Abstract
Background/objectives
Chronic pressure ulcers affect patient health, emotional state, and quality of life, causing considerable morbidity and mortality in addition to contributing to significant health care costs from lengthy hospitalizations to advanced home care and surgical care costs. The conventional treatment of these wounds can be slow due to their chronic inflammatory state and the senescence of local reparative cells. Platelet-rich plasma (PRP) therapy has been growing as a viable treatment alternative for a number of clinical applications and has potential benefit for use in chronic wounds. The sustained release of large quantities of autologous growth factors, cytokines, and other mediators found in PRP plus the favorable mononuclear cell profile of PRP may help us to stimulate wound healing and resolve chronic inflammation.
Methods
Three veterans with spinal cord injury (SCI), presenting with chronic stage IV pressure ulcers, were treated with a sustained release PRP therapy to stimulate wound healing.
Results
PRP treatment consistently resulted in the formation of granulation tissue and improved vascularity for each of the three patients treated, while reducing the overall ulcer area and volume.
Conclusion
The controlled release of growth factors from PRP demonstrated a positive stimulatory effect on the healing rate of chronic pressure ulcers in individuals with SCI.
Keywords: Spinal cord injuries, Pressure ulcers, Tetraplegia, Paraplegia, Wound healing, Platelet-rich plasma therapy
Introduction
Patients suffering from spinal cord injury (SCI) are at lifelong risk of developing pressure ulcers. These ulcers can occur in any setting, the patient's home, hospital, or care facility, and are a cause of great distress for both patients and caregivers alike. These wounds are typically non-healing, resulting in a downward spiral of chronic inflammation, which can be a source of morbidity and even mortality in immobile populations. For this reason, patients presenting with such wounds are commonly subject to lengthy hospitalizations, leading to significant decreases in quality of life, social isolation, emotional stress, and depression.1–3
Chronic ulcers are also a source of great financial burden on the health care system. It has been estimated that 60% of patients with SCI will develop pressure ulcers,2 with an estimated cost of $70 000 to treat a single full-thickness wound.4 Such costs are likely underestimated due to the indirect costs: absence from work, loss of employment, cost of medical transport, assistance with daily living, and self-care and medication expenditures. In all, it has been estimated that nearly $5 billion are spent annually in the United States to treat pressure ulcers.3 Promoting accelerated healing of pressure ulcers would provide an improvement of patient quality of life and reduce the economic impact that chronic wounds have on the health care system.
Platelet-rich plasma (PRP) therapy is a method for collecting and concentrating autologous platelets for the purpose of activating and releasing their growth factor-rich alpha- and dense granules. The discharge of these concentrated granules releases a number of growth factors and cytokines in physiologically relevant ratios (albeit in concentrations several times higher than that of normal blood) that are critical to tissue regeneration and cellular recruitment: platelet-derived growth factor, transforming growth factor-beta, vascular endothelial growth factor, fibroblast growth factor, epidermal growth factor, etc.5–11 It has also been documented that PRP contains a class of anti-inflammatory mediators known as lipoxins, which are known to promote resolution of inflammation.5 Additionally, PRP provides a mononuclear cell content that is more favorable to tissue healing versus the acute inflammatory cell profile in normal blood. PRP therapy is currently in use clinically to stimulate tissue growth and regeneration and has been demonstrated to be effective in accelerating repair in osteochondral defects,6,11,12 tendon/ligament injuries6,10–15), and in chronic skin wounds.7,11,12,16
There have been several methods reported in the literature on successfully delivering PRP to an injury site; most involve the creation of a platelet gel using thrombin6,11,12,17 or CaCl2.6,9,11,12 These PRP gels are then easily applied to wound sites through injection or topical application. However, studies have shown that the use of thrombin as a clotting agent can result in a rapid activation of platelets and a bolus release of growth factors with 70% of the growth factors released within 10 minutes of clotting and nearly 100% released within 1 hour.6 This ‘dumping’ method fails to maximize the cell-stimulating potential of the PRP growth factors as most are cleared before they can take effect.18
The use of a sustained release method for delivering PRP growth factors and cytokines would be highly advantageous in a chronic wound state where senescent cells are prevalent,3 and could benefit from the sustained presence of stimulating factors. Studies performed in vitro have shown that alginate beads were successful in delivering (based on cell proliferation) PRP-derived growth factors and cytokines over the course of 14 days.18 Alginate is a biomaterial, typically derived from seaweed or algae, which is commonly used as a wound dressing in the treatment of pressure ulcers and has a long history of biocompatibility.1,19,20
The purpose of this report is to demonstrate, through a small three-person case study, the results of using CaCl2-activated PRP and PRP containing alginate beads as a delivery vehicle for the sustained release of PRP-derived growth factors and cytokines to stimulate healing in stalled pressure ulcers where conventional treatment methods have failed.
