Abstract
Skin injury is a common side effect of breast-cancer radiation therapy. Although physicians often observe skin toxicity, quantifying its severity remains a challenge. We present a novel quantitative ultrasonic technique to evaluate skin changes associated with radiotherapy. An in vivo study with twelve breast-cancer patients was conducted. All patients received a standard course of post-surgery radiation therapy. Each patient received ultrasound scans to the irradiated breast and the untreated (contra-lateral) breast. Radio-frequency (RF) backscatter signals and B-mode images were acquired simultaneously. To quantify the severity of skin injury, two metrics were calculated from the RF signals: skin thickness and Pearson correlation coefficient of the subcutaneous layer. Comparing to the non-irradiated skin, the average thickness of the irradiated skin increased by 40% (p=0.005) and the average correlation coefficient of the irradiated hypodermis decreased by 35% (p=0.02). This study demonstrates the feasibility of using a non-invasive ultrasonic technique to detect and quantify radiation-induced skin changes.
1. Introduction
The management of breast cancer with surgery (lumpectomy) and radiation therapy has been the preferred standard of care for most women with early-stage breast cancer [1–3]. In spite of the advances in radiation therapy technology, high doses of ionizing radiation often induce acute and late skin effects. Skin effects such as erythema, dermal atrophy, fibrosis, and retraction, may result in undesirable cosmetic changes and pain [4].
During the course of radiation treatment and in follow-up, clinical assessment of radiation toxicity includes visual observation and palpation of the treatment site. Radiation toxicity is often graded using standardized scoring systems, e.g. the Radiation Therapy Oncology Group (RTOG) Radiation Morbidity Scoring Scheme (Table 1) [5]. Although quantitative, these scoring systems are still subjective. In this paper, we present an ultrasonic technique that measures radiation-induced dermal changes.
Table 1.
RTOG Radiation Morbidity Scoring Scheme
| Skin late toxicity | |
|---|---|
| Grade 0 | None |
| Grade 1 | Slight atrophy, pigmentation change, some hair loss |
| Grade 2 | Patchy atrophy, moderate telangiectasia, total hair loss |
| Grade 3 | Marked atrophy, gross telangiectasia |
| Grade 4 | Ulceration |
Any toxicity which caused death is graded 5.
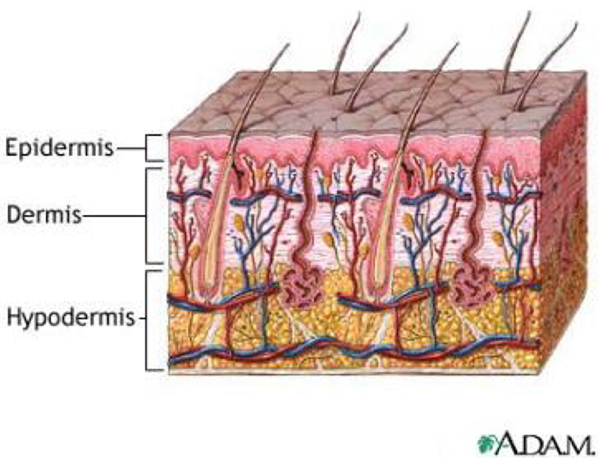
Skin consists of three layers: epidermis, dermis, and hypodermis (subcutaneous layer) (Fig. 1) [6]. Histology studies have shown that radiation induces within these layers endothelial cell loss, vesicular loss, and fibrosis [7]. In our study, we employed a clinical ultrasound scanner to measure the anatomical and structural changes of the irradiated skin. Our technique utilized the backscattered radio-frequency (RF) signals and computed two parameters: the skin thickness and Pearson correlation coefficient of the hypodermis. We have implemented this technology in our clinic and to date twelve breast-cancer patients have been examined.
FIG. 1.
Schematic diagram of the three layers of skin: epidermis, dermis, and hypodermis.
