Abstract
The intermediate and anterior lobes of the pituitary gland are derived from an invagination of oral ectoderm that forms Rathke’s pouch. During gestation proliferating cells are enriched around the pouch lumen, and they appear to delaminate as they exit the cell cycle and differentiate. During late mouse gestation and the post-natal period, anterior lobe progenitors re-enter the cell cycle and expand the populations of specialized, hormone-producing cells. At birth, all cell types are present, and their localization appears stratified based on cell type. We conducted a birth dating study of Rathke’s pouch derivatives to determine whether the location of specialized cells at birth is correlated with the timing of cell cycle exit. We find that all of the anterior lobe cell types initiate differentiation concurrently with a peak between e11.5 and e13.5. Differentiation of intermediate lobe melanotropes is delayed relative to anterior lobe cell types. We discovered that specialized cell types are not grouped together based on birth date and are dispersed throughout the anterior lobe. Thus, the apparent stratification of specialized cells at birth is not correlated with cell cycle exit. Thus, the currently popular model of cell specification, dependent upon timing of extrinsic, directional gradients of signaling molecules, needs revision. We propose that signals intrinsic to Rathke’s pouch are necessary for cell specification between e11.5 and e13.5 and that cell-cell communication likely plays an important role in regulating this process.
Keywords: Pituitary gland, Differentiation, BMP, FGF, Birthdating, Rathke’s pouch
Introduction
The development of the vertebrate neural ectoderm provides a paradigm for understanding the timing of cell specification in relation to the physical location of cell types. Neural progenitors actively divide around the lumen of the neural tube, then exit the cell cycle and migrate towards the periphery where they differentiate into specialized cell types. In the neural tube, the earliest born neurons are located in the ventricular zone, while the later born neurons are located in the superficial marginal zone (Luskin and Shatz, 1985). The development of the mouse pituitary gland from the oral ectoderm is similar to the neural tube in that the progenitors actively divide around the lumen of Rathke’s pouch, the precursor of the pituitary gland (Ikeda and Yoshimoto 1991; Ward et al. 2005). As cells on the ventral side of Rathke’s pouch exit the cell cycle they progress to a CYCLIN-DEPENDENT KINASE INHIBITOR 1C (p57Kip2) positive, CYCLIN E positive transitional stage at the boundary between the lumenal area and the forming anterior lobe (Bilodeau et al., 2009). These transitional cells then form the anterior lobe where they express CYCLIN-DEPENDENT KINASE INHIBITOR 1B (p27Kip1) and intermediate markers of differentiation, such as TBX19 for corticotropes, before differentiating fully to hormone expressing cells (Bilodeau et al., 2009; Ikeda and Yoshimoto, 1991; Ward et al., 2005). It is not known whether the cells actively migrate or are passively extruded from the lumenal area. Cells in the intermediate lobe remain on the dorsal side of the lumen adjacent to the posterior lobe of the pituitary. These intermediate lobe cells differentiate into melanotropes, which process pro-opiomelanocortin (POMC) into melanocyte stimulating hormone (MSH).
The anterior lobe contains specialized cell types that are typified by the hormones that they produce: gonadotropes produce luteinizing hormone (LH) and follicle stimulating hormone (FSH); somatotropes produce growth hormone (GH); corticotropes produce adrenocorticotropin (ACTH), which is processed from POMC; thyrotropes produce thyroid stimulating hormone (TSH); and lactotropes produce prolactin (PRL). At birth the rodent hormone-producing cells in the anterior lobe of the pituitary gland have a stratified appearance such that these specific cell types are enriched in specific locations. For instance, gonadotropes are located at the rostral end of the pituitary, while somatotropes occupy more caudal positions (Baker and Gross, 1978). The anterior pituitary hormone-producing cells are enriched in stereotypical homotypic and heterotypic positions in other vertebrates including birds, fish, amphibians, and reptiles as well (Matsumoto and Ishii, 1992). The grouping of the hormone-producing cells in the pituitary anterior lobe could result from differential timing of cell specification, as in the neural ectoderm, where different cell types occupying different levels in the neural tube are sequentially produced from progenitor cells in the ventricular zone. Alternatively, the organization could result from cell migration to form homotypic networks (Bonnefont et al., 2005).
Cell cycle labeling experiments demonstrate that Rathke’s pouch cells labeled at e11.5 are located at more rostral and ventral locations in the developing anterior lobe than cells labeled at e12.5 (Ward et al., 2005). Unlike the neural ectoderm, which has an inside out ordering of cell types based on age, the anterior pituitary appears to have its earliest born cells located farthest from the lumen. Because gonadotropes occupy the most rostral locations, they should be specified before somatotropes that occupy the more caudal and lateral locations. The prediction that gonadotropes are specified before somatotropes is not intuitive because gonadotropes are the last cell type to express markers of completed differentiation (LH is not detected in gonadotropes until e16.5, while GH is detected in somatotropes at e15.5) (Japon et al., 1994). Gonadotropes likely undergo a stepwise progression of differentiation; the first gonadotrope specific marker, gonadotropin releasing hormone receptor, Gnrhr, is expressed at e14.5 (Holley et al., 2002), followed by Nr5a1 (SF-1) at e15.5 (Barnhart and Mellon, 1994), Lhb at e16.5, and Fshb at e17.5 (Japon et al., 1994). While this stepwise differentiation of gonadotropes takes longer than the differentiation of somatotropes, the more rostral location of gonadotropes predicts an earlier commitment to cell fate.
Neural tube cells that have exited the cell cycle are truly post-mitotic; they do not re-enter the cell cycle. In contrast, the pituitary gland increases in size during the late gestational period through the postnatal period in part through the proliferation of differentiated anterior lobe cell types and progenitors that have seeded the anterior lobe (Carbajo-Perez et al., 1989; Carbajo-Perez and Watanabe, 1990; Taniguchi et al., 2001a, b). However, during gestation, the focus of this study, the anterior lobe has few proliferating cells relative to the peri-lumenal area, and the majority of cells that have begun to differentiate or are fully differentiated are quiescent (Bilodeau et al., 2009; Ward et al., 2005; Ward et al., 2006). For this study references to cell cycle exit will refer to this time period from e11.5 to e17.5, when differentiated cells have a low proliferation rate.
Current models of cell specification in the anterior lobe propose that opposing gradients of FGF and BMP signaling pattern the progenitor cells within Rathke’s pouch before they move to the anterior lobe where they differentiate (Burgess et al., 2002; Wagner and Thomas, 2007). Fgf8 is expressed in the infundibulum, the precursor of the posterior lobe of the pituitary gland, dorsal to Rathke’s pouch, while Bmp2 is expressed in the ventral portion of Rathke’s pouch at e10.5 (Ericson et al., 1998; Treier et al., 1998). Ventral cell types in the anterior lobe, such as gonadotropes, are predicted to arise from ventral locations in Rathke’s pouch that receive high levels of BMP, while dorsal cell types in the anterior lobe, such as somatotropes, are predicted to arise from dorsal locations in Rathke’s pouch that receive high levels of FGF. Thyrotropes located in the caudo-medial region of the anterior lobe should arise from areas with intermediate levels of FGF and BMP in Rathke’s pouch. These models are largely based on gain of function and dominant negative experiments in transgenic mice and studies with pituitary explants (Ericson et al., 1998; Treier et al., 1998).
