Abstract
Green tea polyphenols have emerged over the past two decades as an important dietary factor for health promotion. There is considerable evidence that tea polyphenols, in particular (−)-epigallocatechin-3-gallate (EGCG) inhibit carcinogenesis. However, the mechanisms for the cancer-preventive activity of EGCG are not completely characterized and many features remain to be elucidated. Recently we have identified a cell-surface EGCG receptor and the relating molecules that confer EGCG responsiveness to many cancer cells at physiological concentrations. Here, we review some of the reported mechanisms for the cancer chemopreventive action of EGCG and provide an overview of several molecules that sense and manage the physiological functions of EGCG.
Keywords: green tea, catechins, food factors, sensing, molecular target, 67LR
Introduction
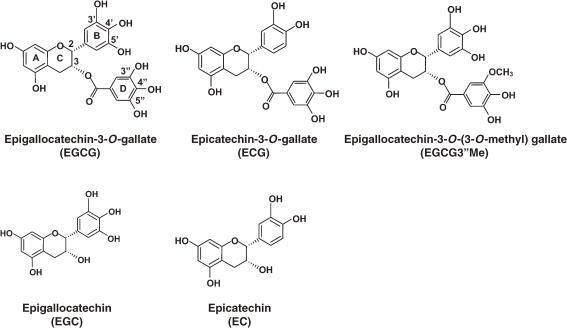
Phytochemicals, compounds made from fruits, vegetables, and grains, possess anti-cancer properties and represent a promising therapeutic approach for the prevention and treatment of many cancers. An extensive amount of research is being conducted on the benefits of phytochemicals. Indeed, there are a number of clinical trials that have evaluated these natural products for their efficacy in preventing disease. Many food items, beverages and dietary constituents have shown anticancer and cancer-preventive activities. Tea is one of the most widely consumed beverages in the world after water. Green tea, black tea, and oolong tea are all derived from the dried leaves of the plant Camellia sinensis and contain an assortment of compounds, the most significant components of which are phytochemicals. Among all teas consumed in the world, green tea is best studied for its health benefits. It has been demonstrated that tea constituents exhibit various biological and pharmacological properties such anti-carcinogenic, anti-oxidative, anti-allergic, anti-virus, anti-hypertensive, anti-atherosclerosis, anti-cardiovascular disease and anti-hypercholesterolemic activities.1–8) Principles for these activities were shown to be a group of polyphenols, catechin. The major green tea catechins are (−)-epigallocatechin-3-gallate (EGCG), (−)-epigallocatechin (EGC), (−)-epicatechin-3-gallate (ECG) and (−)-epicatechin (Fig. 1 ). Tea catechins are characterized by the dihydroxyl or trihydroxyl substitutions on the B ring and the m-5,7-dihydroxyl substitutions on the A ring. The B ring seems to be the principal site of antioxidant reactions and the antioxidant activity is further increased by the trihydroxyl structure in the D ring (gallate) in EGCG and ECG. A typical green tea beverage, prepared in a proportion of 1 g leaf to 100 ml water in a 3-min brew, usually contains 250–350 mg tea solids, and catechins account for 30–42% of the dry weight of the solids.9) Among the green tea catechins, EGCG is the most abundant, representing ∼16.5 wt % of the water extractable fraction of green tea leaves, and most active catechin in various kinds of physiological activities. Because EGCG is not found to a plant except tea, EGCG is regarded as a constituent characterizing green tea. In this review we focused the current understanding of EGCG sensing mechanisms by which EGCG exerts the beneficial health effects.
Figure 1.
Chemical structures of green tea catechins.
Anticancer activities of EGCG in animal models
The cancer-preventive activities of tea and tea constituents have also been studied in different models of oral–digestive tract carcinogenesis.10) In a review of the 147 papers published by 2008, 133 described cancer-preventive or inhibitory effects.11) In the ApcMin/+ mouse model of intestinal tumorigenesis, EGCG solution given as the sole source of drinking fluid, inhibited the spontaneous development of small intestinal tumours.12) In a model using a food carcinogen, PhIP (2-amino-1-methyl-6-phenylimidazo[4,5-6]pyridine), to induce colon carcinogenesis in rats, treatment with EGCG during the post-initiation stage for 15 weeks reduced ACF formation by 71% compared with water-treated controls.13) We also found that tumor growth of B16 melanoma cells was significantly retarded in 0.1% EGCG-administered C57BL/6N mice.14)
Cancer prevention by tea consumption
The effects of tea consumption on the risk of human cancer have been investigated in many epidemiological studies, but the results have been inconclusive.10) A literature search in the PubMed database for papers published up to 2008 showed 127 case–control studies and 90 cohort studies on the relationship between tea consumption and the risk for colon, lung, stomach, breast, prostate, ovarian, pancreatic, kidney, bladder and other cancers.11) Of these studies, only 51 case–control studies and 19 cohort studies showed an inverse association between tea consumption and cancer risk, suggesting a cancer-preventive effect of tea, whereas other studies showed no such association. The inconsistent results of the epidemiological studies were probably due to different confounding factors, difficulties in quantifying tea consumption, varied cancer aetiology in different populations and population heterogeneity. When these factors were effectively managed, a clearer relationship between tea consumption and cancer risk was observed in several studies. A preliminary double-blind, placebo-controlled study on oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia (HG-PIN) suggested a chemopreventive activity of EGCG on prostate cancer.15)
Anti-oxidant and pro-oxidant properties of EGCG
EGCG and other tea polyphenols are well known for their anti-oxidant activities. Among tea catechins, EGCG is the most effective in reacting with the majority of reactive oxygen species (ROS). Tea polyphenols are also strong chelators of metal ions; the chelation of free metal ions prevents the formation of ROS from the auto-oxidation of many compounds. Indeed, they have been demonstrated to inhibit carcinogen-induced DNA damage and tumor promoter-induced oxidative stress.16) These results are consistent with the commonly mentioned idea that tea prevents cancer because tea polyphenols are anti-oxidants. However, it is unclear whether this is a general mechanism for cancer prevention, especially in human carcinogenesis.
There are two major problems in extrapolating results observed in cell lines to animal models. (1) The concentration of the test compound used in cell line systems, for example, EGCG at 20–100 µM, or higher concentrations, are much higher than those observed in the plasma or tissues in experimental animals or humans after ingestion of tea or related tea preparations.17) (2) The oxygen partial pressure in a cell culture system (160 mmHg) is much higher than that in the blood or tissues (<40 mmHg). Under cell culture conditions, EGCG is not stable, with a half-life less than 2 h.18) The half-life can be extended several folds by the addition of superoxide dismutase (SOD), suggesting a role for superoxide radical in the oxidation and polymerization of EGCG.19) Similar to other anti-oxidants, EGCG and other tea polyphenols may also act as pro-oxidants. In the case of EGCG, auto-oxidation generates superoxide anion and hydrogen peroxide and leads to the formation of dimers, such as theasinensins.14) The polyphenolic structure of tea polyphenols also makes them good donors for hydrogen bonding. For example, hydrogen bonding of water molecules to EGCG forms a large hydration shell, which reduces the absorption of EGCG.20–22) This hydrogen bonding capacity also enables tea polyphenols to bind strongly to proteins and nucleic acids. It is not clear whether pro-oxidants produced by EGCG-generated reactions occur in low oxygen partial pressure conditions in vivo in cells, which generally have strong anti-oxidative capacity and low oxygen partial pressure. The difference between in vitro and in vivo systems should be considered in studies attempting to elucidate the mechanisms of action of EGCG.