2. Method and Materials
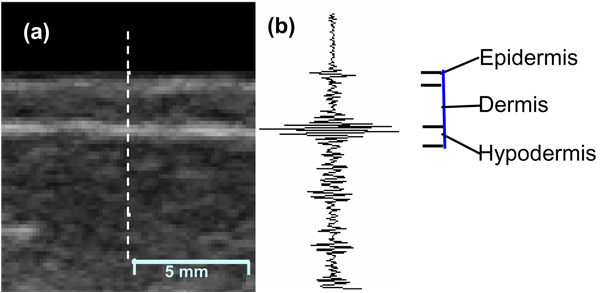
A clinical ultrasound scanner (Sonix RP system) with a linear array probe (L14-5/38) was used for data acquisition. The probe was operated at a nominal frequency of 6 MHz. Conventional B-mode images as well as the backscattered RF signals were captured simultaneously. The B-mode images show anatomical structure and were used to identify the skin layers. The RF data were acquired with a 20 MHz sampling frequency. Figure 2 shows a B-mode breast image and the corresponding RF data at the central line.
FIG. 2.
(a). B-mode breast image showing three skin layers (epidermis, dermis, and hypodermis) and subcutaneous tissue. (b). Backscatter RF signal corresponding to the central line (the dotted line).
Twelve breast-cancer patients who had completed radiation therapy were enrolled in our IRB-approved study. All patients received a standard course of post-surgery radiation: the whole diseased breast received a total dose of 50-50.4 Gy followed by an electron boost of 10–16 Gy at the lumpectomy site. Follow up time was 6 to 94 months with a median time of 22 months. Each patient received ultrasound scans of the irradiated breast and of the untreated contra-lateral breast. The ultrasound examinations took 5 to 10 minutes.
On the post-radiation breast ultrasound B-mode images, we have often observed two phenomena: 1) an overall thickening of the skin and 2) blurring and loss of uniformity within the hypodermis layer. We felt these observations warranted further investigation. In this study, we used two ultrasonic metrics to study the phenomena in a systematic manner.
2.1. Skin thickness estimation
Although skin thickness can be read directly from the B-mode images, these measures are subject to error introduced by the assumption of a constant speed of sound, 1540 m/s, for all tissue types. We calculated skin thickness from the RF signals using a speed of sound specific for breast skin of 1640 m/s [8]. We used the arrival time between the RF echo from the anterior (epidermis) and posterior skin layer (hypodermis). Skin thickness is computed by using the equation, D = v M / 2 fs, where v is the speed of sound, M is the sample points and fs is the sampling frequency. In data acquisition, sample points were collected at .05 µs intervals along the wave propagation direction (depth).
2.2. Hypodermis damage assessment
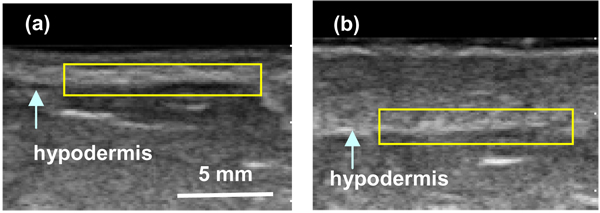
The conventional B-mode images displayed anatomical structure of the breast and were used to identify the hypodermis region of interest (ROI), as shown in Fig. 3. We manually placed each ROI for the analysis. All of the computations were performed on the raw RF data within the ROI. Each ROI consists of m sample points and n scan lines. A Hilbert transform was applied to the RF scan lines. The absolute value of the transformed data for each scan line, si, was then used as a basis for the Pearson correlation coefficient (Equation 1).
FIG. 3.
Ultrasound B-mode breast images with hypodermis ROI demarked (yellow). (a) Non-irradiated (normal) breast, and (b) Irradiated breast.
The Pearson correlation coefficient between scan lines i and j is defined by:
| (1) |
where s̄i and s̄j are the sample means, σi and σj arethe standard deviations and the sum is over m sample points. The average correlation coefficient over n scan lines, ξ = <ξi,j> reflects the variability in the acoustic impedance. Mathematically, the correlation coefficient ξ ranges from 0 to 1, where 0 represents no correlation and 1 represents perfect correlation. For a planar reflector, the correlation coefficient is close to 1. Healthy skin has well-defined layers of epidermis, dermis, and hypodermis. Therefore, we anticipate a higher ξ for normal skin. As cellular fibrosis develops post radiation, hypodermis layer becomes blurred or irregular. This will result in a decrease in the correlation coefficient along the hypodermis.
