Abstract
Objective
Abdominal obesity conveys substantial health risks, in association with high levels of visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT) and an increased proportion of VAT to SAT. The purposes were to determine the influence of spinal cord injury (SCI) on the associations between single axial cross-sectional area (CSA) slices and the average CSA or volumes of VAT and SAT across multi-axial slices of magnetic resonance imaging (MRI); and the relationships relative to the whole body composition and anthropometrics.
Methods
Thirteen healthy male participants with traumatic motor complete SCI underwent fast spin-echo MRI to measure VAT and SAT across multi-axial slices, followed by dual-energy X-ray absorptiometry to measure whole body fat-free mass (FFM) and fat mass (FM). Waist circumference (WC) was also measured in the seated position.
Results
The trunk CSAs of VAT and SAT were 99 ± 51 and 164 ± 69 cm2, respectively, and the ratio of VAT to SAT was 0.68 ± 0.33. The CSAs of VAT and SAT at a single slice strongly predicted the average CSA and modestly predicted the volumes across multi-axial slices. VAT and SAT represented 5.7 ± 1.8% and 9.7 ± 3.2% of the total body FM, respectively. Percent body FFM was negatively related to VAT and SAT volumes, but not to a single axial CSA.
Conclusion
A single slice CSA can modestly predict the volume of multi-axial slices in individuals with SCI, yet it is not related to any of the body composition variables. Increased percent FFM is associated with a reduction in VAT and SAT volumes measured across multi-axial slices. The ratio of VAT to SAT is greater than 0.4, suggesting that individuals with SCI are at high risk of developing metabolic sequelae.
Keywords: Spinal cord injuries, Body composition, MRI, DXA, Subcutaneous and visceral adipose tissue, Obesity
Introduction
Spinal cord injury (SCI) is a devastating medical condition that affects approximately 10 000–11 000 individuals on an annual basis.1 It is widely accepted that there is a decline in fat-free mass (FFM) associated with skeletal muscle atrophy and increase in fat mass (FM) after SCI.2–5 Individuals with SCI have total body FM that exceeds 27%,4–7 and when compared to age and body mass index (BMI)-matched able-bodied (AB) controls, they have 1.13-fold more fat per unit of BMI.5 Altered body composition after SCI is associated with impaired glucose tolerance,8,9 insulin resistance,10–12 dyslipidemia,10,13 osteoporosis,14 metabolic syndrome,15 and consequently, cardiovascular disease.4–16 These pathological adaptations contribute to the increased socioeconomic burden, decreased quality of life and shortened life expectancy after SCI.17 Therefore, studying the factors that may lead to these metabolic sequelae is of paramount importance to this population.
Central or abdominal obesity is commonly associated with deterioration in metabolic, cardiovascular and other health-related variables.18–24 The reported contribution of visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) to different metabolic disorders varies widely25 and is still under investigation after SCI. Much of this variability can be attributed to differences in the methodological accuracy of quantifying VAT and SAT. The distribution of VAT and SAT was previously predicted using waist circumference (WC), the waist-to-hip ratio,21,22,24,26,27 or using more advanced imaging techniques such as ultrasound to measure adipose tissue depth, dual-energy X-ray absorptiometry (DXA) to quantify trunk adiposity,11,28,29 or more recently, highly precise quantitative techniques such as computerized tomography (CT) and magnetic resonance imaging (MRI).30–41 MRI is now identified as the gold-standard technique that could easily quantify the volumes of VAT and SAT.32,35,37,40 Ross and colleagues have demonstrated the use of MRI to capture multi-axial slices to quantify the volumetric distribution of VAT and SAT. Their data support the robustness of a very brief scan MRI protocol to measure VAT and SAT.32–34
The cross-sectional areas (CSAs) of VAT and SAT were recently measured in individuals with SCI using single axial CT scans at the level of L4–L5 or umbilicus.30,31 Despite similarities in BMI and WC between the two studies,30,31 there are wide discrepancies in the values reported in VAT CSA. Edward et al. reported that the range of CSAs of VAT and SAT may vary up to 89 and 87%, respectively, among individuals with SCI.30 The use of a single slice compared to multi-axial slices remains an issue of controversy in the process of quantifying VAT and SAT.37–40 Lee et al. suggested that a single slice can accurately predict SAT or VAT mass.41 Abate et al. showed that measuring SAT at L4–L5 space had the poorest correlation of the SAT volume compared to L5–S1 space.37 This may suggest that the CSA of a single slice may not accurately reflect the magnitude of VAT and SAT after SCI.
We are unaware of any study that has quantified VAT and SAT volumes in individuals with SCI using multi-axial slices. Considering the array of adaptations in body composition after SCI,4,5,22,42 it is unclear if the CSA of a single slice can predict the distribution of VAT and SAT across multi-axial slices. Additionally, we have quantified the distribution of VAT and SAT relative to the whole body FM in individuals with SCI. We hypothesized that a single slice may predict the CSA of multi-axial slices; however, it may not accurately reflect the volumes of VAT and SAT. Additionally, the relative distribution of VAT and SAT would be lower than what has been previously reported in AB controls due to the greater whole body FM in individuals with SCI.
Materials and methods
Participants
Thirteen healthy men with chronic traumatic motor complete SCI (mean ± SD age 35 ± 8 years, body weight 74 ± 13 kg, height 182 ± 7 cm, and BMI 23 ± 4 kg/m2) participated in the study. Only men were chosen to ensure homogeneity of our sample and to reduce possible effects of sex on body composition.26,43 Additionally, compared to women, men accumulate greater quantities of VAT.43 The participants were at least 1 year post-injury with levels of injury ranging from C5 to T11 and American Spinal Injury Association (ASIA) classification A or B (motor complete SCI). We have chosen to study motor complete SCI because the magnitude of body composition adaptations may vary between complete and incomplete SCI.2,3,5 Participants were recruited from the University of Michigan SCI model system (n = 5; C6–T11) and from Indiana University Hospitals (n = 8; C5–T11). Participants signed an informed consent statement that was approved by the local ethics committee at the University of Michigan and Indiana University and they were compensated for participation.
