Abstract
Background/objective
Persons with spinal cord injury (SCI) develop marked bone loss from paralysis and immobilization. Low-intensity vibration (LIV) has shown to be associated with improvement in bone mineral density in post-menopausal women and children with cerebral palsy. We investigated the transmissibility of LIV through the axial skeleton of persons with SCI as an initial approach to determine whether LIV may be used as a clinical modality to preserve skeletal integrity.
Methods
Transmission of a plantar-based LIV signal (0.27 ± 0.11 g; 34 Hz) from the feet through the axial skeleton was evaluated as a function of tilt-table angle (15, 30, and 45°) in seven non-ambulatory subjects with SCI and ten able-bodied controls. Three SCI and five control subjects were also tested at 0.44 ± 0.18 g and 34 Hz. Transmission was measured using accelerometers affixed to a bite-bar to determine the percentage of LIV signal transmitted through the body.
Results
The SCI group transmitted 25, 34, and 43% of the LIV signal, and the control group transmitted 28, 45, and 57% to the cranium at tilt angles of 15, 30, and 45°, respectively. No significant differences were noted between groups at any of the three angles of tilt.
Conclusion
SCI and control groups demonstrated equivalent transmission of LIV, with greater signal transmission observed at steeper angles of tilt. This work supports the possibility of the utility of LIV as a means to deliver mechanical signals in a form of therapeutic intervention to prevent/reverse skeletal fragility in the SCI population.
Keywords: Vibration, Osteoporosis, Spinal cord injuries, Immobilization, Paralysis, Bone loss, Tetraplegia, Paraplegia
Introduction
Osteoporosis below the level of lesion is a major secondary complication of spinal cord injury (SCI). Bone mineral density (BMD) of the long bones may fall below the fracture threshold 1–5 years after acute injury.1 The loss of bone in individuals with SCI is associated with the degree of unloading,2,3 hormonal changes,2,4–6 and duration of injury.3,5,7 The prevalence of fractures is reported to be as high as 21% in the SCI population, and these fractures occur even with minor stress or trauma.4,8,9
Currently, there are limited efficacious, easy-to-apply, non-pharmacological methods to prevent bone loss in persons with SCI. Pharmacological approaches to prevent bone loss have not been generally accepted as efficacious, and they are not routinely administered.10,11 However, low-magnitude mechanical signals of low-intensity vibration (LIV) delivered by an oscillating platform have been shown to be safe,12–15 easy to administer, and anabolic to bone in both animal16–19 and human studies.14,20 This preliminary evidence, although not yet studied in persons with SCI, has demonstrated efficacy in reducing or preventing bone loss in persons with low bone density, as reported in postmenopausal women14 and children with cerebral palsy.20 One of the major potential difficulties of utilizing this intervention is that most individuals with SCI are only able to stand on a vibrating plate with assistive adaptations. As such, these devices used to assist passive standing or in a supine tilt may diminish the transmission of plantar-based signals to the axial skeleton.
If persons with SCI could have LIV efficiently transmitted through the skeleton, then this intervention could be tested for efficacy and potentially be used as a non-pharmacological treatment to prevent bone loss after acute paralysis and immobilization. Because of the technical difficulty of achieving upright posture in persons with SCI, which may be especially the case in those with acute/subacute SCI, delivering LIV in a supine position at various angles of supine tilt appears to offer a practical alternative to mechanically stimulate the skeletal system. The present study investigated the feasibility of providing LIV through the lower appendicular and axial skeleton in persons with SCI. The specific goal of this study was to determine the percent transmission of low-magnitude vibratory signals that were delivered at the foot and measured at the mouth as a function of the degree of tilt, using a standard tilt table.
Methods
The study was reviewed and approved by the institutional review board of our medical center. Subjects who agreed to participate in this study provided written informed consent before performing any of the experimental procedures.
Subjects
Eighteen male subjects participated in the study: eight subjects with SCI and ten able-bodied controls. The SCI group consisted of three subjects with paraplegia and five subjects with tetraplegia [five motor complete (ASIA impairment scale or AIS A and B); three motor incomplete (AIS C)]. All SCI subjects were wheelchair reliant; minor volitional leg movement did not exclude subjects from participation in the study. Persons were excluded from the study if they had a history of severe underlying chronic illness, flexion contractures of the lower extremities, femur or tibia fracture, prior bone disease, alcoholism, seizure disorders, pressure ulcers, implanted rods placed between two or more vertebral segments, pacemakers, implanted cardiac defibrillators, or any other electrical cardiac device. The demographics of the participants are provided (Table 1).
