Abstract
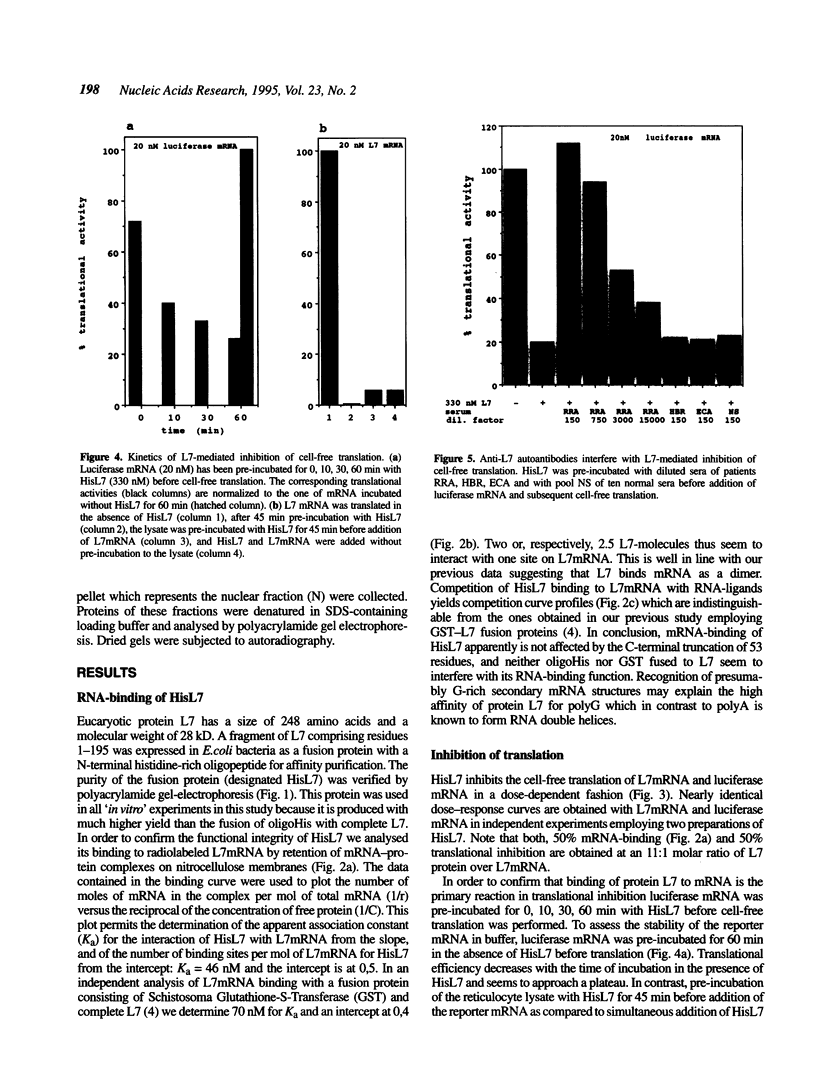
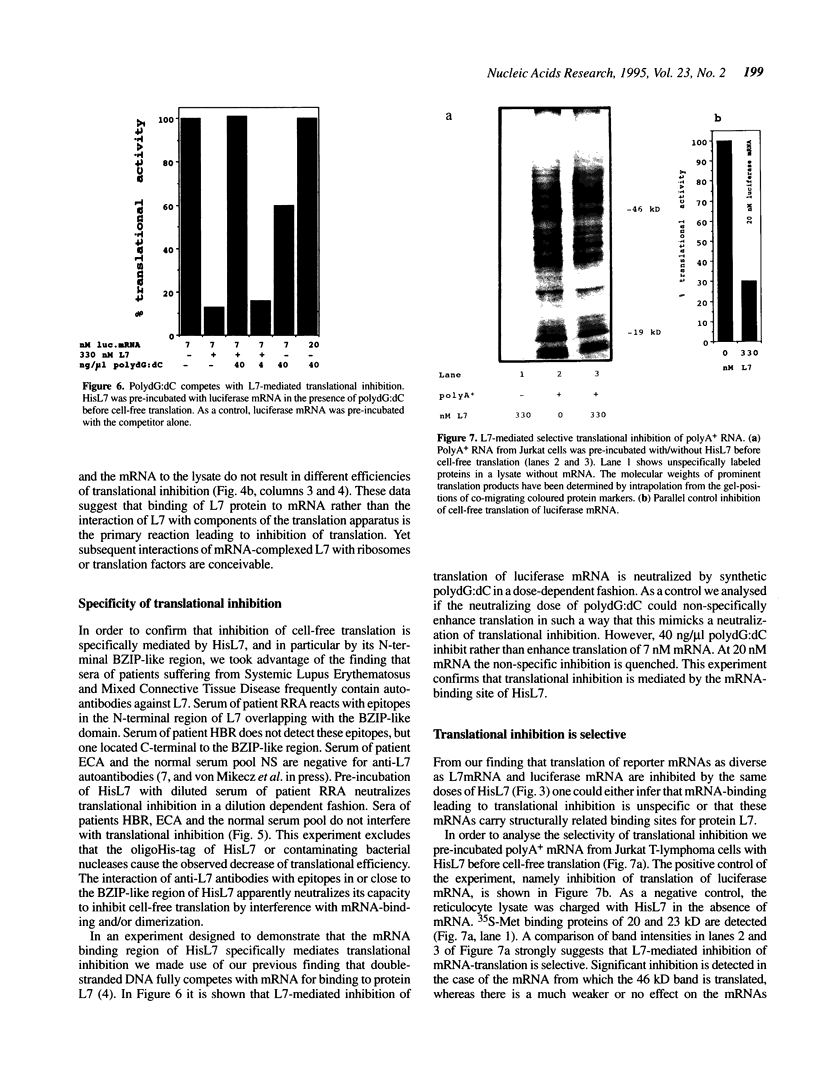
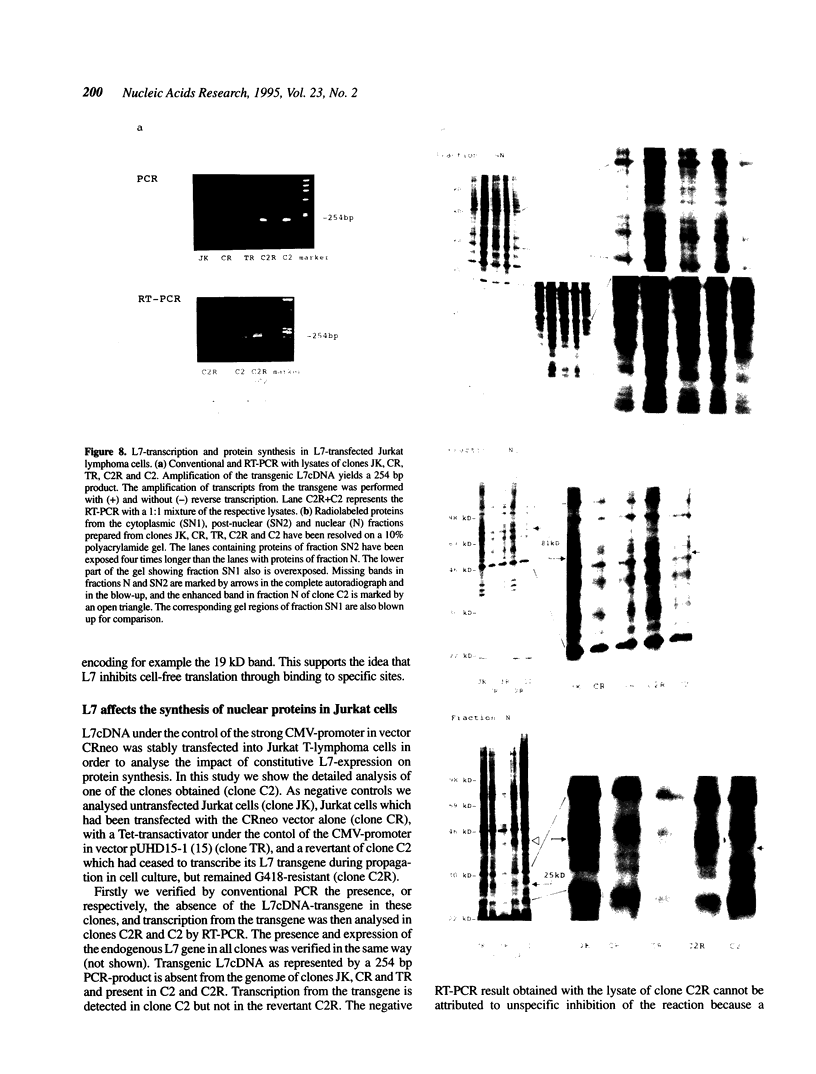
Eucaryotic ribosomal protein L7 carries a 'Basic-Region-Leucine-Zipper (BZIP)'-like region which mediates high-affinity binding to mRNA and 28S rRNA and formation of homodimers [P. Hemmerich et al. (1993) Nucleic Acids Res. 21, 223-231). Its biological function is unknown as yet and no direct L7-equivalent in procaryotes has been found. In this report we show that eucaryotic L7 specifically inhibits the cell-free translation of reporter mRNAs. The interaction of L7 with mRNA is an essential step in this reaction which is inhibitable by antibodies directed against the BZIP-like region of L7, and by competitors of mRNA binding. L7-mediated inhibition of cell-free translation of polyA+ RNA from Jurkat T-lymphoma cells is selective in that the synthesis of a major 46 kD protein is suppressed. Upon stable transfection of L7 cDNA into Jurkat lymphoma cells two major proteins disappear, namely one nuclear protein and one which associates with the nucleus. Our data suggest a regulatory role of protein L7 in the eucaryotic translation apparatus.