Participants were included if they were (1) men aged 18–45 years of age, with the maximum age chosen to avoid any confounding effects of the aging process on body composition; (2) a minimum of 1 year post-injury, due to adaptations in body composition stabilizing by this time5; and (3) the level of injury was C5–T11; individuals with injury above C5 have limited hand functions and are dependent on others to prepare their meals, which may indirectly influence their body composition, body weight, VAT, and SAT.32,34 Lower motor neuron injury is typically found in those with injury below T11 and leads to flaccid paralysis of the involved skeletal muscles that ultimately affects body composition.44
Participants were excluded from the study if they had any of the following characteristics: had cardiovascular disease, hypertension, diabetes, or high lipid profile, were smokers or alcohol abusers, or had pressure ulcers greater than Grade II. Individuals with BMI greater than 30 kg/m2 were excluded because lower BMI cut-off points have been recommended to identify the risk of obesity in people with SCI.5,6 Persons with MRI-incompatible materials such as rods, screws, valves, and stents that were implanted for different medical purposes were also excluded.
Anthropometric variables
All study participants received a physical examination including a resting electrocardiogram and basic vital signs. Body weight was measured while wearing light clothes in a supine position using a hospital bed scale calibrated to 0.1 kg. Height was measured from the same position to the nearest 0.1 cm. BMI was calculated as weight in kg divided by height in m2 (kg/m2). WC (n = 9) was measured using inelastic tape measure at the level of the narrowest part of the torso.22 The measurements were taken in doublets from a seated position in their wheelchairs at the end of expiration without compressing the skin as was previously done.22 All measurements were obtained by the same investigator.
Dual-energy X-ray absorptiometry
DXA was used to study the whole body FFM and FM (kg). Body composition was measured using whole body scans with a Lunar Prodigy Advance scanner (n = 5; Lunar DPX, DXA Scanner; Lunar Inc., Madison, WI, USA) and a Hologic QDR-2000 scanner (n = 8). Selection of two different densitometers was based on the availability of the DXA scanners at the two institutions. To account for any variability, a whole body standard phantom (Body Phantom S/N 1067, Hologic, Inc., Waltham, MA, USA) was scanned 26 times to correct for the source of error from using two different densitometers. The results showed that the between-machine difference was 0.06% for FM and 7% for FFM. Whole body %FM and FFM were calculated after excluding bone tissue and the index of FM to FFM was used to determine relative distribution of FM. The coefficient of variability of two repeated scans on the same participant was less than 3%.
Tissue measurement by MRI
Images were obtained with a 1.5 or 3 T whole body scanner (General Electric Signa scanner, Milwaukee, WI, USA). T1-weighted imaging was performed using a fast spin-echo sequence with the following parameters: axial in-phase/out-phase with a repetition time of 140 ms and echo time of 4.2 and 1.8 ms for the in-phase and the out-phase, respectively; a 46 cm field of view, matrix size of 256 × 256 or 320 × 320, number of excitations = 1 and acquisition time of 4–5 minutes. Transverse slices (0.8 or 1 cm thickness) were acquired every 0.4 or 1 cm gap from the xiphoid process to the femoral heads. Images were acquired in a series of two stacks with L4–L5 used as a separating point. After the acquisition of a localizer sequence, the inter-vertebral space between the fourth and fifth lumbar vertebrae was identified by locating the umbilicus.32–34 To ensure a short breath holding duration, two sets of nine slices were captured. The first set extended superiorly from L4–L5 to the xiphoid process and the second set distally from L4–L5 to the femoral heads. During scanning, participants were asked to take a deep breath in and hold their breath for 10–15 seconds. The breath holding technique was applied to reduce the respiratory motion artifact normally associated with acquisition of MRI in the abdominal region. The use of a fast spin echo and dividing the trunk region into two stacks ensured a short breath holding time for the participants, notably more tolerable for those with higher SCI.
Procedures for acquiring MRI
After arriving for study, participants were transported to the scanner for a non-contrast abdominal MRI. Participants were then asked to fill out an MRI safety checklist to ensure no contraindications. The MRI table was moved outside the magnet room and two persons (one MRI technician and one researcher) assisted in the participant's transfer from the wheelchair to the table. After lying supine, their knees and feet were strapped to ensure neutral position inside the magnet and to avoid incidental movement due to spasms, which may cause image artifact. The movable table was then docked to the magnet with the participant's head slide in first and arms were placed across the chest due to range of motion limitations preventing overhead placements. The technician instructed participants to maintain their position and to avoid movement during scanning. All participants were provided earplugs to protect against magnet noise and a blanket to maintain normal body temperature and to avoid triggering muscle spasms inside the magnet.
Calculation of VAT and SAT area and volume
Images were downloaded to a disk and analyzed on specifically designed software (Win Vessel 2, Ronald Meyer, PhD, Department of Physiology, MSU, East Lansing, MI, USA). The images were automatically segmented into fat (high-intensity), muscle (mid-intensity), and background/bone (low-intensity) regions.3,9,44,45 The CSAs were computed automatically by summing the tissues' pixels and multiplying by pixel surface area (Fig. 1). Pixel surface area is multiplied by (Field of view/Matrix size)2. The volume (cm3) was calculated by multiplying the CSA by the slice thickness and inter-slice space (1.2 or 2 cm). Depending on torso length of the participants, 10–14 transverse images extended distally from the liver to the femoral heads were analyzed. Selection of the images was based on visual distinction of VAT and SAT regions within a single slice. To adjust for differences in torso length among our participants, consecutive slices were averaged in taller individuals and truncal VAT and SAT volumes were calculated by summing the volumes of 10 consecutive slices. The VAT and SAT masses (g) were calculated by multiplying the corresponding volumes (cm3) by fat density (0.92 g/cm2).46
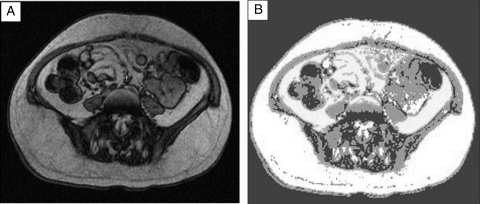
Figure 1.