Methods
Three SCI patients presenting with chronic stage IV pressure ulcers were treated with CaCl2-activated PRP and sustained release PRP therapy to stimulate healing and promote tissue repair. Upon admission, these patients were treated with conventional ulcer care methods including antibiotics for evidence of infection, surgical and bedside debridement with mechanical stimulation, and saline wet to dry dressings. One patient additionally underwent wound vacuum therapy and the use of an allograft skin graft. The ulcers responded to the initial treatments but their healing rates measured by surface area and depth appeared to stall or plateau over time prompting consideration for ulcer stimulation therapy using PRP.
Prior to treatment, a 2% alginate solution18 was created under sterile conditions by adding alginate powder (Acros Chemicals, Geel, Belgium) to sterile water and passing the solution through a luer-lock syringe filter with 0.22 micron diameter pores (Millipore Millex, Billerica, MA, USA) to sterilize the solution. This sterile solution was aliquoted for individual patient use and stored refrigerated at 4°C in sealed sterile containers until use.
At the bedside, peripheral venous blood was drawn (54–112 ml) and PRP was created using a SmartPReP® 2 (Harvest Technologies Corp, Plymouth, MA, USA) centrifugation system as per manufacturer's protocol. Patients were turned to position the sacral or greater trochanter ulcers in the horizontal plane (prone to sidelying) as much as possible. For injection into the ulcer bed and margins, a small volume of PRP (1–5 ml based on ulcer size) was mixed in a 10:1 v/v ratio with 10% CaCl2 to reverse the effect of anticoagulant used during the blood draw and to slowly activate platelets. The CaCl2-activated PRP was injected through a 25-gauge needle along the ulcer margins at 0.5–1-cm intervals in small amounts (<0.1 ml). Similar small volumes of PRP were injected into the ulcer base focusing on the more fibrous-appearing regions of the base lacking granulation tissue. As the injected PRP was exposed to collagen in the ulcer bed, slow conversion to surface clot was observed. The smaller diameter opening margins in two patients with undermined ulcers were not injected to avoid premature closure before infilling of the ulcer bed had occurred.
The remaining PRP was combined with the sterile alginate solution in a 2:1 ratio (based upon unpublished empirical laboratory data) to create PRP:alginate beads for sustained release of growth factors over a 7-day period as they dissolved. A PRP:alginate solution was then slowly added drop-wise through the tip of a 20 ml sterile syringe to a sterile steel bowl containing 70 ml of CaCl2. As Ca2+ ions were transferred from the CaCl2 bath to the PRP:alginate droplets, a solid bead structure was formed. This gelation process was allowed to continue for 5 minutes, and the beads were then filtered through sterile gauze and retained for use in the wound.
All patients received consultation with a staff nutritionist, with an emphasis on high protein meals throughout their hospitalization.
Results
Case 1
Patient 1 is a 38-year-old veteran with a 15-year history of T6 American Spinal Injury Association (ASIA) A paraplegia impairment. Since that time he has had multiple hospitalizations for complications for pressure ulcers over his bilateral greater trochanters, sacrum, and ischium.
Most recently, he was admitted after a period of immobility and change to his pressure relief schedule that resulted in bilateral greater trochanter and posterior pelvis pressure ulcers. Initially he was admitted to an outside hospital where he underwent surgical debridement and was started on IV antibiotics for suspected osteomyelitis. At the time of transfer to our VAMC SCI unit, the pressure ulcer on the right greater trochanter was noted to be stage IV with fibrous and necrotic base measuring 17 × 14 × 4.5 cm3. He underwent surgical debridement in addition to conservative treatment ranging from daily dressing changes using irrigants from Dakin's solution to normal saline and enzymatic debridement using products such as collagenase. Initially there was improvement in ulcer healing with measurements decreasing to 12 × 10 × 2.25 cm3, but over time the healing process slowed prompting consultation for PRP treatment (Fig. 1). Patient pre-albumin levels (mg/dl) are included in Table 1. The patient suffered from hypogonadism and was treated with a transdermal testerone patch throughout treatment.
