Abstract
The authors reviewed contribution of Kumamoto University group to the progress of the studies on transthyretin (TTR)-related familial amyloidotic polyneuropathy (TTR-related FAP) for 42 years (from 1967 to 2009). Andrade (1952) first described a large group of patients with FAP in Portugal and Araki et al. (1967) in second discovered similar FAP patients in Arao, Kumamoto, Japan. Owing to progress in biochemical and molecular genetic analyses, FAP is now believed to occur worldwide. As of today, reports of about 100 different points of single or two mutations, or a deletion in the transthyretin (TTR) gene, have been published. The authors’ group has made pioneer works for study of FAP in the world. The focus on therapy in amylodosis will increase sharply as an impetus in near future, and successful treatments are expected.
Keywords: transthyretin-related familial amyloidotic polyneuropathy (TTR-related FAP), clinicopathology, molecular genetics, epidemiology, diagnostic tests, liver transplantation
Introduction
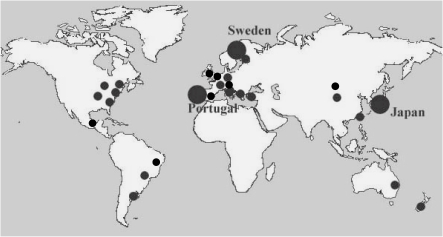
Familial amyloidotic polyneuropathy (FAP) is a hereditary amyloidosis in which amyloidogenic mutated transthyretin (ATTR), apolipoprotein A-I (AApoA-I), and gelsolin (Agel) have been identified as FAP-related amyloidogenic proteins.1) Of these proteins, ATTR is the most common throughout the world.2–4) Andrade first reported a large group of patients with FAP ATTR Val30Met in Portugal in 1952,5) and other large foci have been discovered in Japan by Araki et al.6) and in Sweden by Andersson et al.7) Until 20 years ago, FAP was thought to be a disease restricted to endemic occurrence in those areas. However, owing to progress in biochemical and molecular genetic analyses, this disease is now believed to occur worldwide (Fig. 1 ).2–4)
Figure 1.
Distribution of FAP in the world. Locations of foci of FAP ATTR Val30Met patients described in previous reports and obtained from personal communications are presented.
As of today, reports of about 100 different points of single or double mutations, or a deletion in the TTR gene, have appeared8); the majority of cases involve small kindred or no family history. Of TTR-related FAP types, FAP ATTR Val30Met is the most common, and only FAP ATTR Val30Met is found in large foci of patients, although the reason for this characteristic remains to be elucidated. Recently, several phenotypes of FAP, including the polyneuropathic, oculoleptomeningeal, and cardiac types, have been reported,9–12) and heterogeneity of clinical symptoms is recognized even for the same mutation of the TTR gene.
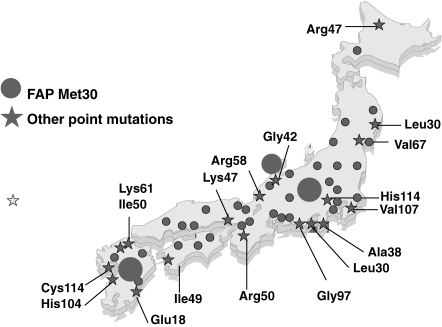
In Japan, Araki et al. first reported a focus of patients with FAP ATTR Val30Met in Arao district, Kumamoto,6) and Kito reported another focus in Nagano in Honshu Island.13) More than 25 points of mutation in the TTR gene have now been reported from the long islands of Japan, from the south to the north (Fig. 2 ).8,14) In this review, we present the history of our FAP research progress by Kumamoto group, and current clinicopathological, biochemical, molecular genetic, and epidemiological aspects of TTR-related FAP. In addition, we introduce a simple new diagnostic procedure for TTR-related FAP.
Figure 2.
Distribution of FAP in Japan. Localization of various types of FAP patients in previous reports are presented in this figure.
1. Background of the discovery of the disease in Japan
The discovery of a new disease in the history of medicine is often regarded as a chance occurrence. FAP was not documented in Japan until 1966, when Araki examined a patient who had been referred by a physician at the Arao City hospital in Kumamoto, Japan.
The patient was suffering from a sensory disturbance of the lower extremities, diarrhea, and impotence. At first, a physician at the Arao City Hospital suspected this patient might have been suffering from subacute myelo-optico-neuropathy (SMON). SMON is a disease of Clioquinol intoxication characterized by sensory disturbance in the lower limbs, pyramidal signs, and occasional visual disturbance preceded by abdominal symptoms. 5-chloro-7-iodo-8-quinolinol (Clioquinol) had been widely used in Japan to treat dysentery and diarrhea. A nationwide survey in 1972 revealed a cumulative total of 11,007 patients with SMON; 618 patients died between July 1967 and December 1981.
Araki found amyloid deposition in the biopsy specimen of the sural nerve taken from the referred patient, and the diagnosis of SMON in this patient could not be substantiated. Family members related to this patient showed similar clinical features. The representative case is as follows.
2. Report of a case6)
1). History
The first patient, a 46-years-old farmer became impotent at age of 40.
Two years later, he began to have a tingling sensation and numbness in the toes, ascending to the levels of both ankles 1 year later.
At age 44, he had constipation and diarrhea alternately. In the meantime, paresthesia and analgesia slowly ascended to the upper thighs and he became incontinent of urine. He became difficult to raise his legs in walking and he stumbled often. About this time, he sometimes experienced dizziness when he suddenly stood up and this episode became frequent. At age 45, he noticed numbness in the hands and diarrhea worsened, with occasional incontinence.
On May 26, 1966, at age 46, he was admitted to Neurological Institute, Kyushu University.
2). Examinations
He was malnourished. The skin of all four extremities was dry and cool. The blood pressure was 115/85 mmHg in the supine position, but 58/10 mmHg in the sitting position. Orthostastic hypotension was marked. Hoarseness was noticed, but other cranial nerves were unremarkable. Muscular atrophy and weakness were detected in all extremities; especially severely in the distal portions of the lower extremities. Deep tendon reflexes were slightly decreased in the upper extremities and absent in the lower. Superficial sensations were decreased in all four extremities, more severely in the periphery, but deep sensations were relatively preserved. Sural nerve biopsy showed amyloid deposition. Muscular atrophy and sensory impairment progressed thereafter. The patient’s high fever continued for 2 months prior to November 13, 1967 when he died of urinary tract infection after 6 years from the onset of impotence.
3). Abnormal laboratory data during the hospitalization
Red blood count was 322 × 104, Electrocardiogram showed 1st degree A-V block, and cerebrospinal fluid obtained by lumbar puncture showed clear, colorless, cell counts 3 (all lymphocytes), and total protein 112 mg/dl. Motor nerve conduction velocities decreased in four limbs. electromyogram showed giant and complex motor unit potential in the limb muscles.