Bioavailability of EGCG
The bioavailability and biotransformation of tea catechins following tea ingestion has been investigated in human volunteers, and a time to reach maximal concentration in the plasma of 1.5 to 2.5 h after consumption of decaffeinated green tea solids (1.5, 3.0, and 4.5 g).23) The catechins levels decreased and were not detectable by 24 h. Whereas EGCG and ECG were not detected in the urine, 90% of the urinary EC and EGC were excreted by 8 h. Most of the ingested EGCG apparently does not get into the blood, and absolute EGCG is preferentially excreted through the bile to the colon. Glucuronidation, sulfation, methylation, and ring-fission metabolism represent the major metabolic pathways for green tea catechins. Plasma EC and EGC were present mainly in the conjugated form such as glucuronide and sulfate conjugates, whereas 77% of the EGCG was in the free form. EGCG has also been shown to undergo methylation. The maximum plasma concentration of 4′,4″-di-O-methyl-EGCG is 20% that of EGCG but the cumulative excretion of 4′,4″-di-O-methyl-EGCG is 10-fold higher than that of EGCG over 24 h.24) Although most of published studies in cell culture systems used 10–100 µM of EGCG, the blood level of EGCG after consuming the equivalent of 2–3 cups of green tea was 0.1–0.6 µM and for an equivalent of 7–9 cups was still lower than 1 µM.25) The rather poor bioavailability of tea catechins needs to be considered when we extrapolate results obtained in vitro to situations in vivo.
The discovery of a cell surface EGCG receptor
It should be noted that most of the effects of EGCG in cell culture systems and cell-free systems have been obtained with considerable high concentrations than observed in the plasma or tissues of animals or in human plasma after administration of green tea or EGCG. As described previously, the pharmacokinetic studies in humans indicate that the peak plasma concentration after single dose of EGCG is <1.0 µM. Furthermore, the intracellular levels of EGCG are much lower than the concentrations observed in the extracellular levels. Searching for high-affinity proteins that bind to EGCG is the first step to understanding the molecular and biochemical mechanisms of the anti-cancer effects of tea polyphenols. Several proteins that can directly bind with EGCG have been identified in vitro models. Using an EGCG-conjugated affinity column, two dimensional electrophoresis and matrix-assisted laser desorption/ionization-time-of-flight mass spectroscopy, vimentin,26) insulin-like growth factor 1 receptor (IGF1R),27) Fyn,28) glucose-regulated protein 78 kDa29) and ZAP7030) are identified as high-affinity EGCG binding proteins. All of these proteins were demonstrated to be important for the inhibitory activity of EGCG in cell lines, but higher EGCG concentrations than the Kd values were needed. For example, vimentin binds to EGCG with a Kd of 3.3 nM, and functional studies showed that EGCG inhibited the phosphorylation of vimentin at Ser55 and Ser50 by Cdc2 (IC50 = 17 µM). The difference in effective concentrations is probably due to the nonspecific binding of EGCG to other proteins, which compete with the target proteins. Therefore, it is not clear whether the activities observed with high EGCG concentrations in vitro can be observed in vivo and the proposed EGCG-binding molecules as mentioned above are responsible for in vivo physiological activities of EGCG.
To elucidate the detail molecular basis for the action of EGCG, it is necessary to identify the molecular target triggering a specific signaling of EGCG. Studies of Toll-like receptors teach us the principal role in the pathogens sensing and the necessity of identification of the specific receptor as a signal initiator for generating cellular responses for understanding the specific cellular signaling of foreign or functional substances. However, the molecular target for physiologically relevant EGCG that can mediate its anti-cancer effect still remained unknown. We found that all-trans-retinoic acid (ATRA) enhances the binding of EGCG to the cell surface of cancer cells when the binding was monitored on the basis of the increase in response units in a surface plasmon resonance (SPR) assay.31) To identify candidates through which EGCG inhibits cell growth, we used a subtraction cloning strategy involving cDNA libraries constructed from cells treated or untreated with ATRA. We isolated a single target that allows EGCG to bind to the cell surface. An analysis of the DNA sequence identified this unknown cell surface candidate as the 67-kDa laminin receptor (67LR). In fact, the expression of this 67LR was enhanced by ATRA treatment.
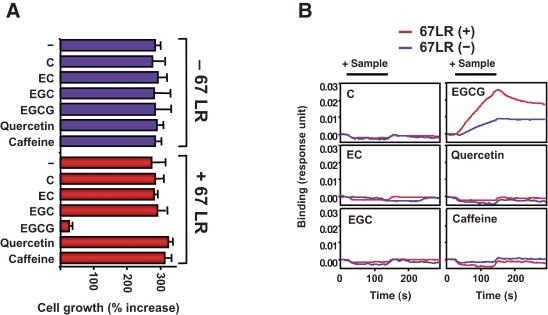
Human lung cancer A549 cells were used to assess how effectively the 67LR mediates EGCG-mediated growth inhibition. Cells transfected with empty vector and treated with EGCG showed no growth inhibition (Fig. 2 ). However, cells transfected with the gene encoding 67LR and treated with EGCG demonstrated considerable inhibition as compared with the cells treated with H2O. We next tested whether the growth inhibitory activity of EGCG correlates with the binding strength of EGCG to the cell surface. We found increased binding of EGCG to the cell surface of cells transfected with 67LR (Fig. 2). EGCG binding to the 67LR-transfected cells was inhibited by treatment with an antibody to 67LR. The predicted Kd value for the binding of EGCG to the 67LR protein is 39.9 nM.31) Most of the 67LR protein was found to exist in the raft fraction rather than the non-raft fraction,32) and this distribution pattern correlated well with the plasma membrane-associated EGCG level after treating the cells with EGCG.33)
Figure 2.
The interactions between tea constituents and 67LR-transfected cells. (A) Growth inhibitory activities of tea constituents (indicated by bars, 5 µmol/L) on A549 cells transfected with either the gene encoding 67LR (red) or vector only (purple) were examined. (B) The interaction between tea constituents (5 µmol/L) and A549 cells transfected with the 67LR vector (red line) or the empty vector (purple line) was measured using a SPR assay.
To investigate whether the 67LR can confer a sensitivity to EGCG at physiologically relevant concentrations, we treated the 67LR-transfected A549 cells with two concentrations of EGCG (0.1 and 1.0 µM); these concentrations are similar to the amount of EGCG found in human plasma after drinking more than two or three cups of tea. The growth of the transfected cells was inhibited at both of these concentrations.31) In addition, this growth-suppressive effect was completely eliminated upon treatment with anti-67LR antibody before the addition of EGCG.
Next, we investigated the effect of oral administration of EGCG on subcutaneous tumor growth in C57BL/6N mice challenged with 67LR-ablated B16 cells.14) We confirmed both silencing of 67LR by stable RNAi in B16 cells and attenuation of the inhibitory effect of 1 µM EGCG on cell growth in 67LR-ablated B16 cells in vitro. Tumor growth was significantly retarded in EGCG-administered mice implanted with the B16 cells harboring a control shRNA, whereas tumor growth was not affected by EGCG in the mice implanted with 67LR-ablated B16 cells, suggesting that 67LR functions as an EGCG receptor not only in vitro but also in vivo. Together, these observations demonstrate that the cell-surface 67LR is the receptor for antitumor action of EGCG at the physiologically relevant concentration. The discovery of EGCG receptor as 67LR has solved some of the discrepancies of the cancer-preventing activity of EGCG between in vitro data and in vivo data.
The 67-kDa laminin receptor
67LR was first discovered by three independent laboratories in 198334–36) through its ability to bind to and be isolated by laminin sepharose. The receptor bound laminin with high affinity with a Kd of 2 nM.34) Consequently, this receptor was named 67LR and, more recently, LAMR1 (laminin receptor-1). Its gene, however, was found to encode a protein of only 37-kDa. The discrepancy between these two molecular weights was later resolved by showing that the 37-kDa gene product serves as a monomeric precursor to a 67-kDa dimmer.37) The exact composition of the 67-kDa dimer and the process by which it is formed remains obscure as evidence supports both a homo38) and a heterodimer.39,40) 37/67-kDa laminin receptor was shown to be acylated by three fatty acids (palmitate, stearate, and oleate38)) and fatty acid synthesis is required for 67-kDa laminin receptor formation.39) Beyond this not much is known about what regulates the dimerization process.