Full text
PDF

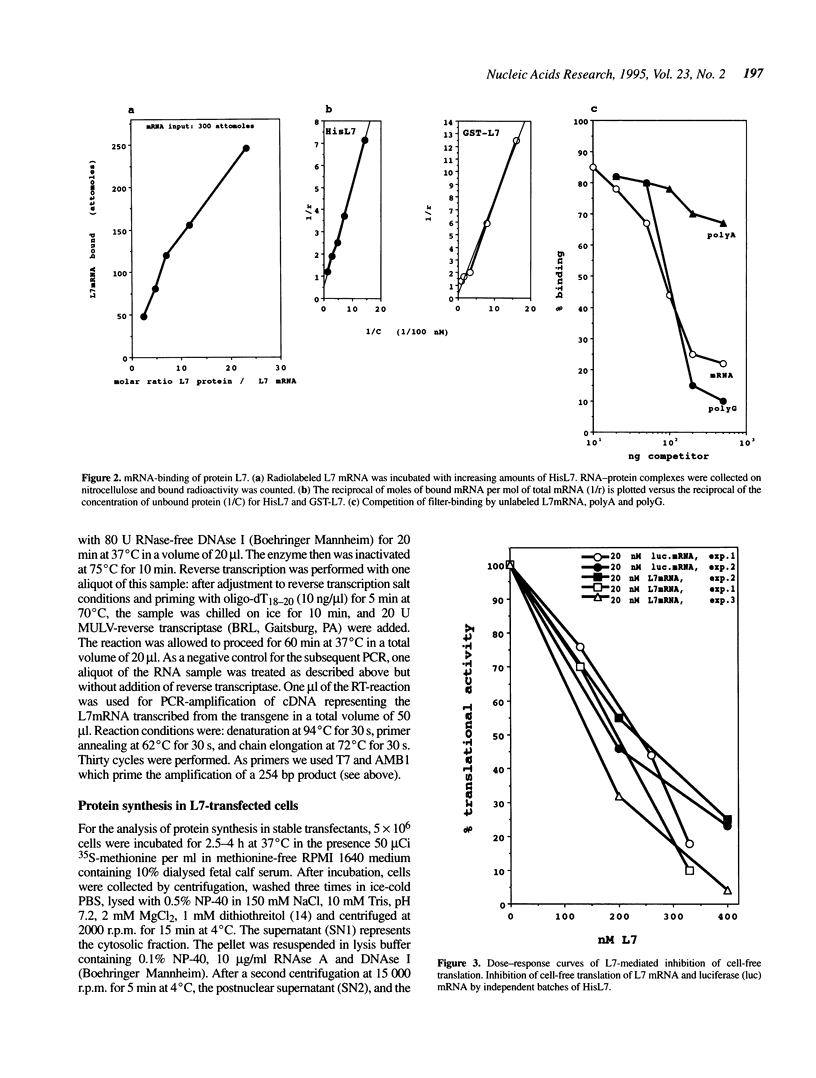


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abovich N., Gritz L., Tung L., Rosbash M. Effect of RP51 gene dosage alterations on ribosome synthesis in Saccharomyces cerevisiae. Mol Cell Biol. 1985 Dec;5(12):3429–3435. doi: 10.1128/mcb.5.12.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaldi F., Pierandrei-Amaldi P. Translational regulation of the expression of ribosomal protein genes in Xenopus laevis. Enzyme. 1990;44(1-4):93–105. doi: 10.1159/000468750. [DOI] [PubMed] [Google Scholar]
- Bowman L. H. The synthesis of ribosomal proteins S16 and L32 is not autogenously regulated during mouse myoblast differentiation. Mol Cell Biol. 1987 Dec;7(12):4464–4471. doi: 10.1128/mcb.7.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher M. P. The eucaryotic aminoacyl-tRNA synthetase complex: suggestions for its structure and function. J Cell Biol. 1984 Aug;99(2):373–377. doi: 10.1083/jcb.99.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossen B., Caughman S. W., Harford J. B., Klausner R. D., Hentze M. W. Translational repression by a complex between the iron-responsive element of ferritin mRNA and its specific cytoplasmic binding protein is position-dependent in vivo. EMBO J. 1990 Dec;9(12):4127–4133. doi: 10.1002/j.1460-2075.1990.tb07635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M., Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N. K., Hentze M. W. Iron regulatory protein prevents binding of the 43S translation pre-initiation complex to ferritin and eALAS mRNAs. EMBO J. 1994 Aug 15;13(16):3882–3891. doi: 10.1002/j.1460-2075.1994.tb06699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell C. M., McKenzie A. R., Patino M. M., Walden W. E., Theil E. C. Ferritin mRNA: interactions of iron regulatory element with translational regulator protein P-90 and the effect on base-paired flanking regions. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4166–4170. doi: 10.1073/pnas.88.10.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerich P., von Mikecz A., Neumann F., Sözeri O., Wolff-Vorbeck G., Zoebelein R., Krawinkel U. Structural and functional properties of ribosomal protein L7 from humans and rodents. Nucleic Acids Res. 1993 Jan 25;21(2):223–231. doi: 10.1093/nar/21.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W. Enzymes as RNA-binding proteins: a role for (di)nucleotide-binding domains? Trends Biochem Sci. 1994 Mar;19(3):101–103. doi: 10.1016/0968-0004(94)90198-8. [DOI] [PubMed] [Google Scholar]