4). Autopsy findings
An autopsy for an FAP patient revealed that prominent amyloid deposition was detected in the peripheral and autonomic nervous systems. The brain and spinal cord were unaffected. Amyloid deposition in the general organs was found chiefly in the kidneys, spleen, and around the walls of small blood vessels.
3. Reports on FAP
In 1967–1968, Araki et al. first reported a large focus of FAP in the area of Arao, Kumamoto, Japan.6) The clinical and pathological findings were similar in both Portuguese and Japanese patients. Since then, there have been several reports of families in Ogawa village region in Nagano prefecture by Kito in 1973 and in other parts of Japan with similar findings. FAP was also reported in Sweden, UK, Germany, Greek, Poland, USA, and others.
4. Identification of amyloid precursor protein
In 1978, Costa et al. reported in FAP of Portuguese origin that the FAP amyloid fibrils are closely related to the 13,745 dalton protein.15) In 1983, Pras et al. reported in Jewish origin that the amyloid protein contains 127 amino acid residues, and was identical to a human prealbumin subunit. In Japan, Tawara et al. (1983) have described the structural identification of an amyloid fibril protein of 14 K dalton in Japanese patients and have elucidated that the amyloid precursor protein is a variant TTR with a single amino acid substitution of varine by methionine at the 30th portion of the amino acid sequence of wild type TTR.16) This was the first discovery of molecular analysis in FAP.
5. TTR related FAP syndromes
Our study group has found three major FAP foci in Kumamoto, Nagasaki, and Fukuoka prefectures in Kyushu, Japan. They show different clinical features.
(1). FAP ATTR Val30Met
One hundred eighty-six patients with FAP ATTR Val30Met were registered from 1967 to 2008 in Arao district, Kumamoto, Japan. The inheritance pattern is autosomal dominant with a high penetrance rate and an equal sex ratio. The symptoms are first recognized when the patient is between 18 and 83 years of age, with a mean age at onset of 35.4 years. The age at onset in this type was found to show anticipation, as seen in FAP patients in other endemic areas.2,9)
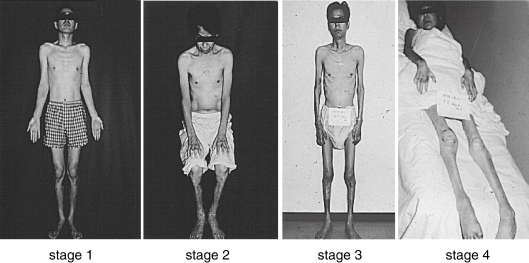
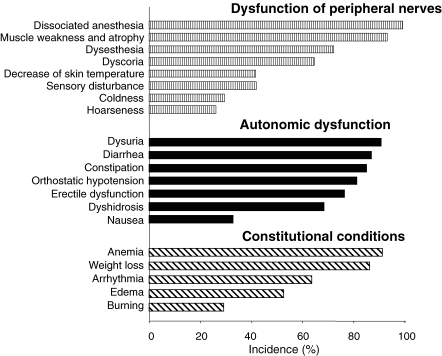
The disease is slowly progressive and reaches the terminal stage in 10.8 years (Fig. 3 ). Evidence of a sensorimotor peripheral neuropathy is usually found in the lower limbs. Dissociation of sensory impairment is common, with pain and temperature sensation being the most severely affected. Autonomic nervous system involvement, such as dyshidorosis, sexual impotence, disturbances of gastrointestinal motility (diarrhea alternating with constipation, orthostatic hypotension, and urinary disturbances) are frequent (Fig. 4 ). Cardiac and renal dysfunction is commonly recognized during the course of the illness. Anemia is also observed.17) Ocular involvement, such as vitreous opacity, kerato-conjunctivitis, glaucoma, and papillary disturbances, are seen.18) In addition, to the most common FAP types, various heterogeneous phenotypes have been observed. Clinical stages of FAP are classified into 4 types with ADL and sensory signs.19)
Figure 3.
FAP patients in each clinical stage. Typical features of FAP patients in each clinical stage as described in ref. 12 are demonstrated in the pictures.
Figure 4.
Clinical manifestations in patients with FAP ATTR Val30Met. One hundred sixty-nine patients with FAP ATTR Val30Met were registered from 1968 to 2003 in Arao district, Kumamoto, Japan.
(2). FAP ATTR Tyr114Cys
This type of FAP was discovered in Shimabara district, Nagasaki, Japan.20) The symptoms were first recognized when the patients were between 31 and 50 years of age, with a mean age at onset of 38.9 years. So far, 35 patients have been confirmed as having FAP ATTR Tyr114Cys. All patients showed decreased visual acuity as initial symptoms. Vitreous opacityes, cardiac failure, and autonomic dysfunction are the main symptoms recognized during the course of the illness. Sensory-dominant polyneuropathy is mild and less common compared with FAP ATTR Val30Met.20) A patient of this genotype was also found in Busan, Korea. Laboratory examinations have revealed extremely low serum TTR levels;21) a conformentional change in ATTR Tyr114Cys may facilitate aggregation of the TTR.
Attention has recently focused on the ocular-leptomeningeal form of FAP (induced by several point mutations in the TTR gene), as well as on the advanced stages of ATTR Val30Met-type FAP.18) Cerebral amyloid angiopathy and ocular amyloidosis are common characteristic clinical features in those types of FAP. Cerebral amyloid angiopathy is characterized by amyloid deposition in the media and adventitia of medium and small arteries, arterioles, and occasionally veins of the cortex and leptomeninges. Typical nervous system manifestation included cerebral infarction, hemorrhage, hydrocephalus, ataxia, spastic paralysis, convulsion, and dementia. These symptoms are often found in several types of TTR-related FAP and lead to classification into oclulomeningeal amyloidosis, in which amyloid deposition is also found in vitreous bodies and other tissues of the eye.22–24)
As the same way as other type of leptomeningeal form of FAP, amyloid deposition was found in the leptomeninges and in perivascular areas of the cerebral vessels and loss of consciousness, transient ischemic attacks, and cerebral bleeding were observed in FAP ATTR Tyr114Cys. The duration of the disease is shorter than that of other types of TTR related FAPs.20)
(3). FAP ATTR Ser50Ile
This type was discovered in Kasuya district, Fukuoka Prefecture in 1972. In addition, we found 13 new cases, and so totally 29 patients in the district. Cardiac failure is a primary clinical manifestation25) (Sakashita et al. 2000) and is a commonly recognized as the first symptom. Sensory-dominant polyneuropathy and autonomic failure are less common than in FAP ATTR Tyr114Cys.