Representative axial MRI slice of L4–L5 space of individuals with C7 motor complete SCI (A) before and (B) after segmenting the image and tracing the region of interest. VAT CSA is the white region segmented within the abdominal wall and SAT CSA is the white region outside the abdominal wall. BMI: 23 kg/m2; WC: 84 cm; VAT: 66 cm2; SAT: 223 cm2.
Reliability
Inter-observer reliability values were used to determine variations in the average CSA of VAT and SAT across multi-axial slices.41 This was assessed by comparing the measurement outcomes of two trained examiners after each had analyzed multi-axial slices of two randomly selected participants. Using the coefficient of variation (CV), the inter-observer error was 13% for VAT and 1.5% for SAT. It should be noted that CV of a single examiner was no more than 3% for VAT and 0.5% for SAT. The reported values were within the acceptable range previously noted in a similar study.41
Statistical analysis
Statistical procedures were performed using SPSS version 15.0 (SPSS, Chicago, IL, USA). Linear regression analysis was used to determine the relationship between single and multi-axial slice measures of VAT and SAT CSAs. Multiple regression analyses were used to determine the variables that could predict VAT or SAT volumes. One-way analysis of variance (ANOVA) was used to identify the statistical differences among three stacks of multi-axial slices from L1–L3, L3–L5, and L5–S1. The first and last stacks had three slices of axial images and L3–L5 had four slices. The arrangement of axial slices into stacks was performed to determine the influence of anatomical sites on the magnitude of VAT and SAT accumulation. Pearson correlation was used to quantify the relationships between VAT or SAT and body composition variables.
Results
Physical characteristics of the participants
Six participants had cervical (C5–C7, 46%) SCI and seven had thoracic (T4–T11, 54%) SCI. Out of the 13 individuals, 10 were ASIA A and the other 3 were ASIA B. The age and BMI ranged from 22 to 45 years and from 16 to 29 kg/m2, respectively. Time since injury was 12 ± 8 years (2–19 years post-injury).
Relationship between VAT and SAT and physical characteristics
Age was positively related to VAT volume (r = 0.60, P = 0.03) with a trend towards VAT CSA (0.54, P = 0.054), but not with SAT or VAT:SAT ratio. Time since injury was positively related to VAT:SAT ratio (r = 0.64, P = 0.019). Table 1 presents the relationships among weight, BMI, and WC and the CSAs and volumes of VAT and SAT. It should be noted that the data of WC on four of the participants were inadvertently lost. WC was related to weight (r = 0.81, P = 0.009), BMI (r = 0.85, P = 0.004), SAT volume, and sum of SAT and VAT volumes, but not to VAT volume (Table 1). Surprisingly, WC was related the SAT CSA at L3 (r = 0.75, P = 0.02) and L3–L4 (r = 0.71, P = 0.03), but not to L4–L5 and L5–S1.
Table 1.
Relationship between CSAs (cm2) and volumes (cm3) of multi-axial slices and physical characteristics
| Cross-sectional areas |
Volumes |
|||||
|---|---|---|---|---|---|---|
| SAT (cm2) | VAT (cm2) | SAT + VAT (cm2) | SAT (cm3) | VAT (cm3) | SAT + VAT (cm3) | |
| Weight (kg) | 0.63 (0.22) | 0.66 (0.016) | 0.80 (0.001) | 0.60 (0.03) | 0.68 (0.01) | 0.75 (0.003) |
| BMI (kg/m2) | 0.61 (0.027) | NS | 0.71 (0.007) | 0.77 (0.002) | 0.70 (0.007) | 0.89 (0.0001) |
| WC (cm) | 0.67 (0.051) | NS | NS | 0.74 (0.02) | NS | 0.68 (0.046) |
BMI: body mass index; WC: waist circumference; NS: non-significant values.
Values shown are Pearson r (P values).
Multi-axial distribution of VAT and SAT
Table 2 presents the distribution of VAT, SAT, and VAT:SAT ratio across multi-axial slices. The average CSAs of VAT and SAT were 99 ± 51 and 165 ± 68 cm2, respectively. The average volumes of VAT and SAT were 1.4 ± 0.6 and 2.4 ± 1 l, respectively. VAT and SAT masses were 1.3 ± 0.6 and 2.2 ± 0.9 kg, respectively. VAT and SAT masses constitute 5.7 ± 1.8% (2.5–8.5%) and 9.7 ± 3.2% (4.5–14%) of the total body FM, respectively. One-way ANOVA did not identify any difference in VAT or SAT CSA after being separated into stacks of axial slices. The CSA of VAT ranged from 81 to 115 cm2 between the inter-vertebral space of L1–L2 to L3 vertebral body and reached another peak towards S1, suggesting that accumulation of VAT is site dependent. SAT distribution showed a peak at L3–L4 space and L4 vertebral body with a decline towards the sacral region. The average ratio of VAT to SAT was 0.68 ± 0.30 with the highest ratio at the sacral region above the femoral heads (Table 2). The high ratios of VAT to SAT suggest that these individuals are at high risk of developing metabolic abnormalities.
Table 2.
