Abstract
Background/objective
Patients with spinal cord injury (SCI) are at risk of acquiring colonization with Clostridium difficile and vancomycin-resistant Enterococcus (VRE) due to prolonged hospitalization and frequent antimicrobial use. We examined the frequency of stool, skin, and environmental contamination with C. difficile and VRE in hospitalized patients with SCI.
Methods
We performed a cross-sectional study of 22 hospitalized patients with SCI with no symptoms of C. difficile infection. Stool samples, skin, and environmental sites were cultured for C. difficile and VRE, and polymerase chain reaction ribotyping was performed for C. difficile isolates. Fisher's exact test was used to compare the proportions of skin and environmental contamination among stool carriers and non-carriers. Univariate analysis was used to assess factors associated with asymptomatic carriage of C. difficile.
Results
Of 22 asymptomatic patients, 11 (50%) were asymptomatic carriers of toxigenic C. difficile and 12 (55%) were carriers of VRE. In comparison with non-carriers, asymptomatic carriers of toxigenic C. difficile had higher rates of skin (45 versus 9%) (P = 0.07) and environmental contamination (55 versus 9%) (P = 0.03) and longer length of stay (median, 57 versus 6 days; P = 0.04). A majority of skin and environmental C. difficile isolates from individuals were identical to isolates from stool. In comparison with non-carriers, patients with VRE stool colonization had non-significant trends toward more frequent skin (27 versus 9%) and environmental (18 versus 9%) contamination.
Conclusion
Asymptomatic stool carriage of toxigenic C. difficile and VRE was common on an acute-care SCI unit. Asymptomatic carriers of toxigenic C. difficile had frequent skin and environmental contamination, suggesting the potential to contribute to transmission.
Keywords: Clostridium difficile, Enterococcus, Vancomycin resistant, Colonization, Environment, Spinal cord injuries, Urinary tract infection
Introduction
Clostridium difficile is the most common cause of healthcare-associated diarrhea in developed countries.1 In recent years, large outbreaks of C. difficile infection (CDI) in North America and Europe have been attributed to the emergence of an epidemic strain (North American pulsed field gel electrophoresis type 1, or NAP1) with unique putative virulence factors and increased resistance to fluoroquinolone antibiotics.2–4 Multi-faceted infection control measures have been effective in reducing or eliminating some outbreaks associated with the epidemic strain.3–5 Control measures typically focus on patients with suspected or documented CDI.1,6 Those individuals are placed in contact precautions until their diarrhea resolves and their rooms are disinfected with a 1:10 dilution of household bleach or other sporicidal agents.6,7 Because many patients with CDI become asymptomatic carriers of toxigenic C. difficile (i.e. continue to shed spores in stool) after their diarrhea has resolved, some experts have recommended that contact precautions be continued until the time of discharge for patients who have recovered from CDI.4,7
Since 2002, the NAP1 strain has become endemic at the Cleveland VA Medical Center.8 A particularly high rate of infection has been observed on our spinal cord injury (SCI) unit (unpublished data). In our long-term care facility, 51% of 68 asymptomatic patients were found to be asymptomatic carriers of toxigenic C. difficile strains, and these carriers frequently had skin and environmental contamination.9 Because patients with SCI share similar risk factors with long-term care facility residents (e.g. prolonged length of stay and frequent antibiotic therapy),6,9 we examined the prevalence of toxigenic C. difficile stool carriage and of skin and environmental contamination in hospitalized patients on our SCI unit. In addition, we examined the prevalence of asymptomatic stool carriage and of skin and environmental contamination with vancomycin-resistant Enterococcus (VRE). VRE is another important healthcare-associated pathogen that shares risk factors with and often coexists with C. difficile.10
Methods
From 24 January through 2 February 2007, we performed a point-prevalence culture survey on the 32-bed SCI unit at the Cleveland Veterans Affairs Medical Center to assess the prevalence of asymptomatic carriage of toxigenic C. difficile and VRE, and the frequency of associated skin and environmental contamination. Patients with diarrhea of unknown etiology were excluded from the culture survey. Two patients with known CDI on the SCI unit during the study period were included for comparison as positive controls for the assessment of skin and environmental contamination; seven additional patients with CDI cared for on the SCI unit during the 2 months after the initial survey were also included to provide additional data on levels of contamination in CDI patients. The patients’ electronic medical record was utilized to obtain the SCI classification, demographic characteristics, coexisting illnesses, fecal incontinence, bowel care regimen, and medications including the use of antibiotics in the preceding 90 days. The Cleveland VA Medical Center's Institutional Review Board approved the study protocol and informed consent was obtained from all subjects.