(4). Other novel mutations in the TTR gene
Including the above-mentioned 3 mutations, a total of 235 FAP patients with three different mutations have been recognized. They are Glu42Gly,26) Val30Leu,27) Tyr114His,28) Ser49Ile,29) and compound heterozygote Arg104His/Val30Met.21) Ando et al. (2000) reported on the first Japanese FAP ATTR Val30Met identical twins.30) ATTR Asn124Ser with colocalization of κ light chain was identified in Italian sample by Bergström et al. who studied in Kumamoto 2007.31)
6. Pathology
A clinicopathological, histo-chemical, immunohistohemical, and ultrastructural study of materials obtained by autopsy or biopsy in 17 patients were performed by Takahashi et al. (1991).32) In the autopsy cases, amyloid deposits were predominant in the peripheral nerve tissues, autonomic nervous system, choroid plexus, cardiovascular system, and kidneys. Amyloid involvement in the anterior and posterior roots of the spinal cord, spinal ganglia, thyroid, and gastrointestinal tract was also frequent. In the cardiac conduction system, amyloid depositions were prominent in the sino-atrial node and in the limbs of the intraventricular bundle. In sural nerve biopsy specimens from patients with early-stage FAP, amyloid depositions were observed in the small vessel walls and the surrounding tissues. In the advanced cases, amyloid deposits were found in the subperineural and/or epineural regions. The numbers of myelinated and unmyelinated nerve decreased markedly, which indicated degenerative changes in Schwann cells. Degenerative changes in the axon, myelin sheath, and Schwann cells and the decrease in collagen fibers paralleled the severity of the peripheral nerve disturbances.
Morphometric data on the number and caliber of myelinated fibers in the sural nerve biopsy specimen from patients with early-stage FAP showed a greater reduction of small-caliber fibers than of large-caliber fibers. In patients with more advanced FAP, the numbers of myelinated fibers were decreased.33) Araki et al. (2000) summarized pathological findings on FAP ATTR Val30Met.34)
7. Biochemical aspects of amyloidogenesis
The amyloid formation working hypothesis established by substantial studies, gave rise to the idea that stabilizing tetrameric TTR is promising method for prevention of amyloid formation. McCutchen et al. first demonstrated this concept by using recombinant wild-type TTR and variant TTR. Normal TTR behaves as a tetramer and binds to retinol binding protein (RBP) and thyroxine (T4) in plasma.35) Tetrameric TTR is not itself amyloidogenic, but dissociation of the tetramer into a compact non-native monomer with low conformational stability can lead to amyloid fibril formation.36) Although several of TTR single-site mutations have a normal tetrameric structure under physiological condition, these mutations significantly destabilize the tetramer. Recent biochemical and pathological studies further revealed that instability of the tetrameric form of TTR by mutation, especially FAP ATTR Val30Met, leads to amyloid formation in the tissues of FAP patients.35) It’s interesting to note that subjects possessing the TTR Thr119Met gene are asymptomatic carriers, and that compound heterozygotes having ATTR Val30Met and TTR Thr119Met genes show very mild FAP symptoms of have no symptoms.37) Alves et al. demonstrated by semidenaturing isoelectric focusing that patients possessing TTR Thr119Met or ATTR Val30Met/TTR Thr119Met genes showed marked TTR tetrameric structural stability.38,39) Terazaki et al. also reported an interesting late-onset compound heterozygote patient with FAP ATTR Val30Met/TTR Arg104His who had very mild and slowly progressive clinical symptoms and whose tetrameric TTR stability was greater than that of the TTR from healthy volunteers.21,38) These evidences suggest that stabilizing the tetrameric TTR, as a potential therapeutic strategy, is a prerequisite for prevention of amyloid formation.40) On the basis of this concept, several treatments were proposed and examined.
(1). Various nonsteroidal anti-inflammatory drugs (NSAIDs) derivertives
Because thyroxine (T4) is one of the most important molecules for stabilizing TTR in tetrameric form, T4-based therapeutic drugs have been proposed. Binding of T4 and their derivatives stabilizes the native conformation of TTR and inhibits amyloid formation in vitro and possibly in vivo. In fact, it has been proposed that, in the cerebral spinal fluid (CSF) where a high concentration of TTR with bound T4 is found, the protein is in the stable tetrameric conformation and does not lead to detectable TTR amyloid formation in the brain.41)
Baures et al. reported that various NSAIDs have potential for stabilizing the tetrameric TTR. Several NSAIDs, which bind and inhibit the cyclooxygenases, also bind to TTR as mimic compounds of T4.41) The structure of NSAIDs resembles the structure of T4 and these drugs bind to TTR via a T4 binding site. Peterson et al. demonstrated that the three-dimensional structure of TTR with flufenamic acid (FLU), a derivative of NSAIDs that is a more efficacious TTR amyloid inhibitor than the natural ligand T4. Difulnisal and flufenamic acid exhibit notable inhibition of both acid-mediated aggregation and urea-mediated tetramer dissociation of the most common disease associated variants. Tojo et al. reported that therapeutic serum concentrations of difulnisal (100–200 µM) stabilized serum variant TTR tetramer more effective than those of fluenamic acid (35–70 µM). Sekijima et al. also demonstrated that oral administration of diflunisal mediates kinetic stabilization of TTR in human serum.42) Furthermore, structurally related derivatives of NSAIDs were also exploited to enhance the selectivity and reduce the adverse effect. Various reports confirmed these findings and this concept is now widely accepted.43)
(2). Cr3+
Recent studies suggested that certain metal ions affect amyloidogenesis in several types of amyloidosis. In FAP, metal ions may influence the stability of the tetrameric form of TTR. Sato et al. therefore investigated whether various metal ions (e.g., Zn2+, Cu2+, Ca2+, Fe3+, Al3+, Cr3+) affect amyloidogenesis of wild-type TTR and ATTR. Among the metal ions, Cr3+ increased the tetrameric stability of both wild-type and ATTR Val30Met, and suppressed TTR amyloidogenesis. It is unlikely that Cr3+ binds to T4-binding sites of TTR, because of its chemical structure. Cr3+ may increase the thermodynamic stability of TTR tetramer by facilitating the binding of T4. Because Cr3+ is a component of health foods that is widely used throughout the world, administration of this metal ion was thought to be a good candidate for therapy. However, at the serum concentration, Cr3+ had no significant effect on TTR stability in serum. While serum Cr3+ concentration will increase after Cr3+ supplementation, it is still very low compared to the concentration of TTR, likely explaining why stabilization is not observed. However, Cr3+ could be useful for treating TTR amyloidosis by enhancing the effects of diflunisal, because the effects of T4 and Cr3+ are not competitive but cooperative.44) Although no obvious effect of Cr3+ on patients was seen, further in vivo evaluation of the effects is needed.
