Abstract
ATP synthase (FoF1) consists of an ATP-driven motor (F1) and a H+-driven motor (Fo), which rotate in opposite directions. FoF1 reconstituted into a lipid membrane is capable of ATP synthesis driven by H+ flux. As the basic structures of F1 (α3β3γδε) and Fo (ab2c10) are ubiquitous, stable thermophilic FoF1 (TFoF1) has been used to elucidate molecular mechanisms, while human F1Fo (HF1Fo) has been used to study biomedical significance. Among F1s, only thermophilic F1 (TF1) can be analyzed simultaneously by reconstitution, crystallography, mutagenesis and nanotechnology for torque-driven ATP synthesis using elastic coupling mechanisms. In contrast to the single operon of TFoF1, HFoF1 is encoded by both nuclear DNA with introns and mitochondrial DNA. The regulatory mechanism, tissue specificity and physiopathology of HFoF1 were elucidated by proteomics, RNA interference, cytoplasts and transgenic mice. The ATP synthesized daily by HFoF1 is in the order of tens of kilograms, and is primarily controlled by the brain in response to fluctuations in activity.
Keywords: FoF1, molecular motor, mitochondria, omics, cytoplasts, bioenergetics
Introduction
All human activity depends on ATP, which is primarily synthesized via mitochondrial oxidative phosphorylation (oxphos). By analogy with glycolytic ATP synthesis, there have been many futile attempts to isolate hypothetical high-energy intermediates, such as phosphoenolpyruvate, that tightly couple respiratory energy to ATP synthesis. In 1961, Mitchell proposed the chemiosmotic hypothesis, which states that in oxphos, the respiratory energy is coupled to an imaginary anisotropic “ATPase system” located in an ion-impermeable membrane (Fig. 1 of Ref. 1) via H+/OH− flux driven by the electrochemical activity ([H+]Left/[H+]Right = 107 × [ATP]/[ADP]) across the membrane.1) At the same time, soluble ATPase, known as coupling factor 1 (F1), was purified from mitochondria in Racker’s laboratory.2) When F1 was bound to F1-deficient mitochondrial membrane, respiratory energy was coupled to ATP synthesis.2) The author then isolated the entire ATP synthase, later called FoF1 (Fig. 1 , right), and reconstituted FoF1 into liposomes capable of converting energy from ATP hydrolysis to that for H+ flux driven by the electrochemical potential of protons across the membrane (ΔμH+), where ΔμH+ = FΔψ − 2.3RTΔpH.3) The H+ flux through FoF1 liposomes was demonstrated first by a pH meter (Fig. 6 of Ref. 3). After the primary chemiosmotic hypothesis1) was thus partially established,3) the subunit-subunit interactions in FoF1 during ATP synthesis became the next research target. Boyer proposed a hypothetical two-subunit model of conformational energy transfer, and finally proposed the rotational hypothesis in 1981,4) based on our report on thermophilic F1 (TF1) subunits with ubiquitous stoichiometry α3β3γδε (Fig. 1, upper left)5) and conformational changes of isolated TF1β from βE (with an empty catalytic site) to βD or βT by binding ADP or ATP, respectively.6,7)
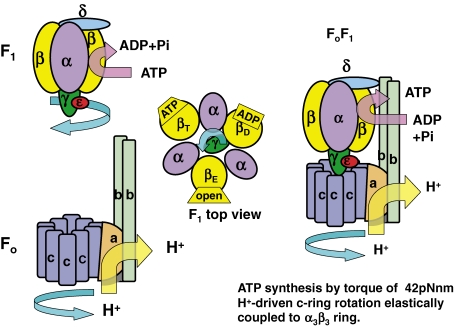
Figure 1.
Basic structure of TF1 (ATP-driven motor), TFo (proton-driven motor) and TFoF1 (proton-driven ATP synthase). Upper left: Side view of TF1 composed of α, β, γ, δ, and ε subunits. The γε turns counterclockwise against the α3β3 hexamer in the ATP hydrolysis direction when viewed from the Fo side. Middle: Top view of TF1 with three different conformations of β subunits. Lower left: Side view of TFo composed of a, b and c subunits. The c10 ring turns clockwise against ab2 in the proton-driven direction. Right: Side view of TFoF1. The central stalk (γε) turns with the c10 rotor clockwise in the ATP synthesis direction when viewed from TFo side.
The objectives of this review are to publish how achievements on thermophilic FoF1 (TFoF1) in Japan have advanced the primary chemiosmotic1) and rotational4) theories, and how our studies on human FoF1 (HFoF1) will contribute to the development of human bioenergetics. To date, ligand-binding α or β subunits have only been isolated and reconstituted into αβ subcomplexes from TF1.5–7) In contrast to active α3β3 hexamer and α1β1 dimer of TF1,7) attempts to reconstitute αβ subcomplexes from E. coli F1 (EF1) and HF1 in vitro has been unsuccessful. However, functional complementation of yeast F1 (YF1) in a quintuple deletion mutant (ΔαΔβΔγΔδΔε) with genes encoding α, β, γ and ε of BF1 strongly suggests the presence of common structure and function of HF1 subunits.8) Thus, the molecular mechanisms by which rotation and catalysis are coupled were mainly elucidated by crucial experiments using TF1 and TFo (ab2c10) to represent all FoF1s.7) The rotational hypothesis4) assumes that on ATP synthesis, the eccentric central stalk (γε) connected to the c10 ring is rotated against the α3β3 hexamer in the clockwise direction, as viewed from the Fo side (Fig. 1, right). This rotation induces cyclic conformational changes of β in the order of βE→βD→βT→βE so as to change the affinity for nucleotides, and finally release ATP to return to βE (Fig. 1, middle and upper right).4,7) In fact, Walker’s X-ray crystallography of most of α3β3γ of BF1 visualized the distinct conformations of β with different nucleotide occupancies (βE, βD and βT).9)
The concept of FoF1 as an H+-driven rotor (γ-c10) and stator (δ-ab2), rotating with a torque of 42 pN nm, was predicted in 1996 (Fig. 1, right).10) The most convincing evidence of the rotational hypothesis was the direct observation of the rotation of an actin filament attached to γ against the fixed α3β3 hexamer by Noji11) in 1997, using the TF1 gene.12) Briefly, the ATP-driven F1 motor rotates clockwise (Fig. 1, upper left) and the H+-driven Fo motor rotates in the anticlockwise direction as viewed from the F1 side (Fig. 1, lower left).7,10,11) Preliminary experiments on “the 120° rotation of the c subunit oligomer” have been reported,13) but this was not sensitive to Fo inhibitor and may represent the γ rotation in the F1 portion of FoF1. In fact, crystallographic analysis of yeast FoF1 (YFoF1) revealed that the ring of c subunits contains 10 protomers, rather than the widely anticipated 12 (4H+ per 120° rotation), and tightly connected to γδε complex.14) Although crystal of EFo is not available, exact experiments on TFoF1 confirmed the c10-ring structure (Fig. 1, right).15) By using single-molecule FRET measuring the change in the distance between the subunits a and c during the rotation (see section 6.1), a 36° sequential stepping mode of the c10-ring rotation in FoF1 was confirmed.16) ATP is synthesized at the clefts between α and β by ΔμH+-driven H+ flux when both motors are connected by central (γε) and peripheral stalks (ab2δ) to the c10-ring in TF1 (Fig. 1).7,14,17) Elucidation of primary structures of E. coli FoF1 (EFoF1)18) and TFoF1,12,19) and site-directed mutagenesis18,20) and suppression of the lost functions18) led to refinements in the rotational hypothesis including elastic power transmission.10)
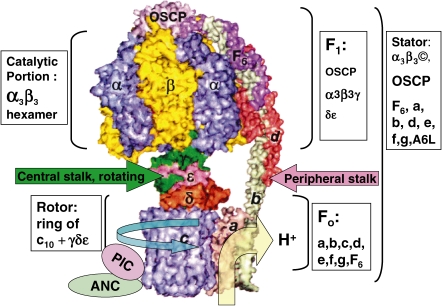
After the primary rotational hypothesis4) was thus partially established,7,10,11,17) research on FoF1 was divided into single molecular elucidation of energy transduction at the atomic level using stable TFoF112,17,20) and biomedical elucidation of human FoF1 (HFoF1)21) at the mitochondrial22) and in vivo levels.23) In contrast to the single operon of bacterial FoF1,12,18,19) animal FoF1 is encoded by both nuclear DNA21,24,25) and mitochondrial DNA.22,26) The primary structures of bovine FoF1 (BFoF1)24,25) and HFoF121,27,28) were sequenced, and cDNAs of the β subunits of BF1 and HF1, for example, were found to share 99% amino acid homology and 94% nucleotide homology (authors report in 1986 quoted in Ref. 21). Although the core structure of TFoF1 (α3β3γδε + ab2c10)7,10,12) (Fig. 1) was conserved in H(B)FoF1, it is more complex due to additional 8 subunits (d, e, f, g, A6L (ATP8), OSCP and F6, plus a natural inhibitor called IF1) (Fig. 2 )21,24,25); subunits δ and ε of TF1 correspond to OSCP (oligomycin sensitivity conferring protein) and the δε complex in HF1, respectively.21) Although mammalian δ subunit is partially homologous to ε subunit of TF1, X-ray crystallographic data of both BF129) and YF114) showed that δε complex interacts with a Rossmann fold in the γ subunit, forming a foot and c10-γ-δ-ε rotates as an ensemble central stalk (Fig. 2),14,29) similar to the ε subunit at the γ subunit in TF1 (Fig. 1).7,17) In fact, the δε complex was dissociated from BF1 by guanidine treatment as a stable heterodimer (Papageorgiou, S., and Solaini, G., 2004, PubMed abstract). So called minor subunits (e, f, g and A6L) of HFo are unlikely to have a role directly in ATP synthesis, but they appear to influence oligomeric state of HFoF1.21,25) Supramolecular structures of HFoF1 include HFoF1 dimer30) and ATP synthasome31) composed of phosphate carrier (PIC), adenine nucleotide carrier (ANC) and HFoF1 (Fig. 2). Moreover, there are tissue differences in HFoF1, with muscle- and liver-type γ subunits in HF1.27) The complex gene structure,21,28) specific regulation systems,21,28) expression and alternative splicing27,32) of HFoF1 and related regulatory genes were elucidated by recent cytoplast technology,22,33) transgenic mice,32) transcriptomics21,32) and proteomics.31,32) Based on the knowledge from these extensive studies,7,17,21) human energetics in physiological activities21) and diseases21,23) have been analyzed.
Figure 2.
Side view of space filling model of HFoF1. As there is high homology among FoF1s from different species,12,17–21) and quintuple yeast deletion YF1 mutant (ΔαΔβΔγΔδΔε) is complemented by genes encoding BF1,8) the major structure of eukaryotic FoF1 is apparently universal.9,14,25,29,73–76) Thus, X-ray crystallography data for FoF1 subunits from different species were taken from RCSB Protein Data Bank (http://www.rcsb.org/pdb/results/results.do?outformat) and assembled according to sequence data for HFoF1.21) No high-resolution structural data are available for subunit a and the hinge region of subunit b. ATP synthasome contains both PIC and ANC.31) PIC: Phosphate carrier; ANC: Adenine nucleotide carrier.
In this article, mechanistic studies of FoF1, including a reconstitution study and crystallography, will be reviewed, and then, more complex mitochondrial cytobiology and human biomedical studies will be described, because mitochondrial structure, neurohormonal control, tissue-specific activity and disease are not found in bacteria. Historical evaluations of contributions made by scientists in mechanistic studies are summarized in excellent reviews (Refs. 4 and 17, and references therein).
1. Isolation of ATP synthase (FoF1) by membrane biology
FoF1 is a membrane protein of oxidative phosphorylation.
In the 1960s, membrane biology was in its infancy, and the many attempts to purify ATP-synthesizing membrane proteins from mitochondria had been unsuccessful. Long before Fleischer’s group extracted phospholipids from the mitochondrial membrane with aqueous-acetone (10% water) and restored electron transport activity by adding back phospholipid micelles in 1962,34) Kakiuchi succeeded in a similar experiment in 1926.35) Okunuki36) succeeded in preparing several cytochromes. Green’s group prepared electron transport components from mitochondria.37) However, even after phospholipids were added back and electron transport activity was restored,34–37) liposomes capable of ΔμH+-driven oxphos, as predicted by Mitchell,1) were not reconstituted.
Membrane proteins were classified into extrinsic and intrinsic proteins.3) Extrinsic proteins, such as cytochrome c36) and F1,2,5) or components of F1, including OSCP3,25) and F63,25) (Figs. 1 and 2), are easily detached from the biomembrane by treatment with ultrasonic irradiation, chelating agents, and chaotropic anions (KI, KCN),3) and can be purified as soluble proteins in the water phase by chromatography and ammonium sulfate fractionation. However, intrinsic proteins including cytochrome oxidase36,37) and Fo3) are hydrophobic and embedded in the lipid bilayer, and require proper detergents for solubilization3) (Fig. 3 , right). In 1966, detailed phase diagrams of phospholipid cholate system were reported,38) and these were useful for solubilizing intrinsic proteins and reconstituting functional biomembrane.3,39,40) The detergent concentration needed to solubilize FoF1 is near its critical micelle concentration.3)
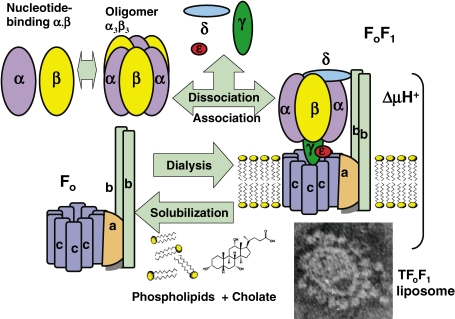
Figure 3.
Reconstitution of FoF1 from subunits and FoF1 liposomes from phospholipids.3,39) Isolated nucleotide-binding α and β subunits6,56) and α3β3 hexamer were obtained by dissociation of TF1 (upper left). Solubilized Fo or FoF1 was mixed with phospholipids and cholate, and dialyzed to reconstitute FoF1 liposomes (bottom right, electron micrograph) capable of proton-driven ATP synthesis.
The F1 reported by Racker’s laboratory2) was not sensitive to an energy transfer inhibitor of oxphos, oligomycin, but became oligomycin sensitive when bound to F1-depleted mitochondrial membrane.41) The oligomycin sensitivity conferring factor (Fo, H+-driven motor, Fig. 1) was characterized as an intrinsic membrane protein,41) and by using cholate, oligomycin-sensitive ATPase, later designated FoF1, was isolated (Fig. 3).3,42) FoF1 was reconstituted from Fo and F1.42) The electron microscopic images of BFoF1 revealed spherical 3H-acetyl-BF1 attached to membrane-embedded BFo.43) In combined morphological-biochemical studies to identify the in situ structure of BFoF1,43) radiolabeled BF1 was added to BFo membrane, and then the structure, radioactivity and ATPase activity were removed in parallel.43) However, mitochondrial F1s, including BF1 and HF1, are unstable and reconstitution of F1 from each isolated subunit was unsuccessful, even with chaperones. Thus, TF17,44) and TFoF17,45) were purified from thermophilic bacillus PS3, and stable TFoF1 was reconstituted from TF1 and TFo (Fig. 1).7,44,45) Recent proteomics using mild detergent extraction of HFoF1 from membrane followed by native gel electrophoresis revealed supramolecular structures including dimeric HFoF130) and ATP synthasome (PIC + ANC)31) (Fig. 2) (see section 9).
2. Reconstitution of FoF1 membrane capable of ATP synthesis by proton flux
FoF1 converts energy of ΔμH+ driven H+ flux into ATP synthesis.