Collection of rectal, skin, and environmental cultures
Rectal cultures were obtained by introducing a sterile swab into the rectum. A previous study has demonstrated that rectal swabs detected 100% of C. difficile carriers with positive stool cultures.11 Skin cultures for C. difficile (groin and chest/abdomen) were obtained by donning sterile gloves and rubbing a standardized 5 × 20 cm2 area with a pre-moistened sterile gauze pad (3 × 3 cm2). The gauze pad was then placed into a sterile specimen cup. Skin cultures for VRE were obtained by rubbing a sterile swab on a standardized 5 × 20 cm2 area. For each patient the C. difficile specimen was obtained prior to the VRE specimen and different areas were cultured for each pathogen. We have previously demonstrated that gauze pads are more sensitive than swabs for the recovery of C. difficile from environmental surfaces,9 whereas gauze pads and swabs yield similar rates of recovery of VRE from skin or the environment (authors’ unpublished data).
Environmental cultures for C. difficile (bed rail and hand grip of trapeze) were obtained by donning sterile gloves and rubbing a sterile pre-moistened gauze pad (3 × 3 cm) on a designated area of each surface (5 × 20 cm for bed rail and the entire inferior surface of the hand grip). Environmental cultures for VRE were obtained by rubbing a sterile pre-moistened swab on a separate 5 × 20 cm area of the bed rail and the entire inferior surface of the hand grip. All cultures were obtained by one of the investigators (DMD) using standardized methods for each subject.
C. difficile microbiologic analysis and molecular typing
Culture specimens were incubated for 48 hours in pre-reduced cycloserine–cefoxitin–fructose (CCF) broth containing 0.1% taurocholic acid and lysozyme 5 mg/l, and then plated onto CCF agar plates containing taurocholic acid and lysozyme and incubated for an additional 48 hours.8,9 Isolates were confirmed to be C. difficile on the basis of typical odor and appearance of colonies and by a positive reaction using C. difficile latex agglutination (Microgen Bioproducts, Camberly, UK). All C. difficile isolates were tested for in vitro cytotoxin production using C. difficile Tox A/B II (Wampole Laboratories, Princeton, NJ, USA), and isolates that did not produce toxin were excluded from the analysis.
For a subset of patients, molecular typing was performed to compare isolates from stool, skin, and environmental sites and to determine the prevalence of epidemic NAP1 strains. Crude DNA was extracted from C. difficile isolates using QIAamp DNA Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. Polymerase chain reaction (PCR) ribotyping was used to genotype C. difficile isolates as previously described.12 PCR was performed to amplify one of the genes for binary toxin (cdtB) using the methods of Terhes et al.13 To assess for partial deletions of the tcdC gene, PCR was performed according to the methods of Spigaglia and Mastrantonio.14 Isolates with partial deletions were identified based on different migration patterns on a 2% agarose gel. For each assay, a known epidemic strain (typed as BI6 using restriction enzyme analysis) was used as a positive control and American Type Culture Collection (ATCC, Manassas, VA, USA) C. difficile 9689 was used as a negative control.
VRE microbiologic analysis
Swab tips were incubated in EnterococcoselTM broth (Becton Dickinson, Sparks, MD, USA) containing vancomycin 20 µg/ml for 48 hours, followed by plating onto Enterococcosel agarTM (Becton Dickinson) containing vancomycin 20 µg/ml for an additional 48 hours. Identification was performed in accordance with Clinical Laboratory Standards Institute guidelines.15
Data analysis
Distributions of clinical and demographic characteristics of asymptomatic carriers and non-carriers (i.e. patients with negative stool cultures) were compared. Unpaired t-test was used for normally distributed data. Fisher's exact test was used for categorical data. Data were analyzed with the use of SPSS statistical software version 10.0 (SPSS Inc., Chicago, IL, USA) and STATA 9.1 (StataCorp, College Station, TX, USA).