(3). Fx-1006A
Tremendous efforts revealed the possibility of stabilization of the tetrameric form of TTR as a potential therapeutic strategy. Ongoing biochemical and pathological studies explored further potentiality of this therapy. It’s noteworthy that Fx-1006A, a potent and selective stabilizer for tetrameric TTR, is underway in a multinational Phase II/III clinical study in patients with FAP worldwide. Moreover, a number of structurally diverse small molecules that bind to the TTR increasing the protein stability and thereafter inhibiting amyloid fibrillogenesis have been tested. In fact, several small molecules, such as plant-derived flavones and xanthones, the synthetic estrogen diethylstilbestrol, 2,4-dinitrophenol (DNP) as well as 3,5-diiodosalicylic acid were shown to have high affinity towards TTR and to inhibit amyloid formation in vitro.45)
8. Molecular genetics
The human TTR gene was localized at 18p 11.1-q 12.3 and structure was first determined by Tsuzuki et al. (1985).46) Mita et al. (1984) cloned cDNAcording for human TTR from cDNA library prepared from human liver.47) A diagnosis of FAP ATTR Val 30 Met can be made by restriction endonuclease Nsi1 with Southern blotting procedures.48) In 100 abnormal TTR genes, 0, 36, 40, and 24 points of mutation were detected in exons 1, 2, 3, and 4, respectively; a deletion in the TTR gene also occurred. The main sites of production of TTR confirmed by in situ hybridization methods are the liver, retinal pigment epithelium, choroid plexus of the brain, α-cells in the pancreatic islets and visceral york sac endoderm.49,50)
9. Animal models
Shimada and Yamamura et al. (1989)51) developed transgenic mice carrying and expressing the human mutant TTR ATTR Val30Met. Yi et al. (1991)52) analyzed amyloid deposition in the mice and found that deposition started in the gastrointestinal tract, cardiovascular system, and kidneys 6 months after birth and extended to various other organs and tissues with advancing age. The pattern of amyloid deposition in the mouse at age 24 months was similar to that observed in human autopsy cases of FAP, except for the absence of amyloid in the choroid plexus and the peripheral and autonomic nervous systems. Amyloid deposited was shown to be composed of human TTR and, in addition, mouse serum amyloid P-component. These results clearly indicate that human variant TTR produced in transgenic mice is deposited as a major component of amyloid fibrils in various organs and tissues. Thus, this animal model is useful for analyzing how amyloid deposition is initiated and proceeds in FAP.
Murakami et al. (1992)53) induced chronic inflammation in the transgenic mice, but secondary amyloidosis was observed, and there were no changes in quantitative amyloid deposition compared with the mice without inflammation.
Our group newly developed the transgenic rat having human ATTR Val30Met gene which express the human variant gene in the liver, retina, and choloid plexus in collaboration with Jichi Medical University research group.54) The transgenic rat is useful for the experiments of liver transplantation, and examining the effect of therapeutic drugs.
10. Clinical genetic testing
For the screening of FAP patients, we first perform mass spectrometric analysis, with a system for serum and cerebrospinal fluid that uses methods such as electron spray ionization-mass spectrometry (ESI-MS)55) and matrix-assisted laser desorption ionization/time-of-flight mass spectrometry (MALDI/TOF-MS).56) With this system, the presence or absence of mutations in the TTR gene can be determined. A shift in the molecular mass caused by substitution of amino acids can be detected as a peak that differs from those identifying the wild-type TTR. Recently, to shorten the time to screen the FAP suspected serum samples, we newly applied protein chip system using surface-enhanced laser desorption/ionization (SELDI/TOF MS). We can detect variant TTRs within 3 hours in a several µl serum samples.57)
Another method, in addition to polymerase chain reaction and restriction endonuclease analysis, has recently been used for screening patients who have a family history or typical clinical manifestations, or who come from an endemic FAP area. By means of the LightCycler method, we can detect the presence or absence of a TTR mutation in 1 hour.
(1). Detection of ATTR Val30Met via LightCycler
LightCycler technology can detect mutations quickly and accurately by using fluorescent hybridization probes and melting curves. For example, an anchor probe (5′-TGTGGCCGTGCATGTGT-3′-FITC, in which the underlined nucleotide is the normal nucleotide) and a sensor probe (LC Red 640-5′-CAGAAAGGCTGCTG ATGACACCTGGGAGCCATTTGCCTCTGGG-3′-OH) were used to detect ATTR Val30Met. Because a single mismatch can significantly reduce the melting temperature of the oligonucleotide, the melting temperature is reduced when the amplified gene encodes ATTR Val30Met. Therefore, it is possible to discriminate among a normal subject, an FAP ATTR Val30Met homozygote, and an FAP ATTR Val30Met heterozygote by using melting curve analysis.8)
(2). Nonisotopic single-strand conformational polymorphism
Single-strand conformational polymorphism (SSCP) was applied with samples with molecular mass shift in TTR to determine the exon in which a mutation was present. However, a conventional type of SSCP requires a radioisotope to perform the procedure. Therefore, to avoid the need for a radioisotope, we recently developed SSCP analysis with capillary electrophoresis (SSCP-CE) in which a forward primer labeled with Cy5 and a microchip (iChip 12; Hitachi Chemical Co., Ltd., Tokyo, Japan) filled with i-S gel 3 (Hitachi Chemical Co., Ltd.) are used. An example of SSCP-CE analysis of an FAP ATTR Tyr114Cys heterozygote is presented herein. As shown in Fig. 3B, an additional peak is observed when the amplified gene encodes for ATTR Tyr114Cys.8)
With the combined diagnostic methods, such as pathologic, mass spectrometric, and molecular genetic examinations, screening of FAP patients become simpler and more accurate. Since those methods can not always reveal all mutations in TTR gene of FAP patients, we determine the presence or absence of a mutation with an auto-sequencer.