Table 1.
Subject characteristics
| Control | SCI | Difference | P value | |
|---|---|---|---|---|
| Count (n) | 10 | 7 | — | — |
| Age (year) | 34 ± 11 | 46 ± 13 | 12 | 0.05* |
| Weight (kg) | 77 ± 8 | 85 ± 19 | 8 | 0.24 |
| Height (m) | 1.74 ± 0.05 | 1.73 ± 0.14 | 0.01 | 0.91 |
| BMI (m/kg2) | 26 ± 2 | 28 ± 4 | 2.7 | 0.11 |
| DOI (year) | n/a | 15 ± 12 | — | — |
BMI = body mass index; DOI = duration of injury.
Values are expressed as mean ± SD.
*Represents significant difference.
Administration of LIV
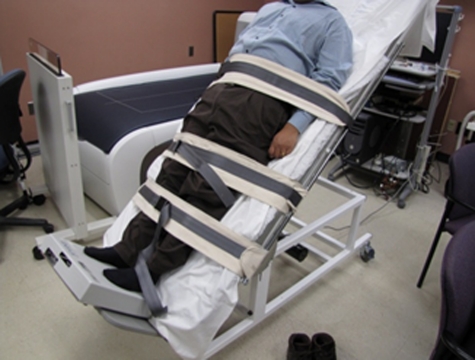
LIV was administered to subjects while lying supine on a tilt table at angles of 15, 30, and 45° (Fig. 1). LIV was delivered using a small oscillating plate, with acceleration fidelity controlled through closed-loop feedback (Modified research device, Juvent Medical, Somerset, NJ). Loading was performed with the subjects in stocking feet flat on the vibrating plate with their legs straight and securely strapped to the tilt table to prevent buckling at higher angles of tilt. Subjects were weighed at each angle of tilt by placing a platform scale beneath the feet of each subject. The tilt table was initially raised to an angle of 45° with a technician assisting each subject onto the scale to ensure that maximal load was achieved; each subject's total weight loaded was recorded at 45, 30, and 15° of tilt as the tilt of the table was reduced back to 0°. The platform scale was then replaced by the LIV platform, and then each subject was loaded in a similar manner as previously described with the platform scale. The percent of plantar-based vibrations that passed through the axial skeleton was measured by clenching down on a bite-bar instrumented with an accelerometer (CXL10HF3, Crossbow Technology, San Jose, CA). The use of a bite-bar to measure vibrations at the head has been reported previously.21
Figure 1.
The setup of a supine study subject tilted to 45° and loaded onto a vibration plate. The vibrating plate is located on the foot rest of the tilt table. The straps to secure the subject have been placed in three locations: below the patella, above the patella, and across the trunk.
LIV was administered at a frequency of 34 Hz and a peak-to-peak (P2P) acceleration of 0.27 ± 11 g (reference: the earth's gravitational field is 1 g or 9.8 m/s2). A subgroup of five SCI and three control subjects participated in additional trials with a higher P2P acceleration of 0.44 ± 0.18 g. Three 30-second measurements were recorded at each angle of tilt using two tri-axial accelerometers: one accelerometer was placed directly on the LIV platform and secured with an adhesive strip and the other was affixed to a bite-bar and held in the mouth. Accelerometer data were recorded at 1000 Hz using a 16-bit data acquisition system.
Outcome measurements
The three vectors representing the axes of movement from both accelerometers were filtered with a fourth-order band-pass Butterworth filter (15–45 Hz). The three vectors of each accelerometer were combined, resulting in one vector for each accelerometer. The two resultant vectors were assumed to be aligned to the direction of the acceleration applied by the LIV platform. This assumption was made because it was not possible to align the axis of the accelerometer at the vibrating plate to the axis of the bite-bar. The resultant vectors were then used to determine the signal transmission through the skeleton.