Expression of the 67LR has been shown to be upregulated in neoplastic cells compared with their normal counterparts and directly correlate with an enhanced invasive and metastatic potential in many malignancies.41,42) The receptor has been implicated in laminin-induced tumor cell attachment and migration, as well as in tumor angiogenesis, invasion, and metastasis.43–45) Surface expression of the 67LR has also been reported to be a dominant laminin-binding protein expressed in neutrophils, macrophages, and monocytes, which suggests that the receptor may play an important role in the regulation of cell adherence via the basement membrane laminin.46–48) It has been reported that the expression of 67LR in mast cells is related to the adhesion to laminin and may contribute to the tissue distribution of mast cells and to mast cell accumulation at sites of tissue injury and inflammation.49,50) 67LR has also been shown to be able to modulate granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling by inhibiting GM-CSF-its receptor (GMR) complex formation through its interaction with GMR.51) Furthermore, it has been suggested that the 67LR expression in human T cells is upregulated by stimulating the neuropeptides GnRH-II and GnRH-I which trigger its homing to specific organs.52)
This protein also acts as a receptor for pathogenic prion protein,53) cytotoxic necrotizing factor 1 from E. coli,54) Sindbis virus,55) Dengue virus,56) and adeno-associated virus subtypes 2, 3, 8, and 9.57) Interestingly, unmodified 37-kDa laminin receptor precurcor (37LRP) is a ribosomal component and homologues of this protein are found in all five kingdoms. 37/67-kDa laminin receptor has been shown to localize to the nucleus and interact with histones in the eukaryotic cell nucleus.58)
Mice that are heterozygous for the Rpsatm1Ells, LAMR1, targeted mutation are viable, fertile, and do not display any gross behavioral abnormalities. Homozygous null mice have an embryonic lethal phenotype, failing to develop past embryonic days 3.5. Heterozygotes exhibit delayed embryonic growth that normalizes postnatally (unpublished data).
The inhibition of cancer cell growth by EGCG through the 67LR
Phosphorylation of the myosin regulatory light chain (MRLC) at Thr18/Ser19 was shown to regulate the association between myosin II and filamentous actin (F-actin). The association of myosin II with F-actin results in the formation of stress fibers in interphase cells and the contractile ring in dividing cells. When human cervical carcinoma HeLa cells were incubated with EGCG, the cells retracted and left intercellular gaps. In addition, disappearance of the stress fibers in the central cell body was observed upon treatment with EGCG and the MRLC phosphorylation was reduced by EGCG treatment. The phosphorylation of MRLC at Thr18/Ser19 has been shown to be necessary for formation of the contractile ring in dividing cells. EGCG treatment for significantly increased the percentage of cells in the G2/M phase.
To analyze whether the suppressive effect of EGCG on the MRLC phosphorylation is mediated by the 67LR, RNAi-mediated gene silencing was utilized to knock down the expression of the 67LR.21) EGCG significantly reduced the phosphorylation of MRLC in the HeLa cells, however in the 67LR-ablated cells, EGCG only slightly reduced the phosphorylation, suggesting that EGCG inhibits the cancer cell growth by reducing the MRLC phosphorylation and this effect is mediated by the 67LR. Epidemiological studies have suggested that the consumption of green tea may decrease colon cancer risk in woman.59) We also found that a physiologically achievable concentration of EGCG inhibited cell cycle progression of human colon adenocarcinoma Caco-2 cells through 67LR.20)
Induction of apoptosis in myeloma and leukaemia cells by EGCG through the 67LR
EGCG has been shown to be able to induce growth arrest and subsequent apoptotic cell death in multiple myeloma (MM) cells and primary patient MM cells in vitro, while having no significant effect on growth normal cells such as peripheral blood mononuclear cells (PBMCs) and fibroblasts.60) Treatment with EGCG (33 mg/kg/d) also led to significant apoptosis in human myeloma cells grown as tumors in SCID mice. The expression of 67LR was significantly elevated in myeloma cell lines and patient samples compared to normal PBMCs. RNAi-mediated inhibition of 67LR expression resulted in abrogation of EGCG-induced apoptosis in myeloma cells, indicating that 67LR plays an important role in mediating EGCG activity in MM while sparing PBMCs.60) Evaluation of changes in gene expression profile indicates that EGCG treatment activates distinct pathways of growth arrest and apoptosis in MM cells by inducing the expression of death-associated protein kinase 2, the initiators and mediators of death receptor-dependent apoptosis (Fas ligand, Fas, and caspase 4), p53-like proteins (p73, p63), positive regulators of apoptosis and NF-κB activation (CARD10, CARD14), and cyclin-dependent kinase inhibitors (p16 and p18). These data demonstrate potent and specific anti-myeloma activity of EGCG and provide the rationale for its clinical evaluation.
EGCG also induces cell death in acute myeloid leukaemia (AML) patient samples while granulocytes and PBMC from healthy donors were not affected by EGCG. AML cells express the 67LR while normal controls does not express the receptor. Moreover, the susceptibility of AML patient samples has been shown to be closely associated with the level of 67LR expression.61)
On the other hand, EGCG has been shown to inhibit cell death of muscle cells. Duchenne muscular dystrophy is a fatal muscle wasting disease caused by the absence of the protein dystrophin. 67LR was seven times more abundant in dystrophic muscle cells compared with normal cells. EGCG protected primary dystrophic muscle cells from oxidative damage induced by hydrogen peroxide in the widely used mdx mouse model but could not protect normal cells.62)
Anti-allergic action of EGCG through the 67LR
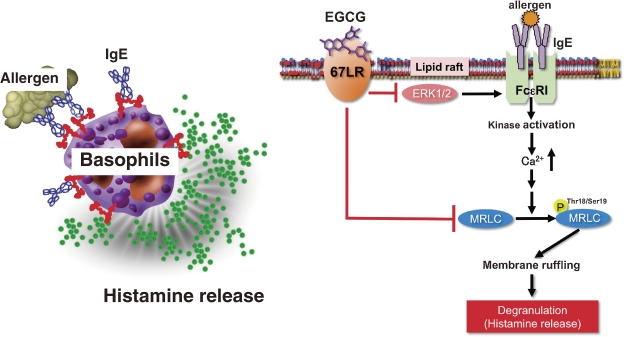
Mast cells and basophils play a central role in immediate allergic reactions mediated by IgE. Binding of multivalent allergens to specific IgE attached to the FcεRI on the surface of mast cells or basophils leads to the release of both preformed and newly generated inflammatory mediators such as histamine. These mediators ultimately cause various symptoms including atopic dermatitis, bronchial asthma, and food allergy.63,64) The early phase of cell activation of mast cells and basophils includes the phosphorylation and activation of protein tyrosine kinases and their substrates, generation of the second messengers such as inositol trisphosphate and diacylglycerol, and elevation of intracellular Ca2+ levels.65,66) The late phase of the activation, which occurs after the influx of Ca2+, includes the fusion of secretory granules with the membrane and dramatic morphological changes due to remodeling of actin cytoskeleton, which undergo extensive membrane ruffling.67–69) We found that EGCG inhibited the calcium ionophore A23187-induced histamine release from the human basophilic KU812 cells and could not inhibit the increase of the intracellular Ca2+ level after stimulation with A23187.70) This result suggested that the effect of EGCG on histamine release occurs after the elevation of the intracellular Ca2+ concentration. Thr18/Ser19 phosphorylation of MRLC has been reported to be temporally correlated with degranulation in the rat basophilic RBL-2H3 cells, and the inhibition of MRLC phosphorylation has been shown to impair the degranulation.71) Although EGC, having no ability to inhibit histamine release, showed no inhibitory effect on MRLC phosphorylation, EGCG clearly reduced the level of phosphorylated MRLC.70) After treatment of KU812 cells with the anti-67LR antibody, cells were incubated with EGCG, and further challenged with A23187. The reductive effect of EGCG on the histamine release was almost completely inhibited in cells treated with the anti-67LR antibody. Experiment using such 67LR-downregulated cells revealed a significant abrogation of the inhibitory effect of EGCG on degranulation. Furthermore, the lowering effect of EGCG on the phosphorylation of MRLC was also inhibited by either treatment with the anti-67LR antibody or 67LR-knockdown. These findings indicate that the inhibitory effect of EGCG on degranulation was caused by a modification of myosin cytoskeleton through the binding of EGCG to 67LR on the cell surface (Fig. 3 ). When the basophilic cells were stimulated with A23187 in the presence of EGCG (25 µmol/L), membrane ruffling was inhibited and a biased F-actin accumulation was observed. Furthermore, this EGCG-induced actin remodeling was abolished in both anti-67LR antibody-treated cells and 67LR-knockdowned cells.70) Our findings indicated that EGCG-induced actin remodeling is caused by lowering MRLC phosphorylation mediated through the binding of EGCG to the 67LR. Thus, these cytoskeletal modifications may have an important role in the inhibition of histamine release by EGCG.