Based on these proposed gradients of FGF and BMP in Rathke’s pouch, the peri-lumenal pattern of progenitor proliferation, and the preferential location of earlier born cells to occupy more rostral positions in the anterior lobe, we predicted that the caudal to rostral location of cell types in the anterior lobe would reflect the relative timing of cell specification. For example, rostrally and ventrally located gonadotropes would exit the cell cycle and begin to differentiate before caudally positioned somatotropes. We tested this prediction by birthdating the anterior lobe cell types. We found that the caudal to rostral location of cell types does not correlate with the timing of cell cycle exit. In addition, cells that are born concurrently are dispersed throughout the anterior lobe, instead of occupying adjacent locations. Therefore, the localized appearance of cells in the pituitary anterior lobe does not result from the sequential release of specialized cell types from the cell cycle. The apparent enrichment of cell types in specific regions of the anterior lobe at birth is more likely a result of cell movement to establish networks of specific cell types than a relationship with timing of cell cycle exit (Bonnefont et al., 2005). Our findings suggest that the model for cell specification in the pituitary gland needs revision. Altering the balance of signaling factors that originate in the ventral diencephalon does not affect cell specification in the anterior lobe (Brinkmeier et al., 2007; Davis and Camper, 2007; Potok et al., 2008; Rizzoti et al., 2004; Zhao et al., 2009). Therefore, we present a new model for pituitary cell specification that focuses on factors that are intrinsic to Rathke’s pouch when cells begin to differentiate between e11.5 to e13.5, including the BMP, WNT, and NOTCH signaling pathways.
Materials and Methods
Mice
Pregnant CD-1 mice were purchased from Charles River and kept at the University of Michigan utilizing protocols approved by the University of Michigan Committee on the Care and Use of Animals. The day a copulation plug was detected was defined as embryonic day 0.5 (e0.5). Pregnant mice received intraperitoneal injections of 100 μg BrdU per gram of body weight (Nowakowski et al., 1989). Pregnant mice were injected for each embryonic day of development between e9.5 and e17.5. All embryos were allowed to develop until e17.5 when they were collected. All embryos were fixed in 3.7% formaldehyde in phosphate buffered saline (PBS), pH 7.5 overnight at 4 C. Embryos were dehydrated and embedded in paraffin (Hogan et al., 1994).
Immunohistochemistry, sampling, and counting
Slides used for immunohistochemistry were dewaxed in xylene and then hydrated through 100% and 95% ethanol before washing in PBS. Please see Table 1 for specifics for each experiment, including antigen retrieval, peroxidase inactivation, blocking, antibodies used, and detection methods.
Table 1.
Experimental details for immunohistochemistry experiements
| Immunohistochemistry experiments with BrdU | ||||||||
|---|---|---|---|---|---|---|---|---|
| Experiment | Citric acid boil | CH3OH:H202 | Block | Primary Antibody | Secondary antibody and detection | Biotin block | Other Primary Antibody | Other secondary and detection |
| BrdU and LH | 5 min | no | TNB1 | 1:100 anti-BrdU, Oxford Biotech | anti-rat FITC | no | 1:500 anti-LH, NHPP2 | anti-guinea pig biotin, Streptavidin Cy3 |
| BrdU and TSH | 5 min | yes | TNB | 1:100 anti-BrdU, Ab Serotec | anti-rat biotin, Streptavidin Cy3 | yes3 | 1:1000 anti-TSH, NHPP | anti-rabbit biotin, TSA FITC |
| BrdU and POMC | 5 min | yes | TNB | 1:100 anti-BrdU, Ab Serotec | anti-rat biotin, Streptavidin Cy3 | yes | 1:1500 anti-POMC, NHPP | anti-rabbit biotin, TSA FITC |
| BrdU and GH | 5 min | yes | TNB | 1:100 anti-BrdU, Ab Serotec | anti-rat biotin, Streptavidin Cy3 | yes | 1:1000 anti-GH, NHPP | anti-human biotin, TSA FITC |
| BrdU and all hormones | 5 min | no | TNB | 1:100 anti-BrdU, Ab Serotec | anti-rat biotin, Streptavidin Cy3 | yes | same as above, combined | anti-guinea pig, rabbit, human, Streptavidin Cy3 |
| BrdU and Sox2 | 10 min | yes | TNB | 1:100 anti-BrdU, Ab Serotec | anti-rat biotin, Streptavidin Cy3 | yes | 1:2000 anti-Sox2, Chemicon | anti-rabbit biotin, TSA FITC |
| Immunohistochemistry experiments without BrdU | ||||||||
|---|---|---|---|---|---|---|---|---|
| TBX19 and POMC | 5 min | no | TNB | 1:1500 anti-POMC, NHPP | anti-rabbit biotin, Streptavidin-488 | yes | 1:200 anti-TBX19, gift from J. Drouin | anti-rabbit biotin, Streptavidin Cy3 |
| NR5A and LH | 5 min | no | TNB | 1:1000 anti-NR5A, gift from G. Hammer | anti-rabbit biotin, Streptavidin-488 | yes | 1:500 anti-LH | anti-guinea pig biotin, Streptavidin Cy3 |
| POU1F1 and GH, TSH | 5 min | yes | TNB | 1:600 anti-POU1F1, gift from S. Rhodes | anti-rabbit biotin, Streptavidin-488 | yes | 1:100 anti-GH, 1:100 anti-TSH | anti-rabbit and anti-human biotin, TSA TRITC |
| pSMAD1/5/8 | 10 min | yes | GBT4 | 1:100 anti-pSMAD, Upstate Biological | anti-rabbit biotin, TSA FITC | no | ||
| pMAPK | 10 min | yes | GBT | 1:100 anti-pMAPK, Cell Signaling | anti-rabbit biotin, TSA FITC | no | ||
Block from Perkin-Elmer TSA-FITC Kit
National Hormone and Pituitary Program
Avidin/Biotin Blocking Kit from Vector Laboratories
10% Goat Serum, 1% BSA, 0.1% Triton X-100
Each e17.5 embryo was embedded in paraffin in a coronal orientation and sectioned at 6 μm in thickness from caudal to rostral. Placing two sections on each slide generally produced 30 slides containing pituitary tissue. Each set of 30 pituitary slides was divided into groups of six slides based on caudal to rostral location. To prevent bias based on a standardized starting point, the first slide picked from each individual in the most caudal group (slides 1 – 6) was picked at random using a random number generator (www.random.org). This number was then used as the starting point for the individual and every 6th slide was then selected to generate a set of five slides that represented each caudal to rostral grouping. This set of five slides was used for one specific hormone antibody and BrdU double immunohistochemistry experiment. Additional double immunohistochemistry experiments were conducted using sets of five slides that followed sequentially from the random starting location. Three embryos were used for each double immunohistochemistry experiment.
Results of the immunohistochemistry experiments were observed on a Leica DMRB microscope at final magnification of 630x and photographed using a Retiga 2000R digital camera. Each image compilation was manually counted using Image Pro Plus software (Media Cybernetics). Due to the large number of samples necessary to conduct this experiment, it was not possible to process all of the slides at the same time. Therefore, the data were expressed as a proportion of the total for each experiment in order to limit the affect of variability inherent between immunohisotchemistry experiments.
Statistical analysis
Proportions of double-labeled cells (# BrdU and hormone double positive cells/total # hormone positive cells) were transformed with the logit-function [logit(p)= ln(p/(1-p)) where p is the proportion of double-labeled cells]. One-way ANOVA with Tukey adjustments was used to compare the means of specific populations, either across time points or locations. Level of significance was set at p < 0.05. Determination of the timing of proliferation used the mean of total BrdU positive cells counted per time point. The distribution of this data was roughly normally distributed and was not transformed. All calculations were performed using SAS 9.1 (SAS Institute).
Results
We tested the hypothesis that the caudal to rostral location of cells within the anterior lobe of the pituitary gland is predictive of the relative time of cell cycle exit. If this hypothesis were true, then rostral cells would be born before caudal cells. BrdU was used to label proliferating cells on each embryonic day of development between e9.5 and e17.5. All embryos were allowed to develop until e17.5, when the majority of anterior pituitary hormones can be detected. The number of BrdU positive cells present at e17.5 is dependent on a variety of factors including the proliferative index of the pituitary, which could differ during development, the amount of BrdU that is retained within cells that continue to divide, the changing size of the developing pituitary gland, and the potential loss of cells during development. Those cells that retain the BrdU likely exited the cell cycle shortly after incorporating the BrdU and began to differentiate. Those cells that continued to proliferate would have diluted BrdU labeling, although it is not clear so how many cell divisions are required before the BrdU is undetectable. The population of BrdU labeled cells should be representative of those cells that exited the cell cycle shortly after incorporating the BrdU for each day that BrdU was injected.