The determination of transmission of the LIV signal from the foot to the head was achieved by calculating the P2P acceleration of the resultant signals. This was performed by calculating the root mean square (RMS) acceleration and, because the signal was sinusoidal, it was converted into P2P accelerations using the equation P2P = RMS × (2√2). The amount of transmission (P2P%Transmission) was determined as a percent of the signal at the mouth (P2Phead) compared to that administered at the vibration plate (P2Pvib), using the equation
P2P%Transmission=(P2Phead/P2Pvib) × 100. The difference in P2P%Transmission between the control and SCI groups permitted calculation of group differences in transmission of the LIV signal for each angle of tilt and for each of the two LIV intensities.
Statistical analysis
The descriptive statistics (means ± standard deviations) are provided (Table 2) for the P2P%Transmission values obtained for the different angles of tilt and LIV intensities for the vibrating plate. Unpaired t-tests were performed to determine the significance of group differences for the demographic variables of age, height, weight, BMI, and percent body weight loaded at the different angles of tilt. A stepwise regression analysis was used to determine the variables that were significant contributors to the transmission of vibrations. The dependent variable was P2P%Transmission. Three independent variables were used: (1) group, contrast coded where the control group was equal to 1 and the SCI group to −1; (2) %BWT, which was the percent of total body weight loaded on the LIV platform; and (3) intensity, which was the P2P acceleration delivered at 0.27 or 0.44 g. Correlation coefficients were used to determine associations among intensity level (0.27 and 0.44 g), tilt angle (15, 30, and 45°), age, height, weight, and BMI with P2P%Transmission. To determine how the groups co-varied on age, height, weight, and/or BMI for percent transmission, analyses of covariance and multiple regression models were developed to control for these variables (covariates) for differences between the groups for percent transmission at each intensity and tilt angle [e.g. Y = % transmission; X1 = weight, X2 = group (contrast coded −1 for SCI and 1 for control as previously described)]. Paired t-tests were used to determine within group differences for percent body weight loaded for each angle of tilt.
Table 2.
Descriptive statistics for the intensity of signal transmission at each angle of tilt
| Tilt angle (deg) | Intensity (g) | Control (%) | SCI (%) | Diff. | P value |
|---|---|---|---|---|---|
| 15 | 0.27 | 28.22 ± 14.62 | 25.25 ± 10.10 | 2.97 | 0.649 |
| 30 | 0.27 | 45.34 ± 22.32 | 34.12 ± 19.00 | 11.22 | 0.296 |
| 45 | 0.27 | 57.49 ± 21.07 | 42.83 ± 25.97 | 14.66 | 0.218 |
| 15 | 0.44 | 24.40 ± 12.91 | 17.56 ± 2.55 | 6.83 | 0.412 |
| 30 | 0.44 | 40.41 ± 19.82 | 23.52 ± 3.62 | 16.89 | 0.206 |
| 45 | 0.44 | 59.45 ± 20.82 | 32.63 ± 2.65 | 26.82 | 0.075 |
Values are expressed as mean ± SD.
Results
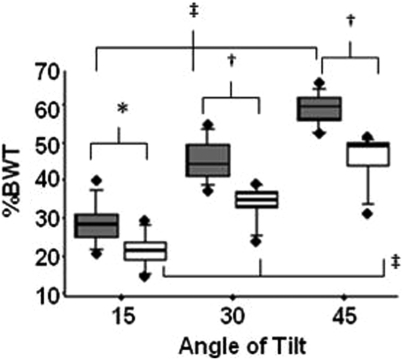
Subjects in the SCI group were older than those in the control group (46 ± 13 versus 34 ± 11, years, P < 0.05); no significant differences were noted between the groups for weight, height, or BMI (Table 1). There were no increases in muscle spasms noted in the SCI group during the intervention. The percent body weight loaded on the vibrating plate was significantly lower in the SCI group at all angles of tilt, despite employing similar procedures for all subjects (Fig. 2). Percent body weight for the control and SCI groups at 15° of tilt was 29 ± 6 and 22 ± 5% (P = 0.015); at 30° of tilt was 46 ± 5 and 35 ± 5% (P < 0.001); and at 45° of tilt was 60 ± 4 and 46 ± 7% (P < 0.001). Both groups significantly increased the percent of body weight loaded for increasing angles of tilt (P < 0.0001).
Figure 2.