Distribution of the VAT, SAT CSAs, and VAT/SAT ratio across multi-axial slices
| L1–L2 | L2 | L2–L3 | L3 | L3–L4 | L4 | L4–L5 | L5 | L5–S1 | S1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| VAT (cm2) | 81.5 ± 59.5 | 95 ± 62 | 105 ± 66 | 115 ± 65 | 112 ± 68 | 99 ± 58 | 97 ± 52 | 86 ± 51 | 94.5 ± 42 | 97 ± 33 |
| SAT (cm2) | 129.5 ± 81 | 148 ± 80 | 166 ± 79 | 181 ± 79 | 188 ± 84 | 189 ± 76 | 190 ± 71 | 169 ± 66 | 147 ± 62 | 131 ± 60 |
| VAT/SAT ratio | 0.70 ± 0.45 | 0.73 ± 0.45 | 0.71 ± 0.44 | 0.7 ± 0.4 | 0.66 ± 0.38 | 0.58 ± 0.33 | 0.57 ± 0.31 | 0.56 ± 0.33 | 0.71 ± 0.3 | 0.89 ± 0.5 |
Values represent means ± SD for the CSA of axial slices. Letter L and S refer to lumbar and sacral vertebrae. The corresponding numbers refer to each vertebral body or inter-vertebral space.
Prediction of multi-axial slices using a single slice
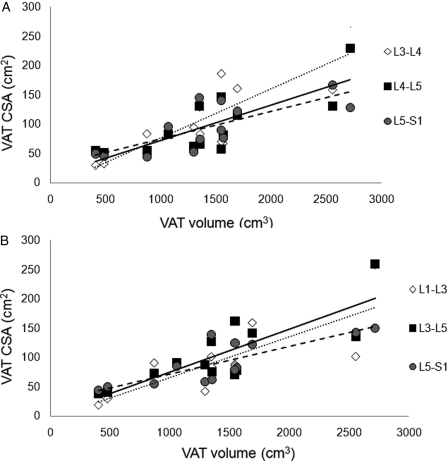
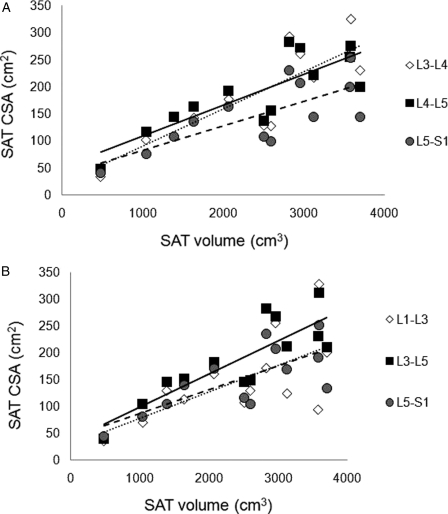
The CSA of a single slice at L3–L4, L4–L5, and L5–S1 modestly predicts VAT volume across multi-axial slices (Fig. 2A). However, the CSAs of L3–L4 (r2 = 0.95, P < 0.0001) and L4–L5 (r2 = 0.92, P < 0.001) strongly predict the average CSA of SAT across multi-axial slices, with a modest prediction at L5–S1 (r2 = 0.57, P < 0.003). The average CSA of the stack of slices between L3–L5 and L5–S1 appeared to be more predictive of VAT volume compared to L1–L3 (Fig. 2B). The CSAs of a single slice at L3–L4, L4–L5, and L5–S1 also appear predictive of SAT volume across multi-axial slices (Fig. 3A). The same slices L3–L4 (r2 = 0.93, P < 0.0001), L4–L5 (r2 = 0.89, P < 0.0001), and L5–S1 (r2 = 0.92, P < 0.003) strongly predict the average CSA of SAT across multi-axial slices. The relationships of the stack of SAT slices at three different regions of the trunk and the SAT volume are presented (Fig. 3B). The L3–L5 region appeared to have the highest magnitude of prediction to the SAT volume (r2 = 0.70, P < 0.0001) compared to L1–L3 (r2 = 0.42, P = 0.01) and L5–S1 (r2 = 0.55, P = 0.004) regions. Whole truncal SAT and VAT were not significantly related at the level of a single slice or across multi-axial slices.
Figure 2.
Prediction of VAT volume using (A) single slice CSA at L3–L4 (dotted line, r2 = 0.67, P < 0.0001), L4–L5 (solid line, r2 = 0.62, P < 0.001), and L5–S1 (dashed line, r2 = 0.56, P < 0.003) spaces and (B) stack of slices CSA at L1–L3 (dotted line, r2 = 0.59, P < 0.002), L3–L5 (solid line, r2 = 0.67, P < 0.001), and L5–S1 (dashed line, r2 = 0.67, P < 0.001).
Figure 3.
Prediction of SAT volume using (A) single slice CSA at L3–L4 (dotted line, r2 = 0.72, P < 0.0001), L4–L5 (solid line, r2 = 0.68, P < 0.0001), and L5–S1 (dashed line, r2 = 0.56, P < 0.003) spaces and (B) stack of slices CSA at L1–L3 (dotted line, r2 = 0.42, P < 0.01), L3–L5 (solid line, r2 = 0.71, P < 0.0001), and L5–S1 (dashed line, r2 = 0.54, P < 0.004).