- Janknecht R., de Martynoff G., Lou J., Hipskind R. A., Nordheim A., Stunnenberg H. G. Rapid and efficient purification of native histidine-tagged protein expressed by recombinant vaccinia virus. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8972–8976. doi: 10.1073/pnas.88.20.8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Rouault T. A., Harford J. B. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell. 1993 Jan 15;72(1):19–28. doi: 10.1016/0092-8674(93)90046-s. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Lin A., Chan Y. L., McNally J., Peleg D., Meyuhas O., Wool I. G. The primary structure of rat ribosomal protein L7. The presence near the amino terminus of L7 of five tandem repeats of a sequence of 12 amino acids. J Biol Chem. 1987 Sep 15;262(26):12665–12671. [PubMed] [Google Scholar]
- Mizuta K., Hashimoto T., Otaka E. Yeast ribosomal proteins: XIII. Saccharomyces cerevisiae YL8A gene, interrupted with two introns, encodes a homolog of mammalian L7. Nucleic Acids Res. 1992 Mar 11;20(5):1011–1016. doi: 10.1093/nar/20.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrutskii B. S., Stapulionis R., Deutscher M. P. Supramolecular organization of the mammalian translation system. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):964–968. doi: 10.1073/pnas.91.3.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Yates J. L., Dean D., Post L. E. Feedback regulation of ribosomal protein gene expression in Escherichia coli: structural homology of ribosomal RNA and ribosomal protein MRNA. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7084–7088. doi: 10.1073/pnas.77.12.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Meyuhas O. Translational control of ribosomal protein production in mammalian cells. Enzyme. 1990;44(1-4):83–92. doi: 10.1159/000468749. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Ryazanov A. G., Ovchinnikov L. P., Spirin A. S. Development of structural organization of protein-synthesizing machinery from prokaryotes to eukaryotes. Biosystems. 1987;20(3):275–288. doi: 10.1016/0303-2647(87)90035-9. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Ulbrich N., Todokoro K., Ackerman E. J., Wool I. G. Characterization of the binding of rat liver ribosomal proteins L6, L7, and L19 to 5 S ribosomal ribonucleic acid. J Biol Chem. 1980 Aug 25;255(16):7712–7715. [PubMed] [Google Scholar]
- White E., Grodzicker T., Stillman B. W. Mutations in the gene encoding the adenovirus early region 1B 19,000-molecular-weight tumor antigen cause the degradation of chromosomal DNA. J Virol. 1984 Nov;52(2):410–419. doi: 10.1128/jvi.52.2.410-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wool I. G. The structure and function of eukaryotic ribosomes. Annu Rev Biochem. 1979;48:719–754. doi: 10.1146/annurev.bi.48.070179.003443. [DOI] [PubMed] [Google Scholar]