The box plots of the percent of total body weight at the foot as a function of angle of tilt of the tilt table for SCI (open) and control (shaded) subjects. Each horizontal line in plot for control and SCI subjects represents a percentile [from bottom to the top: 10th, 25th, 50th, 75th, and 90th percentile for the percent of total body weight (%BWT); the box represents the 25th to the 75th percentiles]. There are significant differences noted between the median values (i.e., middle horizontal line) for the SCI group and the control group (15°: 22.2 ± 4.5 versus 29.4 ± 5.7, P = 0.015; 30°: 34.6 ± 5.0 versus 45.9 ± 5.5, P < 0.001; 45° 46.4 ± 6.9 versus 59.5 ± 4.5, P < 0.001). The solid circles (•) represent outliers. Significant differences between SCI and control groups are represented by *P < 0.05 and † < 0.001. Significant differences within SCI and control groups are represented by ‡P < 0.0001.
Three data sets from one SCI subject with the P2P acceleration at 0.27 g were removed from analysis because these data contained excessive ‘noise’ of an undetermined etiology. After removing this subject's data from analysis, seven participants remained in the SCI group and ten in the control group. All data sets were used in analysis of the trials at the higher LIV intensity of 0.44 g; thus, five participants in the control group and three in the SCI group performed the higher-LIV-intensity trials.
The P2P accelerations recorded at the level of the plate were 0.27 ± 0.11 g at the low-LIV setting and 0.44 ± 0.18 g for the high-LIV setting. The results of transmission for SCI and control groups at the different angles of tilt and two intensities are provided (Table 2). Although the SCI group had significantly lower percent body weight loaded on the plate at each angle of tilt, there was no significant difference between groups in any tilt angle for the percent transmission. In the SCI group, age, height, weight, and BMI were not shown to be related to percent transmission for any angle of tilt. In the control group, at the lower intensity of 0.27 g, weight was found to be significantly inversely related to percent transmission at 15° (r2 = 0.49, P = 0.02), with a trend at 30° (r2 = 0.25, P = 0.15), and not significantly related at 45°(r2 = 0.05, P = 0.54). In a subset of five subjects in the control group at the higher intensity of 0.44 g, at all three tilt angles weight was found to be significantly inversely related to the percent transmission (15°, r2 = 0.88, P = 0.0148; 30°, r2 = 0.80, P = 0.04; and 45°, r2 = 0.94, P < 0.005). Large variability was observed in the signal transmission for both groups at both intensities, with standard deviations as great as 26%.
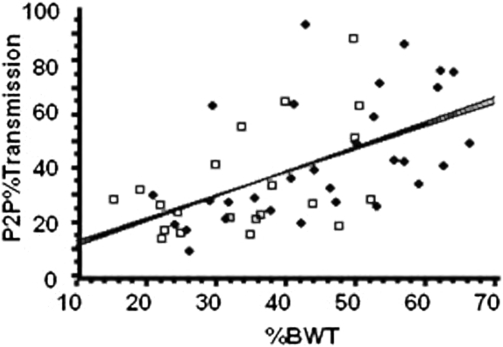
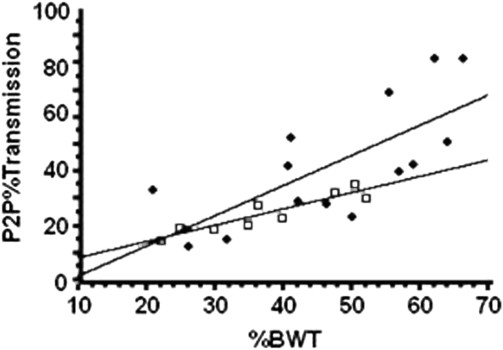
The results from the regression model for the P2P%Transmission are provided (Figs. 3 and 4); an increase in transmission was demonstrated as the percent of body weight increased, irrespective of group membership. The slopes of the regression lines associated with the increase in transmission are 0.923 for the control group and 0.861 for SCI group, during the lower-acceleration (0.27 g) trials, without significant difference between groups (P = 0.98). The slopes of the regression lines at the higher accelerations (0.44 g) were 1.108 for the control group and 0.600 for the SCI group, suggesting a trend for greater percent transmission for the control group than the SCI group (P = 0.1468). However, there was a significant increase in transmission represented by the slopes during the lower intensity (control, P < 0.01; SCI, P < 0.05) and the higher intensity (control, P < 0.05; SCI, P < 0.001).