Immunohistochemistry was used to detect GH for somatotropes, POMC for corticotropes and melanotropes, TSH for thyrotropes, and LH for gonadotropes in conjunction with a BrdU antibody to determine the relative timing of cell cycle exit for each cell type. The lactotrope population was not examined because prolactin expression is estrogen dependent and is not readily detectable until after birth (Ogasawara et al., 2009; Slabaugh et al., 1982). Folliculo-stellate cells are also only detectable post partum, and were not examined in this study (Soji et al., 1997).
Timing of Anterior Lobe Cell Cycle Exit
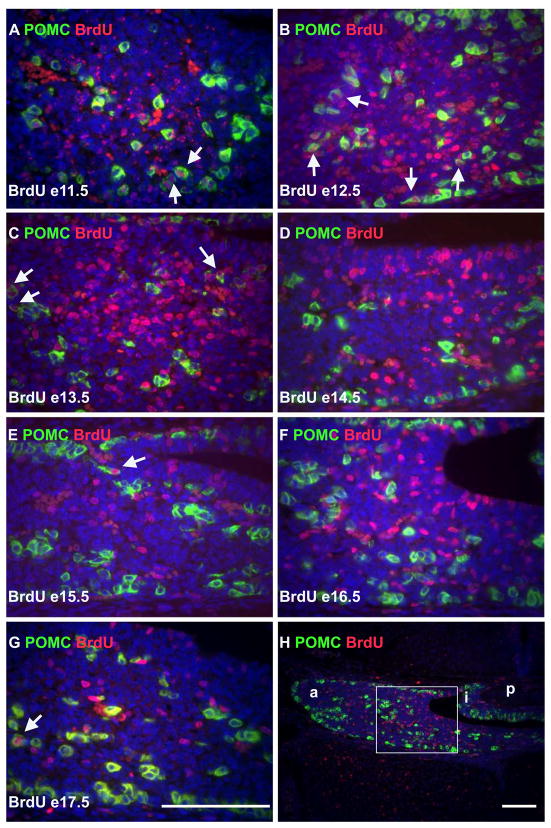
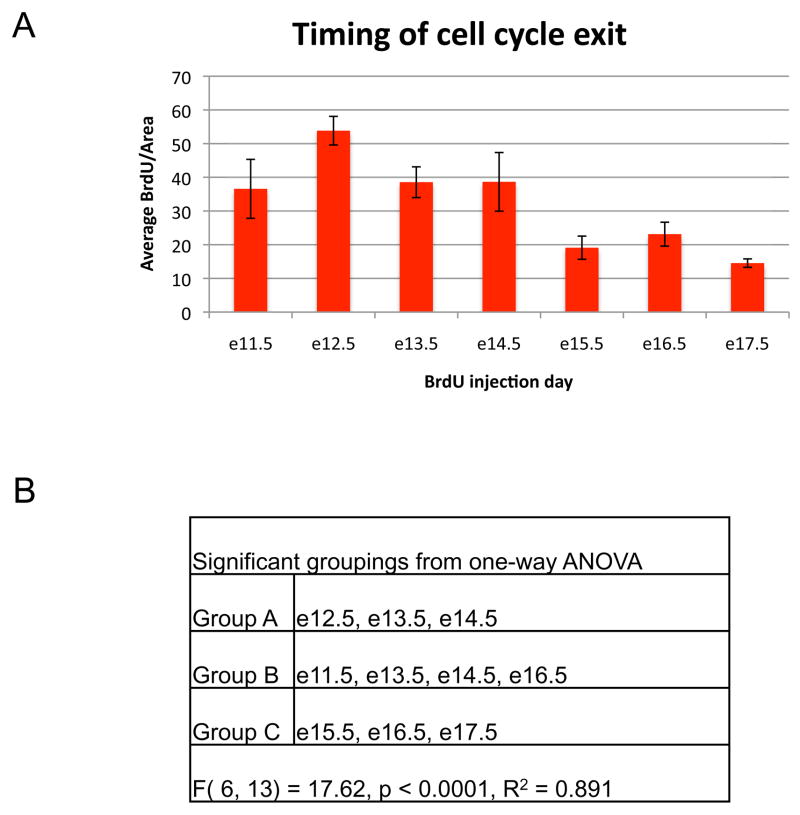
At e17.5 pituitary cells labeled with BrdU at e9.5 and e10.5 were undetectable (data not shown), suggesting that pituitary progenitors underwent numerous cell divisions subsequent to incorporation of label. BrdU incorporated at e11.5 was detectable at e17.5 (Figure 1A, this panel is a double immunohistochemistry for both BrdU and POMC). In order to determine when the majority of cells in the anterior lobe exit the cell cycle the average number of cells that incorporated BrdU per area of anterior lobe at e17.5 was determined for each day of development between e11.5 and e17.5 when BrdU was injected (Figure 2A). One-way ANOVA was used to assess significance at p < 0.05 (Figure 2B). The highest rates of incorporation in the anterior lobe occurred between e11.5 and e14.5. Because anterior lobe cells are generally not dividing, these cells must have exited the cell cycle shortly after incorporating BrdU and begun the differentiation process.
Figure 1.
Corticotropes begin to differentiate early in pituitary organogenesis. (A-G) Immunohistochemistry for POMC (green), and BrdU (red), then counter-stained with DAPI (blue). BrdU was injected on e11.5 (A), e12.5 (B), e13.5 (C), e14.5 (D), e15.5 (E), e16.5 (F), and e17.5 (G) and the assay performed for all images at e17.5. Arrows highlight selected cells that are positive for both POMC and BrdU. All sections are coronal with dorsal towards the top and medial on the right. (H) Lower magnification of F with the area depicted in F boxed. a = anterior lobe; i = intermediate lobe; p = posterior lobe. (A – G) Similar regions of the pituitary in their respective embryos. Similar results were observed for LH, TSH, and GH (Supplemental figures 2–4). Scale bars equal 100 um. Scale bar in G applies to A-F.
Figure 2.