Incorporation of membrane proteins into the lipid bilayer is essential in activity studies in membrane biology. The most difficult step was the reconstitution of liposomes containing active membrane proteins capable of producing ΔμH+.3,39,40) The use of removable detergents, including cholate, was essential in the preparation of BFoF1 (Fig. 3),3,39,40) and after cholate extraction, Triton-X100 was used in the chromatography to purify TFoF1.45) The removal of 14C-cholate during dialysis was estimated by radioactivity,3,40) and tight closure of the liposome membrane was estimated by radioactivity of enclosed 14C-inulin and confirmed by electron microscopy of enclosed ferritin.3,40) Electron microscopy showed liposomal membranes studded with closely neighboring F1 (Fig. 3, lower right).3,43) As several extrinsic proteins in oxphos, including F1, OSCP and F6 were partially lost during the extraction and dialysis of FoF1, the best activity was attained by adding these after dialysis.3,39)
These liposomes showed H+ translocation on addition of ATP,3,40,46) ATP-32Pi exchange reactions3,39,46) or H+-driven ATP synthesis (Fig. 1, right).46) Thus, FoF1-liposome was shown to be the anisotropic “ATPase system” imagined by Mitchell.1) ATP synthesis was sensitive to the combination of valinomycin (K+ ionophore) and nigericin (H+–K+ exchange ionophore), which collapsed ΔμH+.3,39,46)
More stable TFoF1-liposomes synthesized ATP from ADP and Pi with energy from proton flux driven by ΔμH+ formed by ΔpH and Δψ across their membranes.46) Using chloroplasts, ATP synthesis from ADP and Pi was demonstrated by applying ΔpH using acid–base transition47) and by imposing Δψ using an electric pulse.48) However, chloroplasts contain an electron transport system and other components that are energized by either ΔpH or Δψ. Thus, net ATP synthesis by applying ΔpH (acid–base transition) or Δψ (external electric pulse) to purified TFoF1 reconstituted in liposome46) was the most convincing evidence of chemiosmotic theory.1)
The electrical potential between the inside and outside of liposomes formed by K+ diffusion in the presence of valinomycin was calculated as Δψ = RT/F × ln([K+]out/[K+]in). Maximal net ATP synthesis from ADP and Pi was achieved by incubating vesicles in malonate at pH 5.5 with valinomycin, and then rapidly transferring them to a solution of pH 8.4 and 150 mM K+.7,46) To synthesize ATP, the minimal ΔμH+ (= Δψ − 60ΔpH, at 30℃) of 210 mV and optimal ΔμH+ of 290 mV was required.46) The H+-conducting activity of TFo-liposome through TFo was proportional to the imposed ΔμH+ (6H+/sec/103 mV at pH 8.0).49) The pH profile of the rate revealed that a proton, not a hydroxyl ion, was the true substrate.49) Because of the kinetic equivalency between ΔΨ and ΔpH as driving forces of ATP synthesis,46) Δψ is expected to replace ΔμH+. In fact, TFoF1-liposomes irradiated with external electric pulses (760 V/cm, 30 ms, rectangular) catalyzed net ATP synthesis.50) The amount of ATP synthesized increased with the number, voltage and duration of electric pulses.50) The net synthesis of ATP by application of ΔμH+ across the TFoF1-liposomes46,50) firmly established the chemiosmotic hypothesis.1) To directly measure ΔΨ with electrodes on both sides of the membrane, TFoF1 was reconstituted into planar phospholipid bilayers, and the magnitude of the electric current generated upon addition of ATP was shown to follow simple Michaelis–Menten type kinetics, and the Km was found to be 0.14 mM.51) There was no apparent dependence of Km on ΔΨ.51) This observation indicates that ΔμH+ does not directly affect Km to release the ATP formed on TFoF1,7,51) and opens the way for conformational energy transfer in ATP synthesis.
Since the success of the FoF1-liposome,3,39,46) the reconstitution method has been used to analyze the activity of intrinsic proteins including channels, receptors and membrane proteins. Using the liposome method, the important outer membrane diffusion channel known as VDAC, (voltage-dependent anion channel) in mitochondrial transport was isolated in 1980 (see sections 9 and 10).52)
3. Reconstitution of F1 subunit complexes capable of ATP synthesis by torque
3.1. Core components of Fo are a, b, and c subunits and those of F1 are α, β, γ, δ and ε.
The structure of FoF1 is shown in Figs. 1 and 2.7,9,12,18,53,54) Complete reconstitution of F1 (370 kDa), after complete denaturation of all subunits into their primary structures with sodium dodecylsulfate and urea and refolding into tertiary structure,53) from subunits α (55 kDa), β (52 kDa), γ (32 kDa), δ (20 kDa) and ε (14 kDa) was possible only in TF1 (Fig. 3).53) The intermediate core subunit complexes, α1β1 dimer55) and α3β3 hexamer,55) were also only obtained in TF1, although the sequence homologies of core subunits were conserved in HF1 (371 kDa).21) Enzymology revealed that α3β3 hexamer was an oligomer, while α1β1 dimer was a protomer.55) Owing to the stability of TF1, the subcomplexes with chemically modified and mutated subunits were useful for nanotechnology.7,17,54)
TFo (148 kDa) is composed of a (30 kDa), b (17 kDa) and c (8 kDa) in a stoichiometric ratio of 1:2:10 (Figs. 1 and 3).7,12,17) HFo and BFo contain, in addition to the common subunits a (also called ATP6; 25 kDa), b (25 kDa), and c (8 kDa), minor subunits d (19 kDa), e (8 kDa), f (10 kDa), g (11 kDa), F6 (9 kDa) and A6L (also called ATP8; 8 kDa)18,25,29) (Fig. 2). The single stalk BFo obtained by urea-cholate treatment of BFoF1 was reconstituted with externally added 3H-acetyl-F1 and the additional peripheral stalk components OSCP and F6 were demonstrated in 1971.3,39,40) Isolated BFo subunits other than OSCP and F6 are unstable, and their reconstitution has been unsuccessful without presequence and organizing machinery, even with chaperones.21) These BFo subunits were only identified on gel electrophoresis and X-ray crystallography.25,29) FoF1 was seen as a sphere (α3β3 hexamer portion, diameter 12 nm× height 10 nm)43) connected by two stalks (central and peripheral) to a basal piece (subunits a and c10 ring) (Fig. 3, lower right).3,43)
3.2. Protomeric, oligomeric and rotational ATPases: α1β1 dimer, α3β3 hexamer and α3β3γ heptamer.
The isolated α and β subunits of TF1 both have AT(D)P-Mg binding activity accompanied by conformational changes6) without ATPase activity (Fig. 3, left).55,56) The open structure of βE and closed structures of βD and βT in the presence of ligands were confirmed in the isolated thermophilic β using 1H-NMR.56) Both thermophilic α and β subunits were reconstituted to form an active α1β1 dimer by forming a catalytic αβ interface.55) Three α1β1 dimers were reconstituted to form an allosterically active α3β3 hexamer (Fig. 3, upper middle).55) Both the high catalytic activity and formation of F1-bound ATP from ADP + Pi depend on the α3β3γ structure with rotational ATPase.7,11,17) There are six potential nucleotide-binding sites on F1 and α3β3γ: three catalytic sites on β and three noncatalytic sites on α,7,17) as confirmed by X-ray crystallography.9)
Depending on the occupancy of catalytic sites with increasing ATP concentration ([ATP]), there are three types of ATPase activities of F1: uni-site, bi-site and tri-site.4,57) Uni-site catalysis is measured at sub-stoichiometric ATP concentrations ([ATP] < [F1]). Uni-site activity is very low, and the apparent KmATP is less than 20 nM.57) The ATPase activity of the α1β1 dimer of TF157) showed typical Michaelis–Menten kinetics with only one KmATP value of 70 µM, and a Vmax value of 0.1 unit/mg, without the cooperative characteristics of a protomer.57) In contrast, ATPase activity of the α3β3 hexamer showed the cooperative characteristics of an oligomer.58) The apparent KmATPs of oligomeric ATPase of α3β3 at 25℃ were about 150 µM (bi-site) and 490 µM (tri-site).58) KmATPs of rotational ATPase of TF1 (Fig. 3, right) were about 80 µM (bi-site) and 490 µM (tri-site).58) The γ-containing complexes α3β3γ, α3β3γδ and α3β3γε, show common kinetic properties.59) The α3β3 hexamer was inhibited by only one mole of [3H]-3′-O-(4-benzoyl) benzoyl-ADP per hexamer, similarly to both BF1 and TF1.60) Thus, the presence of only one inhibited-β in the hexamer blocked multi-site steady-state ATPase activity. This single-hit inactivation and cooperativity is an inherent property of the symmetrical α3β3, but is not the due to the inhibition of rotation by TF1 or α3β3γ.
3.3. Rotation of the γ subunit in α3β3 hexamer of TF1: One mole ATP hydrolysis at one β subunit drives 120° rotation of γ subunit in a concerted manner.
The rotational hypothesis of FoF14) was also proposed by Oosawa as the “loose coupling mechanism of rotational proton ATPase” based on analogy with the H+-driven flagella motor in 1986.61) The rotation of the γ subunit axis in the cylinder of the α3β3 hexamer in the FoF1 motor with the torque of 42 pN nm was predicted by many lines of evidence.10) The rotation of γ was directly demonstrated in single-molecule studies using α3β3γ from TF1.11) Rotational motion was visualized by attaching a fluorescently labeled actin filament (1–4 µm) to γS107C of artificially induced mutant γ subunit with the biotin-streptavidine bridge. The α3β3 hexamer was immobilized on a glass surface of Ni-nitrilotriacetate by artificially attaching decapolyhistidine to β,11) and the ATP-driven rotation of the γ subunit was found to be anticlockwise when F1 was observed from the Fo side (Fig. 1, upper left).11,17)
The work performed by the rotating γ in a fixed α3β3 is the frictional torque times angle of rotation. The hydrodynamic frictional drag coefficient (ξ) of the actin filament for the propeller rotation is given by ξ = (π/3)ηL3/[ln(L/2r) − 0.447], where, η (10−3 N s m−2) is the viscosity of the medium, L, the length of the actin filament (1–4 µm) and r, the radius of the filament (5 nm).11) The observed rates of filament rotation at 2 mM ATP are 7, 1, and 0.1 revolutions per second, when the lengths of f-actin are 1, 2 and 4 µm, respectively.11) The frictional torque ξω was about 40 pN nm, where ω is the angular velocity.11) Hydrolysis of one ATP molecule drives a 120° rotation of the γ subunit relative to the cylinder of the α3β3 hexamer, and therefore, hydrolysis of three ATP molecules is required for one complete 360° revolution.15,62) In order to analyze rapid rotation by reducing the viscosity resistance of long actin filament, fluorescent gold beads (40 nm) were attached to γ. At nanomolar ATP concentrations, βE waits until the next ATP molecule is bound, and the duration of the pause depends on the ATP concentration (ATP-waiting dwell time).62) ATP binding to βE is the power step that drives the 80° rotation of γ.55) This rotation leads to simultaneous release of ADP from the catalytic site of βD, and hydrolytic cleavage of ATP into ADP and Pi at βT after a pause (catalytic dwell time), and a 40° rotation occurs to complete the 120° rotation.62)
Using mutant β (E190D) of TF1, in the same rotational experiments, the catalytic activity of each β subunit was shown to be coordinated with the other two β subunits to drive rotation of the βE, βD, and βT cycle.63) Hybrid F1 containing one or two mutations with altered catalytic kinetics rotates in an asymmetric stepwise fashion with different dwell times. Analysis of the rotation revealed that for any given β subunit, the subunit binds ATP at 0°, cleaves ATP at approximately 200° and carries out a third catalytic event at approximately 320°. This demonstrates the concerted nature of the F1 complex activity, where all three β subunits participate to drive each 120° rotation of the γ subunit with a 120° phase difference.63)
3.4. Torque-driven ATP synthesis by TF1. ATP is synthesized by mechanical energy applied on the γ subunit without proton flux.
ATP synthesis driven by mechanical energy (Fig. 1) was directly shown by attaching a magnetic bead (diameter = 700 nm, biotinylated) to the γ subunit of α3β3γ of mutant TF1 (C193α, H10-β, and S107Cγ, I210Cγ) on a glass surface, and rotating the bead using electrical magnets.64) After the ATP-driven rotation of the beads was confirmed, the magnet was turned on and several bursts of hundreds of revolutions at 10 Hz were imposed.64) Anticlockwise forced rotation of the γ subunit by the magnetic beads resulted in the appearance of ATP in the medium, as detected by counting the photons emitted from the luciferase–luciferin reaction with a camera (Hamamatsu Photonics).64) This shows that torque working at one particular point (γ) on a protein complex can influence a chemical reaction occurring at physically remote catalytic sites (β), driving the reaction far from equilibrium.64)
4. Genes for TFoF1 and HFoF1: Single operon vs. nuclear and mitochondrial genes
Detailed genetic analysis and site-directed mutagenesis have been reported by Futai using E. coli FoF1 (EFoF1), as E. coli genetics are well understood.18,65) The catalytic, structural and regulatory significance of an amino acid residue in EF1 was elucidated by site-directed mutagenesis.65) However, many crucial experiments, including the planar FoF1 bilayer51) and torque-driven ATP synthesis,64) have not been successful to date with EFoF1, due to its fragility. Thus, a special sequencing method for thermophilic genes was developed.66) The structure of the TFoF1 operon (number of amino acid residues),12,19) I(127)- a(210)- c(72)- b(163)- δ(163)- α(502)- γ(286)- β(473)- ε(132), was similar to that of the EFoF1 operon.18,65)
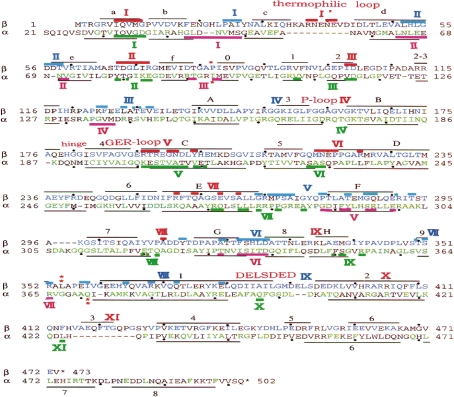
Amino acid residues in the different α, β, and γ subunits from TF1,12,19) HF1,21,27,28) BF124) and EF118,65) are aligned19) and expressed in the format α10, which refers to residue #10 in the α subunit. The residue numbers of amino acid sequences in the α and β subunits of TF1 are shown in Fig. 4 (dots indicate every tenth residue).17,19) Primary structures are homologous, with 59% sequence identity between thermophilic α/human α and 68% between thermophilic β/human β.19) The primary structure of the TF1 β subunit showed homology with 270 residues which are identical in the β subunits from HF1, CF1, and EF1.19) The homologies of the amino acid sequence between BF1 and YF1 were 73%, 79% and 40%, respectively, for the α, β and γ subunits.14) As these YF1 subunits were functionally complemented with corresponding BF1 subunits,8) the essential structure is conserved among YF1, BF1 and HF1 (sequence is nearly identical to that of BF1, but there were polymorphisms in HF1).8) Residues forming reverse turns (Gly and Pro) were highly conserved among the β subunits.19) Conserved residues (green and blue letters in Fig. 4) among TF1, HF1 and EF1 are closely related to catalytic and regulatory functions.19,21,65) The observed substitutions in the thermophilic subunit increased its propensities to form secondary structures, and its external polarity to form tertiary structure.19)
Figure 4.