Figure 3.
The P2P%Transmission for control (solid circles) and SCI (open squares) subjects for the vibrating plate delivering 0.27 g at 34 Hz. P2P%Transmission is the percent of the vibration signal detected at the mouth. %BWT is the percent of body weight loaded on the vibrating plate. The slopes of the regression lines associated with transmission are 0.923 for the control group and 0.861 for the SCI group, which are not significantly different (P = 0.98); there is an increase in transmission with increase %BWT for control (P < 0.01) and SCI (P < 0.05) groups.
Figure 4.
The P2P%Transmission for control (closed circles) and SCI (open squares) subjects for the vibrating plate delivering 0.44 g at 34 Hz. P2P%Transmission is the percent of the vibration signal detected at the mouth. %BWT is the percent of body weight loaded on the vibrating plate. The slopes of the regression lines associated with transmission are 1.108 for the control group and 0.600 for the SCI group, which are not significantly different (P = 0.1468); there is an increase in transmission with increase %BWT for control (P < 0.05) and SCI (P < 0.001) groups.
Discussion
Our study was able to demonstrate the feasibility of delivering LIV through the weight-bearing skeleton of persons with SCI. There was no statistical difference in the transmission of LIV between groups for any angle of tilt, despite significantly lower body weight loaded on the vibrating plate for any angle. SCI and control groups showed an increase in transmission as weight-bearing increased for both LIV intensities with increasing angles of tilt. This indicates that with higher loads obtained with higher degrees of tilt, a greater percent of vibration was transmitted through the body. Thus, the inference that may be made is that increases in transmissibility with steeper angles of tilt may be expected to have a greater therapeutic effect on the long bones of the leg. This report has provided the basis for continued investigation that should focus on the efficacy of the application of LIV in the prevention and/or recovery of bone loss in persons with SCI.
The SCI group demonstrated a lower load at all angles of tilt, which we believe was due, in part, to support from the restraining straps placed to prevent the knees from buckling at increased angles of tilt. The lack of volitional muscle function in the SCI group would result in off-loading a portion of their body weight onto the straps, whereas subjects in the control group had the straps in place but the straps were not required to keep their legs straight and, thus, their weight was more fully loaded on the platform. Furthermore, it is conceivable that the restraining straps may have been placed at differing degrees of tightness, which may have added an additional and variable force that prevented the SCI subjects from fully loading onto the LIV platform. The slightly lower mechanical load applied to those with SCI may not have been sufficient to preclude a therapeutic effect because subjects still had significant increases in load as the angle of tilt increased, with associated increases in transmission of the LIV signal.
Both groups showed high variability in vibration transmission. The cause of this variability in the SCI group had no apparent relationship to age, total body weight, height, BMI, and/or percent of body weight loaded at the different angles of tilt. In the control group, an inverse relationship was observed with increased total body weight associated with reduced signal transmission; none of the other parameters were found to contribute significantly to enhancing, or compromising, transmissibility. Although an effort was made to keep the legs fully extended, the variability in both groups could have been due to slight degrees of flexion at the knee. Albeit this possibility was not addressed in our study, one report had demonstrated a dampening of vibrations with knee flexion,22 and in the general population high degrees of variability in transmission of vibrations have been reported23–25; both SCI and control groups in our study demonstrated large variability of transmission.
Body composition was not determined in our study, but it is appreciated that persons with SCI have muscle atrophy and increased adiposity compared to healthy able-bodied controls for comparable BMIs.26,27 Muscle activation has been shown to increase with the onset of vibration and has been shown to dampen soft tissue resonance due to vibrations and shock waves caused at heel strike during ambulation.28–31 As such, there may have been relatively increased attenuation of the vibrations due to lean tissue in the control group compared to the SCI group. No difference in transmission was evident in persons with motor complete and incomplete lesions, nor was there a difference in transmission for those with paraplegia and tetraplegia. This lack of difference in impulse transmission may have been due to the relatively small sample size. Thus, the combination of the lack of muscle dampening of vibrations (e.g. resulting in increased transmission of signal) and the lower load observed in the SCI group (e.g. resulting in decreased transmission of signal) may have resulted in the net effect of the lack of significant difference in signal transmission between the SCI and control groups.