Relationships between VAT or SAT and body composition variables
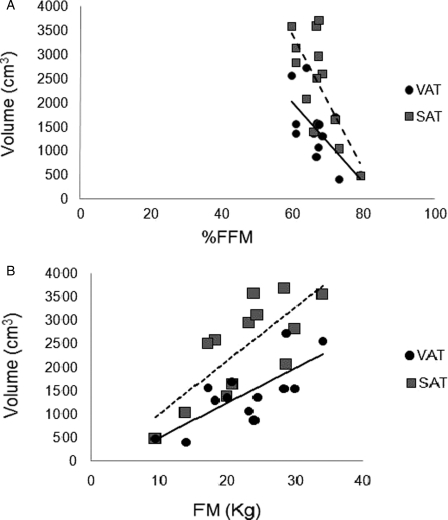
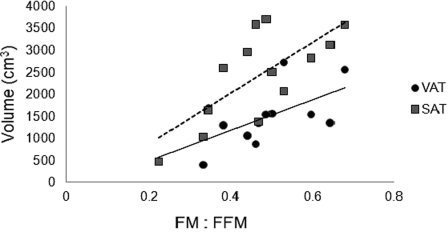
Percent body FFM and FM were 67 ± 5% and 31 ± 6%, respectively, and the index of FM to FFM was 0.47 ± 0.13. Percent body FFM was negatively related to VAT (r = −0.66, P = 0.014) and SAT (r = −0.72, P = 0.005; Fig. 4A) volumes and SAT CSA (r = −0.62, P = 0.02). Whole body FM was positively related to VAT and SAT volumes (r = 0.76, P = 0.003; Fig. 4B), and VAT (r = 0.61, P = 0.02) and SAT (r = 0.69, P = 0.009) CSA across multi-axial slices. Additionally, the ratio of FM to FFM was positively related to VAT (r = 0.66, P = 0.014) and to SAT (r = 0.72, P = 0.006) volumes (Fig. 5), but not to their CSAs. A single slice of SAT CSA at L3–L4, L4–L5, and L5–S1 was not related to any of the body composition variables. A single slice of VAT CSA at L3–L4 was related to FM (r = 0.65, P = 0.016). Finally, multiple regression analyses were used to identify the most significant predictors that could quantify VAT and SAT volumes (Table 3).
Figure 4.
Relationship between SAT and VAT volumes and whole body composition (A) %FFM and (B) absolute body FM. Significant inverse relationship was noted between %FFM and VAT (solid line, r = −0.66, P < 0.01) or SAT (dashed line, r = −0.73, P < 0.005) volume. Whole body FM was positively related to either VAT (solid line, r = 0.7, P < 0.003) or SAT (dashed line, r = 0.76, P < 0.003) volume.
Figure 5.
Relationship between whole body ratio of FM:FFM and VAT (solid line, r = 0.66, P < 0.01) or SAT (dashed line, r = 0.72, P < 0.006) volume.
Table 3.
Multiple regression equations used to identify predictors of VAT and SAT volumes
| Equations | ||
|---|---|---|
| VAT volume (cm3) = | 23 (age) + 21 (weight) + 32.5 (%FM) − 1930 | R2 = 0.63, P = 0.025 |
| 9 (age) − 1.9 (weight) + 37 (%FM) + 6.5 (L3–L4 CSA)* − 648 | R2 = 0.81, P = 0.006 | |
| SAT volume (cm3) = | 25 (weight) + 127 (%FM) − 42 (age) − 1929 | R2 = 0.71, P = 0.009 |
| 4 (weight) + 83 (%FM) − 21 (age) − 7 (L3–L5 CSA)** − 1027. | R2 = 0.81, P = 0.005 |
*It should be noted that that the use of L3, L3–L4, L4, and L4–L5 CSAs is considered a significant predictor for measuring VAT volume.
**The use of a single slice CSA did not improve the prediction of SAT volume compared to the average CSA of the four slices between L3 and L5.
Discussion
The major findings of the study were that (1) the CSA of a single slice at L3–L4, L4–L5, or L5–S1 can strongly predict the average CSA across multi-axial slices and modestly predict the volumes of VAT and SAT; (2) VAT and SAT volumes exhibited expected relationships with %FFM and FM, but these were not evident with the CSA of a single slice; (3) maintaining a relatively high body FFM is associated with less accumulation of VAT and SAT volumes after SCI; (4) increased weight, BMI, and whole body FM are indicative of increased SAT and VAT volumes in people with SCI, but WC should be used with caution; and (5) the unfavorable ratio of VAT to SAT suggests that these individuals are at high risk of developing metabolic abnormalities.
Altered body composition after SCI increases the susceptibility to several deleterious metabolic disorders.8–16 The importance of accurately evaluating body composition after SCI has been a subject of numerous investigations.5–7,11,42 Compared to healthy or several other clinical populations, the contribution of VAT and SAT to altered metabolic and cardiovascular profile after SCI is poorly defined. Therefore, accurately quantifying the magnitude of distribution of VAT and SAT may help us to better understand the contribution to various metabolic pathogenesis after SCI. VAT and SAT CSAs have recently been quantified using a single slice CT scan at the level of L4–L5.30,31 Edward et al.30 suggested that VAT CSA is 120 cm2 with a range of 27–257 cm2. Maruyama et al.31 showed that VAT CSA is 191 cm2 and it can reach 300 cm2. These discrepancies in VAT CSA could possibly be explained by the fact that a single slice CSA cannot always capture the actual magnitude of VAT CSA. There is still growing skepticism that a single slice CSA can accurately predict the CSA of multi-axial slices or VAT and SAT volumes.37,38,40
We have described volumetric quantification of VAT, SAT, and the ratios for the first time in individuals with SCI using multi-axial MRI slices. Previously, multi-axial slices were used to quantify the extent of atrophy and hypertrophy after complete and incomplete SCI.2,3,9,45,47 Moreover, MRI allows non-invasive quantification of adipose tissue such as intramuscular,3,9,45 subfacial, and SAT.9,45,48 In agreement with previous reports, a single CSA was able to accurately predict the average CSA across multi-axial slices; however, it modestly predicts the actual volume. Using a single slice CSA at L3–L4 improved the magnitude of relationship compared to other sites. It appeared that stacked slices between L3 and L5 can best predict VAT or SAT volumes. This may explain previous inconsistent relationships between the metabolic profile and VAT or SAT after using a single axial slice at L4–L5.30,31 The use of MRI to capture multi-axial slice could have been hampered by the following challenges. First, individuals with rods in their back for spinal column fusions could experience complications or produce low-quality MR images. This was overcome by including individuals who have no rods in their back or have short rods that do not interfere with the region of interest causing imaging artifact. The second challenge was the difficulty in executing breath holding techniques, especially in higher level SCI. Selection of a fast spin-echo sequence and dividing the region of interest into stacks of slices allowed for a short breath holding duration.