Peak BrdU incorporation occurs early in pituitary organogenesis. (A) The average number of BrdU positive cells per mm2 of anterior lobe tissue for three individuals was determined for each embryonic day of BrdU injection. Error bars represent the standard deviation. (B) Signficant groupings as determined by one-way ANOVA. F (model degree of freedom, error degree of freedom) = F value
Anterior Lobe Cell Type Distribution
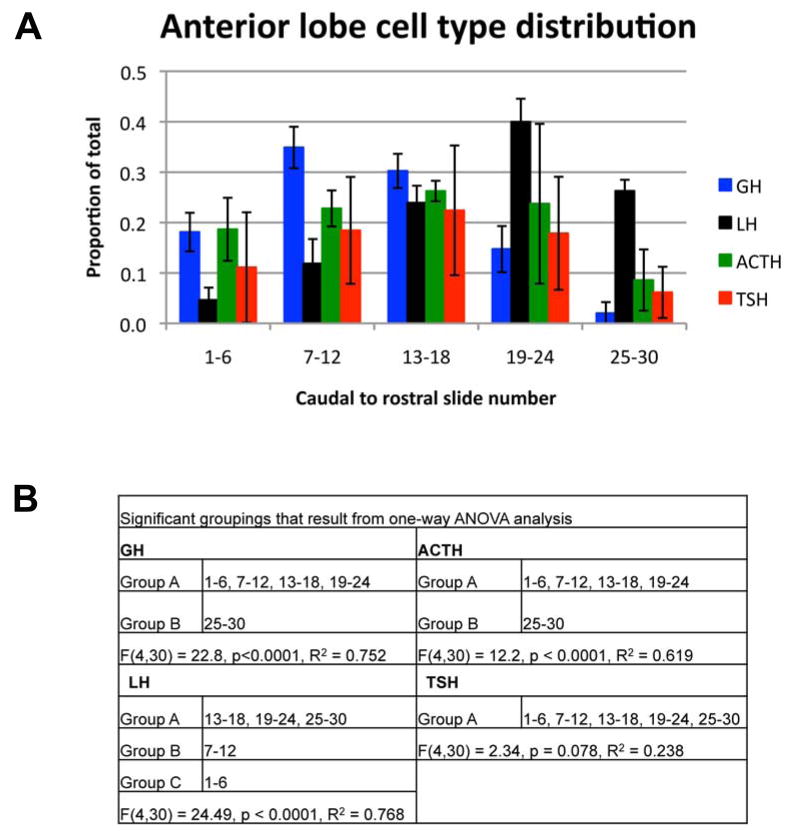
While the regional enrichment of cell types in the pituitary anterior lobe at birth has been reported previously (Baker and Gross, 1978), it has not been quantitatively assessed. Therefore, we quantified the distribution of cell types along the caudal to rostral axis by determining the proportion of the total number of cells located along the axis for each cell type (Figure 3A). Gonadotropes are preferentially located at more rostral locations within the anterior lobe, while somatotropes are the most caudally located cell type (Figure 3B). Corticotropes and thyrotropes occupy more intermediate locations (Figure 3B). About 10% percent of both corticotropes and thyrotropes are located at the rostral end of the pituitary (Figure 3A). This percentage is significantly different from the other locations for corticotropes, while it is not for thyrotropes. Our interpretation is that both corticotropes and thyrotropes are preferentially located in intermediate locations in the anterior lobe with a lower representation at the most rostral end. Cell types are not precisely stratified. Instead, each cell type is enriched in certain locations along the caudal to rostral axis, but they are not excluded from any location.
Figure 3.
Gonadotropes are located more rostrally than somatotropes. (A) The total number of hormone positive cells was determined for each cell type listed. The proportion of the total for each cell type was determined for each caudal to rostral location across the pituitary. Points represent the mean for three individuals for each cell type and error bars represent the standard deviation. (B) Signficant groupings as determined by one-way ANOVA.
Timing of Cell Cycle Exit for Hormone-Producing Cell Types
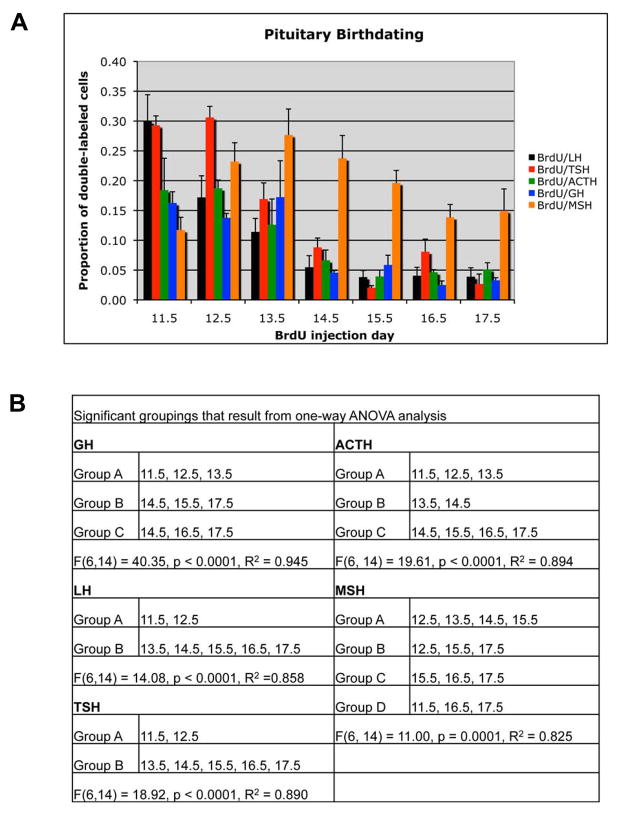
Based on the higher rate of proliferation from e11.5 to 14.5 and the spatial distribution of anterior lobe cell types, we asked whether the birthdates of hormone-producing cells exhibit unique temporal-spatial features. For each anterior and intermediate lobe cell type the timing of cell cycle exit and transition to differentiation was examined by determining the proportion of each hormone-producing cell type that incorporated and retained BrdU between embryonic days e11.5 and e17.5. Figure 1 contains representative data for BrdU and POMC, where BrdU+/POMC+ cells are readily identifiable at e11.5, e12.5, and e13.5 (Figure 1A – C), but are less numerous from e14.5 to e17.5 (Figure 1D – G, see Supplemental figure 1 for immunohistochemistry control experiments). Similar data were observed for LH, TSH, and GH (Supplemental figures 2–4). For each hormone the proportion of BrdU+/Hormone+ cells out of the total number of hormone cells was determined (Figure 4A). One-way ANOVA analysis was used to identify significant groupings for each cell type (Figure 4B). The e11.5 to e12.5 time point is when gonadotropes and thyrotropes are primarily specified. Corticotropes and somatotropes have a broader range of specification from e11.5 to e13.5, which overlaps with the gonadotropes and thyrotropes. There is no significant difference between the proportion of cells specified on each day within these groupings, i.e. similar proportions of somatotropes are likely to be specified on e11.5, e12.5, and e13.5. We conclude that all anterior lobe cell types begin the differentiation process concurrently, rather than in a temporally discrete manner.
Figure 4.
Pituitary gland birthdating reveals that differentiation of melanotropes occurs after anterior lobe cell types. (A) The total number of BrdU and hormone double-labeled cells was determined for each cell type listed, as well as, the total number of hormone positive cells. The bars represent the mean proportion of BrdU and hormone double-labeled cells out of the total hormone population for three individuals for each embryonic day of BrdU injection. Error bars represent the standard deviation. (B) Signficant groupings as determined by one-way ANOVA.
We extended our analysis to include the intermediate lobe melanotropes. Melanotropes do not enter the anterior lobe, but remain on the dorsal side of the lumen. We found that melanotropes exit the cell cycle between e12.5 and e14.5, after the anterior lobe cell types (Figure 4A and B, Supplemental figure 5). This result suggests that Rathke’s pouch progenitors have a preference for producing anterior lobe cell types before intermediate lobe melanotropes.
Anterior Lobe Hormone-Negative Cell Cycle Exit
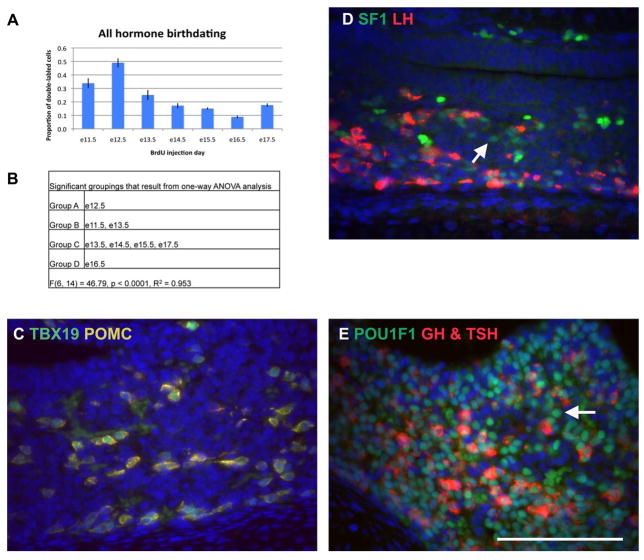
The pituitary contains a population of cells that do not express any detectable hormone (Senovilla et al., 2008). We sought to birthdate this hormone-negative population. We performed immunohistochemistry using the primary antibodies for GH, LH, TSH, and POMC concurrently and visualized the entire hormone-producing population with the same fluorophore. We measured the proportion of double-labeled BrdU and hormone positive cells out of the total BrdU positive cells to reveal when all hormone-producing cell types are born (Figure 5A, Supplemental figure 6). BrdU labeled anterior lobe cells that do not express hormone represent the hormone-negative cell population. As expected based on the results of birthdating with individual anterior lobe hormone antibodies, the largest proportion of BrdU labeled “all hormone” cells arise between e11.5 and e13.5 (Figure 5A and B). While some e17.5 double-labeled “all hormone” cells are specified after e13.5, most of the cells that incorporated BrdU after e13.5 did not express any hormone. The hormone-negative cells maybe a mixture of undifferentiated progenitors and incompletely differentiated, but committed cells.