A limitation of the study was that it is not known which regions of the body attenuated the LIV signal; because vibratory signals reached the cranium, it can be assumed that at least an equal or greater percentage of the signal were received by the lower appendicular and axial skeleton. The assessment of the transmitted signal at a particular region of interest could be used to adjust the vibrations originating at the plate-foot junction to obtain a specific intensity at the region of interest. ‘Dosing’ of a mechanical challenge to the skeletal system may be considerably more difficult to institute or safely tolerated on a daily basis with other rehabilitative weight-bearing interventions than that of LIV.
The lower levels of mechanical signals proposed in this study as a possible intervention to prevent bone loss may be counterintuitive. As we become more knowledgeable concerning what bone is most responsive to, in terms of stimulating mechanical adaptation, it does not appear that a larger signal is necessarily better, especially when considering that larger signals are associated with an increased risk of tissue damage. Other studies that have administered LIV have been shown to be safe,12–15 and these prior reports have demonstrated potential to increase trabecular bone density and improve bone strength in animal studies,16–18,32,33 with benefits to both bone and muscle.14,18 In a rat model of disuse with constant hind limb suspension compared with normal weight-bearing animals, LIV administered at 90 Hz and 0.25 g on the hind limb suspended rats for 10 minutes a day, 5 days per week, successfully maintained normal rates of bone formation; experimental animals had suppressed bone formation rates of 72% compared to weight-bearing control animals.18 In addition, female sheep that received LIV, 20 minutes/day for 5 days/week over 1 year, showed 35% greater bone volume fraction in the distal region of the femur as compared to controls.17
The influence of LIV on the skeletal system of humans has also been examined. In children with cerebral palsy, 6 months of LIV (∼4.4 minutes administered at 90 Hz and 0.3 g for 5 days/week) increased the volumetric trabecular BMD of the proximal tibia by about 6%, while those in the group who used a placebo device lost almost 12%, representing a net benefit approaching 18%.20 In a group of 70 post-menopausal women, who were exposed to LIV administered at 30 Hz and 0.2 g for 20 minutes a day, those who maintained a compliance rate of 86%, BMD was maintained, whereas the placebo group lost 2.13% at the femoral neck.14
In a population that is at high risk for fracture, it is imperative that the stimulation be within a range that will stimulate bone ‘sufficiently’ to prevent bone loss but not reach a level that might precipitate a fracture, which a mechanically based intervention is intended to prevent.15 In our study, the LIV signal administered about one-quarter the acceleration due to gravity for the lower-intensity setting, and about one-half the acceleration due to gravity for the higher-intensity setting to the plantar surface of the foot. The P2P accelerations delivered through the appendicular and axial skeleton to the cranium ranged from 25 to 59% of that administered. Although the LIV signals examined are certainly small compared to what is experienced during strenuous activity, they are also within a range that will not precipitate fractures. LIV in sheep generated P2P strains of no more than five micro strain,34 which is far lower than the 6800 micro strain35 reported as the yield strain of bone, and yet these signals were anabolic to bone.
Further investigation of body composition and vibration attenuation should be performed in the SCI population, as has been reported in the general population.22,29–31 These interventions would be expected to reduce the prevalence of fractures, thus decreasing morbidity and reducing healthcare costs. Quality of life would be improved by increasing employability (e.g. reduction in days absent from employment and income lost) and enhancing personal activities (e.g. recreational endeavors, independence, and ease by which one performs activities of daily living). Individuals with SCI, if spared the development of osteoporosis, may then engage more securely in job-related and personal activities without fear of fracture, which would be a tremendous psychological benefit. Ultimately, it will be necessary to determine whether LIV signals delivered in the supine position has efficacy in the prevention and/or reversal of bone loss in persons with SCI, as well as other populations confined to chronic bed rest.