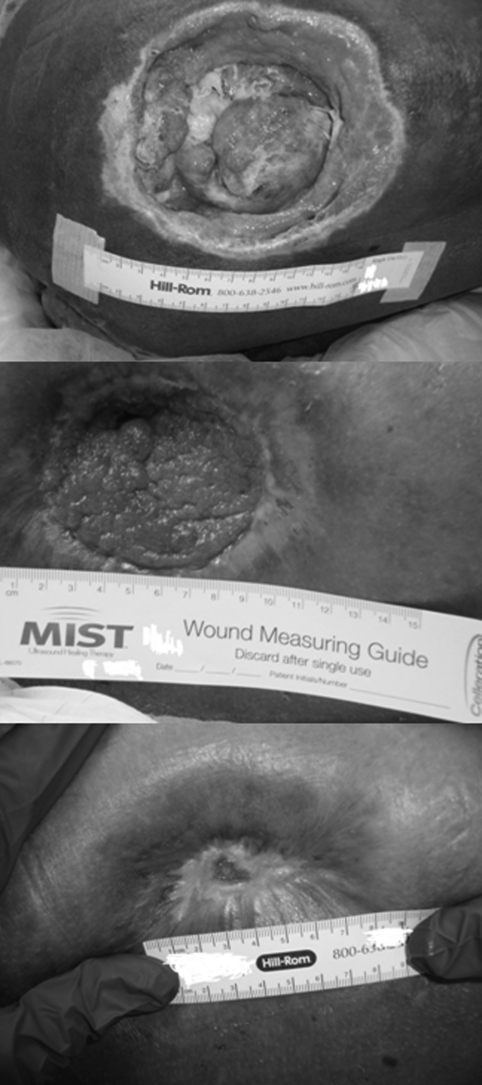
Figure 1.
Photograph of patient 1′s right trochanteric pressure ulcer at the time of admission (top), before treatment 5 at week 13 (middle), and 8 weeks after the 11th PRP treatment (bottom).
Table 1.
Albumin levels (mg/dl) at admission, prior to the first treatment of PRP, and at their highest and lowest points during treatment
| Admission | Prior to the first PRP treatment | Treatment high | Treatment low | |
|---|---|---|---|---|
| Case 1 | 10.7 | 16.8 | 26.8 (31) | 10.0 (16) |
| Case 2 | 27.1 | 24.9 | 44.5 (37) | 9.8 (21) |
| Case 3 | 8.8 | 27.4 | 33.3 (18) | 8.8 (0) |
The week of treatment is given in parentheses.
The patient underwent a total of 11 PRP treatments over a span of 18 weeks (performed weekly or bi-weekly). As described above, these treatments involved injection of activated PRP into the margins of the wound bed as well as packing of the wound with beads of PRP and alginate (2:1 ratio of PRP to alginate) for the sustained release of growth factors. Dressings consisted of a layer of silicone Mepitel dressing (Molnlycke Health Care, Norcross, GA, USA) to hold beads in place while allowing wound exudate to diffuse freely, a double layer of Restore calcium alginate dressing (Hollister Woundcare, Libertyville, IL, USA) to absorb wound exudates, and a sealing layer of Tegaderm topical transparent dressing (3M Healthcare, St. Paul, MN, USA). The top layers of the dressing were changed as needed (typically 2–3 days), while the Mepitel and bead layers were left undisturbed until subsequent PRP treatment.
Over the course of the PRP treatment, the wound became noticeably vascularized and filled with granulation tissue. On one occasion the degree of granulation tissue layering was so robust that silver nitrate treatment to more prominent islands of tissue within the ulcer bed was performed by the wound care team. The results of the changes in wound surface area and volume measurements are shown in Fig. 2. The wound was essentially completely filled in with granulation tissue after eight PRP treatments (beads and injections), with only epithelialization of the new tissue remaining. As such, the subsequent three treatments involved only the injection of activated PRP into the wound bed and along the wound margins. The right trochanter ulcer progressed to the point that outpatient and home care management would be appropriate, but he remained hospitalized due to the presence of other pressure ulcers, notably a stage IV ulcer on the left greater trochanter. This ulcer failed to exhibit any significant healing (granulation formation, increased vascularity, etc.) with continued conservative treatments, and served as a pseudo-control.