(3). Other diagnostic methods
Ando et al. presented a histochemical method of diagnosis of ATTR Val30Met by using FAP patients’ hair and monoclonal antibody supplied by Costa et al.58) Also, Ando et al. demonstrated abundant abnormal ocular vessels in patients with FAP ATTR Val30Met, which has diagnostic value.59)
11. Epidemiology
The presence of patients with TTR-related FAP has been confirmed in more than 30 countries, with FAP ATTR Val30Met patients verified in more than 15 countries. Only FAP ATTR Val30Met has large foci in the world, although the reason for this is not known. Holmgren et al. performed an epidemiological study in the northern part of Sweden and estimated that the number of ATTR Val30Met gene carriers in a total population of 500,000 in the area was approximately 7,500, although the penetrance of the mutation was as low as about 2%.60) To elucidate the reason for this phenomenon, we examined the haplotype studies in the worldwide. The analyses revealed that Swedish FAP patients had different haplotypes compared with those of Japanese and Portuguese. By 2007, more than 3,000 FAP ATTR Val30Met patients from 489 pedigrees had been diagnosed at Centro de Estudos de Paramidoidose in Porto, Portugal. So far, more than 3,000 FAP patients have been registered in Portugal (personal communication). In Japan, more than 400 FAP patients were found in two endemic foci: one is Arao city in Kumamoto prefecture, and the other is Ogawa village in Nagano prefecture. In addition, 44 FAP kindred with Val30Met TTR were traced. They were genealogically independent and were geographically scattered throughout Japan. Recently, the third largest focus of this disease was identified in Noto peninsular, Ishikawa prefecture.
12. Origin of FAP ATTR Val30Met
As described above, in the 1960s, large foci of patients were found in Japan and Sweden in addition to Portugal. These three countries are geographically distant, and a consanguineous relationship between foci has not been identified. The issue of whether there is a common origin in the foci for a mutant allele has not been resolved. Furthermore, Continho hypothesized that a mutant allele in the Portuguese kindred could be one origin of the mutation for FAP foci throughout the world, including Japan, Europe, North and South America, and Africa.61) This hypothesis was based only on well-known historical relations and thus far it has not been scientifically tested. Ohmori et al. compared haplotypes in several foci of FAP patients and discovered that a common founder could be conceivable for Japanese and Portuguese patients, and for Portuguese and Spanish patients, but not for Swedish and other patients. Additional studies of genotypes and phenotypes are needed.
13. Liver transplantation
Liver transplantation has been reported to halt the progression. According to data in the Familial Amyloidotic Polyneuropathy World Transplant Registry (FAPWTR), More than 60 centers in 17 countries have performed orthotopic liver transplantation (OLT) for FAP. By the end of 2008, more than 1,500 patients have undergone OLTs. Survival of the patients has been excellent (overall 5-year survival of 80%) and comparable to the survival for OLT performed for other chronic liver disorders, but a longer follow-up is needed to compare the outcome after OLT with the natural course of the disease. The main cause of death was related to cardiac difficulties (40%).62–68)
In our group, we had 42 FAP patients (ATTR Val30Met patients: 38, Ser50Ile: 1, Tyr114Cys: 3, and Ser33leu: 1) who underwent liver transplantation from 1994 to 2009. More than 90% of the transplanted patients have a full time work, and fortunately 5-year-survival rate is 100%. From 1994 to 1998, liver transplantation was performed using a cadaveric donor, however, from 1999 partial liver transplantation using a living donor was performed: simultaneously 22 patients with severe liver diseases underwent domino liver transplantation using a resected FAP patient’s liver.
The use of sequential liver transplantation with dissected livers from FAP patients started in Portugal; more than 500 patients have received FAP patients’ livers. Several second recipients transplanted FAP patient’s liver started to show FAP symptoms in several years after liver transplantation.69,70) We must carefully follow up such transplanted patients.
14. New therapeutic approaches for FAP
Although liver transplantation is the only therapy to halt the clinical manifestations of transthyretin (TTR) related FAP, the therapy has given rise to have several problems. An alternative treatment is needed. After the precursor protein of amyloid fibrils in FAP was determined in 1983, several different therapeutic approaches have been investigated as an essential therapy for FAP: (1) reduction of variant TTR levels in plasma, (2) down-regulation of TTR gene of mRNA (3) inhibition of amyloid deposition, (4) stabilization of the tetrameric TTR structure and (5) replacement of the variant TTR gene with the normal TTR gene (which can be achieved by liver transplantation or by gene therapy) (Fig. 5 ). In our research group, as gene therapy for variant TTR gene, gene conversion therapy using wild type TTR single stranded oligonucleotides, or gene silencing using siRNA for variant TTR gene has been studied. In addition, effect of antibody therapy for targeting misfiled variant TTR has been examined in vivo. Both projects are now on going, and getting better results in vivo. In near future, these therapeutic projects may be in phase study.
Figure 5.
Working hypothesis for TTR amyloid formation and therapies. On the basis of amyloid formation mechanism, several promising therapies are considering.
Conclusions
TTR-related amyloidosis is not an insignificant disease, and many more affected patients would likely be found if more careful and precise investigations were performed. The collaboration of neurologists, gastroenterologists, ophthalmologists, and cardiologists is needed to make a definitive diagnosis. Although liver transplantation can holt the progression of FAP, it has several serious problems. New therapeutic approaches, such as gene therapy, and antibody therapy should try to be examined.
Abbreviations
- FAP
familial amyloidotic polyneuropathy
- TTR
transthyretin
- ATTR
amyloidogenic TTR
- SMON
subacute myelo-optico-neuropathy
- Clioquinol
5-chloro-7-iodo-8-quinolinol
Biographies
Profile
Shukuro Araki was born in 1927 in Kumamoto, Japan. He graduated from Kumamoto University, Department of Medicine, in 1952 and started his clinical carriers in neurology at Montefiore Medical Center, New York City. After receiving Ph.D. degree in 1960 from Kyushu University, Faculty of Medicine, he studied at the Neurological Institute, New York, USA, under the guidance of Professors H. Houston Merritt and Lewis P. Rowland. (Biochemical study of the neuro-muscular metabolic disorders). He became Associate Professor, Department of Neurology, Kyushu University, Faculty of Medicine, 1963. His life work on FAP started from 1967, when he discovered FAP in Japan. He and his research groups in Kumamoto, Kyushu, Kawasaki and Miyazaki Universities carried out clinicopathological, biochemical, molecular genetic, epidemiological, and therapeutic studies. He was awarded the Takeda Medical Prize in 1994 for study on amyloidosis. His successor Professor Yukio Ando and research group are now carrying out the clinical and experimental research on FAP.
Yukio Ando was born in 1955. He graduated from Kumamoto University, School of Medicine, in 1983 and started his clinical carriers in neurology as well as medicine at Kumamoto University Hospital from the same year. After receiving Ph.D. degree in 1991 (under the guidance of Professors Y. Morino, and Professor S. Araki from Kumamoto University School of Medicine), he studied at First Department of Internal Medicine, under the guidance of Professor S. Araki. From 1996 to 1998, he performed basic and clinical studies on FAP at Department of Medicine, Umea University, Sweden as a visiting professor. He is the professor of Department of Diagnostic Medicine, Graduate School of Medical Sciences, Kumamoto University. He was awarded several prizes, such as Japanese Laboratory Medicine Prize, Japanese Society of Neurological Therapeutics Prize, and Honorary PhD Award in Sweden.