Figure 3.
Model of possible EGCG signaling pathway for anti-allergic actions through 67LR. The suppression of MRLC phosphorylation through the cell-surface binding to the 67LR contributes to the inhibitory effect of EGCG on the histamine release from basophils. The 67LR also mediates the EGCG-induced suppression of FcεRI expression in basophils by reducing ERK1/2 phosphorylation.
FcεRI plays a central role in the induction and maintenance of IgE mediated allergic responses such as atopic dermatitis, bronchial asthma, and food allergy. Analysis of α chain-deficient mice demonstrated that IgE was unable to bind to the cell surface of mast cells, thereby inabling the induction of degranulation through IgE binding.72) Thus, it is expected that the downregulation of FcεRI expression in mast cells and basophils may lead to the attenuation of the IgE-mediated allergic symptoms. We found that EGCG was able to decrease the cell-surface expression of FcεRI in human basophilic KU812 cells. Total cellular expression of the FcεRI α chain decreased upon treatment with EGCG. We also found that EGCG has an ability to inhibit the phosphorylation of the extracellular signal-regulated kinase1/2 (ERK1/2).33) This inhibition was involved in downregulation of FcεRI expression by EGCG. Moreover, the inhibitory effect elicited by EGCG on ERK1/2 was prevented by disruption of lipid rafts. Accordingly, the interaction between EGCG and the lipid rafts is important for EGCG’s ability to downregulate FcεRI expression, and ERK1/2 may be involved in this suppression signal. We also demonstrated that the suppressive effect of EGCG was inhibited by the knockdown of 67LR. Furthermore, the ability of EGCG to decrease the phosphorylation of ERK1/2 was reduced in the 67LR-knocked down cells. These results indicate that the effect of EGCG on ERK1/2 phosphorylation correlates with the expression of 67LR, which implies that the 67LR is the molecule responsible for transducing the EGCG’s downregulatory signaling of the FcεRI (Fig. 3).
The O-methylated derivative of EGCG, (−)-epigallocatechin-3-O-(3-O-methyl)-gallate (EGCG3″Me) and (−)-epigallocatechin-3-O-(4-O-methyl)-gallate (EGCG4″Me), which were isolated from tea leaves such as Tong-ting oolong tea or cultivars ‘Benifuuki’ has been shown to inhibit allergic reactions in vitro.73,74) The inhibitory effects of O-methylated EGCG on mouse type I and IV allergies in vivo more potently than EGCG.73,75) These catechins also strongly inhibited mast cell activation through the prevention of tyrosine phosphorylation (Lyn, Syk and Btk) of cellular protein and histamine/leukotriene release, interleukin-2 secretion after FcεRI cross-linking.76) A double-blind clinical trial to treat allergic cedar pollinosis patients with ‘Benifuuki’ green tea rich in EGCG3″Me was carried out, and promising results have been obtained by using a protocol of drinking of 1.5 g of tea powder with water twice a day for 13 weeks.77) We have been found that EGCG3″Me can inhibit histamine release and suppress the FcεRI expression in human basophilic KU812 cells the same as EGCG.74,78) RNAi-mediated knockdown of 67LR expression resulted in a decreased activity of EGCG3″Me.79) The suppression of MRLC phosphorylation through the cell-surface binding to the 67LR contributes to the inhibitory effect of EGCG3″Me on the histamine release. The 67LR also mediated the EGCG3″Me-induced suppression of FcεRI expression by reducing ERK1/2 phosphorylation.
EGCG is known to be unstable and is degraded easily in animal bodies. On the other hand, EGCG3″Me and EGCG4″Me are absorbed efficiently and are more stable than EGCG in animal and human plasma, suggesting the reason for the methylated derivatives of EGCG having potent inhibitory activities to allergies in vivo. EGCG has been reported to undergo methylation, and (−)-4′-O-methyl-epigallocatechin-3-O-(4-O-methyl) gallate (EGCG4′4″diMe) has been shown to be a major metabolite of EGCG in plasma,80) and EGCG4′4″diMe did not demonstrate a suppressive effect in KU812 cells.81) Although our studies on methylated EGCGs may contribute to the elucidation of physiological activities of EGCG in vivo, further investigation of the relationship between metabolites of EGCG and 67LR is necessary for a better understanding of the molecular basis of EGCG activities in vivo.
Anti-inflammatory action of EGCG through the 67LR
EGCG has been shown to rescue mice from Lipopolysaccharide (LPS)-induced lethal endotoxemia and downregulate inflammatory responses in macrophages.82) LPS is one of the most powerful activators of Toll-like receptor (TLR) 4 signaling, and is also well known to induce production of inflammatory mediators through the receptor. Recently, we found the molecular basis for the downregulation of TLR4 signal transduction by EGCG at 1 µM in macrophages.83) Anti-67LR antibody treatment or RNAi-mediated silencing of 67LR resulted in abrogation of the inhibitory action of EGCG on LPS-induced activation of downstream signaling pathways and target gene expressions in murine macrophages. Additionally, we found that EGCG reduced the TLR4 expression through 67LR. Interestingly, EGCG induced a rapid upregulation of Tollip protein, a nagative regulator of TLR-signaling, and this EGCG action was prevented by 67LR silencing or anti-67LR antibody treatment. RNAi-mediated silencing of Tollip impaired the TLR4 signaling inhibitory activity of EGCG. Taken together, these findings demonstrate that 67LR plays a critical role in mediating anti-inflammatory action of a physiologically relevant EGCG and Tollip expression could be modulated through 67LR in macrophages.
Modulation of insulin action by EGCG through the 67LR
Insulin is a key factor in stimulating fat cell mitogenesis and adipogenesis.84) Green tea polyphenols have been proposed as chemopreventative agents for obesity and modulators of fat cell growth.85,86) In particular, EGCG has been shown to reduce body weight and body fat in vivo.87) EGCG inhibited insulin stimulation of murine preadipocyte proliferation. EGCG also suppressed insulin-stimulated phosphorylation of the insulin receptor-β, insulin receptor (IR) substrates 1 and 2 (IRS1 and IRS2), and mitogen-activated protein kinase pathway proteins. Pretreatment of preadipocytes with 67LR antiserum prevented the effects of EGCG on insulin-stimulated phosphorylation of IRS2, RAF1, and ERK1/2 and insulin-stimulated preadipocyte proliferation. Moreover, EGCG tended to increase insulin-stimulated associations between the 67LR and IR, IRS1, IRS2, and IRS4 proteins. These data suggest that EGCG mediates anti-insulin signaling in preadipocyte mitogenesis via the 67LR. However, higher EGCG concentrations are needed to modulate insulin action. The applicability of this observation remains to be investigated.
Inhibition of tissue factor expression by EGCG through the 67LR in aortic endothelial cells
Tissue factor (TF) is an important trigger of arterial thrombosis.88) EGCG exhibits cardioprotective effects and inhibits TNF-α- and histamine-induced endothelial TF expression and activity.89) In vivo administration of EGCG (30 mg/kg/day) inhibited TF activity in carotid arteries of C57BL6 mice. EGCG decreased TF expression at the transcriptional level and impaired activation of the JNK 1/2. Human aortic endothelial cells expressed 67LR, and 67LR-blocking antibodies blunted the inhibitory effect of EGCG on both TF protein expression and JNK activation. This leads to a prediction that EGCG-elicited 67LR activation inhibits endothelial TF expression by impairing JNK phosphorylation.