Figure 5.
Most cells born after e13.5 do not express any hormones. (A) The total number of BrdU cells double labeled with either ACTH, GH, LH, and TSH was determined, as well as, the total number of BrdU positive cells. The bars represent the mean proportion of double-labeled cells out of the total BrdU positive population for each of three individuals for each embryonic day of BrdU injection. Error bars represent the standard deviation. (B) Significant groupings as determined by one-way ANOVA. (C) Double immunohistochemistry for TBX19 (green) and POMC (yellow). POMC was detected with Cy3, but appears yellow because of the cross-reaction from using two antibodies raised in rabbit. (D) Double immunohistochemistry for NR5A1 (green) and LH (red). Arrow indicates a NR5A1 positive cell that is negative for LH. (E) Double immunohistochemistry for POU1F1 (green) and GH and TSH (red). A Cy3 secondary was used to detect both GH and TSH. Arrow indicates a POU1F1 positive cell that is negative for GH and TSH. Scale bar in E equals 100 μm for C, D, and E.
We assessed the relative proportion of incompletely differentiated, but committed cells by performing a double immunohistochemistry for transcription factors that identify separate lineages and a hormone specific to that lineage. All cells that expressed TBX19 (TPIT) also expressed POMC, indicating that there are few corticotropes that are not fully differentiated (Figure 5C). The gonadotropes were analyzed using an antibody against NR5A1 (SF1) and LH. Most NR5A1 positive cells were also LH positive (Figure 5D). However, a few cells were NR5A1 positive and LH negative, indicating either that these cells will eventually express LH, but have not fully differentiated or that these cells may represent a transitional cell type that expresses both FSH and TSH (Wen et al., 2010). The large proportion of hormone-negative cells cannot be accounted for by the few incompletely differentiated gonadotropes. Next we analyzed the Pit1 lineage by performing a double immunohistochemistry for POU1F1 (PIT1) with GH and TSH combined with the same fluorophore (Figure 5E). Many POU1F1 positive cells were identified that did not express detectable levels of hormone, suggesting that partially differentiated cells of the PIT1 lineage are present in the embryonic pituitary gland. These cells may represent the lactotrope cells that will begin PRL expression after birth and/or somatotropes, which substantially increase in number after birth.
Proliferation of Progenitor Cells
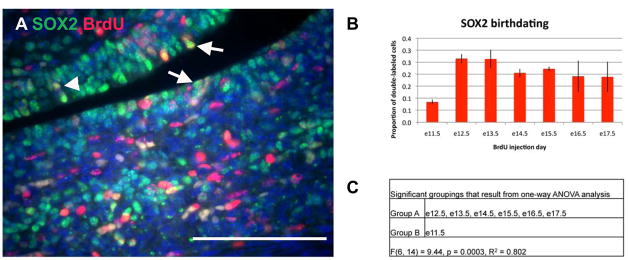
SOX2 is a marker of stem cells in a variety of tissues. In the pituitary, SOX2 marks a population of cells that line the lumen from formation of Rathke’s pouch into adulthood (Chen et al., 2009; Fauquier et al., 2008; Garcia-Lavandeira et al., 2009). Pituispheres cultured from adult pituitaries that can differentiate into all anterior lobe cell types also express SOX2, suggesting that these lumenal SOX2 cells may represent a pituitary stem cell population (Fauquier et al., 2008). SOX2 is also found throughout the anterior lobe, but this population of cells is also SOX9 positive and is thought to represent a population of early progenitor cells (Fauquier et al., 2008). Therefore, we examined the proliferation of SOX2 positive cells that are located adjacent to the lumen in the proposed stem cell niche (Figure 6A). Throughout embryonic pituitary organogenesis, a steady proportion of SOX2 positive cells are in a proliferative state (Figure 6B, Supplemental figure 7).
Figure 6.
SOX2 progenitor cells are constantly dividing throughout pituitary organogenesis. (A) Double immunohistochemistry for SOX2 (green) and BrdU (red). Arrows indicate SOX2 and BrdU double positive cells that are adjacent to the lumen and were counted as potential stem cells. Arrowhead indicates a SOX2 and BrdU double positive cells that is not adjacent to the lumen and was not counted as a potential stem cell. Scale bar equals 100 μm. (B) The total number of BrdU and SOX2 double-labeled cells was determined, as well as, the total number of SOX2 positive cells. The bars represent the mean proportion of BrdU and SOX2 double-labeled cells out of the total SOX2 population for three individuals for each embryonic day of BrdU injection. Error bars represent the standard deviation. (C) Significant groupings as determined by one-way ANOVA.
Spatial Location of BrdU Positive and Hormone Positive Cells
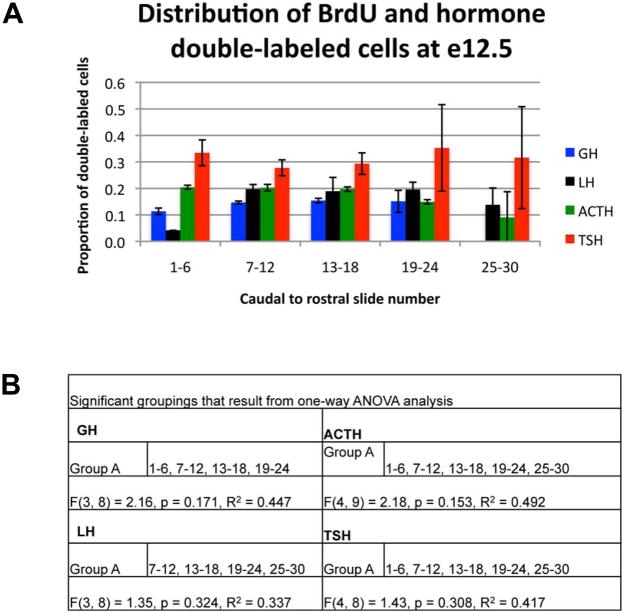
Most of the hormone expressing cell types appear to stop proliferating and differentiate between e11.5 and e14.5 rather than in a sequential pattern of discrete times. Despite this broad range of birthdates, anterior lobe cell types may be stratified based on the time of cell cycle exit. To examine this possibility, we graphed the proportion of BrdU and hormone double-labeled cells out of the total hormone population across the caudal to rostral axis when BrdU was injected on e12.5 (Figure 7A). We found no enrichment of double-labeled cells at any caudal to rostral location for thyrotropes and corticotropes; instead, double-labeled cells for each cell type were distributed across the caudal to rostral axis (Figure 7B). This is also true for somatotropes and gonadotropes except that double-labeled cells were not abundant in the caudal (gonadotropes) or rostral (somatotropes) ends of the pituitary. An even distribution of double-labeled cells for all anterior lobe cell types was also observed on all other days of BrdU incorporation (data not shown).
Figure 7.
Hormone producing cells born on e12.5 are scattered throughout the anterior lobe. (A) The proportion of double-labeled BrdU and hormone positive cells out of the total number of hormone cells was determined for each location across the caudal to rostral axis when BrdU was injected on e12.5. Points represent the mean proportion for three individuals for each hormone indicated. Error bars represent the standard deviation. (C) Significant groupings as determined by one-way ANOVA.