References
- 1).Araki S. (1984) Type I familial amyloidotic polyneuropathy (Japanese type). Brain Dev. 6, 128–133 [DOI] [PubMed] [Google Scholar]
- 2).Benson M.D. (1989) Familial amyloidotic polyneuropathy. Trends Neurosci. 12, 88–92 [DOI] [PubMed] [Google Scholar]
- 3).Benson M.D., Uemichi T. (1996) Transthyretin amyloidosis. Amyloid 3, 44–56 [Google Scholar]
- 4).Ando Y., Araki S., Ando M. (1993) Transthyretin related amyloidosis. Intern. Med. 32, 920–922 [DOI] [PubMed] [Google Scholar]
- 5).Andrade C. (1952) A peculiar form of peripheral neuropathy: Familial generalized amyloidosis with special involvement of the peripheral nerves. Brain 75, 408–427 [DOI] [PubMed] [Google Scholar]
- 6).Araki S., Mawatari S., Ohta M., Nakajima A., Kuroiwa Y. (1968) Polyneurotic amyloidosis in a Japanese family. Arch. Neurol. 18, 593–602 [DOI] [PubMed] [Google Scholar]
- 7).Andersson R. (1976) Familial amyloidosis with polyneuropathy. A clinical study based on patients living in northern Sweden. Acta Med. Scand. Suppl. 590, 1–64 [PubMed] [Google Scholar]
- 8).Ando Y., Nakamura M., Araki S. (2005) Transthyretin related familial amyloidotic polyneuropathy. Arch. Neurol. 62, 1057–1062 [DOI] [PubMed] [Google Scholar]
- 9).Tashima K., Ando Y., Tanaka Y., Uchino M., Ando M. (1995) Change in the age of onset in patients with familial amyloidotic polyneuropathy type I. Intern. Med. 34, 748–750 [DOI] [PubMed] [Google Scholar]
- 10).Benson M.D., Uemichi T. (1996) Transthyretin amyloidosis. Amyloid 3, 44–56 [Google Scholar]
- 11).Connors L.H., Lim A., Prokaeva T., Roskens V.A., Costello C.E. (2003) Tabulation of human transthyretin (TTR) variants, 2003. Amyloid 10, 160–185 [DOI] [PubMed] [Google Scholar]
- 12).Goren H., Steinberg M.C., Farboody G.H. (1980) Familial oculoleptomeningeal amyloidosis. Brain 103, 473–495 [DOI] [PubMed] [Google Scholar]
- 13).Kito S., Itonaga E., Kamiya K., Kishida T., Yamamura Y. (1980) Studies on familial amyloidotic polyneuropathy in Ogawa Village, Japan. Eur. Neurol. 19, 141–151 [DOI] [PubMed] [Google Scholar]
- 14).Ikeda S., Nakazato M., Ando Y., Sobue G. (2002) Familial amyloidotic polyneuropathy in Japan: Clinical and genetic heterogeneity. Neurology 58, 1001–1007 [DOI] [PubMed] [Google Scholar]
- 15).Costa P.P., Figueira A.S., Bravo F.R. (1978) Amyloid fibril protein related to prealbumin in familial amyloidotic polyneuropathy. Proc. Natl. Acad. Sci. USA 75, 4499–4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Tawara S., Nakazato M., Kangawa K., Matsuo H., Araki S. (1983) Identification of amyloid prealbumin variant in familial amyloidotic polyneuropathy (Japanese type). Biochem. Biophys. Res. Commun. 116, 880–888 [DOI] [PubMed] [Google Scholar]
- 17).Asahara K., Ando Y., Tanaka Y., Yi S., Yamashita T., Ando M. (1993) Secondary hypoplastic anemia in patients with familial amyloidotic polyneuropathy. Acta Haematol. 90, 130–135 [DOI] [PubMed] [Google Scholar]
- 18).Ando E., Ando Y., Okamura R., Uchino M., Ando M., Negi A. (1997) Ocular manifestations of familial amyloidotic polyneuropathy type I: long-term follow up. Br. J. Ophthalmol. 81, 295–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Araki S. (1982) Amyloidosis. Asian Med. J. 25, 89–92 [Google Scholar]
- 20).Nakamura M., Yamashita T., Ueda M., Obayashi K., Sato T., Ikeda T., et al. (2005) Neuroradiologic and clinicopathologic features of oculoleptomeningeal type amyloidosis. Neurology 65, 1051–1056 [DOI] [PubMed] [Google Scholar]
- 21).Terazaki H., Ando Y., Misumi S., Nakamura M., Ando E., Matsunaga N., et al. (1999) A novel compound heterozygote (FAP ATTR Arg104His/ATTR Val30Met) with high serum transthyretin (TTR) and retinol binding protein (RBP) levels. Biochem. Biophys. Res. Commun. 264, 365–370 [DOI] [PubMed] [Google Scholar]
- 22).Arpa Gutiérrez J., Morales C., Lara M., Muñoz C., García-Rojo M., Caminero A., et al. (1993) Type I familial amyloid polyneuropathy and pontine haemorrhage. Acta Neuropathol. 86, 542–545 [DOI] [PubMed] [Google Scholar]
- 23).Ushiyama M., Ikeda S., Yanagihara N. (1991) Transthyretin type cerebral amyloid angiopathy in type I familial amyloid polyneuropathy. Acta Neuropathol. 81, 524–528 [DOI] [PubMed] [Google Scholar]
- 24).Sakashita N., Ando Y., Jinnouchi K., Yoshimatsu M., Terazaki H., Obayashi K., et al. (2001) Familial amyloidotic polyneuropathy (ATTR Val30Met) with widespread cerebral amyloid angiopathy and lethal cerebral hemorrhage. Pathol. Int. 51, 476–480 [DOI] [PubMed] [Google Scholar]
- 25).Sakashita N., Ando Y., Obayashi K., Terazaki H., Yamashita T., Takeya M., et al. (2000) Familial amyloidotic polyneuropathy (ATTR Ser50Ile): the first autopsy case report. Virchows Arch. 436, 345–350 [DOI] [PubMed] [Google Scholar]
- 26).Murakami T., Yi S., Yamamoto K., Maruyama S., Araki S. (1992) Familial amyloidotic polyneuropathy: report of patients heterozygous for the transthyretin Gly42 gene. Ann. Neurol. 31, 340–342 [DOI] [PubMed] [Google Scholar]
- 27).Murakami T., Tachibana S., Endo Y., Kawai R., Hara M., Tanase S., et al. (1994) Familial carpal tunnel syndrome due to amyloidogenic transthyretin His 114 variant. Neurology 44, 315–318 [DOI] [PubMed] [Google Scholar]