Results
Of 30 patients admitted to the SCI unit during the study, 2 had CDI and the other 28 had no diarrhea or other changes in bowel habits. Of the 28 subjects with no diarrhea, 22 (78.6%) patients were enrolled and 6 patients were excluded because they were discharged prior to enrollment or were unavailable for cultures due to appointments or procedures. Of the 22 subjects, 11 (50%) had asymptomatic carriage of toxigenic C. difficile in stool and 11 did not. Table 1 shows a comparison of the characteristics of the subjects with and without asymptomatic stool carriage of toxigenic C. difficile. The average length of stay prior to the culture being obtained was significantly longer for the asymptomatic carriers of toxigenic C. difficile versus the non-carriers (median, 57 versus 6 days; P = 0.04). There were non-significant trends toward more frequent indwelling devices (P = 0.08), recent intensive care unit admission, and more frequent antibiotic use within the past 90 days for the asymptomatic carriers of toxigenic C. difficile versus non-carriers.
Table 1.
Baseline characteristics of 22 male SCI patients with no symptoms of CDI, according to stool carriage of C. difficile
| Characteristic (n (%) unless otherwise specified) | Asymptomatic C. difficile carrier (n = 11) | Non-carrier (n = 11) | P value, (unless specified as relative risk (RR)) |
|---|---|---|---|
| Age (median, IQR) | 54 (43–72 | 58 (49–61) | NS |
| Length of stay (median days, IQR) | 57 (9–243) | 6 (1–23) | 0.04 |
| Level of SCI | |||
| Cervical | 3 (27) | 5 (45) | NS |
| Thoracic | 6 (55) | 6 (55) | NS |
| Lumbar or undetermined | 2 (18)* | 0 (0) | NS |
| VRE colonization | 7 (64) | 5 (45) | 1.4 (0.64–3.07) |
| Transfer from outside hospital | 6 (55) | 3 (27) | RR = 2 (0.66–6.04) |
| Long-term care facility resident | 3 (27) | 1 (9) | RR = 3 (0.37–24.6) |
| Infection during admission | 10 (91) | 6 (55) | RR = 1.7 (0.94–2.95) |
| Antibiotics within past 90 days | 9 (82) | 5 (45) | RR = 1.8 (0.89–3.64) |
| Prior C. difficile infection | 2 (18) | 2 (18) | RR = 1 (0.17–5.4) |
| Indwelling device† | 7 (64) | 2 (18) | RR = 3.5 (0.92–13.2) |
| Bowel care regimen including rectal stimulation | 5 (45) | 4 (36) | RR = 1.25 (0.45–3.45) |
| Acid-suppressive medication (proton pump inhibitor or H2 blocker) | 3 (27) | 4 (36) | RR = 0.75 (0.14–3.24) |
| Intensive care admission past 90 days | 3 (27) | 1 (9) | RR = 3 (0.13–3.24) |
IQR = interquartile range; NS = not statistically significant and indicates that the P value was ≥ 0.05.
*One subject had lumbar SCI and the other had multiple sclerosis.
†Indwelling devices included chronic indwelling urinary catheters, tracheostomies, and percutaneous endoscopic gastostomy tubes.
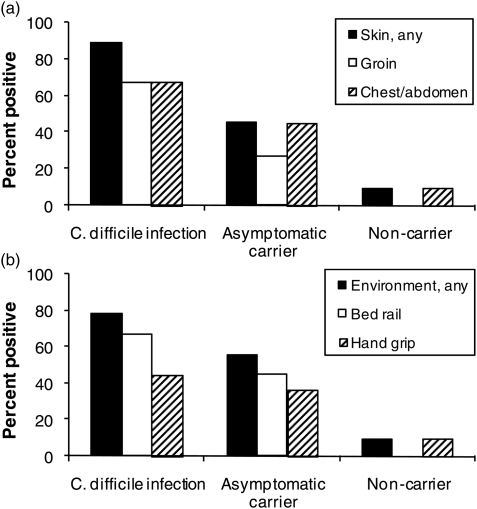
Comparing patients with CDI, asymptomatic carriers of toxigenic C. difficile, and non-carriers, the percentages of positive cultures for any skin site were 89, 45, and 9, respectively, and for any environmental site, it was 78, 55, and 9, respectively (Fig. 1). In comparison with non-carriers, asymptomatic carriers of toxigenic C. difficile had a significantly higher percentage of environmental (P = 0.03) contamination and a trend toward a higher percentage of skin (P = 0.07) contamination. In comparison with CDI patients, asymptomatic carriers of toxigenic C. difficile had trends toward lower percentages of skin (P = 0.07) and environmental (P = 0.24) contamination. Of the 11 patients with negative stool cultures, one had both skin and environmental contamination with C. difficile.