3. Results
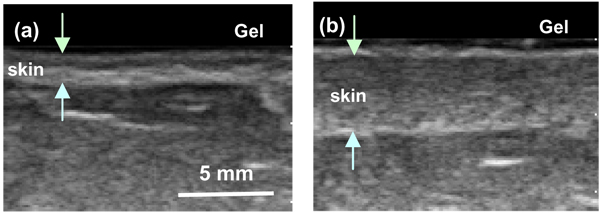
Figure 4 shows the B-mode images of a 57 old breast-cancer patient one year post radiation therapy. Significant skin thickening was observed on the treated breast (Fig. 4b) relative to the untreated breast (Fig. 4a). For all patients, we calculated the thickness, D, for both the irradiated and the contra-lateral (untreated) breasts (Table 2). Out of twelve patients, eleven experienced skin thickening on the irradiated breast. For the skin thickness, the normal skin ranged from 1.93 to 2.75 mm while the irradiated skin ranged from 2.01 to 5.82 mm. The average thickness of the irradiated skin increased to 3.3 ± 1.4 mm over a baseline reading of 2.2 ± 0.4 mm for un-irradiated skin. The average increase in skin thickness was 0.88 mm (40%), with a paired t-test of p = 0.005.
FIG. 4.
B-mode breast images of a patient one year post radiation therapy. (a) Non-irradiated breast (skin thickness 2 mm); and (b) Irradiated breast, (skin thickness 5 mm).
Table 2.
Ultrasonic measurements of the irradiated skin vs. the non-irradiated skin for twelve patients.
| PT | Skin thickness D (mm) |
Correlation coefficient (ξ) |
||
|---|---|---|---|---|
| Normal tissue | Irradiated tissue | Normal tissue | Irradiated tissue | |
| 1 | 1.93 | 2.09 | 0.35 | 0.15 |
| 2 | 2.67 | 5.82 | 0.14 | 0.19 |
| 3 | 2.62 | 4.39 | 0.33 | 0.22 |
| 4 | 2.46 | 4.76 | 0.24 | 0.01 |
| 5 | 2.17 | 4.76 | 0.23 | 0.13 |
| 6 | 2.38 | 2.75 | 0.34 | 0.26 |
| 7 | 2.17 | 2.13 | 0.42 | 0.36 |
| 8 | 2.09 | 3.24 | 0.10 | 0.15 |
| 9 | 2.75 | 3.24 | 0.32 | 0.16 |
| 10 | 1.31 | 2.38 | 0.15 | 0.21 |
| 11 | 1.56 | 1.68 | 0.20 | 0.19 |
| 12 | 1.93 | 2.01 | 0.39 | 0.12 |
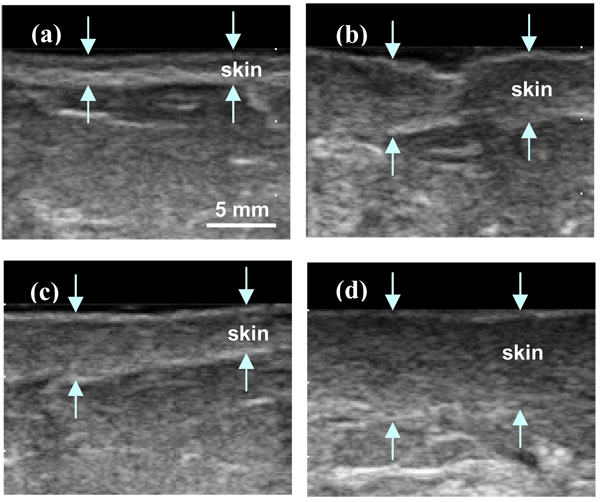
Figure 5 shows B-mode images of normal breast (Fig. 5a) and irradiated breast (Fig. 5b, c and d). We can observe the irregularity of the hypodermis layer on Figures 5b and 5c. These result in the fluctuations in the RF scan-line data. Figure 5d shows the loss of endothelial cells where the hypodermis seems blurred. This results in the amplitude decreases of RF echo signal along the hypodermis boundary. We calculated the Pearson correlation coefficient along the hypodermis layer for irradiated and non-irradiated breast. We manually chose ROI on the B-mode images and RF within the ROI was used for computation (Fig. 3). In this study, all ROIs had the same dimensions of 50 sample points (2 mm depth) by 80 scan lines (12 mm width). For the twelve patients examined, nine had a lower Pearson correlation coefficient in the irradiated breast. The Pearson correlation coefficient of irradiated and non-irradiated hypodermis ranged from 0.01 to 0.36, and 0.10 to 0.42, respectively. The irradiated hypodermis had an average correlation coefficient of 0.18 ± 0.08 vs. the untreated (normal) hypodermis of 0.27 ± 0.10 (Table 2). The average correlation coefficient decreased by 0.09 (35%), with a paired t-test of p = 0.02.
