald WI. Correlation of SEP abnormalities with brain and cervical cord MRI in multiple sclerosis. Brain 1991;114(Pt 1B):663–81 [DOI] [PubMed] [Google Scholar]
- 11.Trop I, Bourgouin PM, Lapierre Y, et al. Multiple sclerosis of the spinal cord: diagnosis and follow-up with contrast-enhanced MR and correlation with clinical activity. AJNR Am J Neuroradiol 1998;19(6):1025–33 [PMC free article] [PubMed] [Google Scholar]
- 12.Held P, Dorenbeck U, Seitz J, Fründ R, Albrich H. MRI of the abnormal cervical spinal cord using 2D spoiled gradient echo multiecho sequence (MEDIC) with magnetization transfer saturation pulse. A T2* weighted feasibility study. J Neuroradiol 2003;30(2):83–90 [PubMed] [Google Scholar]
- 13.Katz BH, Quencer RM, Hinks RS. Comparison of gradient-recalled-echo and T2-weighted spin-echo pulse sequences in intramedullary spinal lesions. AJNR Am J Neuroradiol 1989;10(4):815–22 [PMC free article] [PubMed] [Google Scholar]
- 14.Ikuta F, Zimmerman HM. Distribution of plaques in seventy autopsy cases of multiple sclerosis in the United States. Neurology 1976;26(6 Pt 2):26–8 [DOI] [PubMed] [Google Scholar]
- 15.Maravilla KR, Weinreb JC, Suss R, Nunnally RL. Magnetic resonance demonstration of multiple sclerosis plaques in the cervical cord. AJR Am J Roentgenol 1985;144(2):381–5 [DOI] [PubMed] [Google Scholar]
- 16.Kidd D, Thorpe JW, Thompson AJ, et al. Spinal cord MRI using multi-array coils and fast spin echo. II. Findings in multiple sclerosis. Neurology 1993;43(12):2632–7 [DOI] [PubMed] [Google Scholar]
- 17.Tartaglino LM, Friedman DP, Flanders AE, Lublin FD, Knobler RL, Liem M. Multiple sclerosis in the spinal cord: MR appearance and correlation with clinical parameters. Radiology 1995;195(3):725–32 [DOI] [PubMed] [Google Scholar]
- 18.Nijeholt GJ, van Walderveen MA, Castelijns JA, et al. Brain and spinal cord abnormalities in multiple sclerosis. Correlation between MRI parameters, clinical subtypes and symptoms. Brain 1998;121(Pt 4):687–97 [DOI] [PubMed] [Google Scholar]
- 19.Rocca MA, Mastronardo G, Horsfield MA, et al. Comparison of three MR sequences for the detection of cervical cord lesions in patients with multiple sclerosis. AJNR Am J Neuroradiol 1999;20(9):1710–6 [PMC free article] [PubMed] [Google Scholar]
- 20.Mikulis DJ, Wood ML, Zerdoner OA, Poncelet BP. Oscillatory motion of the normal cervical spinal cord. Radiology 1994;192(1):117–21 [DOI] [PubMed] [Google Scholar]
- 21.Vertinsky AT, Krasnokutsky MV, Augustin M, Bammer R. Cutting-edge imaging of the spine. Neuroimaging Clin N Am 2007;17(1):117–36 Review [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hittmair K, Mallek R, Prayer D, Schindler EG, Kollegger H. Spinal cord lesions in patients with multiple sclerosis: comparison of MR pulse sequences. AJNR Am J Neuroradiol 1996;17(8):1555–65 [PMC free article] [PubMed] [Google Scholar]
- 23.Bianco F. Is fast spin-echo superior to gradient-echo imaging in detecting spinal cord lesions…or not? AJNR Am J Neuroradiol 1996;17(1):194. [PMC free article] [PubMed] [Google Scholar]
- 24.Lycklama G, Thompson A, Filippi M, et al. Spinal-cord MRI in multiple sclerosis. Lancet Neurol 2003;2(9):555–62 [DOI] [PubMed] [Google Scholar]
- 25.van Hecke W, Nagels G, Emonds G, et al. A diffusion tensor imaging group study of the spinal cord in multiple sclerosis patients with and without T2 spinal cord lesions. J Magn Reson Imaging 2009;30(1):25–34 [DOI] [PubMed] [Google Scholar]