Green tea polyphenol sensing molecules
In an attempt to elucidate the pathways involved in the anticancer action of EGCG, we applied genetic suppressor element (GSE) methodology. GSEs are short cDNA fragments encoding peptides acting as dominant inhibitors of protein function or antisense RNAs inhibiting gene expression. GSEs behave as dominant selectable markers for the phenotype associated with the repression of the gene from which they derived, thus allowing identification of this gene. For identifying genes mediating cell sensitivity to EGCG, we selected GSEs conferring resistance to EGCG. To search for the mediators of EGCG-induced cell growth inhibition in B16 mouse melanoma cells, we utilized a targeted genetic screen with a GSE complementary DNA library. Among genetic elements protecting cells from EGCG-induced cell growth inhibition, we isolated a GSE that encoded the N terminus of eukaryotic translation elongation factor 1A (eEF1A). eEF1A is an important component of the eukaryotic translation apparatus and is also known as a multifunctional protein that is involved in a large number of cellular processes.90)
To investigate the role of eEF1A in EGCG-induced cell growth inhibition, we used stable RNAi to silence eEF1A expression in B16 cells. Remarkably, silencing of eEF1A attenuated the inhibitory effect of 1 µM EGCG on cell growth.14) In contrast, overexpression of eEF1A enhanced the inhibitory effects of 1 µM EGCG on cell growth. This concentration is similar to the amount of EGCG found in human plasma after drinking more than two or three cups of green tea. Given this, we investigated the effect of oral administration of EGCG on subcutaneous tumor growth in C57BL/6N mice challenged with eEF1A-ablated B16 cells.14) Tumor growth was significantly retarded in EGCG-administered mice implanted with the B16 cells harboring a control shRNA, whereas tumor growth was not affected by EGCG in the mice implanted with eEF1A-ablated B16 cells, indicating that eEF1A is involved in EGCG-induced cancer prevention. These results support our conclusion that eEF1A serves as a mediator for EGCG-induced cancer prevention.
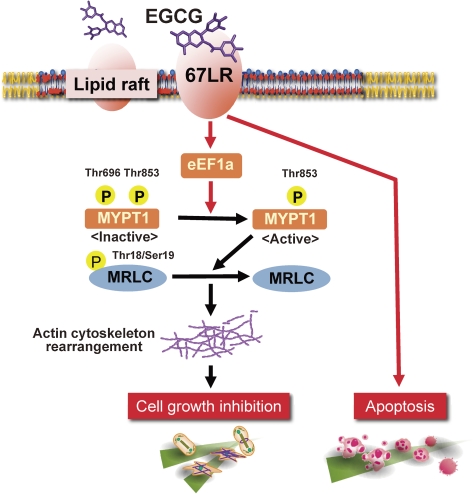
As described previously, EGCG-induced cell growth inhibition may result from the reduction of the phosphorylation of MRLC at Thr-18/Ser-19 through 67LR.21) The activity of myosin phosphatase is known to be inhibited by phosphorylation of its targeting subunit MYPT1 at Thr-696 and Thr-853.91) We tested the effect of EGCG on the phosphorylation of MYPT1 at Thr-696 and Thr-853. Intriguingly, although the phosphorylation level at Thr-853 was unaffected by EGCG, EGCG induced the dephosphorylation of MYPT1 at Thr-696. Further, this effect correlated with EGCG-induced reduction of the MRLC phosphorylation, suggesting that EGCG activates myosin phosphatase by reducing the MYPT1 phosphorylation level at Thr-696. Next, we investigated whether MYPT1 is involved in anticancer action of EGCG in vivo. In B16 cells, physiological concentrations of EGCG reduced the MYPT1 phosphorylation at Thr-696 and the MRLC phosphorylation. We confirmed both silencing of MYPT1 by stable RNAi in B16 cells and attenuation of the inhibitory effect of 1 µM EGCG on cell growth in MYPT1-ablated B16 cells in vitro. We tested the effect of oral administration of EGCG on subcutaneous tumor growth in C57BL/6N mice challenged with MYPT1-ablated B16 cells.14) Tumor growth was significantly retarded in EGCG-administered mice implanted with the B16 cells harboring a control shRNA, whereas tumor growth was not affected by EGCG in the mice implanted with MYPT-1-ablated B16 cells, suggesting that MYPT1 is indispensable for EGCG-induced cancer prevention.
In both 67LR-ablated HeLa cells and eEF1A-ablated HeLa cells, the inhibitory effect of EGCG on both the phosphorylation of MYPT1 at Thr-696 and the phosphorylation of MRLC was attenuated. In addition, EGCG-induced actin cytoskeleton rearrangement was no longer observed in MYPT1-, eEF1A-, or 67LR-ablated HeLa cells. The involvement of MYPT1 in downstream EGCG-triggered signaling from both 67LR and eEF1A was further documented by confirming abrogation of 1 µM EGCG-induced reduction of the MYPT1 phosphorylation level at Thr-696 and the MRLC phosphorylation in 67LR- or eEF1A-ablated B16 cells. These results suggest that MYPT1 is involved in downstream EGCG signaling from both 67LR and eEF1A (Fig. 4 ). It has been reported that MYPT1 binds to eEF1A,92) and more than half of the total eEF1A (>60%) binds to the actin cytoskeleton.93) Characterizing the mechanisms by which EGCG induces reduction of the MYPT1 phosphorylation at Thr-696 and reorganization of actin cytoskeleton through eEF1A should help in more precise understanding of cytoskeleton organization.
Figure 4.
The signaling pathways that sense and respond to EGCG through 67LR are depicted. After EGCG binding to lipid raft-associated 67LR, through eEF1A, the phosphorylation of MYPT1 at Thr-696 but not Thr-853 is reduced, which leads to the activation of myosin phosphatase. The activated myosin phosphatase dephosphorylates its substrates (e.g. MRLC), and actin cytoskeleton rearrangement is induced. The alteration of actin cytoskeleton might lead to cell growth inhibition. EGCG also induces apoptosis in the 67LR-expressing cells derived from multiple myeloma and acute myeloid leukaemia patient samples.
Conclusions
Beneficial health effects by edible phytochemicals is now considered to be an inexpensive, readily applicable, acceptable, and accessible approach to cancer control and management,94) however, little is known about the mechanism of the chemopreventive action of most phytochemicals. Here we described that 67LR is a critical sensor molecule to respond to EGCG and mediate the beneficial activities of this phyrochemical. eEF1A and MYPT1 are also EGCG-sensing molecules for EGCG-elicited cancer prevention at physiological concentrations. More definitive information on the relationship between EGCG sensing-pathways and beneficial effects of EGCG ingestion will emerge from cohort studies and human intervention trials. We hope that this review will have wide-ranging implications, as many of the issues discussed here might also be applicable to studies of other dietary ingredients.
Profile
Hirofumi Tachibana was born in 1964 and started his research career in 1987 with studies on the cellular immunology and antibody engineering in the Graduate School of Genetic Resources Technology at the Kyushu University, after graduating from the Faculty of Agriculture at the Kyushu University. He succeeded in the functional modification of human monoclonal antibodies via the light chain glycosylation. He obtained his Ph.D. in Agriculture Science from The Kyushu University in 1993, working on the human antibody engineering. He was promoted to assistant professor in 1991 and lecturer in 1994 at the Graduate School of Genetic Resources Technology Kyushu University. In 1996, he was promoted to associate professor of the Department of Food Science and Technology Kyushu University. He focused on the physiological mechanisms of the functional food factors at a molecular level. He identified some sensory systems that respond to the functional food factors. Especially, he succeeded in the discovery of green tea polyphenol receptor (2004). He was awarded The Japan Bioscience, Biotechnology and Agrochemistry Society Award for the Encouragement of Young Scientists in 1998, the Agricultural Sciences of Japan Award in 2004, the JSPS Prize in 2006, and the Japanese Association for Food Immunology Award in 2010.