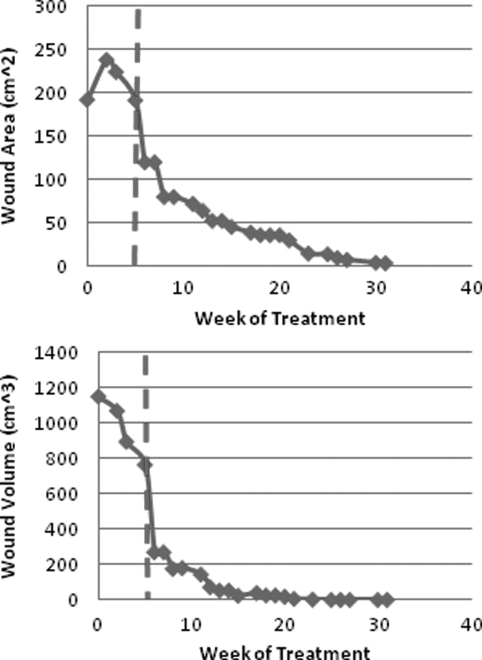
Figure 2.
Graph of changes in wound surface area (top) and volume (bottom) for case 1 following treatment with PRP. The dashed line indicates onset of PRP treatment.
Case 2
Patient 2 is a 51-year-old veteran with a 1-year history of C4 level ASIA A tetraplegia impairment. He was initially treated at an outside hospital for medical and surgical stabilization of his injuries and transferred to our SCI unit for rehabilitation care.
At the time of admission to our VAMC SCI unit, he had an unstageable sacral pressure ulcer that measured 6 × 4 × 0.5 cm3 with yellow slough that covered the base of the wound bed. The wound care team began following treatment of the patient and began twice daily irrigations of the wound with Dakin's 0.25% and applications of collagenase, along with placement in a pressure reduction bed. The dimensions of the wound did not show significant change with conservative treatment and the depth of the wound continued to increase over the next several weeks. The depth of the wound advanced to 4 cm with an additional 5 cm of undermining before wound vacuum therapy was initiated. Wound vacuum therapy was continued for 2 months and wound size decreased to 3 × 3 × 3 cm3 with 4.5 cm of undermining. Additionally, a cryopreserved human skin allograft with both epidermis and dermis layers (Theraskin™ Soluble Systems, LLC, Newport News, VA, USA) was placed on the wound to promote healing. However, healing progress stalled and consultation for PRP was requested. Patient pre-albumin levels (mg/dl) are included in Table 1.
The patient underwent a total of 10 PRP treatments that consisted primarily of controlled release PRP:alginate beads due to the deeply undermined nature of the wound. Six of the treatments included CaCl2-activated PRP injections into the ulcer cavity base. At each treatment the wound was irrigated and packed to capacity with PRP beads following PRP injection if done. Platelet-poor plasma (PPP), a side product of the dual centrifugation process containing growth factors and fibrin, was gelled by combining it with human recombinant thrombin (Recothrom, ZymoGenetics, Seattle, WA, USA, 10:1 v/v ratio) to hold the beads in place inside the wound. Dressings and changes were identical to case 1.
The results of this treatment are shown in Fig. 3. Due to the undermined nature of this wound, it was not possible to determine an accurate surface area measurement, so only wound volume was reported. As the wound depth decreased the volume measurement became less accurate and we elected to use measured saline infilling of the ulcer cavity before overflow to assess the volume.
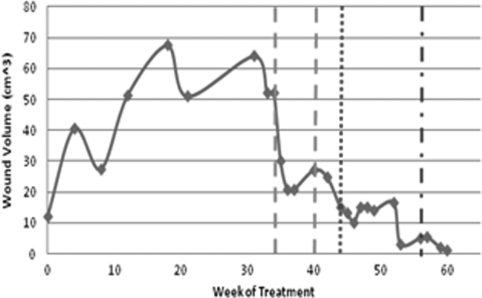
Figure 3.
Graph of changes in wound volume for case 2 following treatment with wound vacuum therapy (dashed lines) and PRP treatment (dotted line). The mixed (dash and dot) line indicates the point at which saline infilling was used for volume measurement.
Case 3
Patient 3 is a 61-year-old male veteran with a 30-year history of C7 ASIA A tetraplegia impairment. He has had multiple hospitalizations for related complications, most commonly for pressure ulcers. He was admitted to our SCI unit with a stage IV right gluteal ulcer measuring 5 × 5 cm2. Patient pre-albumin levels (mg/dl) are included in Table 1. The patient suffered from hypogonadism, which was not treated during the duration of treatment.