Aligned amino acid sequences12,19) and secondary structure elements71) of α and β subunits in TF1. Solid black lines indicate folds, and these were classified into α-helices (A–H, 1–8) and β-sheets (a–f, 0–8). The labels for folds are provided only for the β subunit, except for the three C-terminal α-helices in the α subunit. Dots indicate every tenth residue. I–XI: areas of αβ contact. Red: catalytic contact areas of β. Pink: catalytic contact areas of α. Blue: non-catalytic contact areas of β. Green: non-catalytic contact areas of α. Colored bars indicate contact residues in TβE. Sequences are divided by red asterisks (*) to indicate the three domains.
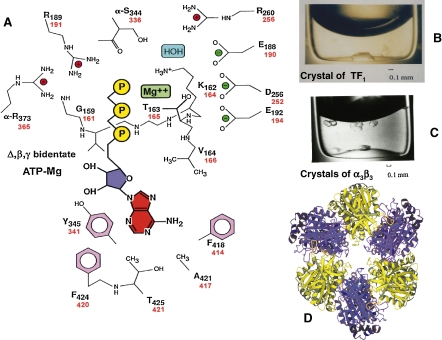
Chemical and genetic modification of residues in F1 revealed a nucleotide-binding P-loop (-GGAGVGKT-; thermophilic β158–165 corresponds to bovine β156–163) (Figs. 4, 5A ).7,9,12,19,54) Long before the X-ray crystallographic elucidation of the P-loop,9) site-directed mutagenesis of the TF1 gene20) to induce thermophilic βK164I and thermophilic αK175I, identified an essential role for lysine residues in the catalysis (Fig. 5A, red letters).20) The proton abstracting thermophilic βE190 (Fig. 5A) localized in the GER-loop (-VGER-) (Fig. 4)7,19,54) was also predicted by TF1 mutagenesis producing thermophilic βE190Q.67) These mutant TF1 subunits produced an α3β3γ complex that was suitable for experiments on torque-driven ATP synthesis.64) Species-specific residues (black letters in Fig. 4) may have phylogenic components, including thermophilic loops (-ARNENEV-) (Fig. 4, first line I′) that render TF1 stable.19) Since determining the nucleotide sequence of TFoF1,7,12,19) numerous rotating ATP synthases of thermophilic F-type or V-type (vacuolar ATPase) have been sequence and characterized.65,68)
Figure 5.
Crystals of TF1 and α3β3, and X-ray crystallography data for the catalytic center of F1. A. Catalytic center of the β subunit of BF1 (black text indicates residue number)9) and TF1 (red text indicates residue numbers).12) Except for αR373 and αS344, all of the amino acid residues are present in β. Residues 159–164 are part of the P-loop surrounding the triphosphate residue of ATP. B. Crystals of TF1.70) C. Crystals of α3β3.71) D. Top view of the crystallographic structure of α3β3.71)
In contrast to the single operon TFoF1, the gene structure of HFoF1 is highly complex21,26,28); most subunits are encoded by nuclear DNA, with signal peptides to target this protein to the mitochondrial inner membrane,21,27,28) but subunits a and A6L of HFo are encoded by mitochondrial DNA.21,26) The complete sequence of the 16,569-base pair human mitochondrial DNA contains genes for 12S and 16S rRNAs, 22 tRNAs, ATPase subunits 6 (corresponding to the a subunit of HFo) and 8 (corresponds to A6L of HFo), and 11 other protein coding genes.26)
5. Crystallographic analysis of FoF1: Detailed structure of H+-driven and ATP-driven motors
5.1. Crystallization and analysis at Photon Factory.
The most detailed structural information for amino acid residues in a protein is obtained by crystallographic analysis. In 1977, a two-dimensional crystal of TF1 showed the pseudo-hexagonal structure of α3β3.69) Three-dimensional crystals of TF1 (Fig. 5B) and α3β3 hexamer (Fig. 5C) were obtained using dye-ligand chromatography columns.70,71) The high resolution power of the Photon Factory synchrotron (for TF1 α3β3, 0.32 nm resolution at Tsukuba) revealed the detailed structure of α3β3γ,9) α3β3 (Fig. 5D),71) the c-ring,14,72) the peripheral stalk,25) central stalk29) and the stator (Fig. 2).73) X-ray crystallography (0.325 nm resolution) of the α3β3γε complex of EF1 was also recently reported (Ducan, T.M., personal communication, 2010). The peripheral stalk consists of a continuous curved α-helix about 16 nm in length in the single b-subunit, augmented by the predominantly α-helical d and F6 (Fig. 2, right).25)
5.2. Crystallography of Fo.
The c subunits form a ring around a central pore.14,72) The numbers of the c subunit in the Fo ring differ depending on the species: in CFo, it is 14,72) while that for YFo14) and TFo is 10.15) The conserved carboxylates E61 of CFo (corresponds to E56 of TFo) involved in proton transport, are 1.06–1.08 nm apart in the c-ring rotor, which rotates relative to the membrane anchored a subunit. The torque-generating unit consists of the interface between the rotating c-ring and the flanking stator a subunit.14,72) The ring rotor is driven by the sequential protonation and reprotonation of E61. Residues adjacent to the conserved E61 residues show increased hydrophobicity and reduced hydrogen bonding.72) Upon deprotonation, the conformation of E61 is changed to another c subunit and becomes fully exposed to the periphery of the ring.72) Reprotonation of E61 by a conserved R in the adjacent a subunit returns the E61 to its initial conformation.72) Genetically modified TFoF1s, each containing a c subunit dimer (c2) to a dodecamer (c12), were prepared by genetical cross-linking.15) Among these, TFoF1s containing c2, c5, or c10 showed ATP-synthesis and other activities, but those containing c9, c11 or c12 did not. Thus, the c-ring of functional TFoF1 is a decamer (c10).15) When TF1 was removed from the modified TFoF1s, TFos containing only c2, c5 or c10 worked as proton channels.15) In fact, a 36° step size of proton-driven c10-ring in FoF1 was confirmed.16) The c10 ring in YFo14) and functional complementation of the YF1-deleted yeast mutant with BF1 genes strongly suggests the presence of the c10 ring in HFoF1.8)
5.3. Crystallography of catalytic site of F1.
The basic ground state of F1 without nucleotides was shown on crystallography of the thermophilic α3β3 hexamer,55) in which three α1β1 dimers55) were arranged in three-fold symmetry, 12 nm across and 10 nm high (Fig. 5D).71) The first detailed crystallography of nucleotide-bound α3β3γ (partial) of BF1 was determined at 0.28-nm resolution by Walker’s group.9) In the structure of BF1 crystallized in the presence of ligand (AMPPNP:ADP:Pi = 50:1:0, and Mg2+), the three catalytic β subunits differed in conformation and in bound nucleotide. There were four unhydrolyzable ATP analogue (AMPPNP) molecules, three in equal three α subunits, one in β (βT) and one ADP in β (βD); the remaining β was empty (βE).9)
The ATP-binding site of βT is surrounded by the residues shown in Fig. 5A (black numbers for BF1 are identical to those of HF1, red numbers for TF1).9,71) In the P-loop of βT of TF1, the essential K16420) forms hydrogen bonds with the phosphate of the nucleotide, and the oxygen of T165 coordinates with Mg2+, while in the GER-loop, E190 interacts with water (Fig. 5A).9,71) The catalytic activity of β requires α that supplies thermophilic α-R365 and thermophilic α-S336 to the ATP-binding site at the αβ interface (Fig. 5A).9,71,74) The positive charge of α-R365 stabilizes the β-phosphate of AT(D)P, and R256, R191 and K164 of thermophilic β interact with the negative charges of the phosphates of AT(D)P (Fig. 5A). The cross-linking of thermophilic βY341 with azido-ATP7,54) predicted a hydrophobic interaction between the adenine ring, and Y341, F414 and F420 (Fig. 5A).9,71)
5.4. Basic structure of α3β3 is rendered asymmetric by addition of γ and/or nucleotides.
The basic structure of F1 is a symmetrical α3β3 hexamer that is composed of three pairs of alternating αE and βE (Fig. 5D).71) However, the asymmetry induced by introduction of γ and/or AT(D)P to α3β3 hexamer is critical in the mechanism of ATP synthesis.9) The nucleotide-free βE in both the F1 crystal9) and solution56) has an open structure (Fig. 6 ) that is essentially identical to βE in the nucleotide-free α3β3 hexamer.71) Both βT and βD, as well as αT, assume closed structures (Fig. 6, direction of the open arrow).9) Interconversion of the open-close conformational states of β is achieved by addition of AT(D)P to the isolated βE of TF1.6,56) However, nucleotide-free YF1 contained βD and βT structures similar to those of nucleotide-bound BF1.75) This suggests that βγ interactions at the three contact points (Fig. 6, middle, γ1–3), including interaction of Arg residue at position 75 (γR75) with βDE395 in the DELSEED sequence of BF1 to change the mutual conformation,10,14) are as important as nucleotide occupancy in converting open βE into the closed β.75) As genes for α, β and γ of YF1 in the α-β-γ deleted mutant yeast are complemented with those of BF1,8) the functional residues are essentially equal between YF1 and B(H)F1. Occupancy of the catalytic site by ATP or ADP can be mimicked by convenient BeF3-ADP complexes that bind to the catalytic sites of βT and βD.76) The structure is representative of an intermediate in the reaction pathways.76) The conformational change of β induced by γ-rotation is essential for ATP synthesis (ATP release from the catalytic site), while that induced by ATP-binding to β is necessary to elicit torque on γ.74,75)
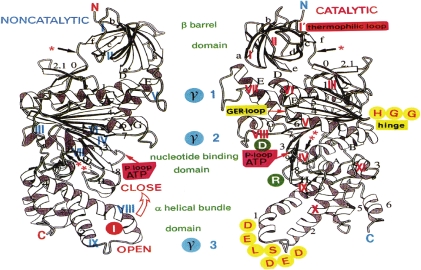
Figure 6.
Three-dimensional structures of TβE. Three-fold axis is vertical, so that views are towards the αβ subunit interfaces. Left: non-catalytic interface (blue I–IX indicates contact areas). Right: catalytic interface (red I–XI indicates contact areas). Green letters: domain names, with domain borders being marked by red asterisks. D and R in green circles are TβD331 and TβR333, respectively, at the entrance to the crevice of the P-loop. I in red circle is TβI386 of ββ contact. H and G in yellow circles indicate hinge residues in Tβ (179–181).74)
5.5. Three domains in α and β: β barrel, nucleotide-binding and α-helical bundle domains.
The overall molecular structure of α and β can be divided into three domains9,71,75): an N-terminal β barrel (Fig. 6, top N to *), a central nucleotide-binding domain (Fig. 6, middle * to **), and a C-terminal α-helical bundle (Fig. 6 bottom ** to C).7) The locations of amino acid residues in the α-helices (Fig. 4, solid black lines A–H, 1–8) and β-sheets (Fig. 4, solid black lines a–f, 0–8)71,74) are compared in the three-dimensional structure of thermophilic β (Fig. 6). As the amino acid sequence of BF124) is nearly identical to that of HF1 (99% homology in β),19,21) the following discussion on BF1 is also applicable to HF1. Superposition of the overall crystallographic structures of thermophilic βE and bovine βE or bovine α revealed that the folding of these structures is very similar.9,71) The β barrel domains (Fig. 4, Tα 21–94, Tβ1–82) contain six β strands (Fig. 6. plate form arrows, βa–βf).74) The nucleotide-binding domains (T α95–371, Tβ83–354) consist of nine-stranded β-sheets surrounded by eight α-helices (Fig. 6, αA to αH) and a small antiparallel β-sheet. The C-terminal α-helical bundle domain of α (thermophilic α375–502) contains six helices (Fig. 4, α1, 2, 4, 6–8), while that of β (theromophilic β355–473) consists of six α-helices (Fig. 4, α1–6). Thus, the largest difference between α and β is found in the C-terminal region,9,74) and the DELSD(E)ED sequence localized in this region was shown to be the most important βγ contact area.10)
5.6. Catalytic and non-catalytic αβ interfaces and conformational change.
The crystal structure of BF19) and α3β3 of TF171) indicates that, in general, the conserved residues lie on the αβ interfaces, as shown by detailed homology search among TF1, HF1, CF1 and EF1.19) There are two types of αβ interface that contain either a catalytic site or non-catalytic site.9,74,75) The area where pairs of residues connecting the αβ interface are located is defined as contact area. Contact residue pairs within a limit of 0.40 nm across the αβ interfaces in BF1 and the α3β3 hexamer of TF1 were analyzed by a computerized atom search using the CCP4 Suite: Program Contact.74) The contact areas composed of homologous residue pairs found in both TF1 and BF1 were defined as homological contact areas. The contact areas found only in one species, such as the thermophilic loop of TF1 (I′ in Fig. 4) were defined as species specific contact areas. These areas are expressed as primary structure in Fig. 4. The contact areas are located in both β and α at catalytic (red and pink bars in Fig. 4), and non-catalytic (blue and green bars in Fig. 4) interfaces. There are seven catalytic (red I–III, V–VIII in Figs. 4 and 6) and non-catalytic (blue I–VII in Figs. 4 and 6) contact areas on the open β form (βE). The number of contact areas on closed β (βD and βT) increased to 11 (red I–XI in Fig. 6) and 9 (blue I–IX in Fig. 6), respectively, in the catalytic and non-catalytic interfaces. The barrel domain harbors the universal contact areas I and II (Fig. 6, upper), and the common electrostatic bond in II is thermophilic βR72–thermophilic αE67 (=human βR71–human αE67).9,71) At the catalytic nucleotide-binding domain, areas III, V, VI, VII and VIII are universally detected (Fig. 4). However, in TβE, the P-loop contact area IV is latent, in contrast to that area in thermophilic αE (Fig. 6). Human αR373 interacts with oxygen in the β- and γ-P of ATP bound at IV.9) In V, the common electrostatic bonds are thermophilic βR193–αD339. In VI and VII of βT and βD, human αF299–βM222 and human αS344–βR260, respectively, interact. However, we identified no direct contact in the α-helical bundle domain in βE of TF1.74) In the catalytic αβ interface of human βE, the contact areas (17.6 nm2) are homologous to those of thermophilic β, while the areas in human βD (30.3 nm2) and human βT (22.0 nm2) are increased to 11 and 10, respectively. This is caused by the 30° upward motion of the C-terminal domains.
5.7. Catalytic sites.
The catalytic αβ interface is located on the left side of β in the α3β3 hexamer (Fig. 5D), and the structure of catalytic domain (Fig. 5A) is strictly conserved among species.7,9,71,74) The catalytic domain accommodates the P-loop located between sheet 3 and helix B (Tβ163–178) and the conserved thermophilic βE190 (=human βE188) in the GER loop localized between β-sheet 4 and N-terminal end of α-helix C9) (Fig. 4, GER, and Fig. 6, middle). As predicted by X-ray crystallography of AMPPNP-BF1,9) NMR analysis revealed that thermophilic βR191 (Fig. 5A, upper left) forms a hydrogen bond with the γ-phosphate of ATP.56) Pi is shown to bind the catalytic domain of βE,62,75) which is identical to the sulfate binding site of βE in α3β3 by βK164 and αR365.71) The structure of the active metal-ATP complex in TF1 at the catalytic domain was shown to be Δ, β, γ-bidentate Mg-ATP.77) (Rp)-[βγ-18O, γ-18O]ATPγS was hydrolyzed by TF1 in H217O, and the resulting inorganic [16O, 17O, 18O] thiophosphate was shown to have an Rp configuration.77) The reaction thus proceeds with inversion of configuration at the phosphorous, and a direct in-line nucleophilic attack of the 17O in water on the γ-phosphate of ATP via the pentavalent intermediate state.77) The ordered water molecules that carry out nucleophilic attack on the γ-phosphate of ATP during hydrolysis are 0.26 nm from the nucleotide analogue, beryllium, in the βD and 0.38 nm away in βT, strongly indicating that βD is the catalytically active conformation.76)
Adjacent to the P-loop, there is a hinge point, -HGG (179–181), between α-helix B and β-sheet 4 (Figs. 4, hinge, and Fig. 6, right). The conformational change in the hinge should be transmitted to the DELSDED sequence in α-helix I. The hinge motion of the C-terminal domain containing the DELSDED sequence in thermophilic βE, rotated away from the core axis from 110° (close) to 144° (open)56) In the reverse reaction, the resulting widening of the P-loop–thermophilic βE190 distance causes the release of Mg-ATP from the catalytic site.