Figure 1.
C. difficile skin (A) and environmental (B) contamination among study groups. Cultures of skin and environmental surfaces were collected concurrently with stool cultures from patients with C. difficile infection (9 patients), asymptomatic fecal carriers (11 patients), and non-carriers (i.e. negative stool cultures) (11 patients).
Of the 11 asymptomatic carriers of toxigenic C. difficile, 1 (9%) was found to be a carrier of the NAP1 strain and the other 10 had non-epidemic strains. All 12 of the skin isolates from 6 asymptomatic carriers of toxigenic C. difficile were identical to concurrent stool isolates by PCR-ribotyping. Of 11 environmental isolates from 5 asymptomatic carriers of toxigenic C. difficile that were subjected to typing, 9 (82%) were identical to concurrent stool isolates.
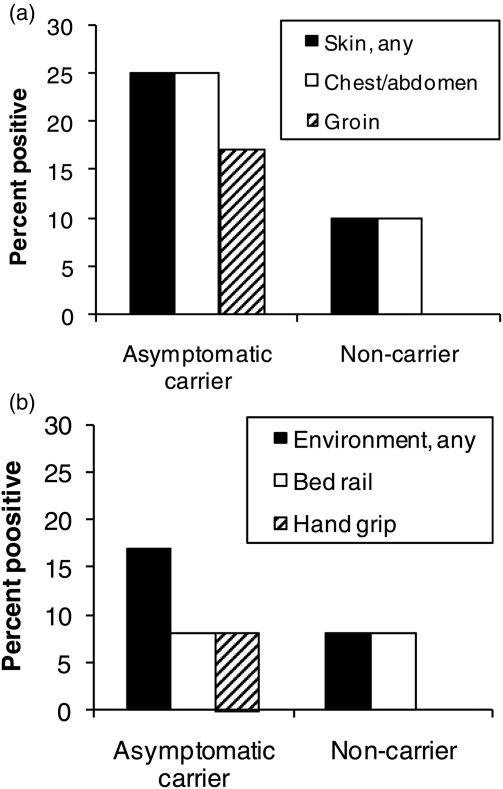
Of the 22 subjects, 12 (55%) were found to have unsuspected colonization of their stool with VRE. Of the 11 asymptomatic carriers of toxigenic C. difficile, 7 (64%) were also carriers of VRE. As shown in Fig. 2, the proportions of positive skin and environmental contamination were higher for the patients with stool colonization versus those with negative stool cultures, but the differences were not statistically significant.
Figure 2.
VRE skin (A) and environmental (B) contamination among study groups. Cultures of skin and environmental surfaces were collected concurrently with stool cultures from asymptomatic fecal VRE carriers (12 patients) and non-carriers (i.e. negative stool cultures) (10 patients).
Discussion
In a setting in which the NAP1 strain has become endemic, we found that 50% of subjects on the acute-care SCI unit at the Cleveland VA Medical Center were asymptomatic carriers of toxigenic C. difficile. These patients frequently had contamination of skin and environmental sites, and molecular typing demonstrated that most skin and environmental isolates were identical to concurrent stool isolates. Unsuspected stool colonization with VRE was also common among the study patients, with frequent skin and environmental contamination. It is likely that the requirement for assistance with bowel care may contribute to frequent skin and environmental contamination among carriers of healthcare-associated pathogens on SCI units. Because asymptomatic carriers of toxigenic C. difficile outnumbered patients with CDI by greater than five-fold, our findings suggest that these individuals could contribute significantly to transmission on SCI units.