Initial wound care treatment consisted of irrigation of the wound with Anasept spray and application of collagenase with daily dressing changes and packing of the undermining volume that had developed. The patient was also placed in a pressure reduction bed. Over the next 2 months, the size of the ulcer changed to 3.5 × 2 × 3.25 cm3 with 6 cm of undermining. Due to the perceived stalling of the healing process PRP treatment was initiated.
The patient has undergone a total of five PRP treatments consisting primarily of beads, due to the undermined nature of the wound, as well as two wound ulcer cavity base injections with CaCl2-activated PRP. The bead protocol used was identical to that of case 2, with gelled PPP used in the wound to hold the beads in place. Dressings and changes were identical to those used in case 1.
The results of this treatment are shown in Fig. 4. Due to the undermined nature of this ulcer, it was not possible to determine an accurate surface area measurement, so only ulcer volume was reported and we used saline filling of the ulcer cavity to estimate volume.
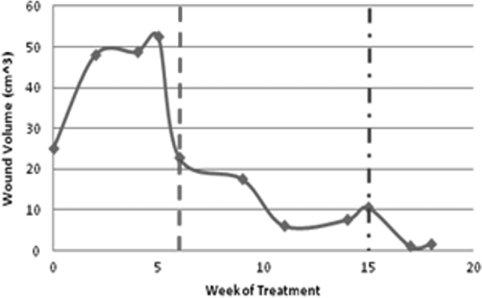
Figure 4.
Graph of changes in wound volume for case 3 following treatment with PRP. Onset of PRP treatment is marked with a dashed line, while the mixed (dash and dot) line indicates the point at which saline infilling was used for volume measurement.
Discussion
The results of the right trochanter ulcer's response to the PRP therapies described in case 1 support the hypothesis that this stimulation therapy can trigger an ulcer healing response when the degree of healing appears to have stalled. The ulcer bed more rapidly demonstrated margin in-growth, granulation tissue development, vascularization and epithelialization. With all three ulcers, the appearance of ulcer bed bleeding during dressing changes and ulcer palpation was noted and is seen as a positive indicator for wound healing. The right trochanter ulcer, without undermining, progressed after eight alginate bead therapies, providing sustained growth factor and mononuclear cell release combined with wound margin and base injections with CaCl2-activated PRP. Once the ulcer base had in-filled, three additional CaCl2-activated PRP treatments to the wound margins and base resulted in an ulcer that could be managed more conservatively in a community setting.
The two patients with undermined ulcers responded to the treatment with considerable volume reduction in the ulcer size but the degree of undermining and trend toward full closure appears to be slower than our experience with the non-undermined ulcer. Both ulcers were in more pressure likely areas (buttocks, sacrum) that might have contributed to the slower in-growth. Additionally, the degree of undermining and cavity volume suggests an overall lack of local cells to promote tissue in-growth through growth factor stimulation. The senescent state and reduced vascularity of these ulcers, coupled with a hypothetical reduction in peripheral blood stem cell content in chronic SCI as seen in other chronic diseases such as depression, hypothyroidism and atherosclerotic disease, may contribute to further delays in ulcer healing despite growth factor stimulation.21–23
However, both undermined ulcers demonstrated a reduction in volume and an increase in granulation tissue with vascularity to suggest that they might respond to surgical flap care with more favorable responses than prior to the PRP treatments.
Conclusion
The use of PRP therapy, involving a combination of sustained and immediate release of growth factors, appeared to stimulate acceleration of healing in three stalled pressure ulcers in hospitalized SCI patients. Significant in-growth and closure of one ulcer that was large but without undermining characteristics took place to the point where that ulcer would not require further in-hospital care. Two pressure ulcers with deep undermining below the ulcer opening demonstrated a reduction in volume along with more granulation tissue in-growth and vascularity. A larger-scale study is indicated to further our understanding of the role of PRP therapy in the treatment of chronic pressure ulcers in patients with SCI.