Crystallographic analysis of the F1–IF1 complex (IF1: natural inhibitory peptide) at 0.28-nm resolution revealed that IF1 binds in the αD–βD interface and opens the catalytic site.78) Inhibitor studies on F1 are thus important in understanding the formation of dimeric HFoF1 and prevention of futile ATP loss when ΔμH+ is decreased, as described in section 10.
6. Nanotechnological analysis of TFoF1 by single-molecule imaging: Dynamic movement of FoF1
X-ray crystallography of F1 and FoF1 is a static snapshot of inhibited ATPase crystallized in the presence of AMPPNP9) or BeF3,76) or in the absence of nucleotides.71,75) These crystals do not represent the transient movement of subunits of TF1 during γ-rotation, or activity of TFoF1 in a liposome.3,39,46) Thus, the dynamics of rotating TF179) or ATP synthesis in FoF1-liposomes80) must be measured by using TF1 containing mutant β subunits,63) and also using modalities such as fluorometry79,80) or NMR.56)
The efficiency of florescence resonance energy transfer (FRET) between a donor and an acceptor fluorophore depends on their distance.16,79,80) If two fluorophores are bound to appropriate amino acid residues in rotor and stator subunits, relative subunit movements can be observed in real time by confocal microscopy.16,79,80)
6.1. Nanomotor movement analysis by single-molecule FRET.
In a single TF1 molecule fixed on a glass surface,11,63) a donor fluorophore (Cy3) was bound to one of the three βs and an acceptor fluorophore (Cy5) was bound to the protruding portion of γ, and single pair (Cys3–Cys5) FRET was performed to estimate the waiting conformation during ATP hydrolysis.79) As Cy3- and Cy5-maleimide are bound to cysteine residues, site-directed mutagenesis [α(C193S), β(S205C) and γ(S107C)] was performed to bind Cy5 to β, and to bind Cy3 to γ, and to prevent binding of Cy3 and Cy5 to α (residue numbers of α and β are indicated in Fig. 4).79) The sole cysteine in a mutant subcomplex of TF1, α(C193S)3β(His-10 tag at N terminus) 3γ (S107C), was labeled with Cy5-maleimide. The (Cy5-γ)TF1 was incubated with Cy3-β(S205C) at 1:10 at 45℃ for 2 days, and the free β subunit was removed on a size exclusion column.79) The energy of the laser beam (532 nm) on Cy3 was transferred to Cy5 and emitted light (670 nm) when the Cy3–Cy5 distance was small, while only Cy3 light (570 nm) was emitted when the Cy3–Cy5 distance was great. FRET yield changed cyclically as γ rotated and the Cy3–Cy5 distances were estimated during the conformation change.79) The distance between the two dyes changed continuously as 5.7, 7.9, and 7.9 nm during rotation at low ATP concentrations, and the conformational change corresponded to the ATP-waiting state of TF1.79)
The relative subunit movement during ATP synthesis has also been measured by FRET between two fluorophores bound to a stator subunit (b-subunit) and a rotor subunit (γ- or ε- or c-subunit) (Fig. 1).62,80) The labeled FoF1 was reconstituted in the liposome (one FoF1 per liposome) and ΔμH+ was applied, so that FoF1 carried out H+-driven ATP synthesis.46) Analysis of the time course of FRET efficiency in the FoF1-liposome showed the rotation of γ- and ε-subunit relative to b-subunit in 120° steps,80) and that of the c10-ring in 36° steps.16) The β motions through an attached fluorophore, concomitantly with the 80° and 40° substep rotations of γ in the same single molecules, showed the sequence of conformations that each β undergoes in three-step bending, an approximately 40° counterclockwise turn followed by two approximately 20° clockwise turns, occurring in synchronization with two substep rotations of γ.80) The results indicate that most previous crystal structures mimic the conformational set of three βs in the catalytic dwells,80) while the previously described set of βE, βD and βT was revealed in the ATP-waiting dwells.80) These fluorescent studies thus bridge the gap between the chemical and mechanical steps in FoF1. Starting from the ATP-waiting dwell (0°), the 80° and 40° substeps of γ rotation are induced by ATP binding and ADP release, and ATP hydrolysis and Pi release, respectively.62,79–81)
6.2. H+/ATP ratio and elastic power transmission in FoF1.
One of the unsolved questions in the mechanistic study is the analog–digital conversion of energy in FoF1. The electrochemical energy of the H+ current1) through FoF1 in liposomes3) and planar bilayers,51) and the electric,48,50) magnetic64) and mechanical energy of the rotation11,62) are all analog quantities. The numbers of ATP molecules synthesized and protons transported are digital quantities. Thus, the elastic power transmission in FoF1 analog/digital conversion during the γ-rotation was predicted in 1996.10) Oosawa also proposed a loose coupling mechanism in which the number of protons necessary for the synthesis of one ATP is not an integer but varies depending on the environmental conditions.61) As one proton is translocated by one shift of c10 in the c-ring of TFoF1,15,16) and one ATP is synthesized per β subunit of TF1, the inevitable consequence is noninteger ratios of rotation step sizes for TFoF1 (120°/36°) and for H+/ATP (10:3).14,15,74) This step-mismatch necessitates elastic twisting of TFoF1 during rotation and elementary events in catalysis.7,74) The H+/ATP ratio in FoF1 addresses this analog/digital problem. Fos with c10 ring are present in organisms that maintain ΔμH+ mainly in the form of Δψ,14,15) whereas Fos with c14 ring are mostly found in species with ΔμH+ existing predominantly in the form of ΔpH rather than Δψ.72) H+/ATP ratios of 4.7 and 3.3 are thus expected for CFoF1 (with 14c/3β)72) and E(T)FoF1 (with 10c/3β),14–16) respectively. To confirm the effects of c/β subunit ratios on H+/ATP ratio, pH of the internal phase of the reconstituted FoF1-liposomes was equilibrated with acidic medium.46,47) Then, an acid-base transition46) was induced by adding alkaline medium to the liposomes to produce ΔpH across the membrane, and the initial rate of ATP synthesis was measured with luciferase.82) From the shift in the equilibrium ΔpH as a function of Q (= [ATP]/([ADP][Pi]), the standard Gibbs free energy for phosphorylation, ΔGp0′, and the H+/ATP ratio were determined.82) The results were as follows: ΔGp0′ = 38 ± 3 kJ/mol and H+/ATP = 4.0 ± 0.2 for CFoF1; and H+/ATP = 4.0 ± 0.3 for EFoF1. This indicates that the thermodynamic H+/ATP ratio is the same and that it differs from the subunit c/β stoichiometric ratio.82) However, in order to estimate actual energetics, the very low turnover rates (<1 ATP/s) in this experiment82) need to be examined under different physiological conditions (>100 ATP/s).
The site of analog–digital conversion by elasticity7) was estimated by direct measurement of the torsional stiffness.83) Most parts of F1, particularly the central γ shaft in F1, and the long eccentric bearing had high stiffness (torsional stiffness κ > 750 pN nm).83) One domain of the rotor, namely, where the globular portions of γ and ε contact the c-ring, was more compliant (κ congruent with 68 pN nm).83) The γ-induced or nucleotide-dependent open–close conversion of conformation is an inherent property of an isolated β, and energy and signals are transferred through αβ interfaces.7,11,74) Rotation of the central shaft γ in α3β3 hexamer is assumed to be driven by domain motions of the βs. These β motions were directly observed through an attached fluorophore by FRET79,80) and NMR.56)
The mechanisms underlying the open(βE)–close(βT) motion were investigated for β subunit of TF1 in solution, using mutagenesis and NMR.56) The hydrogen bond networks involving side chains of K164 (162 for human β is shown in parentheses), T165(163), R191(189), D252(256), D311(315) and R333(337) in the catalytic region (Fig. 5A, red text for TF1 and black text for HF1)74) are significantly different for the ligand-bound and free β subunits.56) The role of each amino acid residue was examined by A (alanine) substitution.56) The chemical shift perturbation of backbone amide signals of the segmentally labeled β(mutant)s indicated stepwise propagation of the open/close conversion on ligand binding.56) Upon ATP binding, the open/close conformation change regulated by hydrogen-bond switching from K164/D252 to T165/D252 (Fig. 5A, right upper, red test) would take place in the thermophilic β subunit because ATP-binding is the major driving force for the first 80° rotation.62) The resulting closing motion of the hinge (-HGG-) between α-helix B and β-sheet 4 (Fig. 6, right) generates the torque of γ rotation through the DELSD(E)ED contact point (Fig. 6, bottom).10,56) Although the time scale of atomic fluctuations is in the order of tens of nanoseconds, molecular simulation will solve the detailed movements of residues in α3β3 during the γ rotation (order of milliseconds) in the future.
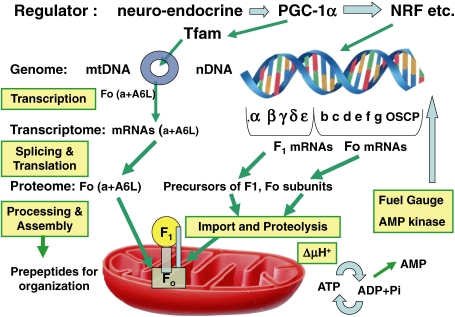
7. Biogenesis of human FoF1: Regulated expression, splicing, import and assembly
The biogenesis of HFoF1 is an intricate process,21) starting from transcription,84–86) splicing of nuclear encoded subunits (Fig. 7 ),27,32) and translation of mitochondrial DNA-encoded subunits (Fig. 8 )22,26,87) and nuclear DNA-encoded precursor peptides for all F1 subunits (α, β, γ, δ and ε)21,86) and 7 Fo subunits (b, c, d, e, f, g and OSCP),21) followed by targeting, importing and processing of nuclear DNA-encoded precursor peptides in the mitochondrion (Fig. 9 ).21,86) Precursor importing requires both ΔμH+ to drive translocation and specific carrier proteins in the outer and inner mitochondrial membrane, as well as general chaperones present in the cytosol and mitochondrial matrix.86) Processing of the presequence of precursor requires a specific protease,86) and after the removal of the presequence, nuclear-encoded subunits are assembled into HFoF1 with two mtDNA-encoded Fo subunits (a and A6L).87) Targeting the presequence of three isoforms of subunit c liberated after proteolysis is required for the assembly of cytochrome oxidase.88)
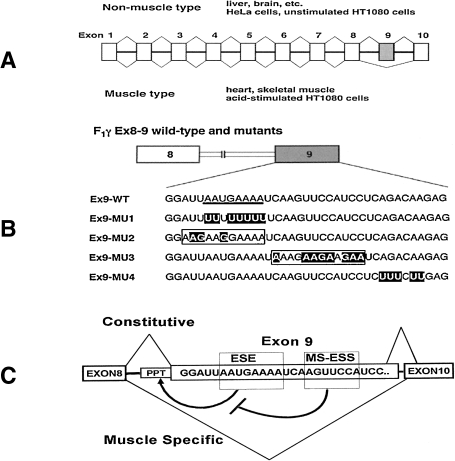
Figure 7.
Alternate splicing of human F1γ pre-mRNA. A. Exons in the F1 γ pre-mRNA are expressed in boxes. Exon number 8 (hatched box) is included in liver and excluded in the muscle. B. Schematic representation of wild-type and mutant F1γ exons 8–9 (Ex8–9).27) Heavy underline in Ex9-wild-type indicates ESE element. Mutated nucleotides are indicated by outlined letters. In Ex9-MU2 and Ex9-MU, boxed letters indicate that these sequences are predicted to act as ESE elements. C. Selection of F1γ exon 9 is regulated by two cis-acting regulatory elements in the same exon.90) Purine-rich ESE promotes exon 9 inclusion, which is repressed by MS-ESS under muscle-specific conditions; PPT: Polypyrimidine tract.
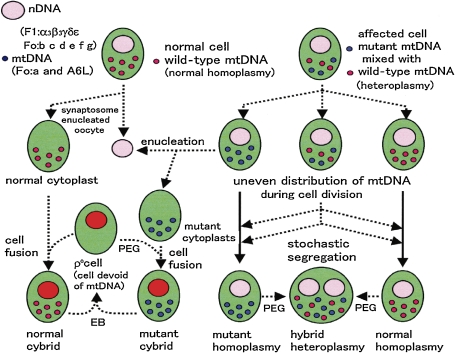
Figure 8.
Homoplasmy, heteroplasmy, cytoplasts and ρo cells.87) Small red circles indicate wild-type mtDNA and small blue circles indicate mutant mtDNA; large red circles indicate nDNA, and green ovals indicate cells. EB: ethidium bromide used to remove mtDNA; PEG: polyethylene glycol used to fuse cells or cytoplasts.
Figure 9.
Regulation of HF1Fo biosynthesis21,86): Transcription, splicing, translation, targeting, import, processing and assembly. PGC-1α: Peroxisome proliferator-activated receptor-γ coactivator-1α; Tfam: mitochondrial transcription factor A; NRF: nuclear respiratory factor; Circle: mitochondrial DNA.
As both the amino acid and nucleotide sequences of subunits in HFoF1 are available in internet databases, only physiologically important points in the biogenesis of HFoF1 will be reviewed here.21,28,86,89) In contrast to prokaryotic FoF1, including TFoF1,7) proteomics have revealed a large universe of pseudogene products,21,84) splice variants (muscle-type F1γ) (Fig. 7),27,32,90) post-translational modifications (phosphorylated HFoF1, etc.),91) dimeric HFoF1,30) a supramolecular complex called ATP synthasome (Fig. 2),31) and ectopic HFoF1 (Fig. 10 , right).92)
Figure 10.
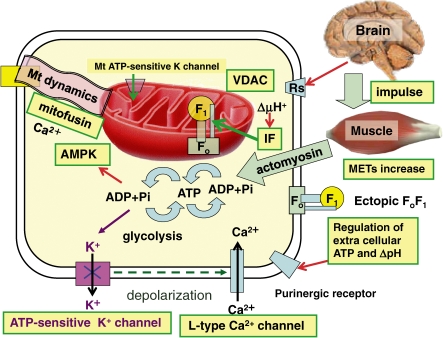
Regulation of HF1Fo activity: Neuronal impulse from brain to human activity driven by ATP by an intricate regulation system. AMPK, AMP kinase; ecto-F1Fo, ectopic or cell surface F1Fo; IF, ATPase inhibitor; METs, metabolic equivalents of exercise intensity; Rs, receptors for neuro-endocrine transmitters; VDAC, voltage-dependent anion channel.