VAT and SAT masses were diluted by an increase in whole body FM after SCI and especially FM in the lower extremities, which constitute approximately 6% and 10% of total body FM, respectively. In AB controls, abdominal SAT represents 20–23% and VAT represents 10–13% of total body FM in lean and obese individuals.18 Significant correlations were found between whole body FM and the two compartments of VAT and SAT. These relationships provide supporting evidence that an FM equal to or greater than 20 kg or 30% of the whole body FM may result in VAT CSA greater than 100 cm2. A previous epidemiological study that used a single slice showed that in men with VAT greater than 71 cm2, there is a risk of coronary artery calcification that may predict coronary artery diseases.27 A novel finding of the current study is that high %FFM is associated with a reduction in VAT and SAT volumes. Similarly, intramuscular fat has been shown to be reduced after evoking skeletal muscle hypertrophy to the paralyzed muscles using neuromuscular electrical stimulation.45 Moreover, a strong relationship was recently observed between whole body FFM and basal metabolic rate in individuals with SCI.7 These findings may highlight the importance of developing countermeasures that could help in increasing skeletal muscle size or maintaining FFM in this population.45,47 Previous work showed that resistance training results in reduction in VAT or SAT volumes, with preferential loss in VAT.32–34 Relatively high FFM could be associated with increased testosterone or growth hormone that influences the distribution of VAT or SAT.49
Although BMI has been shown to underestimate the %FM in individuals with SCI,5,6,42 the current study showed that increase in body weight or BMI is associated with increase in VAT and SAT. Moreover, WC was previously used to investigate central adiposity and the associated metabolic abnormalities after SCI.11,22 In recreationally active athletes with SCI, WC was found to be related to insulin area under the curve and lipid profile.11 In that study, the authors found a negative relationship between trunk FM and insulin sensitivity. Although WC was recently found to be highly correlated with VAT after SCI,30 similar to previous reports34,35 we could not replicate these findings. WC was only related with SAT volume and the sum of SAT and VAT, but not VAT. WC measurement was carried out in the seated position, possibly redistributing tissue into the waist region because of an absence of normal abdominal wall musculature. Limiting measurements to only the seated position without including a measurement in the supine position may have played a role in the lack of correlation with VAT. Losing WC data on four participants may also have affected the magnitude of the relationships.
The ratio of VAT to SAT suggests that these individuals are at high risk of developing metabolic disorders. Previously, a VAT-to-SAT ratio greater than 0.4 was suggested as cut-off points for those at risk of developing metabolic disorders.23 MRI was recently used to quantify VAT and SAT distribution in those with type 2 diabetes. Those with worse insulin resistance have a greater VAT and lower SAT phenotype profile.48 We have observed a reduction in SAT CSA and an increase in the VAT CSA towards the sacral region similar to what has recently been reported in those with type 2 diabetes. It is unclear if the relative distribution of VAT to SAT is impacted by the level of SCI. One month post-injury, VAT increased in the T3 SCI rats but not in the T10 rats.36 Future trials should investigate the effects of the level of injury on the distribution of VAT and SAT, especially in those with T9 or lower SCI. Moreover, the presumptive validity and reliability of DXA at L1–L4 should be determined compared to multi-axial MRI slices of VAT and SAT.
Limitations
Data collections at two different institutions resulted in the use of two different sources of densitometers. Five participants were scanned on a Lunar and the rest were scanned on a Hologic scanner. In a previous study that measured body composition in individuals with SCI in relation to controls, it was suggested that Hologic overestimated FM compared to a Lunar scanner and that the primary sites of difference were the arms and legs.5 We scanned a whole body phantom to adjust for any source of error between the two scanners; the results showed that the outcomes between both scanners are too trivial to influence any of the studied relationships. However, it is worth mentioning that differences between machines for FM and FFM in humans are likely to be greater than those determined by phantom measurements alone. Another limitation is the use of two different magnets with differing magnetic field strength (1.5 T vs. 3 T), which may have introduced a source of error. Spatial quantification of VAT and SAT depends primarily on the relaxation properties of lipid during MRI. de Bazelaire et al. showed insignificant difference in the relaxation time of SAT between 1.5 and 3T.50 The lack of an AB control group may have limited the ability to compare the results of MRI abdominal VAT and/or SAT for the general population reported by other investigators. Nevertheless, there are two studies that have made direct comparisons in VAT and SAT between individuals with SCI and AB controls. The results showed that individuals with SCI had 40% greater VAT, but insignificant difference in SAT CSA.30,31 According to our research findings, the MRI technique can be applied to measure regional body composition adaptations in individuals with SCI. A metallic implant that extends down to L1–L2 may possibly cause visual artifacts that impede the clarity of the images to be analyzed. However, several research groups have successfully applied the techniques to measure skeletal muscle size,2,3 intramuscular fat,9 visceral and subcutaneous fat. In the current study, the technique appeared to be successful as long as there were no interfering implanted rods or screw and plates within the region of interest.
Conclusions
The CSA of a single slice at the level of L3–L4, L4–L5, and L5–S1 can modestly predict SAT and VAT volumes across multi-axial slices. The findings suggest that a single slice CSA does not correlate with body composition similar to the volume of VAT or SAT. The significance of this finding is highlighted in the inverse relationship that was found between %FFM and both VAT and SAT volumes. VAT and SAT represent only 6 and 10%, respectively, of the whole body FM. This may suggest that both compartments are diluted by the higher percentage of FM in individuals with SCI. However, the ratio of VAT to SAT distribution suggests that individuals with SCI are at high risk of developing metabolic disorders.
Acknowledgments
We wish to thank all participants and Dr Ronald Meyer for providing the software that was used in the analysis. The present work was funded by an internal grant from the office of Research Support Funds Grant at Indiana University, Office of Special Education and Rehabilitative Services National Institute on Disability and Rehabilitation (NIDRR) Grant # H133P03000 and General Clinical Research Center (NIH Funding to the University of Michigan). We also thank Dr Kirk Cureton for his insightful comments on the manuscript.