There are limited data on the epidemiology of C. difficile in SCI units. There are a few case reports of CDI in SCI units,16–18 but we are not aware of previous studies that examined rates of asymptomatic carriage in hospitalized patients with SCI. Marciniak et al.18 did investigate the prevalence of toxigenic C. difficile carriage upon admission to a rehabilitation unit that included spinal cord injury patients and found a rate of 16%. Our rate was significantly higher, possibly due to the fact that patients were cultured at various points of hospitalization, with many of the carriers having been hospitalized for weeks to months. It is also possible that the high prevalence of asymptomatic carriage of toxigenic C. difficile could be in part due to ineffective or insufficient environmental cleaning on the ward. However, it is notable that the point-prevalence study was conducted several months after implementation of a facility-wide cleaning intervention that included the use of sodium hypochlorite (i.e. a 1:10 dilution of household bleach) for routine disinfection of all hospital rooms.19 The cleaning intervention resulted in significant reductions in C. difficile and VRE contamination in isolation rooms,19 and with a reduction in the incidence of CDI from 15 to 10 cases per 1000 patient discharges (authors’ unpublished data). We also cannot exclude the possibility that poor compliance with hand washing or glove use contributed to the high prevalence of asymptomatic carriage of toxigenic C. difficile. Based on Infection Control Department surveillance data, the rate of compliance with hand hygiene recommendations on the ward during the year of the study was ∼70%. However, monitoring was only performed during daytime shifts with no evening or weekend monitoring, and no monitoring of glove use was performed.
In our previous study in long-term care, we found that recent antibiotic use and previous CDI were significant risk factors for asymptomatic carriage of toxigenic C. difficile.8 In the present study, we found that 82% of asymptomatic carriers of toxigenic C. difficile had received recent antibiotic therapy, but only 18% had a previous history of CDI. There was a significant association between increased length of stay and asymptomatic carriage of toxigenic C. difficile. Previous studies have demonstrated that increased length of stay is a risk for CDI.6 We also found that presence of indwelling devices was much more common in asymptomatic carriers of toxigenic C. difficile versus non-carriers (64 versus 18%), but this difference was not statistically significant (P = 0.08). Others have recently associated the presence of indwelling devices with more frequent colonization with healthcare-associated antibiotic-resistant pathogens, including VRE and methicillin-resistant Staphylococcus aureus.20,21 The association between devices and colonization by healthcare-associated pathogens may in part be due to confounding factors such as frequent antibiotic use and increased exposure to healthcare workers.20,21
Our study had several limitations. Our population included only male veterans, and therefore additional studies are needed in other settings. The short study period and small number of subjects has the potential to overestimate the actual prevalence of asymptomatic carriage of toxigenic C. difficile in this population, as we may not have been able to study a representative sample of the population. The small number of subjects also limits our ability to analyze the various potential risk factors for asymptomatic carriage of toxigenic C. difficile in this population, and may account for the lack of a significant association between prior antibiotic use and C. difficile carriage. Although the association between length of stay and asymptomatic carriage is plausible and consistent with previous studies,6 this finding should also be interpreted with caution given the small sample size. Another limitation is the use of broth enrichment cultures, which does not allow the quantification of organisms on skin or in the environment. Therefore, our findings do not allow a comparison of the burden of spores associated with CDI versus asymptomatic carriers of toxigenic C. difficile. Finally, although our findings suggest that asymptomatic carriers may contribute to the transmission of C. difficile, molecular typing of strains that are acquired by patients will be necessary to confirm that strains from asymptomatic carriers are being transmitted.
Conclusion
We found that asymptomatic carriage of toxigenic C. difficile and VRE was common among with SCI, and carriers frequently had skin and environmental contamination. Further studies are indicated to determine whether these findings can be generalized to other SCI units.
Acknowledgements
This work was supported by a Merit Review Grant from the Department of Veterans Affairs to C.J.D.
Conflict of interest statement
Dr Donskey has received research support from ViroPharma, Merck, Optimer, Elan, and Ortho-McNeil. All other authors: no conflicts.