7.1. Expression of HFoF1 genes.
The gene structure of the HF1α subunit is 14 kbp in length and contains 12 exons interrupted by 11 introns.21,84) Primer extension and S1 mapping analysis showed the presence of multiple transcription initiation sites in the HF1α gene.84) The 5′-flanking region of the HF1α gene has an unconserved GC-rich region, including several binding motifs of transcriptional factors, such as Sp1, AP-2 and GCF. The basal promoter activity was located near the GC-rich region. Comparison of the 5′-upstream region of the HF1α gene with those of the genes for BF1α, HF1β85) and HF1γ27) indicated three common sequences (CS1, CS2 and CS3) in the regulatory regions,21) suggesting that putative cis-elements coordinate the expression of the three subunit genes for HF1.84–86) The enhancer activities derived from 5′-deletion mutants of a HF1α-CAT (chloramphenicol acetyltransferase) chimeric gene were different in cell lines from four different human tissues, thus suggesting the existence of cell type-specific gene regulation.21)
The HF1β gene is 14 kbp in length and contains 10 exons, with the first exon corresponding to the non-coding region and most of the presequence, which targets this protein to the mitochondria.85,86) Eight Alu repeating sequences including inverted repeats were found in the 5′-upstream region and introns. An S1 nuclease protection experiment revealed two initiation sites for transcription. Three CCAAT boxes were found between the two initiation sites, and two GC boxes were located in the 5′-upstream region. Promoter activity was estimated by the CAT method and an enhancing structure for transcription was detected between nucleotides -400 and -1100 in the upstream region.86)
The system coordinating expression of nuclear-coded mitochondrial proteins was investigated by examining the 5′-flanking region of the HF1β gene. In one of the enhancing regions, a consensus sequence was found for the genes of other mitochondrial proteins, such as those for cytochrome c1 and the pyruvate dehydrogenase α-subunit.21,86) The characteristics of this enhancing element were examined by introducing a synthetic oligonucleotide element into the CAT plasmid with a deleted enhancing element. The resulting plasmid showed full recovery of promoter activity, and this activity was independent of the orientation or location of the insert. Therefore, this enhancer may be common to the nuclear genes of some mitochondrial proteins involved in energy transduction.21)
The functions of subunits in mitochondrial FoF1 were confirmed by expression of genes in deletion mutants, and their functional complementation.8) The genes encoding BF1 subunits (except for those of δ) were expressed in a quintuple yeast YF1 deletion mutant (ΔαΔβΔγΔδΔε) after introduction of a chimeric BF1 subunit gene construct that uses the YF1 transcriptional promoter and termination sites, as well as the presequence for YF1.8) Expression of the α-, β-, γ-, or ε-subunit of BF1 complemented the corresponding individual mutations in YF1. All BF1 subunits (with chimeric δ) expressed in yeast produced an F1 that was purified to a specific activity of about half of that of original BF1.8) These results indicate that the molecular machinery required for the targeting, proteolysis and assembly of the mitochondrial FoF1 is conserved from yeast to humans (Fig. 9).8) Similarly, bovine OSCP and some Fo components have been functionally complemented (quoted in Ref. 8). In one functional study, the gene for γ subunit (atp-3) was partially blocked by RNA interference (RNAi) in Caenorhabditis elegans, and ATP levels decreased from 15 nmol/mg protein (control) down to 4 nmol/mg, and the behavior extended the lifespan.93) However, tissue differentiation is absent in yeast, alternate splicing must be studied in human cells.27,32)
7.2. Tissue-specific splicing of HF1γ: Analysis using transgenic mice and minigenes.
Mammalian FoF1 is characterized by tissue-specific expression of the F1 gene, which was analyzed using transgenic mice32) and minigenes in cultured human cells.90) The muscle-specific isoform of HF1γ was generated by alternative splicing, and exon 9 was found to be lacking in skeletal muscle and heart tissue (Fig. 7, A).27) Using transgenic mice,32,90) the alternative splicing of exon 9 was shown to require de novo protein synthesis of a cis-acting element on the spliced exon of HF1γ gene. An HF1γ wild-type minigene, containing the full-length gene from exons 8 to 10, and two mutants were prepared; one mutant involved a pyrimidine-rich substitution on exon 9, whereas the other was a purine-rich substitution (abbreviated as HF1γ Pu-del and HF1γ Pu-rich mutants, respectively).90) Pu-del inhibited exon inclusion, indicating that a Pu-del mutation disrupts an exonic splicing enhancer. On the other hand, Pu-rich blocked muscle-specific exon exclusion.90)
Transgenic mice bearing both mutant minigenes were then analyzed for their splicing patterns in tissues.90) Based on an analysis of HF1γ Pu-del minigene transgenic mice, the purine nucleotide in this element was shown to be necessary for exon inclusion in non-muscle tissue. In contrast, analysis of HF1γ Pu-rich minigene mice revealed that the HF1γ Pu-rich mutant exon had been excluded from heart and skeletal muscles in these transgenic mice, despite the fact that mutation of the exon inhibited muscle-specific exon exclusion in myotubes at early embryonic stages.32) These results suggest that the splicing regulatory mechanism underlying HF1γ pre-mRNA differs between myotubes and myofibers during myogenesis and cardiogenesis.32)
A detailed mutational analysis of exon 9 (Fig. 7, B) revealed a purine-rich exonic splicing enhancer (ESE) element (5′-AAUGAAAA-3′) functioning ubiquitously, with the exception of muscle tissue. An exonic negative regulatory element responsible for muscle-specific exclusion of exon 9 was discovered using both in vitro and in vivo splicing systems.32,90) Mutation analyses on the HF1γ Ex8-9 minigene using a supplementation assay demonstrated that the muscle-specific negative regulatory element is positioned in the middle region of exon 9, immediately downstream from ESE. Detailed mutation analyses identified a muscle-specific exonic splicing silencer (MS-ESS) (5′-AGUUCCA-3′) responsible for exclusion of exon 9 in vivo and in vitro (Fig. 7, C).90) This element was shown to cause exon 9 skipping of in vivo splicing systems.90) Although there are three variants of the c subunit88) and several alternate splice variants in the human mitochondrial fusogenic proteins (mitofusin 1, 2),87) the γ subunit of HF1 is the only well-characterized variant in FoF1.86,90)
8. Mitochondrial cytology of HFoF1: cytoplasts lack nDNA and ρo cells lack mtDNA
HFoF1 is encoded by both mitochondrial DNA (mtDNA)22,26,33) and nuclear DNA (nDNA) (Fig. 9).21,86) In order to analyze the roles played by mtDNA and nDNA, mtDNA-less cells (ρo cells)22,33) and nDNA-less cells (cytoplasts) were developed (Fig. 8).87,94) Using ethidium bromide, mtDNA was removed and the resulting ρo cells became strictly dependent on glycolysis to compensate for the oxphos that supplies ATP.33,87) Thus, a glucose medium is essential for ρo cells.22) Cytoplasts are enucleated cells that contain mitochondria (Fig. 8, upper left), with examples being enucleated oocytes,87) synaptosomes94) and platelets.94) Since DNA sequence of an individual differs from each other owing to the genetic polymorphism of mtDNA,21) personal collection of mtDNA is essential to elucidate mitochondrial diseases. Human mitochondria with intact mtDNA have been directly isolated from postmortem platelets.94) Expression of nDNA-encoded HFoF1 subunits was not affected in ρo cells,87) while that of mtDNA-encoded HFo subunits (Foa and A6L) was lost in cytoplasts, as nDNA-encoded mitochondrial transcription factor A (Tfam)95) was lacking (Fig. 9).87) The ρ0 cells have no respiratory chain, because of the loss of mtDNA-encoded subunits of cytochromes and NADH dehydrogenase.26,87) Despite the absence of oxphos, ρo cells require mitochondrial compartments with a sufficient ΔμH+ for energy driven transport of matrix components.87,96) The essential ΔμH+ of ρo cells is maintained by the electrogenic exchange of ATP4− for ADP3− by ANC.96) To energize the inner membrane, α3β3 (ATPase active)55) in the matrix of ρo cells regenerates ADP from translocated ATP.96)
The term heteroplasmy refers to cells that contain a mixture of mtDNAs with different sequences (Fig. 8, upper right), whereas homoplasmy means that 100% of their mtDNA has an identical sequence (Fig. 8, upper left).22,87) Cybrids were formed by cytoplast fusion with ρo cells using polyethylene glycol (PEG) (Fig. 8, bottom).87,94) The majority of pathogenic mtDNA mutations are heteroplasmic, with mutated and wild-type mtDNA coexisting in the same cell (Fig. 8, upper right).86,87) Owing to the absence of protecting histones, mtDNA is highly susceptible to mutations that result in heteroplasmy. Mutations in the tRNA gene of mtDNA often block translation and cause complete deletion of mtDNA-encoded proteins (syn− mutation), including HFo subunits.87)
During development, cell division unevenly distributes heteroplasmic mtDNA into daughter cells and eventually segregates homoplasmic cells with wild-type and syn− mutant mtDNA (Fig. 8, bottom right). This stochastic segregation of the syn− mutation results in the syn− mutant concentrated tissues and causes mitochondrial diseases, including mitochondrial diabetes.87) The homoplasmic syn− cells lack oxphos and depend on glycolysis. Under the influence of polymorphisms in mtDNA and nDNA, a vicious circle of reactive oxygen species will damage cells. However, mitochondrial transfer from wild-type homoplasmic cytoplasts by fusion to form cybrids will normalize the diseased cells97) and syn− mutation will be alleviated by mitochondrial fusion.87,97) We analyzed heteroplasmy and polymorphisms related to diabetes and its complementation by mitofusins.87) Mitochondria in human cells are visualized as a network or as filaments that undergo continuous changes in shape and in localization within the cells. Mitochondrial fusion proteins including mitofusins87) and OPA198) that may work as natural PEG in Fig. 8,87) and regulate both mitochondrial fusion and metabolism. We characterized splice variants of human mitofusins (also called hfzo 1, 2 and 3)87) as well as OPA1s (Fig. 10, upper right).98) We analyzed complementation by fusogenic proteins, and the lost tRNA in mitochondria of syn− mtDNA inside heteroplasmic cells was complemented by wild-type tRNA in normal mitochondria by fusing wild-type and syn− mitochondria with mitofusins.87) The mitofusin genes were expressed mainly in post-mitotic brain and muscle, thus complementing mutated mtDNA that is not removed during cell division.87)
In order to analyze a mitochondrial disease, pure nuclear transfer was carried out from ρ0 HeLa cells to the fibroblast lines from a patient with cardiomyopathy, and their nuclear hybrid clones were isolated.22) A normal fibroblast line from the fetus and a respiration-deficient fibroblast line from the patient were used as positive and negative controls, respectively.22) By this method, many mitochondrial diseases devoid of HFo have been elucidated87) and mitochondrial gene therapy for heteroplasmic patients was developed using cytoplasts from a normal fetal cells and the cybrids (Fig. 8).97)
The most frequent mutation in mtDNA-encoded HFo gene is NARP (neuropathy, ataxia, and retinitis pigmentosa), caused by a mutation at L156 in the a subunit of HFo (Fig. 2).99) A mutation conferring a milder phenotype (L156P) leads to a 30% reduction in H+ flux, and a similar loss in ATP synthesis. The more severe mutation (L156R) also leads to a 30% reduction in H+ flux, but ATP synthesis is abolished.99) With the L156P mutation, rotation of the c-ring may be slowed, but coupling of ATP synthesis to H+ flux is maintained (Fig. 1, lower left, subunit a), whereas with the L156R mutation, H+ flux is uncoupled, because the transmembrane helix III of Foa is unable to span the membrane. The L156R mutant has ATPase activity,99) because the α3β3 complex portion of F1 is intact, and increased proton permeability through the defective Fo cannot maintain ΔμH+ to inhibit ATP-driven H+ translocation (i.e., uncoupling).3,46)
8.1. Coordination of nuclear and mitochondrial DNA.
The tissue activity of FoF1 mainly relies on mitochondrial biogenesis encoded by both nDNA and mtDNA (Fig. 9). A number of transcriptional modulators have been implicated in the regulation of mitochondrial biogenesis and oxphos activity.84–89) To understand the nDNA–mtDNA interactions in human cells, we identified the nuclear transcription factors that are common to the expression of these gene products. As Tfam encoded by nDNA is essential for both the initiation of transcription and the replication of mtDNA, we cloned and sequenced the human Tfam gene.95) There were sequences in the 5′-upstream regulatory region of Tfam common to those in HF1β.95) In the absence of mtDNA-coded Fo subunits, expression of other FoF1 subunits was not affected, but most nDNA-coded subunits other than α and β of FoF1 could not be assembled.87,96) For in vivo analysis of this regulation, transmitochondrial mice carrying various proportions of deletion mutant mtDNA (ΔmtDNA) were generated by introducing ΔmtDNA from cultured cells into the fertilized eggs of mice.100) The great advantage of transmitochondrial mice is that they share exactly the same nDNA background and their genetic variations are restricted to the proportions of pathogenic mtDNA.100)
Transcription factors in the expression of HFoF1 include PPARγ coactivator 1α (PGC-1α), in cooperation with several factors, such as peroxisome proliferator-activated receptor (PPAR), nuclear respiratory factors 1 and 2 (NRF-1 and NRF-2), or the specificity protein 1 (Sp1), a ubiquitous transcription factor known to regulate the constitutive expression of oxphos genes (Fig. 10, upper).86) PGC-1α is a master modulator of gene expression in human tissues and enhances the activity of PPARα in skeletal muscle.101) Mitochondrial transcription is directed by a small number of nucleus-encoded factors, including Tfam.95) Expression of these factors is coordinated with that of nuclear respiratory proteins through the action of PGC-1 family coactivators.101)
Using these cytological methods (Fig. 8), transcriptomics and proteomics (Fig. 9), human bioenergetics at both cell level (Fig. 10) and in vivo levels were elucidated.
9. Supramolecular structure of HFoF1: Dimeric HFoF1, ATP synthasome and ecto-HFoF1
Mitochondrial FoF1, including HFoF1, is typically isolated as a monomeric complex that contains 16 protein subunits21) and the natural inhibitor protein (IF1) (Fig. 2).78) However, mitochondrial FoF1 was isolated in dimeric and higher oligomeric states using digitonin for one step mild solubilization followed by blue native (BN) or clear native (CN) electrophoresis.102) Recent developments in proteomics have revealed HFoF1 in its natural supramolecular state.30,31,102) Single bands in the gel can be analyzed by proteomics approaches including immunoprecipitation,103) and mass spectrometry to identify the amino acid sequence of the components.103) Electron microscopy of these oligomeric mitochondrial FoF1 particles was reported in 1972 (Fig. 2C in Ref. 3). Dimeric and trimeric FoF1 were purified from mammalian mitochondria in five different tissues by BN electrophoresis and CN electrophoresis,30,102) and these were active, thus suggesting that oligomeric FoF1 is constitutive in mitochondria. Using BN electrophoresis, two membrane proteins (6.8 kDa proteolipid and diabetes-associated protein) that had previously been removed during purification were shown to be stoichiometrically associated with FoF1,30) and this may provide insight for further functional investigations.30)
In situ mitochondrial ΔμH+ was directly estimated by rhodamine 123, which is accumulated in mitochondria depending on ΔμH+.103) The futile ATP hydrolytic activity of HFoF1 during ischemia that lowers ΔμH+, is prevented by IF1.78) Bovine IF1 is an α-helical dimer and residues 1–37 of IF1 open the catalytic interface between αD–βD.78) Atomic force microscopy images show how these FoF1 molecules form dimers with a characteristic 15-nm distance between the axes of their rotors through stereo-specific interactions of the membrane-embedded portions of their stators.104) A different interaction surface is responsible for the formation of rows of oligomers, suggesting the role of subunits e and g of HFo in dimerization.104) Some dimers have a different morphology, with a 10-nm stalk-to-stalk distance, in line with FoF1s, which are thus accessible to IF1.104) Dimeric or polymeric HFoF1 is related to morphology of cristae104) under the influence of OPA1.98)
A channel protein, porin, is now known as VDAC (voltage-dependent anion channel) and is the most abundant protein in the mitochondrial outer membrane.52) VDAC helps ATP/ADP exchange by forming a complex with ANC (ANC–VDAC complex).103) Finally, a supramolecular structure called ATP synthasome composed of FoF1, ANC, PIC and perhaps VDAC was isolated and characterized (Fig. 2).31) Parallel immuno-electron microscopic studies revealed the presence of PIC and ANC located non-centrally in the basepiece, and other studies indicated an ATP synthase/PIC/ANC stoichiometry near 1:1:1 (Fig. 2).31) Collectively, these findings support a mechanism in which the entry of the substrates ADP and Pi into mitochondria, the synthesis of ATP on HFoF1, and the release and exit of ATP are localized in a supramolecular structure in a highly coordinated system.