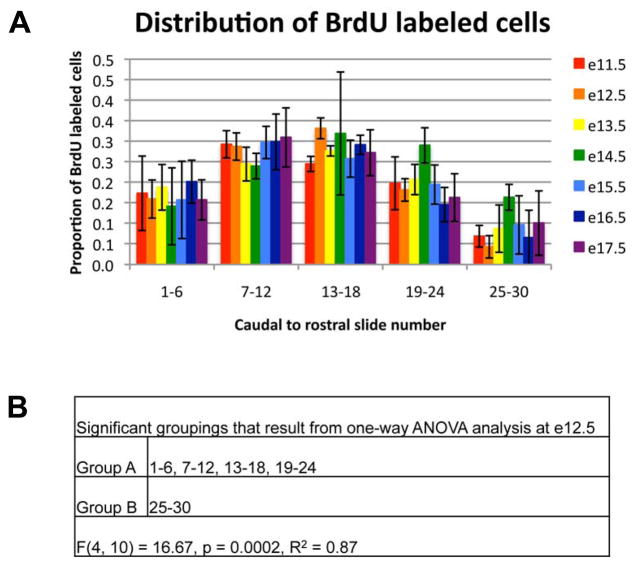
In order to extend the spatial analysis of differentiated anterior lobe cells, we examined the caudal to rostral distribution of anterior lobe cells by embryonic day of BrdU incorporation. For instance, cells born on e11.5 should be found more rostrally than cells born on e13.5, regardless of cell type, if the anterior lobe is stratified based on the timing of cell cycle exit. We found that BrdU positive cells are uniformly distributed across the pituitary for all days of BrdU incorporation (Figure 8A and B). Therefore, the timing of cell cycle exit does not result in a spatial pattern of specialized anterior lobe cell types across the caudal to rostral axis.
Figure 8.
Anterior lobe cells that differentiate concurrently are dispersed throughout the caudal to rostral axis of the anterior lobe. The total number of BrdU positive cells was determined for each day when BrdU was injected from e11.5 to e17.5. The proportion of the total for each day of BrdU injection was determined for each caudal to rostral location across the pituitary. Points represent the mean for three individuals for each cell type. Error bars represent the standard deviation. (C) Significant groupings as determined by one-way ANOVA for the e12.5 BrdU injection day.
Distribution of BrdU Positive Cells in the Dorsal to Ventral and Medial to Lateral Axes
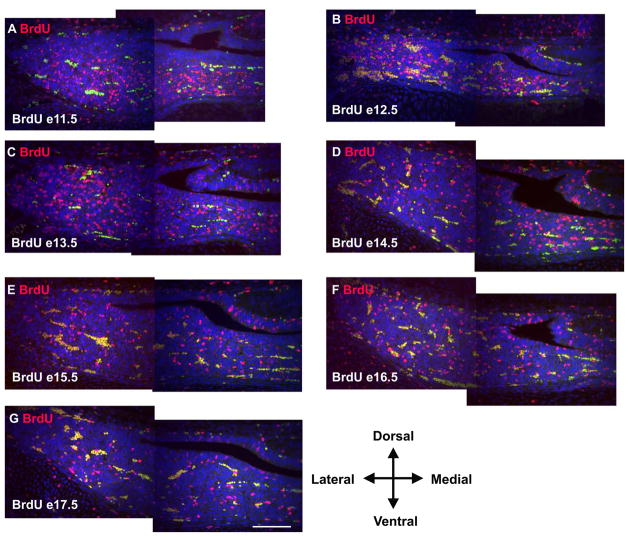
The gradients of FGF and BMP that are thought to regulate cell specification in the pituitary are distributed across the dorsal to ventral axis of Rathke’s pouch (Ericson et al., 1998; Treier et al., 1998). Therefore, the dorsal to ventral axis may be the more appropriate axis for examining the stratification of the anterior lobe. In order to address this possibility we examined the location of BrdU positive cells in coronal sections of e17.5 embryos for BrdU injected at each embryonic day between e11.5 and e17.5 (Figure 9A – G). We did not observe an enrichment of BrdU positive cells on either the dorsal or ventral side, regardless of the date of BrdU injection. Nor did we observe an enrichment of BrdU cells on the medial to lateral axis. Therefore, the enrichment of specific cell types within regions of the anterior pituitary gland at birth does not result from the ordered release from the cell cycle of cell types during pituitary organogenesis. The distribution of cell types must result from a mechanism unrelated to the timing of cell cycle exit.
Figure 9.
Anterior lobe cells that differentiate concurrently are also dispersed throughout the dorsal to ventral and medial to lateral axes. BrdU was injected on e11.5 (A), e12.5 (B), e13.5 (C), e14.5 (D), e15.5 (E), e16.5 (F), and e17.5 (G) and immunohistochemistry was preformed at e17.5 for all images. Autofluorescent blood cells appear yellow as the image is a composite of exposures taken using both red and green filters. All images are coronal with medial on the right. Each image represents one half of the pituitary gland. Cells incorporating BrdU at each time point are distributed throughout the gland at e17.5 without being enriched in any preferred location. Scale bar equals 100 μm for all images.
Temporal and Spatial Analysis of BMP and FGF signaling
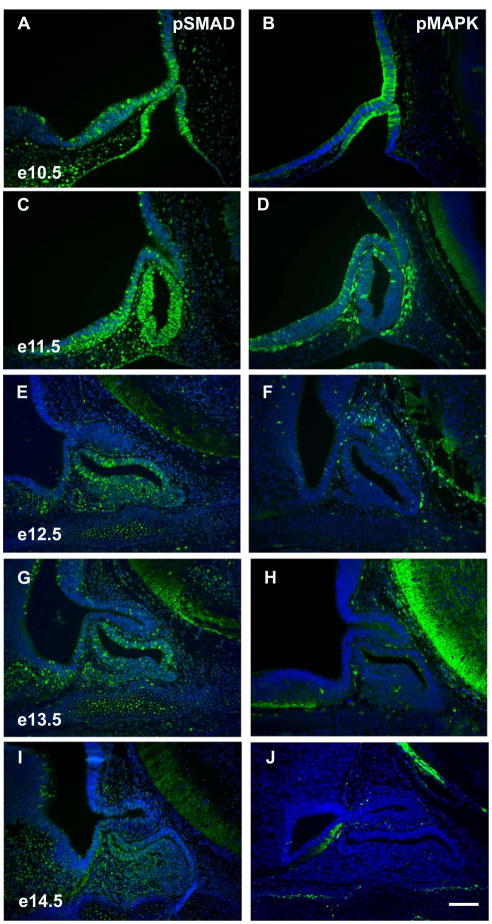
We have shown that anterior lobe cell types exit the cell cycle between e11.5 and e13.5. If gradients of BMP and FGF signaling are necessary for specifying anterior lobe cell types (Burgess et al., 2002; Wagner and Thomas, 2007), then these signaling pathways should be present in the pituitary at this time. We examined the phosphorylation status of SMADs 1, 5, and 8 (pSMAD), common intracellular transducers of BMP signaling, to assess the existence of signaling gradients (Brinkmeier et al., 2007; Davis and Camper, 2007). At e10.5, pSMAD is enriched on the ventral side of Rathke’s pouch in a domain consistent with Bmp2 expression (Figure 10A) (Ericson et al., 1998; Treier et al., 1998). By e11.5, pSMAD is found throughout Rathke’s pouch, consistent with the expansion of Bmp2 throughout Rathke’s pouch at this time (Figure 10C) (Treier et al., 1998). A uniform distribution of pSMAD is also evident at e12.5 except that scattered cells in the lumenal area and forming anterior lobe have more intense staining (Figure 10E). This pattern persists at e13.5, but there is an overall decrease in pSMAD intensity (Figure 10G). At e14.5, cells on the dorsal side of the lumen have little to no pSMAD staining, while the anterior lobe maintains a fairly uniform level of pSMAD signal (Figure 10I). Therefore, there is little evidence for a gradient of BMP signaling when anterior lobe cell types exit the cell cycle between e11.5 and e13.5. Instead, BMP signaling is found throughout Rathke’s pouch at this time.