References
- 1.Poutanen SM, Simor AE. Clostridium difficile-associated diarrhea in adults. CMAJ 2004;171(1):51–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonald LC, Killgore GE, Thompson A, Owens RC, Kazakova SV, Sambol SP, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med 2005;353(23):2433–41 [DOI] [PubMed] [Google Scholar]
- 3.Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med 2005;353(23):2442–9 [DOI] [PubMed] [Google Scholar]
- 4.Muto CA, Blank MK, Marsh JW, Vergis EN, O'Leary MM, Shutt KA, et al. Control of an outbreak of infections with the hypervirulent Clostridium difficile BI strain in a University Hospital using a comprehensive bundle approach. Clin Infect Dis 2007;45(10):1266–73 [DOI] [PubMed] [Google Scholar]
- 5.Valiquette L, Cossette B, Garant MP, Diab H, Pepin J. Impact of a reduction in high-risk antibiotics on the course of an epidemic of Clostridium difficile-associated disease caused by the hypervirulent NAP1/027 strain. Clin Infect Dis 2007;45Suppl 2:S112–21 [DOI] [PubMed] [Google Scholar]
- 6.Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol 1995;16(8):459–77 [DOI] [PubMed] [Google Scholar]
- 7.Dubberke ER, Gerding DN, Classen D, Arias KM, Podgorny K, Anderson DJ, et al. Strategies to prevent Clostridium difficile infections in acute care hospitals. Infect Control Hosp Epidemiol 2008;29Suppl 1:S81–92 [DOI] [PubMed] [Google Scholar]
- 8.Al-Nassir WN, Sethi AK, Nerandzic MM, Bobulsky G, Jump RL, Donskey CJ. Comparison of clinical and microbiological response to treatment of Clostridium difficile-associated disease with metronidazole and vancomycin. Clin Infect Dis 2008;47(1):56–62 [DOI] [PubMed] [Google Scholar]
- 9.Riggs MM, Sethi AK, Zabarsky TF, Eckstein EC, Jump RL, Donskey CJ. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin Infect Dis 2007;45(8):992–8 [DOI] [PubMed] [Google Scholar]
- 10.Donskey CJ. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin Infect Dis 2004;39(2):219–26 [DOI] [PubMed] [Google Scholar]
- 11.McFarland LV, Coyle MB, Kremer WH, Stamm WE. Rectal swab cultures for Clostridium difficile surveillance studies. J Clin Microbiol 1987;25(11):2241–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bidet P, Lalande V, Salauze B. Comparison of PCR-ribotyping, arbitrarily primed PCR, and pulsed-field gel electrophoresis for typing Clostridium difficile. J Clin Microbiol 2000;38(7):2484–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terhes G, Urban E, Soki J, Hamid KA, Nagy E. Community-acquired Clostridium difficile diarrhea caused by binary toxin, toxin A, and toxin B gene-positive isolates in Hungary. J Clin Microbiol 2004;42(9):4316–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spigaglia P, Mastrantonio P. Molecular analysis of the pathogenicity locus and polymorphism in the putative negative regulator of toxin production (TcdC) among Clostridium difficile isolates. J Clin Microbiol 2002;40(9):3470–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7 Wayne, PA: National Committee for Clinical Laboratory Standards; 2005 [Google Scholar]
- 16.Johnson DK, Balmaseda MT. Pseudomembranous colitis in spinal cord injury. Arch Phys Med Rehabil 1985;66(6):394–6 [PubMed] [Google Scholar]
- 17.Bahadursingh AN, Vagefi PA, Longo WE. Fulminant Clostridium difficile colitis in a patient with spinal cord injury: a case report. J Spinal Cord Med 2004;27(3):266–8 [DOI] [PubMed] [Google Scholar]
- 18.Marciniak C, Chen D, Stein AC, Semik PE. Prevalence of Clostridium difficile colonization at admission to rehabilitation. Arch Phys Med Rehabil 2006;87(12):1086–90 [DOI] [PubMed] [Google Scholar]
- 19.Eckstein BD, Adams DA, Eckstein EC, Rao A, Sethi AK, Yadavalli GK, et al. Reduction of Clostridium difficile and vancomycin-resistant Enterococcus contamination of environmental surfaces after an intervention to improve cleaning methods. BMC Infect Dis 2007;7:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mody L, Maheshwari S, Galecki A, Kauffaman CA, Bradley SF. Indwelling device use and antibiotic resistance in nursing homes: identifying a high-risk group. J Am Geriatr Soc 2007;55(12):1921–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mody L, Kauffman CA, Donabedian S, Zervos M, Bradley SF. Epidemiology of Staphylococcus aureus colonization in nursing home residents. Clin Infect Dis 2008;46(9):1368–73 [DOI] [PMC free article] [PubMed] [Google Scholar]