9.1. Ectopic HFoF1: plasma membrane localization in lipid rafts.
HFoF1 is located not only on the mitochondrial inner membrane, but also on the cell surface. Extracellular ATP synthesized by the ectopic HFoF1 is not an energy source but a regulator for various cellular responses that are initiated by purinergic receptors (P2X and P2Y) and signaling processes and are terminated by breakdown of ATP by ectonucleotidases (Fig. 10, right). By using 3H-ADP, net 3H-ATP synthesis by cell surface HFoF1 was confirmed (to rule out ATP + AMP synthesis by adenylate kinase). ATP synthesis was inhibited by membrane-impermeable HFoF1-specific inhibitors (angiostatin and piceatannol) and anti-HF1 antibody.105) Immunoprecipitation indicated that ectopic HFoF1 and a surface protein of endothelial cells, caveolin-1, are physically associated.105) Adipocyte ectopic HFoF1 may contain Fo, as it is inhibited by oligomycin and influenced by a proton conductor (uncoupler) (quoted in Ref. 92). HFoF1 is selectively localized in lipid rafts with other mitochondrial proteins. Lipid rafts are detergent-resistant membrane microdomains enriched in cholesterol and caveolin-1. Intracellular traffic may translocate HFoF1 containing α, β, γ, b, d, F6 and OSCP from mitochondria to lipid rafts.92)
The ectopic HFoF1 has been implicated in numerous activities, including the mediation of intracellular pH, cellular response to antiangiogenic agents, and cholesterol homeostasis as a receptor for apolipoprotein A-1.106) HFoF1 is expressed on the surface of endothelial cells, where it binds angiostatin, regulates surface ATP levels, and modulates endothelial cell proliferation and differentiation via purinergic receptor (Fig. 10, right).106)
Ectopic HFoF1 is closely related to obesity.107) Expression of the α subunit of ectopic HFoF1 is markedly increased during adipocyte differentiation. Treatment of differentiated adipocytes with inhibitors of HFoF1 or antibodies against α and β subunits of HFoF1 leads to a decrease in cytosolic lipid accumulation.107) Apolipoprotein A-I binds to the β subunit of ectopic HFoF1 and its inhibition decreases the production of lipid droplets.107) Depletion of plasma membrane cholesterol with methyl-beta-cyclodextrin disrupts lipid rafts and abolishes co-localization of HFoF1 with caveolin-1, which results in a marked reduction in shear stress-induced ATP release.105) Endothelial cells release ATP from ectopic HFoF1 in response to shear stress, a mechanical force generated by blood flow, and the ATP released modulates cell functions through activation of downstream signals to purinergic receptors.105) These results suggest that the localization and targeting of HFoF1 to caveolin-1/lipid rafts is critical for shear stress-induced ATP release by endothelial cells.105)
It remains uncertain how Fo components encoded by mtDNA are translocated to rafts as ectopic HFoF1, particularly in the form of intact HFoF1 (Fig. 10, right). However, inter-organellar traffic of mitochondrial proteins was clearly demonstrated using green fluorescent protein.103) These mitochondrial dynamics will be discussed in the final section. The energy source (ΔμH+) to synthesize ATP by ectopic HFoF1 may not be respiration, but the K+channel dependent resting potential of plasma membrane is inside negative. These ectopic FoF1 activities have never been reported in prokaryotic FoF1, although FoF1-like V-type ATPase is widely distributed in prokaryotic and mammalian membrane structures to transport ions.65,68) In mammalian tissues, some proteins involved in energy metabolism may exert entirely different functions; cytochrome c, for example, is an electron carrier but also serves as the central signal protein in apoptosis.
10. In vivo ATP synthesis: FoF1 in human bioenergetics and diseases
The function and survival of all organisms is dependent on the dynamic control of energy metabolism. Energy demand is matched to ATP supply by FoF1 and glycolysis (Fig. 10, bottom).7,21) The increase in ADP + Pi produced by ATP consumption results in the instant ΔμH+-driven ATP synthesis by FoF1 and increases in electron transport activity to compensate ΔμH+ (respiratory control). The increase in ADP is amplified as AMP increase by myokinase reaction (2ADP = ATP + AMP). If ATP synthesis by FoF1 is not enough, especially when oxygen supply is limited by high metabolic equivalents (METs >5), increased AMP/ATP ratio activates phosphofructokinase to compensate ATP by glycolysis. Although the regulatory mechanism of TFoF1 is basically ubiquitous,7,108) HFoF1 is specialized for human activity. One of the characteristics of HFoF1 among other FoF1s is genetic polymorphisms during evolution, particularly in mtDNA,87,109) that cause ethnic and interindividual differences in physical activity, aging and disease susceptibility.87,109) Voluntary will of the brain triggers muscle contraction and other organ specific activities that consume about 50 kg of ATP per day, but METs change from 0.9 to 15 in a normal adult (Fig. 10, right). Direct measurement of in vivo HFoF1 activity and AMP/ATP ratio is possible by using 31P magnetic resonance spectroscopy revealed that ATP synthase flux correlated with O2 uptake (METs) and insulin sensitivity.110) The increase in METs is mainly caused by actomyosin contraction (Fig. 10, right), and muscle-specific γ subunit splice variants of HFoF1 are seen during myogenesis and cardiogenesis (Fig. 7).32,90) The resulting change in substrates to increase lactic acid can be assessed by 1H magnetic resonance spectroscopy.110) Lactic acidemia is present not only in individuals undergoing high MET exercise, but also in the majority of patients with mitochondrial disorders, including impaired HFo, and cells isolated from such patients, similarly to ρ0 cells, require glucose medium (Fig. 8).22,87) Mitochondrial disorders can be due to defects in nDNA directly affecting the assembly or function of HFoF1 and respiratory chain, defects in mtDNA affecting HFo and the respiratory chain or nDNA influencing mtDNA structure and viability.21,87)
The regulatory mechanism of HFoF1 including four independent inhibitory sites111) are more complex than those of TFoF1.7,108) AMP-activated protein kinase (AMPK) activates both oxphos and glycolysis, functioning as a ‘fuel gauge’ to monitor AMP/ATP ratio (Fig. 10, left).112) ATP-sensitive K+ channels both in the plasma membrane and in mitochondria also monitors ATP levels to regulate cellular activities.87,113) Increases in ATP concentration close the K+ channel, and the resulting depolarization opens L-type Ca2+ channels (Fig. 10, bottom). Increased intracellular Ca2+ activates many metabolic processes and proteins for mitochondrial dynamics and secretion. For example, in the β-cells of mitochondrial myopathy, ATP-sensitive K+ channels are not closed and defective Ca2+-dependent insulin secretion results in mitochondrial diabetes.87) Mitochondrial ATP-sensitive K+ channels regulate energy transfer through their regulation of intermembrane space volume and are accordingly essential for the inotropic response during periods of high METs.113) Although the target residues in HFoF1 and their signal routes have not yet been determined, mitochondrial ATP-sensitive K+ channels are closely related to kinases including protein kinase Cε.113) In fact, detailed proteomics have revealed, for example, phosphorylation of αS76, βT213, βS529, γY44 or γY52, and acetylation of αK132, βK133, γK79 in mammalian FoF1.91) The monomeric form of HFoF1 contains a phosphorylated γ (γY52) that is not present in the dimeric form.91)
In contrast to bacteria, ATP synthesis by HFoF1 requires exchange of Pi + ADP and ATP between cytosol and mitochondria by PIC and ANC, which are organized as the ATP synthasome (Fig. 2).31) VDAC52) forming a complex with ANC also plays an important role in cytoplasma-mitocondrial communication.103) As mammalian cells are about 1000 times as large as bacteria, and a mitochondrion is as large as a bacterium, mitochondrial dynamics are essential to distribute synthesized ATP (Fig. 10, upper left).114) The concept of mitochondrial dynamics includes the movement of mitochondria along the cytoskeleton, the regulation of mitochondrial morphology and distribution, and connectivity mediated by tethering and fusion/fission events.114) The relevance of these events in HFoF1 activity has been unraveled after the identification of mitofusin87) and OPA1.98) Subjects with diabetes showed reduced expression (by 26%) of mitofusin 2 and a 39% reduction in the α-subunit of FoF1.115) Chronic exercise led to increases in VDAC, and the α-subunit of FoF1 in muscle from control subjects and from those with diabetes.115) Acute exercise caused a 4-fold increase in PGC-1α expression in muscle from control subjects, but not in those with diabetes.115)
10.1. Inhibition of HFoF1 activity and diseases related to bioenergetics.
In the energy metabolism of whole human body,114,116) if ΔμH+ is too low, ATP production by HFoF1 cannot meet demand (mitochondrial diseases),22,87) and if it is too high, reactive oxygen species (ROS) are produced.23,89,116) Oxphos is linked to disease through a lack of energy, excess ROS production, or a combination of both,89,116) and diseases caused by mitochondrial dysfunction include diabetes, cancer, neurodegenerative disorders and ischemia-reperfusion injury.23,89,116)
Because of its complex structure, HFoF1 is inhibited by over 250 natural and synthetic inhibitors.117) In the absence of torque-driving energy, HFoF1 switches from an ATP synthase to an ATP hydrolase, and this occurs during myocardial ischemia. The degree of ATP inefficiently hydrolyzed during ischemia may be as high as 50–90%.118) At the start of FoF1 study, oligomycin was shown to inhibit Fo,3,39–42) and this portion of FoF1 was therefore designated “oligomycin sensitivity conferring factor”. Oligomycin cannot be used to treat myocardial ischemia, as it would reduce ATP synthesis in normal tissue.3) Only when cellular pH is decreased below 6.8 under ischemia, IF178) inhibits ATPase at the αβ interface of HF1.78) The restoration of ΔμH+ favoring ATP synthesis displaces IF1 from the αβ interface. However, IF1 does not completely block hydrolase activity. BMS-199264 selectively inhibits ATPase activity during ischemia while having no effect on ATP synthesis, and enhances the recovery of contractile function following reperfusion.118) IF1, ectopic HFoF1 and the opener of mitochondrial ATP-sensitive K+ channel113) protect cardiomyocytes from ischemic/reperfusion damage.
As HFoF1 supplies most ATP needed for human activity, further study will provide useful insight for emergency medicine,118) mitochondrial cardiomyopathy22,87) and obesity-related chronic diseases.23,115,116)
Conclusions
The excellent work to date on ATP synthases (FoF1) has been reviewed based on data obtained in studies on TFoF1 and HFoF1. The chemiosmotic theory1) was firmly established by ΔμH+-driven ATP synthesis in TFoF1 liposomes (Figs. 1 and 3),7,46) and the rotational theory4) was established by crucial observations of γ-rotation10,11,17) and ATP synthesis on externally added torque to rotate γ in TF1 (Fig. 1).64) TF1 is the only F1 sufficiently stable to be consistently analyzed by reconstitution, crystallography, mutagenesis, and nanotechnology for torque-driven ATP synthesis.7) Crystallographic analysis using BF1 (Figs. 2 and 5)9,25) and TF1 (Figs. 4–6),71,74) site-directed mutagenesis using EFoF118,65) and TFoF1 (Figs. 5 and 6),12,20,63) and dynamic nanotechnology79) have contributed to elucidating elastic7,74) and loose61) energy coupling. Based on the fundamental mechanism of ATP synthesis in TFoF1,7,108) and functional complementation of YF1-deleted yeast with BF1 genes,8) human bioenergetics was developed by research on HFoF1 using plasmids,21,28,86,97) transgenic mice (Fig. 7),23,90,100) cytoplasts (Fig. 8)33,87) and omics (Fig. 9).21,84,86,87) HFoF1 differed from TFoF1 in complex structure (Fig. 2), mitochondrial genetics (Fig. 8),87) organ specificity (Fig. 7)90) and intricate biogenesis (Fig. 9).86) The complex regulation of HFoF1 has been shown to be essential for daily human activity that is triggered by the brain (Fig. 10), thus human bioenergetics is also applicable to emergency medicine113,118) and obesity/diabetes23,116) and mitochondrial diseases.87,97)
Acknowledgments
The author extends thanks to Profs. M. Yoshida, T. Hamamoto, H. Endo, S. Ohta, E. Muneyuki, H. Hirata, M. Yohda and N. Sone, with whom he has worked in the Department of Biochemistry, Jichi Medical School since 1972, for their excellent work. The author would also like to thank Prof. E. Racker for his excellent advice. This study was supported by several Grants-in-Aid (1973–2008), and the “High-Tech Research Center” Project for Private Universities matching fund subsidy (1999–2008; for Y.K.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Abbreviations
- AMPPNP
5′-adenylyl-imindo diphosphate
- ANC
Adenine nucleotide carrier
- α, β, γ, δ and ε
subunits of F1
- βE, βD, and βT
β subunit of F1 with an empty catalytic site, with bound ADP or with bound ATP, respectively
- a, b, c, d, e, f and g
A6L, subunits of Fo
- c10
decamer ring of the c subunit of Fo
- FoF1
ATP synthase
- F1
ATP-driven motor of FoF1
- Fo
H+-driven motor of FoF1
- BF1, CF1, EF1, HF1, TF1 and YF1
F1 from bovine mitochondria, chloroplasts, E. coli, human mitochondria, thermophilic bacillus PS3 and yeast, respectively
- Δψ
membrane potential
- ΔμH+
electrochemical potential difference of protons across the membrane
- F
Faraday constant
- FRET
florescence resonance energy transfer
- IF
F1 inhibitor
- mtDNA
mitochondrial DNA
- nDNA
nuclear DNA
- OPA-1
optic atrophy 1
- Oxphos
oxidative phosphorylation
- Pi
inorganic phosphate
- PIC
Phosphate carrier
- P-loop
-G-X-X-X-X-G-K-T- sequence of the α and β subunits of F1
- R
gas constant
- VDAC
voltage-dependent anion channel. For nomenclature of the amino acid residues in subunits α, β, γ, etc., of F1, “βK35”, for example, refers to a lysine (K) residue located at #35 in the β subunit of F1. The equivalent residues in different F1s are expressed as thermophilic βK164 (=human βK162). Mutations are abbreviated as “βK164I”, which means “residue K164 in the β subunit is changed to I164”.
Profile
Yasuo Kagawa was born in 1932. He graduated from the Medical School at the University of Tokyo in 1957, and after completing an internship at St. Luke’s Hospital, he received his PhD from the Graduate School of the University of Tokyo in 1962. He continued his bioenergetics research as a Fulbright scholar at the Public Health Research Institute of New York in 1963, as an assistant at the University of Tokyo in 1965, as a professor at Shinshu University in 1968, as a visiting professor of Biochemistry and Molecular Biology at Cornell University in 1970, and, since 1972, as a professor at Jichi Medical School (JMS), where his studies on F1Fo ATP synthase have garnered international attention. After retiring in 1998, Dr. Kagawa was appointed Professor Emeritus at JMS and joined Kagawa Nutrition University (KNU) as a professor of Medical Chemistry. He became the vice president of KNU in 1999. He has also served as managing editor of several international journals, and was a committee member of the International Union of Biochemistry and Molecular Biology. Dr. Kagawa has received several awards, including the Medical Research Prize from the Japanese Medical Association in 1985, the Medal with Purple Ribbon in 1996, and the Order of the Sacred Treasure, Gold Rays with Neck Ribbon from the Government of Japan in 2006.