References
- 1.DeVivo MJ, Go BK, Jackson AB. Overview of the national spinal cord injury statistical center database. J Spinal Cord Med 2002;25(4):355–38 [DOI] [PubMed] [Google Scholar]
- 2.Castro MJ, Apple DF, Jr, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol 1999;80(4):373–8 [DOI] [PubMed] [Google Scholar]
- 3.Gorgey AS, Dudley GA. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord 2007;45(4):304–9 [DOI] [PubMed] [Google Scholar]
- 4.Kocina P. Body composition of spinal cord injured adults. Sports Med 1997;23(1):48–60 [DOI] [PubMed] [Google Scholar]
- 5.Spungen AM, Adkins RH, Stewart CA, Wang J, Pierson RN, Jr, Waters RL, et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol 2003;95(6):2398–407 [DOI] [PubMed] [Google Scholar]
- 6.Clasey JL, Gater DR., Jr A comparison of hydrostatic weighing and air displacement plethysmography in adults with spinal cord injury. Arch Phys Med Rehabil 2005;86(11):2106–13 [DOI] [PubMed] [Google Scholar]
- 7.Gorgey AS, Chiodo AE, Zemper ED, Hornyak JE, Rodriguez GM, Gater DR. Relationship of spasticity to soft tissue body composition and the metabolic profile in persons with chronic motor complete spinal cord. J Spinal Cord Med 2010;33(1):6–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duckworth WC, Jallepalli P, Solomon SS. Glucose intolerance in spinal cord injury. Arch Phys Med Rehabil 1983;64(3):107–10 [PubMed] [Google Scholar]
- 9.Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA. Intramuscular fat and glucose tolerance after spinal cord injury – a cross-sectional study. Spinal Cord 2004;42(12):711–6 [DOI] [PubMed] [Google Scholar]
- 10.Bauman WA, Spungen AM. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med 2001;24(4):266–77 [DOI] [PubMed] [Google Scholar]
- 11.Mojtahedi MC, Valentine RJ, Arngrimsson SA, Wilund KR, Evans EM. The association between regional body composition and metabolic outcomes in athletes with spinal cord injury. Spinal Cord 2008;46(3):192–7 [DOI] [PubMed] [Google Scholar]
- 12.Karlsson AK. Insulin resistance and sympathetic function in high spinal cord injury. Spinal Cord 1999;37(7):494–500 [DOI] [PubMed] [Google Scholar]
- 13.Bauman WA, Spungen AM, Zhong YG, Rothstein JA, Petry C, Gordon SK. Depressed serum high density lipoprotein cholesterol levels in veterans with spinal cord injury. Paraplegia 1992;30(10):697–703 [DOI] [PubMed] [Google Scholar]
- 14.Demirel G, Yilmaz H, Paker N, Onel S. Osteoporosis after spinal cord injury. Spinal Cord 1998;36(12):822–5 [DOI] [PubMed] [Google Scholar]
- 15.Nelson MD, Widman LM, Abresch RT, Stanhope K, Havel PJ, Styne DM, et al. Metabolic syndrome in adolescents with spinal cord dysfunction. J Spinal Cord Med 2007;30Suppl 1:S127–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauman WA, Spungen AM. Coronary heart disease in individuals with spinal cord injury: assessment of risk factors. Spinal Cord 2008;46(7):466–76 [DOI] [PubMed] [Google Scholar]
- 17.DeVivo MJ. Causes and costs of spinal cord injury in the United States. Spinal Cord 1997;35(12):809–13 [DOI] [PubMed] [Google Scholar]
- 18.Abate N, Garg A, Peshock RM, Stray-Gundersen J, Grundy SM. Relationships of generalized and regional adiposity to insulin sensitivity in men. J Clin Invest 1995;96(1):88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abate N, Garg A. Heterogeneity in adipose tissue metabolism: causes, implications and management of regional adiposity. Prog Lipid Res 1995;34(1):53–70 [DOI] [PubMed] [Google Scholar]
- 20.Boivin A, Brochu G, Marceau S, Marceau P, Hould FS, Tchernof A. Regional differences in adipose tissue metabolism in obese men. Metabolism 2007;56(4):533–40 [DOI] [PubMed] [Google Scholar]
- 21.Ross R, Shaw KD, Rissanen J, Martel Y, de Guise J, Avruch L. Sex differences in lean and adipose tissue distribution by magnetic resonance imaging: anthropometric relationships. Am J Clin Nutr 1994;59(6):1277–85 [DOI] [PubMed] [Google Scholar]
- 22.Buchholz AC, Bugaresti JM. A review of body mass index and waist circumference as markers of obesity and coronary heart disease risk in persons with chronic spinal cord injury. Spinal Cord 2005;43(9):513–8 [DOI] [PubMed] [Google Scholar]
- 23.Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism 1987;36(1):54–9 [DOI] [PubMed] [Google Scholar]
- 24.Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med 2002;162(18):2074–9 [DOI] [PubMed] [Google Scholar]
- 25.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest 2004;113(11):1582–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuk JL, Lee S, Heymsfield SB, Ross R. Waist circumference and abdominal adipose tissue distribution: influence of age and sex. Am J Clin Nutr 2005;81(6):1330–4 [DOI] [PubMed] [Google Scholar]
- 27.Snell-Bergeon JK, Hokanson JE, Kinney GL, Dabelea D, Ehrlich J, Eckel RH, et al. Measurement of abdominal fat by CT compared to waist circumference and BMI in explaining the presence of coronary calcium. Int J Obes Relat Metab Disord 2004;28(12):1594–9 [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro-Filho FF, Faria AN, Kohlmann O, Jr, Ajzen S, Ribeiro AB, Zanella MT, et al. Ultrasonography for the evaluation of visceral fat and cardiovascular risk. Hypertension 2001;38(3):713–7 [DOI] [PubMed] [Google Scholar]