- 28).Murakami T., Atsumi T., Maeda S., Tanase S., Ishikawa K., Mita S., et al. (1992) A novel transthyretin mutation at position 30 (Leu for Val) associated with familial amyloidotic polyneuropathy. Biochem. Biophys. Res. Commun. 187, 397–403 [DOI] [PubMed] [Google Scholar]
- 29).Nakamura M., Yamashita T., Ando Y., Asl K.H., Tashima K., Ohlsson P.I., et al. (1999) Identification of a new transthyretin variant (ILe49) in familial amyloidotic polyneuropathy using electrospray ionization mass spectrometry and non-isotopic RNase cleavage assay. Hum. Hered. 49, 186–189 [DOI] [PubMed] [Google Scholar]
- 30).Ando Y., Ohtsu Y., Terazaki H., Kibayashi K., Nakamura M., Ando E., et al. (2000) Japanese monozygotic twins with familial amyloidotic polyneuropathy (FAP) (ATTR Val30Met). Amyloid 7, 133–136 [DOI] [PubMed] [Google Scholar]
- 31).Bergström J., Patrosso M.C., Colussi G., Salvadore M., Penco S., Lando G., et al. (2007) A novel type of familial transthyretin amyloidosis, ATTR Asn124Ser, with co-localization of κ light chains. Amyloid 14, 141–145 [DOI] [PubMed] [Google Scholar]
- 32).Takahashi K., Yi S., Kimura Y., Araki S. (1991) Familial amyloidotic polyneuropathy type I in Kumamoto, Japan: a clinico-pathologic, histochemical, immunohistochemical, and ultrastructural study. Hum. Pathol. 22, 519–527 [DOI] [PubMed] [Google Scholar]
- 33).Takahashi K., Sakashita N., Ando Y., Suga M., Ando M. (1997) Late onset type I familial amyloidotic polyneuropathy: presentation of three autopsy cases in comparison with 19 autopsy cases of the ordinary type. Pathol. Int. 47, 353–359 [DOI] [PubMed] [Google Scholar]
- 34).Araki S., Yi S. (2000) Pathology of familial amyloidotic polyneuropathy with TTR met 30 in Kumamoto, Japan. Neuropathology 20Suppl, S47–S51 [DOI] [PubMed] [Google Scholar]
- 35).McCutchen S.L., Colon W., Kelley J.W. (1993) Transthyretin mutation Leu-55-Pro alters tetrameric stability and increases amyloidogenicity. Biochemistry 32, 12119–12127 [DOI] [PubMed] [Google Scholar]
- 36).Kelly J.W., Lansbury P.T., Jr. (1994) A chemical approach to elucidate the mechanism of transthyretin and β-protein amyloid formation. Amyloid 1, 186–205 [Google Scholar]
- 37).Longo Alves I., Hays M.T., Saraiva M.J. (1997) Comparative stability and clearance of [Met30]transthyretin and [Met119]transthyretin. Eur. J. Biochem. 249, 662–668 [DOI] [PubMed] [Google Scholar]
- 38).Almedida M.R., Alves I.L., Terazaki H., Ando Y., Saraiva M.J. (2000) Comparative studies of two transthyretin variants with protective effects on familial amyloidotic polyneuropathy: TTR R104H and TTR T119M. Biochem. Biophys. Res. Commun. 270, 1024–1028 [DOI] [PubMed] [Google Scholar]
- 39).Alves I.L., Divino C.M., Schussler G.C., Altland K., Almeida M.R., Palha J.A., et al. (1993) Thyroxine binding in a TTR Met 119 kindred. J. Clin. Endocrinol. Metab. 77, 484–488 [DOI] [PubMed] [Google Scholar]
- 40).Almeida M.R., Damas A.M., Lans M.C., Brouwer A., Saraiva M.J. (1997) Thyroxine binding to transthyretin Met 119. Comparative studies of different heterozygotic carriers and structural analysis. Endocrine 6, 309–315 [DOI] [PubMed] [Google Scholar]
- 41).Baures P.W., Oza V.B., Peterson S.A., Kelly J.W. (1999) Synthesis and evaluation of inhibitors of transthyretin amyloid formation based on the non-steroidal anti-inflammatory drug, flufenamic acid. Bioorg. Med. Chem. 7, 1339–1347 [DOI] [PubMed] [Google Scholar]
- 42).Tojo K., Sekijima Y., Kelly J.W., Ikeda S. (2006) Diflunisal stabilizes familial amyloid polyneuropathy-associated transthyretin variant tetramers in serum against dissociation required for amyloidogenesis. Neurosci. Res. 56, 441–449 [DOI] [PubMed] [Google Scholar]
- 43).Sekijima Y., Kelly J.W., Ikeda S. (2008) Pathogenesis of and therapeutic strategies to ameliorate the transthyretin amyloidoses. Curr. Pharm. Des. 14, 3219–3230 [DOI] [PubMed] [Google Scholar]
- 44).Sato T., Ando Y., Susuki S., Mikami F., Ikemizu S., Nakamura M., et al. (2006) Chromium (III) ion and thyroxine cooperate to stabilize the transthyretin tetramer and suppress in vitro amyloid fibril formation. FEBS Lett. 580, 491–496 [DOI] [PubMed] [Google Scholar]
- 45).Pater M. (2009) Spotlight focuses on protein-misfolding thera. Nat. Biotechnol. 27, 874. [DOI] [PubMed] [Google Scholar]
- 46).Tsuzuki T., Mita S., Maeda S., Araki S., Shimada K. (1985) Structure of the human prealbumin gene. J. Biol. Chem. 260, 12224–12227 [PubMed] [Google Scholar]
- 47).Mita S., Maeda S., Shimada K., Araki S. (1984) Cloning and sequence analysis of cDNA for human prealbumin. Biochem. Biophys. Res. Commun. 124, 558–564 [DOI] [PubMed] [Google Scholar]
- 48).Mita S., Maeda S., Ide M., Tsuzuki T., Shimada K., Araki S. (1986) Familial amyloidotic polyneuropathy diagnosed by cloned human prealbumin cDNA. Neurology 36, 298–301 [DOI] [PubMed] [Google Scholar]
- 49).Herbert J., Wilcox J.N., Pham K.T., Fremeau R.T., Jr., Zeviani M., Dwork A., et al. (1986) Transthyretin: a choroid plexus-specific transport protein in human brain. The 1986 S. Weir Mitchell Award. Neurology 36, 900–911 [DOI] [PubMed] [Google Scholar]
- 50).Jacobsson B., Carlström A., Platz A., Collins V.P. (1990) Transthyretin messenger ribonucleic acid expression in the pancreas and in endocrine tumors of the pancreas and gut. J. Clin. Endocrinol. Metab. 71, 875–880 [DOI] [PubMed] [Google Scholar]