References
- 1.Pressure ulcer prevention and treatment following spinal cord injury: a clinical practice guideline for health-care professionals. The Consortium of Spinal Cord Medicine, ed. Paralyzed Veterans of America, 2000:80 [DOI] [PubMed] [Google Scholar]
- 2.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 2004;9:283–9 [DOI] [PubMed] [Google Scholar]
- 3.Medina A, Scott PG, Ghahary A, Tredget EE. Pathophysiology of chronic nonhealing wounds. J Burn Care Rehabil 2005;26(4):306–19 [DOI] [PubMed] [Google Scholar]
- 4.Garber SL, Rintala DH. Pressure ulcers in veterans with spinal cord injury: a retrospective study. J Rehabil Res Dev 2003;40(5):433–41 [DOI] [PubMed] [Google Scholar]
- 5.El-Sharkawy H, Kantarci A, Deady J, Hasturk H, Liu H, Alshahat M, et al. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol 2007;78(4):661–9 [DOI] [PubMed] [Google Scholar]
- 6.Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med 2009;37(11):2259–72 [DOI] [PubMed] [Google Scholar]
- 7.Rozman P, Bolta Z. Use of platelet growth factors in treating wounds and soft-tissue injuries. Acta Dermatovenerol Alp Panonica Adriat 2007;16(4):156–65 [PubMed] [Google Scholar]
- 8.Everts PA, Knape JT, Weibrich G, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol 2006;38(2):174–87 [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez M, Anitua E, Orive G, Mujika I, Andia I. Platelet-rich therapies in the treatment of orthopaedic sport injuries. Sports Med. 2009;39(5):345–54 [DOI] [PubMed] [Google Scholar]
- 10.Creaney L, Hamilton B. Growth factor delivery methods in the management of sports injuries: the state of play. Br J Sports Med. 2008;42:314–20 [DOI] [PubMed] [Google Scholar]
- 11.Alsousou J, Thompson M, Hulley P, Noble A, Willett K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br 2009;91(8):987–96 [DOI] [PubMed] [Google Scholar]
- 12.Anitua E, Sanchez M, Orive G, Andia I. Delivering growth factors for therapeutics. Trends Pharmacol Sci 2008;29(1):37–41 [DOI] [PubMed] [Google Scholar]
- 13.Lyras DN, Kazakos K, Verettas D, et al. The effect of platelet-rich plasma gel in the early phase of patellar tendon healing. Arch Orthop Trauma Surg. 2009;129(11):1577–82 [DOI] [PubMed] [Google Scholar]
- 14.Murray MM, Spindler KP, Abreu E, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res 2007;25:81–91 [DOI] [PubMed] [Google Scholar]
- 15.Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med 2006;34(11):1774–8 [DOI] [PubMed] [Google Scholar]
- 16.Anitua E, Aguirre JJ, Algorta J, et al. Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. J Biomed Mater Res B 2008;84(2):415–21 [DOI] [PubMed] [Google Scholar]
- 17.Kocaoemer A, Kern S, Kluter H, Bieback K., Human AB. Serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells 2007;25(5):1270–8 [DOI] [PubMed] [Google Scholar]
- 18.Lu HH, Vo JM, Chin HS, et al. Controlled delivery of platelet-rich plasma-derived growth factors for bone formation. J Biomed Mater Res A 2008;86(4):1128–36 [DOI] [PubMed] [Google Scholar]
- 19.Wang N, Adams G, Buttery L, Falcone FH, Stolnik S. Alginate encapsulation technology supports embryonic stem cells differentiation into insulin-producing cells. J Biotechnol 2009;144(4):304–12 [DOI] [PubMed] [Google Scholar]
- 20.Zimmermann H, Zimmermann D, Reuss R, et al. Towards a medically approved technology for alginate-based microcapsules allowing long-term immunoisolated transplantation. J Mater Sci Mater Med 2005;16(6):491–501 [DOI] [PubMed] [Google Scholar]
- 21.Shakoor SK, Aldibbiat A, Ingoe LE, et al. Endothelial progenitor cells in subclinical hypothyroidism: the effect of thyroid hormone replacement therapy. J Clin Endocrinol Metab 2010;95(1):319–22 [DOI] [PubMed] [Google Scholar]
- 22.Fadini GP, Coracina A, Baesso I, et al. Peripheral blood CD34+KDR+ endothelial progenitor cells are determinants of subclinical atherosclerosis in a middle-aged general population. Stroke 2006;37(9):2277–82 [DOI] [PubMed] [Google Scholar]
- 23.Dome P, Teleki Z, Rihmer Z, et al. Circulating endothelial progenitor cells and depression: a possible novel link between heart and soul. Mol Psychiatry 2009;14(5):523–31 [DOI] [PubMed] [Google Scholar]