References
- 1).Lambert J.D., Yang C.S. (2003) Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutat. Res. 523–524, 201–208 [DOI] [PubMed] [Google Scholar]
- 2).Chisaka T., Matsuda H., Kubomura Y., Mochizuki M., Yamahara J., Fujimura H. (1988) The effect of crude drugs on experimental hypercholesteremia: mode of action of (−)-epigallocatechin gallate in tea leaves. Chem. Pharm. Bull. (Tokyo) 36, 227–233 [DOI] [PubMed] [Google Scholar]
- 3).Cao Y., Cao R. (1999) Angiogenesis inhibited by drinking tea. Nature 398, 381. [DOI] [PubMed] [Google Scholar]
- 4).Kuriyama S., Shimazu T., Ohmori K., Kikuchi N., Nakaya N., Nishino Y., Tsubono Y., Tsuji I. (2006) Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA 296, 1255–1265 [DOI] [PubMed] [Google Scholar]
- 5).Sazuka M., Murakami S., Isemura M., Satoh K., Nukiwa T. (1995) Inhibitory effects of green tea infusion on in vitro invasion and in vivo metastasis of mouse lung carcinoma cells. Cancer Lett. 98, 27–31 [PubMed] [Google Scholar]
- 6).Bors W., Saran M. (1987) Radical scavenging by flavonoid antioxidants. Free Radic. Res. Commun. 2, 289–294 [DOI] [PubMed] [Google Scholar]
- 7).Sano M., Takahashi Y., Yoshino K., Shimoi K., Nakamura Y., Tomita I., Oguni I., Konomoto H. (1995) Effect of tea (Camellia sinensis L.) on lipid peroxidation in rat liver and kidney: a comparison of green and black tea feeding. Biol. Pharm. Bull. 18, 1006–1008 [DOI] [PubMed] [Google Scholar]
- 8).Hodgson J.M., Puddey I.B., Burke V., Beilin L.J., Jordan N. (1999) Effects on blood pressure of drinking green and black tea. J. Hypertens. 17, 457–463 [DOI] [PubMed] [Google Scholar]
- 9).Khan N., Mukhtar H. (2007) Tea polyphenols for health promotion. Life Sci. 81, 519–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Ju J., Lu G., Lambert J.D., Yang C.S. (2007) Inhibition of carcinogenesis by tea constituents. Semin. Cancer Biol. 17, 395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Yang C.S., Wang X., Lu G., Picinich S.C. (2009) Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 9, 429–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Ju J., Hong J., Zhou J.N., Pan Z., Bose M., Liao J., Yang G.Y., Liu Y.Y., Hou Z., Lin Y., Ma J., Shih W.J., Carothers A.M., Yang C.S. (2005) Inhibition of intestinal tumorigenesis in Apc min/+ mice by (−)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 65, 10623–10631 [DOI] [PubMed] [Google Scholar]
- 13).Carter O., Wang R., Dashwood M., Orner G.A., Fischer K.A., Löhr C.V., Pereira C.B., Bailey G.S., Williams D.E., Dashwood R.H. (2007) Comparison of white tea, green tea, epigallocatechin-3-gallate, and caffeine as inhibitors of PhIP-induced colonic aberrant crypts. Nutr. Cancer 58, 60–65 [DOI] [PubMed] [Google Scholar]
- 14).Umeda D., Yano S., Yamada K., Tachibana H. (2008) Green tea polyphenol epigallocatechin-3-gallate signaling pathway through 67-kDa laminin receptor. J. Biol. Chem. 283, 3050–3058 [DOI] [PubMed] [Google Scholar]
- 15).Bettuzzi S., Brausi M., Rizzi F., Castagnetti G., Peracchia G., Corti A. (2006) Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 66, 1234–1240 [DOI] [PubMed] [Google Scholar]
- 16).Higdon J.V., Frei B. (2003) Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 43, 89–143 [DOI] [PubMed] [Google Scholar]
- 17).Yang C.S., Maliakal P., Meng X. (2002) Inhibition of carcinogenesis by tea. Annu. Rev. Pharmacol. Toxicol. 42, 25–54 [DOI] [PubMed] [Google Scholar]
- 18).Hong J., Lu H., Meng X., Ryu J.H., Hara Y., Yang C.S. (2002) Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (−)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 62, 7241–7246 [PubMed] [Google Scholar]
- 19).Hou Z., Sang S., You H., Lee M.J., Hong J., Chin K.V., Yang C.S. (2005) Mechanism of action of (−)-epigallocatechin-3-gallate: auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res. 65, 8049–8056 [DOI] [PubMed] [Google Scholar]
- 20).Umeda D., Yano S., Yamada K., Tachibana H. (2008) Involvement of 67-kDa laminin receptor-mediated myosin phosphatase activation in antiproliferative effect of epigallocatechin-3-O-gallate at a physiological concentration on Caco-2 colon cancer cells. Biochem. Biophys. Res. Commun. 371, 172–176 [DOI] [PubMed] [Google Scholar]
- 21).Umeda D., Tachibana H., Yamada K. (2005) Epigallocatechin-3-O-gallate disrupts stress fibers and the contractile ring by reducing myosin regulatory light chain phosphorylation mediated through the target molecule 67 kDa laminin receptor. Biochem. Biophys. Res. Commun. 333, 628–635 [DOI] [PubMed] [Google Scholar]
- 22).Fujimura Y., Umeda D., Yano S., Maeda-Yamamoto M., Yamada K., Tachibana H. (2007) The 67kDa laminin receptor as a primary determinant of anti-allergic effects of O-methylated EGCG. Biochem. Biophys. Res. Commun. 364, 79–85 [DOI] [PubMed] [Google Scholar]
- 23).Yang C.S., Chen L., Lee M.J., Balentine D., Kuo M.C., Schantz S.P. (1998) Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol. Biomarkers Prev. 7, 351–354 [PubMed] [Google Scholar]
- 24).Lambert J.D., Yang C.S. (2003) Mechanisms of cancer prevention by tea constituents. J. Nutr. 133, 3262S–3267S [DOI] [PubMed] [Google Scholar]
- 25).Lee M.J., Wang Z.Y., Li H., Chen L., Sun Y., Gobbo S., Balentine D.A., Yang C.S. (1995) Analysis of plasma and urinary tea polyphenols in human subjects. Cancer Epidemiol. Biomarkers Prev. 4, 393–399 [PubMed] [Google Scholar]
- 26).Ermakova S., Choi B.Y., Choi H.S., Kang B.S., Bode A.M., Dong Z. (2005) The intermediate filament protein vimentin is a new target for epigallocatechin gallate. J. Biol. Chem. 280, 16882–16890 [DOI] [PubMed] [Google Scholar]
- 27).Li M., He Z., Ermakova S., Zheng D., Tang F., Cho Y.Y., Zhu F., Ma W.Y., Sham Y., Rogozin E.A., Bode A.M., Cao Y., Dong Z. (2007) Direct inhibition of insulin-like growth factor-I receptor kinase activity by (−)-epigallocatechin-3-gallate regulates cell transformation. Cancer Epidemiol. Biomarkers Prev. 16, 598–605 [DOI] [PubMed] [Google Scholar]
- 28).He Z., Tang F., Ermakova S., Li M., Zhao Q., Cho Y.Y., Ma W.Y., Choi H.S., Bode A.M., Yang C.S., Dong Z. (2008) Fyn is a novel target of (−) epigallocatechin gallate in the inhibition of JB6 C141 cell transformation. Mol. Carcinog. 47, 172–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Ermakova S.P., Kang B.S., Choi B.Y., Choi H.S., Schuster T.F., Ma W.Y., Bode A.M., Dong Z. (2006) (−)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucoseregulated protein 78. Cancer Res. 66, 9260–9269 [DOI] [PubMed] [Google Scholar]
- 30).Shim J.H., Choi H.S., Pugliese A., Lee S.Y., Chae J.I., Choi B.Y., Bode A.M., Dong Z. (2008) (−)-Epigallocatechin gallate regulates CD3-mediated T cell receptor signaling in leukemia through the inhibition of ZAP-70 kinase. J. Biol. Chem. 283, 28370–28379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Tachibana H., Koga K., Fujimura Y., Yamada K. (2004) A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol. 11, 380–381 [DOI] [PubMed] [Google Scholar]
- 32).Fujimura Y., Yamada K., Tachibana H. (2005) A lipid raft-associated 67kDa laminin receptor mediates suppressive effect of epigallocatechin-3-O-gallate on FcεRI expression. Biochem. Biophys. Res. Commun. 336, 674–681 [DOI] [PubMed] [Google Scholar]