FIG. 5.
B-mode breast images of non-irradiated (normal) skin (a), and irradiated skin (b), (c), and (d). (The arrows demarcate the region of the skin.)
Overall, both ultrasonic metrics demonstrated significant changes for the irradiated skin. It should be noted that there are large patient variations in normal tissue, as shown with the contra-lateral measures (Table 2). It should also be noted that the two ultrasonic metrics are independent. In other words, those patients that experience dramatic skin thickening post-radiation therapy may not have substantial change in the Pearson correlation coefficient of hypodermis.
4. Discussions
Radiation therapy causes injury to the normal tissue inside the treatment field. Although physicians can recognize the skin toxicity, quantifying the severity presents a challenge [9–10]. To address this clinical need, several groups have investigated the use of ultrasonic measurements, e.g. integrated backscatter, attenuation, and viscoelasticity, to characterize irradiated skin [11, 12]. These ultrasonic parameters may yield reproducible and objective measures of early and late radiation-induced skin injury.
We are conducting a series of multi-modality imaging studies to gain a better understanding of acute and late tissue toxicity in radiotherapy. In this paper, we report our experience on the use of quantitative ultrasonic imaging. Our methodology is motivated by our clinical observations on the B-mode images of post-radiation breast-cancer patients.
We propose a new approach to analysis of ultrasound RF echo signals to extract skin architectural features for the detection of skin injury associated with radiation. Two ultrasonic metrics, skin thickness and the correlation coefficient of hypodermis, were used. In our in vivo study with twelve post-radiation breast-cancer patients, significant changes were found in both parameters of irradiated skin relative to the non-irradiated skin.
The thickening of dermis post-radiation is very well-known and it can be measured directly from the B-mode images. We computed the skin thickness using RF signals. B-mode images are based on a constant speed of sound 1540 m/s for soft tissue, including skin, fat, and muscle. However, studies have shown that breast skin has a higher sound speed of 1640 m/s [8]. Our technique uses this speed, therefore produces more accurate measurement of skin thickness.
We introduced a novel ultrasonic parameter, the Pearson correlation coefficient, to measure the correlation of the RF signals along the hypodermis layer. As a result of radiation toxicity such as cellular fibrosis, the irradiated hypodermis exhibited more irregularity in thickness and loss of contrast on the B-mode images. Mathematically, the RF echo signals would be more random within the hypodermis region. As expected, the irradiated hypodermis had lower Pearson correlation coefficient compare to the normal hypodermis.
Our ultrasonic method measures the skin thickness and the damage to the hypodermal layer. These ultrasonic metrics directly relate to the architectural features of the skin. The structural features are often related to the physiological state of the skin. Our initial study suggests that we could achieve an objective evaluation and follow-up of radiation-induced skin changes.
Although this technique is currently being developed to evaluate radiation-induced skin changes in breast, it can be easily adapted to measure skin toxicity for other anatomical sites, such as head and neck. For future work, we will evaluate the reliability and sensitivity of our ultrasonic method. This pilot study provided us a basis for the design of future clinical study. We will continue to explore new ultrasonic parameters to better quantify the severity of radiation-induced normal tissue injury.