- 51).Shimada K., Maeda S., Murakami T., Nishiguchi S., Tashiro F., Yi S., et al. (1989) Transgenic mouse model of familial amyloidotic polyneuropathy. Mol. Biol. Med. 6, 333–343 [PubMed] [Google Scholar]
- 52).Yi S., Takahashi K., Naito M., Tashiro F., Wakasugi S., Maeda S., et al. (1991) Systemic amyloidosis in transgenic mice carrying the human mutant transthyretin (Met30) gene. Pathologic similarity to human familial amyloidotic polyneuropathy, type I. Am. J. Pathol. 138, 403–412 [PMC free article] [PubMed] [Google Scholar]
- 53).Murakami T., Yi S., Maeda S., Tashiro F., Yamamura K., Takahashi K., et al. (1992) Effect of serum amyloid P component level on transthyretin-derived amyloid deposition in a transgenic mouse model of familial amyloidotic polyneuropathy. Am. J. Pathol. 141, 451–456 [PMC free article] [PubMed] [Google Scholar]
- 54).Ueda M., Ando Y., Hakamata Y., Nakamura M., Yamashita T., Obayashi K., et al. (2007) A novel transgenic rat with human amyloidogenic transthyretin V30M. Biochem. Biophys. Res. Commun. 352, 299–304 [DOI] [PubMed] [Google Scholar]
- 55).Ando Y., Ohlsson P.I., Suhr O., Nyhlin N., Yamashita T., Holmgren G., et al. (1996) A new simple and rapid screening method for variant transthyretin (TTR) related amyloidosis. Biochem. Biophys. Res. Commun. 228, 480–483 [DOI] [PubMed] [Google Scholar]
- 56).Ranlov I., Ando Y., Ohlsson P.I., Holmgren G., Ranlov P.J., Suhr O.B. (1997) Rapid screening for amyloid-related variant forms of transthyretin is possible by electrospray ionization mass spectrometry. Eur. J. Clin. Invest. 27, 956–959 [DOI] [PubMed] [Google Scholar]
- 57).Ueda M., Misumi Y., Mizuguchi M., Nakamura M., Yamashita T., Sekijima Y., et al. (2009) SELDI-TOF Mass spectrometry evaluation of variant transthyretins for diagnosis and pathogenesis of familial amyloidotic polyneuropathy. Clin. Chem. 55, 1223–1227 [DOI] [PubMed] [Google Scholar]
- 58).Ando Y., Anan I., Suhr O., Holmgren G., Costa P.M. (1998) Detection of a variant protein in hair: new diagnostic method in Portuguese type familial amyloid polyneuropathy. BMJ 316, 1500–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Ando E., Ando Y., Maruoka S., Sakai Y., Watanabe S., Yamashita R., et al. (1992) Ocular microangiopathy in familial amyloidotic polyneuropathy, type I. Graefes Arch. Clin. Exp. Ophthalmol. 230, 1–5 [DOI] [PubMed] [Google Scholar]
- 60).Holmgren G., Costa P.M., Andersson C., Asplund K., Steen L., Beckman L., et al. (1994) Geographical distribution of TTR met30 carriers in northern Sweden: discrepancy between carrier frequency and prevalence rate. J. Med. Genet. 31, 351–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Continho, P., Macedo, E., Estacio, A., Saraiva, A., Costa, P.P. and Saraiva, M.J.M. (1988) Periodic plasma exchanges in treatment of familial amyloid polyneuropathy: Preliminary results. In Amyloid and Amyloidosis. (eds. Isobe, T., Araki, S., Uchino, F., Kito, S. and Tsubura, E.). Plenum Press, New York, pp. 845–849. [Google Scholar]
- 62).Ericzon B.G., Holmgren G., Lundgren E., Suhr O.B. (1999) New structural information and update on liver transplantation in transthyretin-associated amyloidosis. Report from the 4th International Symposium on Familial Amyloidotic Polyneuropathy and Other Transthyretin Related Disorders & the 3rd International Workshop on Liver Transplantation in Familial Amyloid Polyneuropathy, Umea Sweden, June 1999. Amyloid 7, 145–147 [DOI] [PubMed] [Google Scholar]
- 63).Skinner M., Lewis W.D., Jones L.A., Kasirsky J., Kane K., Ju S.T., et al. (1994) Liver transplantation as a treatment for familial amyloidotic polyneuropathy. Ann. Intern. Med. 120, 133–134 [DOI] [PubMed] [Google Scholar]
- 64).Takei Y., Ikeda S., Hashikura Y., Ikegami T., Kawasaki S. (1999) Partial-liver transplantation to treat familial amyloid polyneuropathy: Follow-up of 11 patients. Ann. Intern. Med. 131, 592–595 [DOI] [PubMed] [Google Scholar]
- 65).Ando Y., Tanaka Y., Ando E., Yamashita T., Nishida Y., Tashima K., et al. (1995) Effect of liver transplantation on autonomic dysfunction in familial amyloidotic polyneuropathy type I. Lancet 345, 195–196 [DOI] [PubMed] [Google Scholar]
- 66).Suhr O., Danielsson A., Rydh A., Nyhlin N., Hietala S.O., Steen L. (1996) Impact of gastrointestinal dysfunction on survival after liver transplantation for familial amyloidotic polyneuropathy. Dig. Dis. Sci. 41, 1909–1914 [DOI] [PubMed] [Google Scholar]
- 67).Lendoire J., Trigo P., Aziz H., Cueto G., Ando Y., Tashima K., et al. (1999) Liver transplantation in transthyretin familial amyloid polyneuropathy: First report from Argentina. Amyloid 6, 297–300 [DOI] [PubMed] [Google Scholar]
- 68).Ando Y., Terazaki H., Haraoka K., Tajiri T., Nakamura M., Obayashi K., et al. (2002) Presence of autoantibody against ATTRVal30Met after sequential liver transplantation. Transplantation 73, 674–675 [DOI] [PubMed] [Google Scholar]
- 69).Stangou A.J., Hawkins P.N. (2004) Liver transplantation in transthyretin-related familial amyloid polyneuropathy. Curr. Opin. Neurol. 17, 615–620 [DOI] [PubMed] [Google Scholar]
- 70).Goto T., Yamashita T., Ueda M., Ohshima S., Yoneyama K., Nakamura M., et al. (2006) Iatrogenic amyloid neuropathy in a Japanese patient after sequential liver transplantation. Am. J. Transplant. 6, 2512–2515 [DOI] [PubMed] [Google Scholar]