- 33).Fujimura Y., Tachibana H., Yamada K. (2004) Lipid raft-associated catechin suppresses the FcεRI expression by inhibiting phosphorylation of the extracellular signal-regulated kinase1/2. FEBS Lett. 556, 204–210 [DOI] [PubMed] [Google Scholar]
- 34).Rao N.C., Barsky S.H., Terranova V.P., Liotta L.A. (1983) Isolation of a tumor cell laminin receptor. Biochem. Biophys. Res. Commun. 111, 804–808 [DOI] [PubMed] [Google Scholar]
- 35).Malinoff H.L., Wicha M.S. (1983) Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. J. Cell Biol. 96, 1475–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Lesot H., Kuhl U., Mark K.V. (1983) Isolation of a laminin-binding protein from muscle cell membranes. EMBO J. 2, 861–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Rao C.N., Castronovo V., Schmitt M.C., Wewer U.M., Claysmith A.P., Liotta L.A., Sobel M.E. (1989) Evidence for a precursor of the high-affinity metastasis-associated murine laminin receptor. Biochemistry 28, 7476–7486 [DOI] [PubMed] [Google Scholar]
- 38).Landowski T.H., Dratz E.A., Starkey J.R. (1995) Studies of the structure of the metastasis-associated 67 kDa laminin binding protein: fatty acid acylation and evidence supporting dimerization of the 32 kDa gene product to form the mature protein. Biochemistry 34, 11276–11287 [DOI] [PubMed] [Google Scholar]
- 39).Butò S., Tagliabue E., Ardini E., Magnifico A., Ghirelli C., van den Brûle F., Castronovo V., Colnaghi M.I., Sobel M.E., Ménard S. (1998) Formation of the 67-kDa laminin receptor by acylation of the precursor. J. Cell. Biochem. 69, 244–251 [DOI] [PubMed] [Google Scholar]
- 40).Hundt C., Peyrin J.M., Haïk S., Gauczynski S., Leucht C., Rieger R., Riley M.L., Deslys J.P., Dormont D., Lasmézas C.I., Weiss S. (2001) Identification of interaction domains of the prion protein with its 37-kDa/67-kDa laminin receptor. EMBO J. 20, 5876–5886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Menard S., Tagliabue E., Colnaghi M.I. (1998) The 67 kDa laminin receptor as a prognostic factor in human cancer. Breast Cancer Res. Treat. 52, 137–145 [DOI] [PubMed] [Google Scholar]
- 42).Sanjuán X., Fernández P.L., Miquel R., Muñoz J., Castronovo V., Ménard S., Palacín A., Cardesa A., Campo E. (1996) Overexpression of the 67-kD laminin receptor correlates with tumour progression in human colorectal carcinoma. J. Pathol. 179, 376–380 [DOI] [PubMed] [Google Scholar]
- 43).Mafune K., Ravikumar T.S. (1992) Anti-sense RNA of 32-kDa laminin-binding protein inhibits attachment and invasion of a human colon carcinoma cell line. J. Surg. Res. 52, 340–346 [DOI] [PubMed] [Google Scholar]
- 44).Vande Broek I., Vanderkerken K., De Greef C., Asosingh K., Straetmans N., Van Camp B., Van Riet I. (2001) Laminin-1-induced migration of multiple myeloma cells involves the high-affinity 67 kD laminin receptor. Br. J. Cancer 85, 1387–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Tanaka M., Narumi K., Isemura M., Abe M., Sato Y., Abe T., Saijo Y., Nukiwa T., Satoh K. (2000) Expression of the 37-kDa laminin binding protein in murine lung tumor cell correlates with tumor angiogenesis. Cancer Lett. 153, 161–168 [DOI] [PubMed] [Google Scholar]
- 46).Huard T.K., Malinoff H.L., Wicha M.S. (1986) Macrophages express a plasma membrane receptor for basement membrane laminin. Am. J. Pathol. 123, 365–370 [PMC free article] [PubMed] [Google Scholar]
- 47).Yoon P.S., Boxer L.A., Mayo L.A., Yang A.Y., Wicha M.S. (1987) Human neutrophil laminin receptors: activation-dependent receptor expression. J. Immunol. 138, 259–265 [PubMed] [Google Scholar]
- 48).Singer I.I., Scott S., Kawka D.W., Kazazis D.M. (1989) Adhesomes: specific granules containing receptors for laminin, C3bi/fibrinogen, fibronectin, and vitronectin in human polymorphonuclear leukocytes and monocytes. J. Cell Biol. 109, 3169–3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Thompson H.L., Burbelo P.D., Segui-Real B., Yamada Y., Metcalfe D.D. (1989) Laminin promotes mast cell attachment. J. Immunol. 143, 2323–2327 [PubMed] [Google Scholar]
- 50).Thompson H.L., Burbelo P.D., Metcalfe D.D. (1990) Regulation of adhesion of mouse bone marrow-derived mast cells to laminin. J. Immunol. 145, 3425–3431 [PubMed] [Google Scholar]
- 51).Chen J., Carcamo J.M., Borquez-Ojeda O., Erdjument-Bromage H., Tempst P., Golde D.W. (2003) The laminin receptor modulates granulocyte-macrophage colony-stimulating factor receptor complex formation and modulates its signaling. Proc. Natl. Acad. Sci. USA 100, 14000–14005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Chen A., Ganor Y., Rahimipour S., Ben-Aroya N., Koch Y., Levite M. (2002) The neuropeptides GnRH-II and GnRH-I are produced by human T cells and trigger laminin receptor gene expression, adhesion, chemotaxis and homing to specific organs. Nat. Med. 8, 1421–1426 [DOI] [PubMed] [Google Scholar]
- 53).Gauczynski S., Peyrin J.M., Haïk S., Leucht C., Hundt C., Rieger R., Krasemann S., Deslys J.P., Dormont D., Lasmézas C.I., Weiss S. (2001) The 37-kDa/67-kDa laminin receptor acts as the cell-surface receptor for the cellular prion protein. EMBO J. 20, 5863–5875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Kim K.J., Chung J.W., Kim K.S. (2005) 67-kDa laminin receptor promotes internalization of cytotoxic necrotizing factor 1-expressing Escherichia coli K1 into human brain microvascular endothelial cells. J. Biol. Chem. 280, 1360–1368 [DOI] [PubMed] [Google Scholar]
- 55).Wang K.S., Kuhn R.J., Strauss E.G., Ou S., Strauss J.H. (1992) High-affinity laminin receptor is a receptor for Sindbis virus in mammalian cells. J. Virol. 66, 4992–5001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Thepparit C., Smith D.R. (2004) Serotype-specific entry of dengue virus into liver cells: identification of the 37-kilodalton/67-kilodalton high-affinity laminin receptor as a dengue virus serotype 1 receptor. J. Virol. 78, 12647–12656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Ludwig G.V., Kondig J.P., Smith J.F. (1996) A putative receptor for Venezuelan equine encephalitis virus from mosquito cells. J. Virol. 70, 5592–5599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58).Akache B., Grimm D., Pandey K., Yant S.R., Xu H., Kay M.A. (2006) The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J. Virol. 80, 9831–9836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Yang G., Shu X.O., Li H., Chow W.H., Ji B.T., Zhang X., Gao Y.T., Zheng W. (2007) Prospective cohort study of green tea consumption and colorectal cancer risk in women. Cancer Epidemiol. Biomarkers Prev. 16, 1219–1223 [DOI] [PubMed] [Google Scholar]
- 60).Shammas M.A., Neri P., Koley H., Batchu R.B., Bertheau R.C., Munshi V., Prabhala R., Fulciniti M., Tai Y.T., Treon S.P., Goyal R.K., Anderson K.C., Munshi N.C. (2006) Specific killing of multiple myeloma cells by (−)-epigallocatechin-3-gallate extracted from green tea: biologic activity and therapeutic implications. Blood 108, 2804–2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Britschgi A., Simon H.U., Tobler A., Fey M.F., Tschan M.P. (2010) Epigallocatechin-3-gallate induces cell death in acute myeloid leukaemia cells and supports all-trans retinoic acid-induced neutrophil differentiation via death-associated protein kinase 2. Br. J. Haematol. 149, 55–64 [DOI] [PubMed] [Google Scholar]