5. Conclusion
We have developed a novel quantitative ultrasonic imaging technique to measure radiation-induced skin changes. Our ultrasonic metrics, skin thickness and the correlation coefficient of the subcutaneous layer, directly measure the characteristic architectural properties of the skin. These assessments are non-invasive and objective. It may provide a reliable tool to monitor acute and late skin toxicity in radiation therapy.
Acknowledgement
The authors would like to thank Drs. Lianjie Huang and Zheng Feng Lu for the helpful discussions. This research was supported in part by National Cancer Institute Grant CA114313, Columbia University Women at Risk, Sindab pilot award and Varian Medical Systems.
Reference
- 1.Coles CE, Moody AM, Wilson CB, Burnet NG. Reduction of radiotherapy-induced late complications in early breast cancer: the role of intensity-modulated radiation therapy and partial breast irradiation. Part I – normal tissue complications. Clin Oncol (R Coll Radiol) 2005;17:16–24. doi: 10.1016/j.clon.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, Aguilar M, Marubini E. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 3.Geiger AM, Thwin SS, Lash TL, Buist DS, Prout MN, Wei F, Field TS, Ulcickas Yood M, Frost FJ, Enger SM, Silliman RA. Recurrences and second primary breast cancers in older women with initial early-stage disease. Cancer. 2007;109:966–974. doi: 10.1002/cncr.22472. [DOI] [PubMed] [Google Scholar]
- 4.Back M, Ahern V, Delaney G, Graham P, Steigler A, Wratten C. Absence of adverse early quality of life outcomes of radiation therapy in breast conservation therapy for early breast cancer. Australas Radiol. 2005;49:39–43. doi: 10.1111/j.1440-1673.2005.01392.x. [DOI] [PubMed] [Google Scholar]
- 5.Dische S, Warburton MF, Jones D, Lartigau E. The recording of morbidity related to radiotherapy. Radiother Oncol. 1989;16:103–108. doi: 10.1016/0167-8140(89)90026-1. [DOI] [PubMed] [Google Scholar]
- 6.Lafferty M, Panella S. ADAM Interactive Anatomy. Atlanta, GA: A.D.A.M. Software, Inc; 1997. [Google Scholar]
- 7.Archambeau J, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiat Oncol Biol Phys. 1995;31:1171–1185. doi: 10.1016/0360-3016(94)00423-I. [DOI] [PubMed] [Google Scholar]
- 8.Moran CM, Bush NL, Bamber JC. Ultrasonic propagation properties of excised human skin. Ultrasound Med Biol. 1995;21:1177–1190. doi: 10.1016/0301-5629(95)00049-6. [DOI] [PubMed] [Google Scholar]
- 9.Kraus-Tiefenbacher U, Bauer L, Scheda A, Fleckenstein K, Keller A, Herskind C, Steil V, Melchert F, Wenz F. Long-term toxicity of an intraoperative radiotherapy boost using low energy X-rays during breast-conserving surgery. Int J Radiat Oncol Biol Phys. 2006;66:377–381. doi: 10.1016/j.ijrobp.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 10.White J, Joiner MC. Toxicity from radiation in breast cancer. Cancer Treat Res. 2006;128:65–109. doi: 10.1007/0-387-25354-8_5. [DOI] [PubMed] [Google Scholar]
- 11.Warszawski A, Rottinger EM, Vogel R, Warzawski N. 20 MHz ultrasonic imaging for quantitative assessment and documentation of early and late postradiation skin reactions in breast cancer patients. Radiother Oncol. 1997;47:241–247. doi: 10.1016/s0167-8140(97)00201-6. [DOI] [PubMed] [Google Scholar]
- 12.Huang YP, Zheng YP, Leung SF, Mak AFT. Reliability of measurement of skin ultrasonic proproties in vivo: a potential technique for assessing irradiated skin. Skin Res Technol. 2007;13:55–61. doi: 10.1111/j.1600-0846.2006.00191.x. [DOI] [PubMed] [Google Scholar]