References
- 1).Mitchell P. (1961) Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191, 144–148 [DOI] [PubMed] [Google Scholar]
- 2).Pullman M.E., Penefsky H.S., Datta A., Racker E. (1960) Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble, dinitrophenol-stimulated adenosine triphosphatase. J. Biol. Chem. 235, 3322–3329 [PubMed] [Google Scholar]
- 3).Kagawa Y. (1972) Reconstitution of oxidative phosphorylation. Biochim. Biophys. Acta 265, 297–338 [PubMed] [Google Scholar]
- 4).Boyer P.D. (1997) The ATP synthase-a splendid molecular machine. Annu. Rev. Biochem. 66, 717–749 [DOI] [PubMed] [Google Scholar]
- 5).Yoshida M., Sone N., Hirata H., Kagawa Y., Ui N. (1979) Subunit structure of adenosine triphosphatase. Comparison of the structure in thermophilic bacterium PS3 with those in mitochondria, chloroplasts, and Escherichia coli. J. Biol. Chem. 254, 9525–9533 [PubMed] [Google Scholar]
- 6).Ohta S., Tsuboi M., Oshima T., Yoshida M., Kagawa Y. (1980) Nucleotide binding to isolated alpha and beta subunits of proton-translocating adenosine triphosphatase with circular dichroism. J. Biochem. 87, 1609–1617 [DOI] [PubMed] [Google Scholar]
- 7).Kagawa Y. (1999) Biophysical studies on ATP synthase. Adv. Biophys. 36, 1–25 [DOI] [PubMed] [Google Scholar]
- 8).Puri N., Lai-Zhang J., Meier S., Mueller D.M. (2005) Expression of bovine F1-ATPase with functional complementation in yeast Saccharomyces cerevisiae. J. Biol. Chem. 280, 22418–22424 [DOI] [PubMed] [Google Scholar]
- 9).Abrahams J.P., Leslie A.G., Lutter R., Walker J.E. (1994) Structure at 2.8 A resolution of F1-ATPase from bovine heart mitochondria. Nature 370, 621–628 [DOI] [PubMed] [Google Scholar]
- 10).Kagawa Y., Hamamoto T. (1996) The energy transmission in ATP synthase: from the γ-c rotor to the α3β3 oligomer fixed by OSCP-b stator via the βDELSEED sequence. J. Bioenerg. Biomembr. 28, 421–431 [DOI] [PubMed] [Google Scholar]
- 11).Noji H., Yasuda R., Yoshida M., Kinosita K., Jr. (1997) Direct observation of the rotation of F1-ATPase. Nature 386, 299–302 [DOI] [PubMed] [Google Scholar]
- 12).Ohta S., Yohda M., Ishizuka M., Hirata H., Hamamoto T., Otawara-Hamamoto Y., et al. (1988) Sequence and over-expression of subunits of adenosine triphosphate synthase in thermophilic bacterium PS3. Biochim. Biophys. Acta 933, 141–155 [DOI] [PubMed] [Google Scholar]
- 13).Sambongi Y., Iko Y., Tanabe M., Omote H., Iwamoto-Kihara A., Ueda I., et al. (1999) Mechanical rotation of the c subunit oligomer in ATP synthase (F0F1): direct observation. Science 286, 1722–1724 [DOI] [PubMed] [Google Scholar]
- 14).Stock D., Leslie A.G.W., Walker J.E. (1999) Molecular architecture of the rotary motor in ATP synthase. Science 286, 1700–1705 [DOI] [PubMed] [Google Scholar]
- 15).Mitome N., Suzuki T., Hayashi S., Yoshida M. (2004) Thermophilic ATP synthase has a decamer c-ring: indication of noninteger 10:3 H+/ATP ratio and permissive elastic coupling. Proc. Natl. Acad. Sci. USA 101, 12159–12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Düser M.G., Zarrabi N., Cipriano D.J., Ernst S., Glick G.D., Dunn S.D., et al. (2009) 36° step size of proton-driven c-ring rotation in FoF1-ATP synthase. EMBO J. 28, 2689–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Yoshida M., Muneyuki E., Hisabori T. (2001) ATP synthase—a marvelous rotary engine of the cell. Nat. Rev. Mol. Cell Biol. 2, 669–677 [DOI] [PubMed] [Google Scholar]
- 18).Futai M., Noumi T., Maeda M. (1989) ATP synthase (H+-ATPase): results by combined biochemical and molecular biological approaches. Annu. Rev. Biochem. 58, 111–136 [DOI] [PubMed] [Google Scholar]
- 19).Kagawa Y., Ishizuka M., Saishu T., Nakao S. (1986) Stable structure of thermophilic proton ATPase beta subunit. J. Biochem. 100, 923–934 [DOI] [PubMed] [Google Scholar]
- 20).Yohda M., Ohta S., Hisabori T., Kagawa Y. (1988) Site-directed mutagenesis of stable adenosine triphosphate synthase. Biochim. Biophys. Acta 933, 156–164 [DOI] [PubMed] [Google Scholar]
- 21).Kagawa Y., Hamamoto T., Endo H., Ichida M., Shibui H., Hayakawa M. (1997) Genes of human ATP synthase: Their roles in physiology and aging. Biosci. Rep. 17, 115–146 [DOI] [PubMed] [Google Scholar]
- 22).Isobe K., Ito S., Hosaka H., Iwamura Y., Kondo H., Kagawa Y., et al. (1998) Nuclear-recessive mutations of factors involved in mitochondrial translation are responsible for age-related respiration deficiency of human skin fibroblasts. J. Biol. Chem. 273, 4601–4606 [DOI] [PubMed] [Google Scholar]
- 23).Kagawa Y., Yanagisawa Y., Hasegawa K., Suzuki H., Yasuda K., Kudo H., et al. (2002) Single nucleotide polymorphism of thrifty genes for energy metabolism: evolutionary origins and prospects for intervention to prevent obesity-related diseases. Biochem. Biophys. Res. Commun. 295, 207–222 [DOI] [PubMed] [Google Scholar]
- 24).Walker J.E., Fearnley I.M., Gay N.J., Gibson B.W., Northrop F.D., Powell S., et al. (1985) Primary structure and subunit stoichiometry of F1-ATPase from bovine mitochondria. J. Mol. Biol. 184, 677–701 [DOI] [PubMed] [Google Scholar]
- 25).Walker J.E., Dickson V.K. (2006) The peripheral stalk of the mitochondrial ATP synthase. Biochim. Biophys. Acta 1757, 286–296 [DOI] [PubMed] [Google Scholar]
- 26).Anderson S., Bankier A.T., Barrell B.G., de Bruijn M.H., Coulson A.R., Drouin J., et al. (1981) Sequence and organization of the human mitochondrial genome. Nature 290, 457–465 [DOI] [PubMed] [Google Scholar]
- 27).Matsuda C., Endo H., Ohta S., Kagawa Y. (1993) Gene structure of human mitochondrial ATP synthase gamma-subunit. Tissue specificity produced by alternative RNA splicing. J. Biol. Chem. 268, 24950–24958 [PubMed] [Google Scholar]
- 28).Ohta S., Tomura H., Matsuda K., Kagawa Y. (1988) Gene structure of the human mitochondrial adenosine triphosphate synthase beta subunit. J. Biol. Chem. 263, 11257–11262 [PubMed] [Google Scholar]
- 29).Gibbons C., Montgomery M.G., Leslie A.G., Walker J.E. (2000) The structure of the central stalk in bovine F1-ATPase at 2.4 A resolution. Nat. Struct. Biol. 7, 1055–1061 [DOI] [PubMed] [Google Scholar]
- 30).Krause F., Reifschneider N.H., Goto S., Dencher N.A. (2005) Active oligomeric ATP synthases in mammalian mitochondria. Biochem. Biophys. Res. Commun. 329, 583–590 [DOI] [PubMed] [Google Scholar]
- 31).Chen C., Ko Y., Delannoy M., Ludtke S.J., Chiu W., Pedersen P.L. (2004) Mitochondrial ATP synthasome: three-dimensional structure by electron microscopy of the ATP synthase in complex formation with carriers for Pi and ADP/ATP. J. Biol. Chem. 279, 31761–31768 [DOI] [PubMed] [Google Scholar]
- 32).Ichida M., Hakamata Y., Hayakawa M., Ueno E., Ikeda U., Shimada K., et al. (2000) Differential regulation of exonic regulatory elements for muscle-specific alternative splicing during myogenesis and cardiogenesis. J. Biol. Chem. 275, 15992–16001 [DOI] [PubMed] [Google Scholar]
- 33).Hayashi J., Ohta S., Kagawa Y., Kondo H., Kaneda H., Yonekawa H., et al. (1994) Nuclear but not mitochondrial genome involvement in human age-related mitochondrial dysfunction. Functional integrity of mitochondrial DNA from aged subjects. J. Biol. Chem. 269, 6878–6883 [PubMed] [Google Scholar]
- 34).Fleischer S., Brierley G., Klouwen H., Slautterback D.B. (1962) Studies of the electron transfer system. XLVII. The role of phospholipids in electron transfer. J. Biol. Chem. 237, 3264–3272 [PubMed] [Google Scholar]
- 35).Kakiuchi S. (1927) The significance of lipoids in the oxygen consuming activity of tissues. 1. The oxygen consuming activity of tissue and the mitochondrial structure. J. Biochem. 7, 263–265 [Google Scholar]
- 36).Okunuki K. (1940) Ueber die Rolle der Diaphorase in der Wechsel-wirkung zwischen dem Cytochrom b und dem Dehydrasesystem. Proc. Imp. Acad. (Tokyo) 16, 144–148 [Google Scholar]
- 37).Green D.E., Hatefi Y. (1961) The mitochondrion and biochemical machines. Science 133, 13–19 [DOI] [PubMed] [Google Scholar]
- 38).Small D.M., Bourges M.C., Dervichian D.G. (1966) The biophysics of lipidic associates I. The ternary systems lecithin-bile salt-water. Biochim. Biophys. Acta 125, 563–581 [PubMed] [Google Scholar]
- 39).Kagawa Y., Racker E. (1971) Partial resolution of the enzymes catalyzing oxidative phosphorylation. XXV. Reconstitution of vesicles catalyzing 32Pi-adenosine triphosphate exchange. J. Biol. Chem. 246, 5477–5487 [Google Scholar]
- 40).Kagawa Y., Kandrach A., Racker E. (1973) Partial resolution of the enzymes catalyzing oxidative phosphorylation. XXVI. Specificity of phospholipids required for energy transfer reactions. J. Biol. Chem. 248, 676–684 [PubMed] [Google Scholar]
- 41).Kagawa Y., Racker E. (1966) Partial resolution of the enzymes catalyzing oxidative phosphorylation. VIII. Properties of a factor conferring oligomycin sensitivity on mitochondrial adenosine triphosphatase. J. Biol. Chem. 241, 2461–2466 [PubMed] [Google Scholar]
- 42).Kagawa Y., Racker E. (1966) Partial resolution of the enzymes catalyzing oxidative phosphorylation. IX. Reconstruction of oligomycin-sensitive adenosine triphosphatase. J. Biol. Chem. 241, 2467–2474 [PubMed] [Google Scholar]
- 43).Kagawa Y., Racker E. (1966) Partial resolution of the enzymes catalyzing oxidative phosphorylation. X. Correlation of morphology and function in submitochondrial particles. J. Biol. Chem. 241, 2475–2482 [PubMed] [Google Scholar]
- 44).Yoshida M., Sone N., Hirata H., Kagawa Y. (1975) A highly stable adenosine triphosphatase from a thermophilic bacterium. Purification, properties and reconstitution. J. Biol. Chem. 250, 7910–7916 [PubMed] [Google Scholar]
- 45).Sone N., Yoshida M., Hirata H., Kagawa Y. (1975) Purification, properties of a dicyclohexylcarbodiimide-sensitive adenosine triphosphatase from a thermophilic bacterium. J. Biol. Chem. 250, 7917–7923 [PubMed] [Google Scholar]
- 46).Sone N., Yoshida M., Hirata H., Kagawa Y. (1977) Adenosine triphosphate synthesis by electrochemical proton gradient in vesicles reconstituted from purified adenosine triphosphatase and phospholipids of thermophilic bacterium. J. Biol. Chem. 252, 2956–2960 [PubMed] [Google Scholar]
- 47).Jagendorf A.T. (1967) Acid-base transitions and phosphorylation by chloroplasts. Fed. Proc. 26, 1361–1369 [PubMed] [Google Scholar]
- 48).Witt H.T., Schlodder E., Gräber P. (1976) Membrane-bound ATP synthesis generated by an external electrical field. FEBS Lett. 69, 272–276 [DOI] [PubMed] [Google Scholar]
- 49).Okamoto H., Sone N., Hirata H., Yoshida M., Kagawa Y. (1977) Purified proton conductor in proton translocating adenosine triphosphatase of a thermophilic bacterium. J. Biol. Chem. 252, 6125–6131 [PubMed] [Google Scholar]
- 50).Rögner M., Ohno K., Hamamoto T., Sone N., Kagawa Y. (1979) Net ATP synthesis in H+-ATPase macroliposomes by an external electric field. Biochem. Biophys. Res. Commun. 91, 362–367 [DOI] [PubMed] [Google Scholar]
- 51).Muneyuki E., Kagawa Y., Hirata H. (1989) Steady state kinetics of proton translocation catalyzed by thermophilic FoF1-ATPase reconstituted in planar bilayer membranes. J. Biol. Chem. 264, 6092–6096 [PubMed] [Google Scholar]
- 52).Zalman L.S., Nikaido H., Kagawa Y. (1980) Mitochondrial outer membrane contains a protein producing nonspecific diffusion channels. J. Biol. Chem. 255, 1771–1774 [PubMed] [Google Scholar]
- 53).Yoshida M., Sone N., Hirata H., Kagawa Y. (1977) Reconstitution of adenosine triphosphatase of thermophilic bacterium from purified individual subunits. J. Biol. Chem. 252, 3480–3485 [PubMed] [Google Scholar]
- 54).Kagawa Y., Hamamoto T. (1997) Intramolecular rotation in ATP synthase: dynamic and crystallographic studies on thermophilic F1. Biochem. Biophys. Res. Commun. 240, 247–256 [DOI] [PubMed] [Google Scholar]
- 55).Kagawa Y., Ohta S., Harada M., Sato M., Itoh Y. (1992) The α3β3 and α1β1 complexes of ATP synthase. Ann. N. Y. Acad. Sci. 671, 366–376 [DOI] [PubMed] [Google Scholar]
- 56).Yagi H., Kajiwara N., Iwabuchi T., Izumi K., Yoshida M., Akutsu H. (2009) Stepwise propagation of the ATP-induced conformational change of the F1-ATPase β subunit revealed by NMR. J. Biol. Chem. 284, 2374–2382 [DOI] [PubMed] [Google Scholar]
- 57).Saika K., Yoshida M. (1995) A minimum catalytic unit of F1-ATPase shows non-cooperative ATPase activity inherent in a single catalytic site with a Km 70 µM. FEBS Lett. 368, 207–210 [DOI] [PubMed] [Google Scholar]
- 58).Miwa K., Yoshida M. (1989) The α3β3 complex, the catalytic core of F1-ATPase. Proc. Natl. Acad. Sci. USA 86, 6484–6487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Paik S.R., Yokoyama K., Yoshida M., Ohta T., Kagawa Y., Allison W.S. (1993) The TF1-ATPase and ATPase activities of assembled α3β3γ, α3β3γδ, and α3β3γε complexes are stimulated by low and inhibited by high concentrations of rhodamine 6G whereas the dye only inhibits the α3β3, and α3β3δ complexes. J. Bioenerg. Biomembr. 25, 679–684 [DOI] [PubMed] [Google Scholar]
- 60).Aloise P., Kagawa Y., Coleman P.S. (1991) Comparative Mg2+-dependent sequential covalent binding stoichiometries of 3′-O-(4-benzoyl)benzoyl adenosine 5′-diphosphate of MF1, TF1, and the α3β3 core complex of TF1. The binding change motif is independent of the F1 γδε subunits. J. Biol. Chem. 266, 10368–10376 [PubMed] [Google Scholar]