Conclusion
This study has demonstrated the feasibility of delivering low-intensity mechanical signals through the lower appendicular and axial skeleton to SCI and able-bodied control subjects while supine. Significant and controllable LIV signals were transmitted from the feet to the mouth, with transmissibility increasing with increasing degrees of tilt. No significant differences were observed between the SCI and control groups in the percent transmission of vibratory signal, suggesting that the weight-bearing skeleton would receive the mechanical challenges delivered by these high-frequency, low-magnitude stimuli. Similar increases in transmissibility were demonstrated in both groups as the apparent load increased at higher angles of tilt. These observations support the use of LIV as a possible non-invasive, non-pharmacological method to be studied in the prevention or reduction of bone loss in persons with SCI. Future studies are needed to investigate the effectiveness of long-term exposure to low-intensity LIV on preservation of BMD in persons with acute, subacute, or chronic SCI, and/or those immobilized and unable to weight-bear due to another etiology, resulting in long-term bed rest. Thus, studies should be performed to investigate the effects of body composition, load, frequency, intensity, and duration, on regional BMD and bone micro-architecture.
Acknowledgements
This study was funded by the Department of Veterans Affairs Rehabilitation Research & Development Center of Excellence for the Medical Consequences of Spinal Cord Injury Grant (# B2648C). Support was also provided by NIH AR 43498 (CTR).
References
- 1.Szollar S, Martin E, Sartoris D, Parthemore J, Deftos L. Bone mineral density and indexes of bone metabolism in spinal cord injury. Am J Phys Med Rehabil 1998;77(1):28–35 [DOI] [PubMed] [Google Scholar]
- 2.Jiang S, Jiang L, Dai L. Mechanisms of osteoporosis in spinal cord injury. Clin Endocrinol 2006;65(5):555–65 [DOI] [PubMed] [Google Scholar]
- 3.Bauman W, Spungen A, Wang J, Pierson R, Jr, Schwartz E. Continuous loss of bone during chronic immobilization: a monozygotic twin study. Osteoporos Int 1999;10(2):123–7 [DOI] [PubMed] [Google Scholar]
- 4.Giangregorio L, McCartney N. Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med 2006;29(5):489–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauman W, Wecht J, Kirshblum S, Spungen A, Morrison N, Cirnigliaro C, et al. Effect of pamidronate administration on bone in patients with acute spinal cord injury. J Rehabil Res Dev 2005;42(3):305–13 [DOI] [PubMed] [Google Scholar]
- 6.Bauman W, Spungen A, Wang J, Pierson R, Schwartz E. Relationship of fat mass and serum estradiol with lower extremity bone in persons with chronic spinal cord injury. Am J Physiol Endocrinol Metab 2006;290(6):1098–103 [DOI] [PubMed] [Google Scholar]
- 7.Clasey J, Janowiak A, Gater D. Relationship between regional bone density measurements and the time since injury in adults with spinal cord injuries. Arch Phys Med Rehabil 2004;85(1):59–64 [DOI] [PubMed] [Google Scholar]
- 8.Comarr A, Hutchinson R, Bors E. Extremity fractures of patients with spinal cord injuries. Am J Surg 1962;103:732–9 [DOI] [PubMed] [Google Scholar]
- 9.Ragnarsson K, Sell G. Lower extremity fractures after spinal cord injury: a retrospective study. Arch Phys Med Rehabil 1981;62(9):418–23 [PubMed] [Google Scholar]
- 10.Bryson J, Gourlay M. Bisphosphonate use in acute and chronic spinal cord injury: a systematic review. J Spinal Cord Med 2009;32(3):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashe M, Craven C, Eng J, Krassioukov A. Prevention and treatment of bone loss after a spinal cord injury: a systematic review. Top Spinal Cord Inj Rehabil 2007;13(1):123–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torvinen S, Kannus P, Sievänen H. Effect of four-month vertical whole body vibration on performance and balance. Med Sci Sports Exerc 2002;34(9):1523. [DOI] [PubMed] [Google Scholar]
- 13.Torvinen S, Kannus P, Sievänen H, Pasanen M, Kontulainen S, Nenonen A, et al. Effect of 8-month vertical whole body vibration on bone, muscle performance, and body balance: a randomized controlled study. J Bone Miner Res 2003;18(5):876–84 [DOI] [PubMed] [Google Scholar]