- 62).Dorchies O.M., Wagner S., Buetler T.M., Ruegg U.T. (2009) Protection of dystrophic muscle cells with polyphenols from green tea correlates with improved glutathione balance and increased expression of 67LR, a receptor for (−)-epigallocatechin gallate. Biofactors 35, 279–294 [DOI] [PubMed] [Google Scholar]
- 63).Metzger H. (1992) The receptor with high affinity for IgE. Immunol. Rev. 125, 37–48 [DOI] [PubMed] [Google Scholar]
- 64).Ravetch J.V., Kinet J.P. (1991) Fc receptors. Annu. Rev. Immunol. 9, 457–492 [DOI] [PubMed] [Google Scholar]
- 65).Turner H., Kinet J.P. (1999) Signalling through the high-affinity IgE receptor FcεRI. Nature 402, B24–B30 [DOI] [PubMed] [Google Scholar]
- 66).Rivera J. (2002) Molecular adapters in FcεRI signaling and the allergic response. Curr. Opin. Immunol. 14, 688–693 [DOI] [PubMed] [Google Scholar]
- 67).Choi O.H., Adelstein R.S., Beaven M.A. (1994) Secretion from rat basophilic RBL-2H3 cells is associated with diphosphorylation of myosin light chains by myosin light chain kinase as well as phosphorylation by protein kinase C. J. Biol. Chem. 269, 536–541 [PubMed] [Google Scholar]
- 68).Pfeiffer J.R., Seagrave J.C., Davis B.H., Deanin G.G., Oliver J.M. (1985) Membrane and cytoskeletal changes associated with IgE-mediated serotonin release from rat basophilic leukemia cells. J. Cell Biol. 101, 2145–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69).Edgar A.J., Bennett J.P. (1997) Circular ruffle formation in rat basophilic leukemia cells in response to antigen stimulation. Eur. J. Cell Biol. 73, 132–140 [PubMed] [Google Scholar]
- 70).Fujimura Y., Umeda D., Kiyohara Y., Sunada Y., Yamada K., Tachibana H. (2006) The involvement of the 67 kDa laminin receptor-mediated modulation of cytoskeleton in the degranulation inhibition induced by epigallocatechin-3-O-gallate. Biochem. Biophys. Res. Commun. 348, 524–531 [DOI] [PubMed] [Google Scholar]
- 71).Ludowyke R.I., Peleg I., Beaven M.A., Adelstein R.S. (1989) Antigen-induced secretion of histamine and the phosphorylation of myosin by protein kinase C in rat basophilic leukemia cells. J. Biol. Chem. 264, 12492–12501 [PubMed] [Google Scholar]
- 72).Dombrowicz D., Flamand V., Brigman K.K., Koller B.H., Kinet J.P. (1993) Abolition of anaphylaxis by targeted disruption of the high affinity immunoglobulin E receptor α chain gene. Cell 75, 969–976 [DOI] [PubMed] [Google Scholar]
- 73).Sano M., Suzuki M., Miyase T., Yoshino K., Maeda-Yamamoto M. (1999) Novel antiallergic catechin derivatives isolated from oolong tea. J. Agric. Food Chem. 47, 1906–1910 [DOI] [PubMed] [Google Scholar]
- 74).Tachibana H., Sunada Y., Miyase T., Sano M., Maeda-Yamamoto M., Yamada K. (2000) Identification of a methylated tea catechin as an inhibitor of degranulation in human basophilic KU812 cells. Biosci. Biotechnol. Biochem. 64, 452–454 [DOI] [PubMed] [Google Scholar]
- 75).Suzuki M., Yoshino K., Maeda-Yamamoto M., Miyase T., Sano M. (2000) Inhibitory effects of tea catechins and O-methylated derivatives of (−)-epigallocatechin-3-O-gallate on mouse type IV allergy. J. Agric. Food Chem. 48, 5649–5653 [DOI] [PubMed] [Google Scholar]
- 76).Maeda-Yamamoto M., Inagaki N., Kitaura J., Chikumoto T., Kawahara H., Kawakami Y., Sano M., Miyase T., Tachibana H., Nagai H., Kawakami T. (2004) O-methylated catechins from tea leaves inhibit multiple protein kinases in mast cells. J. Immunol. 172, 4486–4492 [DOI] [PubMed] [Google Scholar]
- 77).Maeda-Yamamoto M., Ema K., Shibuichi I. (2007) In vitro and in vivo anti-allergic effects of ‘benifuuki’ green tea containing O-methylated catechin and ginger extract enhancement. Cytotechnology 55, 135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78).Fujimura Y., Tachibana H., Maeda-Yamamoto M., Miyase T., Sano M., Yamada K. (2002) Antiallergic tea catechin, (−)-epigallocatechin-3-O-(3-O-methyl)-gallate, suppresses FcepsilonRI expression in human basophilic KU812 cells. J. Agric. Food Chem. 50, 5729–5734 [DOI] [PubMed] [Google Scholar]
- 79).Fujimura Y., Umeda D., Yano S., Maeda-Yamamoto M., Yamada K., Tachibana H. (2007) The 67kDa laminin receptor as a primary determinant of anti-allergic effects of O-methylated EGCG. Biochem. Biophys. Res. Commun. 364, 79–85 [DOI] [PubMed] [Google Scholar]
- 80).Lambert J.D., Yang C.S. (2003) Mechanisms of cancer prevention by tea constituents. J. Nutr. 133, 3262S–3267S [DOI] [PubMed] [Google Scholar]
- 81).Yano S., Fujimura Y., Umeda D., Miyase T., Yamada K., Tachibana H. (2007) Relationship between the biological activities of methylated derivatives of (−)-epigallocatechin-3-O-gallate (EGCG) and their cell surface binding activities. J. Agric. Food Chem. 55, 7144–7148 [DOI] [PubMed] [Google Scholar]
- 82).Li W., Ashok M., Li J., Yang H., Sama A.E., Wang H. (2007) A major ingredient of green tea rescues mice from lethal sepsis partly by inhibiting HMGB1. PLoS ONE 2, e1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83).Byun E.-H., Fujimura Y., Yamada K., Tachibana H. (2010) TLR 4 signaling inhibitory pathway induced by green tea polyphenol epigallocatechin-3-gallate through 67-kDa laminin receptor. J. Immunol. 185, 33–45 [DOI] [PubMed] [Google Scholar]
- 84).Green H., Kehinde O. (1975) An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell 5, 19–27 [DOI] [PubMed] [Google Scholar]
- 85).Kao Y.H., Hiipakka R.A., Liao S. (2000) Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology 141, 980–987 [DOI] [PubMed] [Google Scholar]
- 86).Kao Y.H., Hiipakka R.A., Liao S. (2000) Modulation of obesity by a green tea catechin. Am. J. Clin. Nutr. 72, 1232–1241 [DOI] [PubMed] [Google Scholar]
- 87).Lin J.K., Lin-Shiau S.Y. (2006) Mechanisms of hypolipidemic and anti-obesity effects of tea and tea polyphenols. Mol. Nutr. Food Res. 50, 211–217 [DOI] [PubMed] [Google Scholar]
- 88).Mackman N., Tilley R.E., Key N.S. (2007) Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler. Thromb. Vasc. Biol. 27, 1687–1693 [DOI] [PubMed] [Google Scholar]
- 89).Holy E.W., Stämpfli S.F., Akhmedov A., Holm N., Camici G.G., Lüscher T.F., Tanner F.C. (2010) Laminin receptor activation inhibits endothelial tissue factor expression. J. Mol. Cell. Cardiol. 48, 1138–1145 [DOI] [PubMed] [Google Scholar]
- 90).Negrutskii B.S., El’skaya A.V. (1998) Eukaryotic translation elongation factor 1 alpha: structure, expression, functions, and possible role in aminoacyl-tRNA channeling. Prog. Nucleic Acid Res. Mol. Biol. 60, 47–78 [DOI] [PubMed] [Google Scholar]
- 91).Hartshorne D.J., Ito M., Erdodi F. (2004) Role of protein phosphatase. J. Biol. Chem. 273, 5542–5548 [DOI] [PubMed] [Google Scholar]
- 92).Izawa T., Fukata Y., Kimura T., Iwamatsu A., Dohi K., Kaibuchi K. (2000) Elongation factor-1 α is a novel substrate of rho-associated kinase. Biochem. Biophys. Res. Commun. 278, 72–78 [DOI] [PubMed] [Google Scholar]
- 93).Edmonds B.T., Wyckoff J., Yeung Y.G., Wang Y., Stanley E.R., Jones J., Segall J., Condeelis J. (1996) Elongation factor-1 α is an overexpressed actin binding protein in metastatic rat mammary adenocarcinoma. J. Cell Sci. 109, 2705–2714 [DOI] [PubMed] [Google Scholar]
- 94).Surh Y.J. (2003) Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 3, 768–780 [DOI] [PubMed] [Google Scholar]