- 29.Glickman SG, Marn CS, Supiano MA, Dengel DR. Validity and reliability of dual-energy X-ray absorptiometry for the assessment of abdominal adiposity. J Appl Physiol 2004;97(2):509–14 [DOI] [PubMed] [Google Scholar]
- 30.Edwards LA, Bugaresti JM, Buchholz AC. Visceral adipose tissue and the ratio of visceral to subcutaneous adipose tissue are greater in adults with than in those without spinal cord injury, despite matching waist circumferences. Am J Clin Nutr 2008;87(3):600–7 [DOI] [PubMed] [Google Scholar]
- 31.Maruyama Y, Mizuguchi M, Yaginuma T, Kusaka M, Yoshida H, Yokoyama K, et al. Serum leptin, abdominal obesity and the metabolic syndrome in individuals with chronic spinal cord injury. Spinal Cord 2008;46(7):494–9 [DOI] [PubMed] [Google Scholar]
- 32.Ross R, Rissanen J. Mobilization of visceral and subcutaneous adipose tissue in response to energy restriction and exercise. Am J Clin Nutr 1994;60(5):695–703 [DOI] [PubMed] [Google Scholar]
- 33.Ross R, Rissanen J, Pedwell H, Clifford J, Shragge P. Influence of diet and exercise on skeletal muscle and visceral adipose tissue in men. J Appl Physiol 1996;81(6):2445–55 [DOI] [PubMed] [Google Scholar]
- 34.Ross R, Rissanen J, Hudson R. Sensitivity associated with the identification of visceral adipose tissue levels using waist circumference in men and women: effects of weight loss. Int J Obes Relat Metab Disord 1996;20(6):533–8 [PubMed] [Google Scholar]
- 35.Thomas EL, Saeed N, Hajnal JV, Brynes A, Goldstone AP, Frost G, et al. Magnetic resonance imaging of total body fat. J Appl Physiol 1998;85(5):1778–85 [DOI] [PubMed] [Google Scholar]
- 36.Inskip J, Plunet W, Ramer LM, Ramsey JB, Yung A, Kozlowski P, et al. Cardiometabolic risk factors in experimental spinal cord injury. J Neurotrauma 2010;27(1):275–85 [DOI] [PubMed] [Google Scholar]
- 37.Abate N, Garg A, Coleman R, Grundy SM, Peshock RM. Prediction of total subcutaneous abdominal, intraperitoneal, and retroperitoneal adipose tissue masses in men by a single axial magnetic resonance imaging slice. Am J Clin Nutr 1997;65(2):403–8 [DOI] [PubMed] [Google Scholar]
- 38.Han TS, Kelly IE, Walsh K, Greene RM, Lean ME. Relationship between volumes and areas from single transverse scans of intra-abdominal fat measured by magnetic resonance imaging. Int J Obes Relat Metab Disord 1997;21(12):1161–6 [DOI] [PubMed] [Google Scholar]
- 39.Schoen RE, Thaete FL, Sankey SS, Weissfeld JL, Kuller LH. Sagittal diameter in comparison with single slice CT as a predictor of total visceral adipose tissue volume. Int J Obes Relat Metab Disord 1998;22(4):338–42 [DOI] [PubMed] [Google Scholar]
- 40.Thomas EL, Bell JD. Influence of undersampling on magnetic resonance imaging measurements of intra-abdominal adipose tissue. Int J Obes Relat Metab Disord 2003;27(2):211–8 [DOI] [PubMed] [Google Scholar]
- 41.Lee S, Janssen I, Ross R. Interindividual variation in abdominal subcutaneous and visceral adipose tissue: influence of measurement site. J Appl Physiol 2004;97(3):948–54 [DOI] [PubMed] [Google Scholar]
- 42.Gater DR., Jr Obesity after spinal cord injury. Phys Med Rehabil Clin N Am 2007;18(2):333–51 [DOI] [PubMed] [Google Scholar]
- 43.Enzi G, Gasparo M, Biondetti PR, Fiore D, Semisa M, Zurlo F. Subcutaneous and visceral fat distribution according to sex, age, and overweight, evaluated by computed tomography. Am J Clin Nutr 1986;44(6):739–46 [DOI] [PubMed] [Google Scholar]
- 44.Gorgey AS, Dudley GA. Spasticity may defend skeletal muscle size and composition after incomplete spinal cord injury. Spinal Cord 2008;46(2):96–102 [DOI] [PubMed] [Google Scholar]
- 45.Gorgey AS, Shepherd C. Skeletal muscle hypertrophy and decreased intramuscular fat after unilateral resistance training in spinal cord injury. A case report. J Spinal Cord Med 2010;33(1):90–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siri WE. Body composition from fluid spaces and density: analysis of methods. 1961. Nutrition 1993;9(5):480–91; discussion 480, 492 [PubMed] [Google Scholar]
- 47.Dudley GA, Castro MJ, Rogers S, Apple DF., Jr A simple means of increasing muscle size after spinal cord injury: a pilot study. Eur J Appl Physiol Occup Physiol 1999;80(4):394–6 [DOI] [PubMed] [Google Scholar]
- 48.Gallagher D, Kelley DE, Yim JE, Spence N, Albu J, Boxt L, et al. Adipose tissue distribution is different in type 2 diabetes. Am J Clin Nutr 2009;89(3):807–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kvorning T, Andersen M, Brixen K, Madsen K. Suppression of endogenous testosterone production attenuates the response to strength training: a randomized, placebo-controlled, and blinded intervention study. Am J Physiol Endocrinol Metab 2006;291(6):E1325–32 [DOI] [PubMed] [Google Scholar]
- 50.de Bazelaire CM, Duhamel GD, Rofsky NM, Alsop DC. MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology 2004;230(3):652–9 [DOI] [PubMed] [Google Scholar]