Figure 10.
pSMAD signaling, but not pMAPK signaling, is observed in Rathke’s pouch during pituitary cell specification. Immunohistochemistry for phosphorylated Smad 1, 5, and 8 (A, C, E, G, and I) and phosphorylated Mapk (B, D, F, H, and J). Time points assayed are e10.5 (A, B), e11.5 (C, D), e12.5 (E, F), 13.5 (G, H), and e14.5 (I, J). All sections are sagittal with dorsal at the top and rostral at the left. Scale bar equals 100 μm for all images.
In order to characterize the affect of FGF signaling on Rathke’s pouch, we examined the phosphorylation status of MAPK (pMAPK), an intracellular mediator of FGF signaling, from e10.5 to e14.5 in the pituitary (Davis and Camper, 2007). At e10.5, pMAPK immunoreactivity is enriched on the dorsal side of Rathke’s pouch (Figure 10B), adjacent to the forming infundibulum, where Fgf8 and Fgf10 are expressed (Ericson et al., 1998; Treier et al., 1998; Yamasaki et al., 1996). At e11.5, pMAPK has decreased, but is still dorsally located (Figure 10D). From e12.5 to e14.5 pMAPK is not detectable in the forming pituitary gland (Figure 10F, H, and J). A gradient of FGF signaling is apparent at e10.5 before cell specification is evident. Between e11.5 to e13.5, when anterior lobe cells exit the cell cycle, little to no FGF signal is detectable in Rathke’s pouch.
Discussion
Current models for pituitary cell specification predict that the stratified appearance of cell types in the anterior lobe results from an ordered specification of cell types that develop from interacting gradients of BMP and FGF (Burgess et al., 2002; Wagner and Thomas, 2007). These models also predict that cells born concurrently will occupy the same location within the anterior lobe. Our results demonstrate that this is not the case. The enrichment of cell types at specific positions along the caudal to rostral axis in newborn mice does not result from an ordered cell cycle exit for each cell type. Instead, cells that exit the cell cycle concurrently are dispersed throughout the anterior lobe of the pituitary. Therefore, the enrichment of cell types in specific regions of the anterior lobe results from a mechanism other than the timing of cell cycle exit, and may occur through active or passive cell movements, formation of networks, or control of final differentiation by lateral inhibition.
Timing of Cell Cycle Exit
Our results indicate that the majority of the anterior lobe hormone-producing cells exit the cell cycle between e11.5 and e13.5. These results are in close agreement with a birthdating study of anterior pituitary cell types that labeled cycling cells with tritiated thymidine (Seuntjens and Denef, 1999). Seuntjens and Denef did not examine cell specification at e11.5, but they determined that the anterior lobe cell types are specified between e12.5 and e13.5. Our two reports are in agreement: cell cycle exit for all anterior lobe cell types occurs concurrently. Individual cell types do not exit the cell cycle at discrete time points, as suggested by the enrichment of cell types in regions of the anterior lobe at birth. Our analysis is more extensive, considering anterior lobe cells that do not produce hormone, intermediate lobe melanotropes, and SOX2 expressing progenitor cells. The majority of cells in the anterior pituitary that do not express detectable levels of hormone at e17.5 express POU1F1. We predict that these POU1F1 positive, hormone-negative cells are likely to be lactotropes that are primed to produce PRL after birth and somatotropes, which increase in number postnatally. In addition, the hormone-negative population contains a few gonadotropes and is likely to contain a variety of other cell types that were not detected in our assay, including multipotential progenitors, folliculo-stellate cells, and endothelial cells.
While the anterior lobe cell types exit the cell cycle concurrently, the intermediate lobe melanotropes are delayed in cell cycle exit until after the anterior lobe cell types. The NOTCH signaling pathway is necessary for maintaining the progenitor state of cells in the developing pituitary gland. In the absence of Hes1, a key intracellular transducer of NOTCH signals, cells in the pituitary gland prematurely differentiate, which leads to a hypoplastic pituitary (Kita et al., 2007; Raetzman et al., 2007). In addition, somatotropes are ectopically formed in the intermediate lobe at the expense of melanotropes (Raetzman et al., 2007). This conversion of melanotropes to somatotropes can be explained by the timing of cell specification. If the pituitary has a pool of progenitors that gives rise to all anterior and intermediate lobe cell types, then premature differentiation will reduce the size of this common progenitor pool (Kita et al., 2007; Raetzman et al., 2007). The anterior lobe cell types will be preferentially generated because of they are specified earlier than the intermediate lobe melanotropes. Therefore, the progenitor pool may be depleted before melanotropes arise from the common progenitor pool. While cells located in the intermediate lobe have the potential to form multiple anterior lobe cell types (Pulichino et al., 2003), it appears that cell specification likely relies on other cues intrinsic to Rathke’s pouch as premature release from the cell cycle is not permissive for the formation of all cell types (Kita et al., 2007; Raetzman et al., 2007).
Recent reports demonstrate that SOX2 positive cells that line the lumen of the pituitary are a progenitor/stem cell population for the anterior pituitary (Chen et al., 2009; Fauquier et al., 2008; Garcia-Lavandeira et al., 2009). We found that these cells are in a steady proliferative state throughout pituitary organogenesis. The proliferative status of these SOX2 positive cells is consistent with the idea that they are a progenitor cell population that yields a variety of pituitary cell types. We expect that the proliferative rate of SOX2 positive cells declines after birth as this population of cells transitions to a “stem cell”-like function necessary for maintaining the pituitary gland in response to physiological need.
Distribution of Anterior Lobe Hormone-Producing Cells
We analyzed the location of anterior lobe cell types based on when they begin to exit the cell cycle and differentiate. We anticipated that cells that exit the cell cycle at the same time would be closely associated with each other along the rostral to caudal axis. Instead, we found that cells that exit the cell cycle concurrently are scattered throughout the anterior lobe of the pituitary. Therefore, the mechanism for enriching cell types in regions of the anterior lobe is not correlated with cell cycle release. Instead directed cell movements may enrich cell networks. Bonnefont et al. demonstrated that the somatotropes preferentially coalesce in the lateral wings of the anterior pituitary and that these networks of somatotropes are very dynamic and change over time as physiological demand changes (Bonnefont et al., 2005). The mechanism of producing this network is unknown, but we speculate that the active movement of cells from the lumenal area and into the anterior lobe is necessary for producing this network.
The nature of the signals that lead to the formation of cell-type specific networks in the pituitary is an unanswered question. Interestingly, releasing hormones from the hypothalamus may play a role in forming or maintaining these networks. Navratil et al. (2007) have shown that gonadotropin releasing hormone (GnRH) causes gonadotropes to undergo cell movement both in vitro and in situ and that gonadotropes will actively move toward GnRH (Navratil et al., 2007). GnRH attraction may stimulate movement of gonadotropes towards the vascular system in order to enhance release of gonadotropins into the blood stream. Localized release of GnRH within the pituitary could cause or reinforce the gonadotrope network at the rostral end of the pituitary.
Cell Cycle Exit and Cell Fate Commitment
At e13.5 there is a zone of non-cycling, undifferentiated progenitors, primarily on the ventral side of the lumen. These cells express p57Kip2 and CYCLIN E and do not express transcription factors diagnostic for anterior lobe cell types, such as TBX19, SF1, or POU1F1. As these cells differentiate they express p27Kip1 and the signature transcription factors of differentiation. Differentiated cells expressing p27Kip1 are found in more rostral locations in the anterior lobe (Bilodeau et al., 2009). Our data demonstrate that the majority of the anterior lobe cells exit the cell cycle between e11.5 and e13.5, although it does not indicate where or when the commitment into specific cell types occurs. The transitional zone identified by Bilodeau et al. is likely to be the correct time and place for cell fate commitment in the anterior lobe. Our data are in agreement with the interpretation that the last embryonic mitotic division for the majority of the hormone-producing cells occurs prior to the appearance of p57Kip2 at e13.5. Commitment to a specific cell type for these cell cycle arrested cells may occur in this transitional zone. As these cells differentiate not only do they turn on cell type specific genes, but also actively arrange themselves into networks within the anterior lobe.