- 61).Oosawa F., Hayashi S. (1986) The loose coupling mechanism in molecular machines of living cells. Adv. Biophys. 22, 151–183 [DOI] [PubMed] [Google Scholar]
- 62).Feniouk B.A., Yoshida M. (2007) Regulatory mechanisms of proton-translocating FoF1-ATP synthase. Results Probl. Cell Differ. 45, 279–308 [DOI] [PubMed] [Google Scholar]
- 63).Ariga T., Muneyuki E., Yoshida M. (2007) F1-ATPase rotates by an asymmetric, sequential mechanism using all three catalytic subunits. Nat. Struct. Mol. Biol. 14, 841–846 [DOI] [PubMed] [Google Scholar]
- 64).Itoh H., Takahashi A., Adachi K., Noji H., Yasuda R., Yoshida M., et al. (2004) Mechanically driven ATP synthesis by F1-ATPase. Nature 427, 465–468 [DOI] [PubMed] [Google Scholar]
- 65).Futai, M., Wada, Y. and Kaplan, J.H. (eds.) (2004) Handbook of ATPases. Wiley-VCH, Germany. pp. 1–468. [Google Scholar]
- 66).Kagawa Y., Nojima H., Nukiwa N., Ishizuka M., Nakajima T., Yasuhara T., et al. (1984) High guanine plus cytosine content in the third letter of codons of an extreme thermophile. DNA sequence of the isopropylmalate dehydrogenase of Thermus thermophilus. J. Biol. Chem. 259, 2956–2960 [PubMed] [Google Scholar]
- 67).Ohtsubo M., Yoshida M., Ohta S., Kagawa Y., Yohda M., Date T. (1987) In vitro mutated β subunits from the F1-ATPase of the thermophilic bacterium, PS3, containing glutamine in place of glutamic acid in positions 190 or 201 assembles with the α and γ subunits to produce inactive complexes. Biochem. Biophys. Res. Commun. 146, 705–710 [DOI] [PubMed] [Google Scholar]
- 68).Imamura H., Nakano M., Noji H., Muneyuki E., Ohkuma S., Yoshida M., et al. (2003) Evidence for rotation of V1-ATPase. Proc. Natl. Acad. Sci. USA 100, 2312–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69).Wakabayashi T., Kubota M., Yoshida M., Kagawa Y. (1977) Structure of ATPase (coupling factor TF1) from a thermophilic bacterium. J. Mol. Biol. 117, 515–519 [DOI] [PubMed] [Google Scholar]
- 70).Shirakihara Y., Yohda M., Kagawa Y., Yokoyama K., Yoshida M. (1991) Purification by dye-ligand chromatography and a crystallization study of the F1-ATPase and its major subunits, β and α, from a thermophilic bacterium, PS3. J. Biochem. 109, 466–471 [DOI] [PubMed] [Google Scholar]
- 71).Shirakihara Y., Leslie A.G., Abrahams J.P., Walker J.E., Ueda T., Sekimoto Y., et al. (1997) The crystal structure of the nucleotide-free α3β3 subcomplex of F1-ATPase from the thermophilic Bacillus PS3 is a symmetric trimer. Structure 5, 825–836 [DOI] [PubMed] [Google Scholar]
- 72).Vollmar M., Schlieper D., Winn M., Büchner C., Groth G. (2009) Structure of the c14 rotor ring of the proton translocating chloroplast ATP synthase. J. Biol. Chem. 284, 18228–18235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Dickson V.K., Silvester J.A., Fearnley I.M., Leslie A.G., Walker J.E. (2006) On the structure of the stator of the mitochondrial ATP synthase. EMBO J. 25, 2911–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74).Kagawa Y., Hamamoto T., Endo H. (2000) The α/β interfaces of α1β1, α3β3, and F1: Domain motions and elastic energy stored during γ rotation. J. Bioenerg. Biomembr. 32, 471–484 [DOI] [PubMed] [Google Scholar]
- 75).Kabaleeswaran V., Shen H., Symersky J., Walker J.E., Leslie A.G., Mueller D.M. (2009) Asymmetric structure of the yeast F1 ATPase in the absence of bound nucleotides. J. Biol. Chem. 284, 10546–10551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76).Kagawa R., Montgomery M.G., Braig K., Leslie A.G., Walker J.E. (2004) The structure of bovine F1-ATPase inhibited by ADP and beryllium fluoride. EMBO J. 23, 2734–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77).Senter P., Eckstein F., Kagawa Y. (1983) Substrate metal adenosine 5′-triphosphate chelate structure and stereochemical course of reaction catalyzed by the adenosine triphosphatase from the thermophilic bacterium PS3. Biochemistry 22, 5514–5518 [Google Scholar]
- 78).Cabezón E., Montgomery M.G., Leslie A.G., Walker J.E. (2003) The structure of bovine F1-ATPase in complex with its regulatory protein IF1. Nat. Struct. Biol. 10, 744–750 [DOI] [PubMed] [Google Scholar]
- 79).Yasuda R., Masaike T., Adachi K., Noji H., Itoh H., Kinosita K., Jr. (2003) The ATP-waiting conformation of rotating F1-ATPase revealed by single-pair fluorescence resonance energy transfer. Proc. Natl. Acad. Sci. USA 100, 9314–9318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80).Galvez E.M., Zimmermann B., Rombach-Riegraf V., Bienert R., Gräber P. (2007) Fluorescence resonance energy transfer in single enzyme molecules with a quantum dot as donor. Eur. Biophys. J. 37, 1367–1371 [DOI] [PubMed] [Google Scholar]
- 81).Masaike T., Koyama-Horibe F., Oiwa K., Yoshida M., Nishizaka T. (2008) Cooperative three-step motions in catalytic subunits of F1-ATPase correlate with 80 degrees and 40 degrees substep rotations. Nat. Struct. Mol. Biol. 15, 1326–1333 [DOI] [PubMed] [Google Scholar]
- 82).Steigmiller S., Turina P., Gräber P. (2008) The thermodynamic H+/ATP ratios of the H+-ATPsynthases from chloroplasts and Escherichia coli. Proc. Natl. Acad. Sci. USA 105, 3745–3750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83).Sielaff H., Rennekamp H., Wächter A., Xie H., Hilbers F., Feldbauer K., et al. (2008) Domain compliance and elastic power transmission in rotary FoF1-ATPase. Proc. Natl. Acad. Sci. USA 105, 17760–17765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84).Akiyama S., Endo H., Inohara N., Ohta S., Kagawa Y. (1994) Gene structure and cell type-specific expression of the human ATP synthase alpha subunit. Biochim. Biophys. Acta 1219, 129–140 [DOI] [PubMed] [Google Scholar]
- 85).Tomura H., Endo H., Kagawa Y., Ohta S. (1990) Novel regulatory enhancer in the nuclear gene of the human mitochondrial ATP synthase beta-subunit. J. Biol. Chem. 265, 6525–6527 [PubMed] [Google Scholar]
- 86).Kagawa Y., Ohta S. (1990) Regulation of mitochondrial ATP synthesis in mammalian cells by transcriptional control. Int. J. Biochem. 22, 219–229 [DOI] [PubMed] [Google Scholar]
- 87).Sato A., Endo H., Umetsu K., Sone H., Yanagisawa Y., Saigusa A., et al. (2003) Polymorphism, heteroplasmy, mitochondrial fusion and diabetes. Biosci. Rep. 23, 313–337 [DOI] [PubMed] [Google Scholar]
- 88).Vives-Bauza C., Magrane J., Andreu A.L., Manfredi G. (2010) Novel role of ATPase subunit c targeting peptide beyond mitochondrial import. Mol. Biol. Cell 21, 131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89).Hüttemann M., Lee I., Pecinova A., Pecina P., Przyklenk K., Doan J.W. (2008) Regulation of oxidative phosphorylation, the mitochondrial membrane potential, and their role in human disease. J. Bioenerg. Biomembr. 40, 445–456 [DOI] [PubMed] [Google Scholar]
- 90).Hayakawa M., Sakashita E., Ueno E., Tominaga S., Hamamoto T., Kagawa Y., et al. (2002) Muscle-specific exonic splicing silencer for exon exclusion in human ATP synthase gamma-subunit pre-mRNA. J. Biol. Chem. 277, 6974–6984 [DOI] [PubMed] [Google Scholar]
- 91).Kane L.A., Van Eyk J.E. (2009) Post-translational modifications of ATP synthase in the heart: biology and function. J. Bioenerg. Biomembr. 41, 145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92).Champagne E., Martinez L.O., Collet X., Barbaras R. (2006) Ecto-F1Fo ATP synthase/F1 ATPase: metabolic and immunological functions. Curr. Opin. Lipidol. 17, 279–284 [DOI] [PubMed] [Google Scholar]
- 93).Dillin A., Hsu A.L., Arantes-Oliveira N., Lehrer-Graiwer J., Hsin H., Fraser A.G., et al. (2002) Rates of behavior and aging specified by mitochondrial function during development. Science 298, 2398–2401 [DOI] [PubMed] [Google Scholar]
- 94).Ito S., Ohta S., Nishimaki K., Kagawa Y., Soma R., Kuno S.Y., et al. (1999) Functional integrity of mitochondrial genomes in human platelets and autopsied brain tissues from elderly patients with Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 95, 2099–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95).Tominaga K., Akiyama S., Kagawa Y., Ohta S. (1992) Upstream region of a genomic gene for human mitochondrial transcription factor 1. Biochim. Biophys. Acta 1131, 217–219 [DOI] [PubMed] [Google Scholar]
- 96).Smith C.P., Thorsness P.E. (2005) Formation of an energized inner membrane in mitochondria with a gamma-deficient F1-ATPase. Eukaryot. Cell 4, 2078–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97).Kagawa Y., Hayashi J.-I. (1997) Gene therapy of mitochondrial diseases using human cytoplasts. Gene Ther. 4, 6–10 [DOI] [PubMed] [Google Scholar]
- 98).Satoh M., Hamamoto T., Seo N., Kagawa Y., Endo H. (2003) Differential sublocalization of the dynamin-related protein OPA1 isoforms in mitochondria. Biochem. Biophys. Res. Commun. 300, 482–493 [DOI] [PubMed] [Google Scholar]
- 99).Solaini G., Harris D.A., Lenaz G., Sgarbi G., Baracca A. (2008) The study of the pathogenic mechanism of mitochondrial diseases provides information on basic bioenergetics. Biochim. Biophys. Acta 1777, 941–945 [DOI] [PubMed] [Google Scholar]
- 100).Nakada K., Sato A., Sone H., Kasahara A., Ikeda K., Kagawa Y., et al. (2004) Accumulation of pathogenic DeltamtDNA induced deafness but not diabetic phenotypes in mito-mice. Biochem. Biophys. Res. Commun. 323, 175–184 [DOI] [PubMed] [Google Scholar]
- 101).Chanséaume E., Morio B. (2009) Potential mechanisms of muscle mitochondrial dysfunction in aging and obesity and cellular consequences. Int. J. Mol. Sci. 10, 306–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102).Meyer B., Wittig I., Trifilieff E., Karas M., Schägger H. (2007) Identification of two proteins associated with mammalian ATP synthase. Mol. Cell. Proteomics 6, 1690–1699 [DOI] [PubMed] [Google Scholar]
- 103).Kasashima K., Ohta E., Kagawa Y., Endo H. (2006) Mitochondrial functions and estrogen receptor-dependent nuclear translocation of pleiotropic human prohibitin 2. J. Biol. Chem. 281, 36401–36410 [DOI] [PubMed] [Google Scholar]
- 104).Buzhynskyy N., Sens P., Prima V., Sturgis J.N., Scheuring S. (2007) Rows of ATP synthase dimers in native mitochondrial inner membranes. Biophys. J. 93, 2870–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105).Yamamoto K., Shimizu N., Obi S., Kumagaya S., Taketani Y., Kamiya A., et al. (2007) Involvement of cell surface ATP synthase in flow-induced ATP release by vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 293, H1646–H1653 [DOI] [PubMed] [Google Scholar]
- 106).Chi S.L., Pizzo S.V. (2006) Cell surface F1Fo ATP synthase: a new paradigm? Ann. Med. 38, 429–438 [DOI] [PubMed] [Google Scholar]
- 107).Arakaki N., Kita T., Shibata H., Higuti T. (2007) Cell-surface H+-ATP synthase as a potential molecular target for anti-obesity drugs. FEBS Lett. 581, 3405–3409 [DOI] [PubMed] [Google Scholar]
- 108).von Ballmoos C., Wiedenmann A., Dimroth P. (2009) Essentials for ATP synthesis by F1F0 ATP synthases. Annu. Rev. Biochem. 78, 649–672 [DOI] [PubMed] [Google Scholar]
- 109).Takasaki S. (2009) Mitochondrial haplogroups associated with Japanese centenarians, Alzheimer’s patients, Parkinson’s patients, type 2 diabetic patients and healthy non-obese young males. J. Genet. Genomics 36, 425–434 [DOI] [PubMed] [Google Scholar]
- 110).Kacerovsky-Bielesz G., Chmelik M., Ling C., Pokan R., Szendroedi J., Farukuoye M., et al. (2009) Short-term exercise training does not stimulate skeletal muscle ATP synthesis in relatives of humans with type 2 diabetes. Diabetes 58, 1333–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111).Gledhill J.R., Walker J.E. (2006) Inhibitors of the catalytic domain of mitochondrial ATP synthase. Biochem. Soc. Trans. 34, 989–992 [DOI] [PubMed] [Google Scholar]
- 112).Steinberg G.R., Kemp B.E. (2009) AMPK in Health and Disease. Physiol. Rev. 89, 1025–1078 [DOI] [PubMed] [Google Scholar]
- 113).Costa A.D., Garlid K.D. (2009) MitoKATP activity in healthy and ischemic hearts. J. Bioenerg. Biomembr. 41, 123–126 [DOI] [PubMed] [Google Scholar]
- 114).Liesa M., Palacín M., Zorzan A. (2009) Mitochondrial dynamics in mammalian health and disease. Physiol. Rev. 89, 799–845 [DOI] [PubMed] [Google Scholar]
- 115).Hernández-Alvarez M.I., Thabit H., Burns N., Shah S., Brema I., Hatunic M., et al. (2009) Subjects with early-onset type 2 diabetes show defective activation of the skeletal muscle PGC-1α/mitofusin-2 regulatory pathway in response to physical activity. Diabetes Care 33, 645–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116).Kagawa Y., Cha S., Hasegawa K., Hamamoto T., Endo H. (1999) Regulation of energy metabolism in human cells in aging and diabetes: FoF1, mtDNA, UCP, and ROS. Biochem. Biophys. Res. Commun. 266, 662–676 [DOI] [PubMed] [Google Scholar]
- 117).Hong S., Pedersen P.L. (2008) ATP synthase and the actions of inhibitors utilized to study its roles in human health, disease, and other scientific areas. Microbiol. Mol. Biol. Rev. 72, 590–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118).Grover G.J., Malm J. (2008) Pharmacological profile of the selective mitochondrial F1F0 ATP hydrolase inhibitor BMS-199264 in myocardial ischemia. Cardiovasc. Ther. 26, 287–296 [DOI] [PubMed] [Google Scholar]