- 14.Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res 2004;19(3):343–51 [DOI] [PubMed] [Google Scholar]
- 15.Kiiski J, Heinonen A, Järvinen T, Kannus P, Sievänen H. Transmission of vertical whole body vibration to the human body. J Bone Miner Res 2008;23(8):1318–25 [DOI] [PubMed] [Google Scholar]
- 16.Garman R, Gaudette G, Donahue L, Rubin C, Judex S. Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J Orthop Res 2007;25(6):732–40 [DOI] [PubMed] [Google Scholar]
- 17.Rubin C, Turner A, Muller R, Mittra E, McLeod E, Lin W, et al. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J Bone Miner Res 2002;17(2):349–57 [DOI] [PubMed] [Google Scholar]
- 18.Rubin C, Xu G, Judex S. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. FASEB J 2001;15(12):2225–9 [DOI] [PubMed] [Google Scholar]
- 19.Rubin C, Turner A, Bain S, Mallinckrodt C, Mcleod K. Anabolism. Low mechanical signals strengthen long bones. Nature 2001;412(6847):603–4 [DOI] [PubMed] [Google Scholar]
- 20.Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res 2004;19(3):360–9 [DOI] [PubMed] [Google Scholar]
- 21.Paddan G, Griffin M. A review of the transmission of translational seat vibration to the head. J Sound Vib 1998;215(4):863–82 [Google Scholar]
- 22.Rubin C, Pope M, Fritton JC, Magnusson M, Hansson T, McLeod K. Transmissibility of 15-hertz to 35-hertz vibrations to the human hip and lumbar spine: determining the physiologic feasibility of delivering low-level anabolic mechanical stimuli to skeletal regions at greatest risk of fracture because of osteoporosis. Spine 2003;28(23):2621–727 [DOI] [PubMed] [Google Scholar]
- 23.Mansfield N, Griffin M. Non-linearities in apparent mass and transmissibility during exposure to whole-body vertical vibration. J Biomech 2000;33(8):933–41 [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto Y, Griffin M. Dynamic response of the standing human body exposed to vertical vibration: influence of posture and vibration magnitude. J Sound Vib 1998;212(1):85–107 [Google Scholar]
- 25.Harazin B, Grzesik J. The transmission of vertical whole-body vibration to the body segments of standing subjects. J Sound Vib 1998;215(4):775–87 [Google Scholar]
- 26.Spungen A, Adkins R, Stewart C, Wang J, Pierson R, Waters R, et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol 2003;95(6):2398–407 [DOI] [PubMed] [Google Scholar]
- 27.Spungen A, Wang J, Pierson R, Bauman W. Soft tissue body composition differences in monozygotic twins discordant for spinal cord injury. J Appl Physiol 2000;88(4):1310–5 [DOI] [PubMed] [Google Scholar]
- 28.Verschueren S, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S, et al. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Miner Res 2004;19(3):352–9 [DOI] [PubMed] [Google Scholar]
- 29.Wakeling J, Liphardt A, Nigg B. Muscle activity reduces soft-tissue resonance at heel-strike during walking. J Biomech 2003;36(12):1761–9 [DOI] [PubMed] [Google Scholar]
- 30.Cardinale M, Lim J. Electromyography activity of vastus lateralis muscle during whole-body vibrations of different frequencies. J Strength Cond Res 2003;17(3):621–4 [DOI] [PubMed] [Google Scholar]
- 31.Wakeling J, Nigg B, Rozitis A. Muscle activity damps the soft tissue resonance that occurs in response to pulsed and continuous vibrations. J Appl Physiol 2002;93(3):1093–103 [DOI] [PubMed] [Google Scholar]
- 32.Christiansen B, Silva M. The effect of varying magnitudes of whole-body vibration on several skeletal sites in mice. Ann Biomed Eng 2006;34(7):1149–56 [DOI] [PubMed] [Google Scholar]
- 33.Rubin C, Mcleod K. Promotion of bony ingrowth by frequency-specific, low-amplitude mechanical strain. Clin Orthop Relat Res 1994;(298):165–74 [PubMed] [Google Scholar]
- 34.Rubin C, Turner A, Mallinckrodt C, Jerome C, McLeod K, Bain S. Mechanical strain, induced noninvasively in the high-frequency domain, is anabolic to cancellous bone, but not cortical bone. Bone 2002;30(3):445–52 [DOI] [PubMed] [Google Scholar]
- 35.Carter D, Caler W, Spengler D, Frankel V. Fatigue behavior of adult cortical bone: the influence of mean strain and strain range. Acta Orthop 1981;52(5):481–90 [DOI] [PubMed] [Google Scholar]