Early in rodent development, the proliferating cells appear to line the lumen of Rathke’s pouch and the majority of anterior lobe cells appear to have exited the cell cycle (Bilodeau et al., 2009; Ikeda and Yoshimoto, 1991; Ward et al., 2005). During the post-natal period, many differentiated, hormone expressing cells start proliferating as the pituitary grows (Carbajo-Perez et al., 1989; Taniguchi et al., 2001a, b). This post-natal growth period is driven in part by the mitogenic effects of the hypothalamic releasing hormones (Alba and Salvatori, 2004; Muglia et al., 1995; Shibusawa et al., 2000). Because the hypothalamic releasing hormones do not have a mitogenic affect on anterior lobe cell types before birth, different factors must stimulate re-entry into the cell cycle during development. The nature of these stimulating factors is not yet known, but they may be introduced by the vascular network, which develops in the rodent anterior lobe around e14.5 (Szabo and Csanyi, 1982). Consistent with this idea, Prop1 mutants have a deficient vascular network and have a hypoplastic anterior pituitary at birth, while Pou1f1 mutants have an intact vascular network and a normal sized anterior pituitary at birth (Ward et al., 2006).
The commitment to a specific cell type may occur while the Rathke’s pouch progenitors are still cycling in the peri-lumenal area before they enter the transition zone. This idea would be similar to the commitment of somites to specific vertebral column morphologies based on their anterior to posterior location and corresponding Hox gene expression (Kieny et al., 1972; Nowicki and Burke, 2000). This scenario is supported by the proposed gradients of FGF and BMP signaling at e10.5 in Rathke’s pouch, which may establish different identities within the pouch for each cell type using a graded series of transcription factors expressed across the pouch, including Foxl2, Isl1, and Pou3f4 on the ventral side and Nkx3-1, Six3, and Prop1 on the dorsal side (Treier et al., 1998).
Gradients in Rathke’s Pouch
We examined the expression of pMAPK and pSMAD to determine the temporal and spatial activity of FGF and BMP gradients in the developing pituitary. The only time that a gradient of these intracellular factors is observed in Rathke’s pouch is at e10.5. At this time point Bmp2 expression is initiated in the ventral portion of Rathke’s pouch in a domain consistent with pSMAD expression. This gradient is lost at e11.5 as Bmp2 expression expands to encompass the entire pouch (Treier et al., 1998). Similarly, a gradient of pMAPK is detected at e10.5, but decreases afterwards. Anterior lobe cell types exit the cell cycle between e11.5 to e13.5, when gradients of FGF and BMP signaling are not detectable. Therefore, it seems unlikely that these proposed gradients would affect cell fate at this time point. While pMAPK and pSMAD are certainly well known intracellular transducers of cell signaling, they are not the only intracellular transducers of these signaling factors. Therefore, it is possible that the presence of a gradient could be observed through other intracellular signaling factors. However, given the expression of Bmp2 throughout Rathke’s pouch at e11.5, and the continued expression of Bmp4 in the infundibulum at e14.5 in a domain coincident with Fgf 8 and Fgf10 expression (Davis and Camper, 2007; Treier et al., 1998), it is unlikely that opposing gradients of BMP and FGF are driving cell fate commitment between e11.5 to e13.5. However, Rathke’s pouch does have regionally expressed transcription factors, suggesting that a gradient of activity is in effect. Other extrinsic signaling pathways including SHH or retinoic acid could potentially establish the restricted expression of these transcription factors.
The absence of a FGF or BMP gradient from e11.5 to e13.5 does not indicate whether commitment to cell fate occurs before or after cell cycle exit. However, in vivo analyses that alter ventral diencephalon expression of BMP and FGF signaling, such as noggin−/−, Tcf7l2−/−, Wnt5a−/−, Sox3−/−, and Lhx2−/− mice, demonstrate that altering the level of BMP and FGF signaling prior to e11.5 can change the size, location, and shape of Rathke’s pouch, but does not alter cell specification per se (Brinkmeier et al., 2007; Davis and Camper, 2007; Potok et al., 2008; Rizzoti et al., 2004; Zhao et al., 2009). Therefore, we believe that cell specification in Rathke’s pouch occurs through intrinsic signals beginning at e11.5. Because BMP2 is expressed throughout Rathke’s pouch at e11.5 it could play a role in cell specification. The NOTCH signaling pathway is another intrinsic pathway that affects cell specification. In fact, loss of Hes1, a transducer of NOTCH signaling, causes a cell fate switch of melanotropes to somatotropes (Raetzman et al., 2007). Recent results in zebrafish demonstrate that NOTCH can control the number of cells specified in different domains of the anterior pituitary. For instance, NOTCH signaling can promote lactotrope specification at the expense of the corticotropes in the most anterior domain and somatotropes at the expense of thyrotropes in the posterior domain of the anterior lobe (Dutta et al., 2008). A similar role for NOTCH signaling in mice has not been explored completely, but it is clear that NOTCH signaling plays diverse roles in the mouse pituitary. Hes6 is expressed in anterior lobe in a domain separate from Hes1, suggesting that Hes6 may play a role in the differentiation process while Hes1 maintains the progenitor pool (Raetzman et al., 2004). Also, the NOTCH ligand Dll3 is expressed in the forming corticotropes and may play a role in their differentiation, although it is not essential (Raetzman et al., 2004).
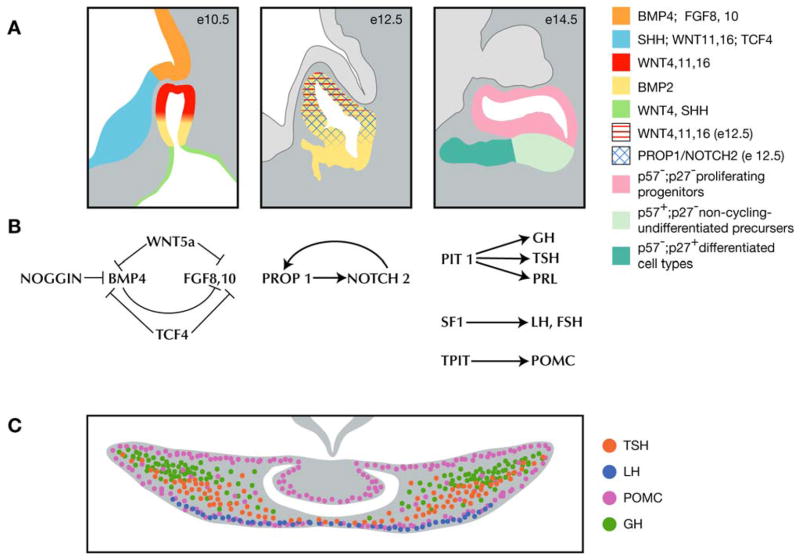
We propose a new model for pituitary gland development that incorporates our determination of the timing of cell cycle exit for hormone-producing cells with the need to identify intrinsic factors necessary for cell specification (Figure 11). At e9.5 Bmp4 expressed in the ventral diencephalon induces the oral ectoderm to invaginate to form Rathke’s pouch (Takuma et al., 1998). FGF signaling from the ventral diencephalon is necessary to ensure the continued proliferation of Rathke’s pouch (De Moerlooze et al., 2000; Takuma et al., 1998). A careful balance of BMP, FGF, and WNT signaling in the ventral diencephalon results in the proper location and size of Rathke’s pouch (Brinkmeier et al., 2007; Davis and Camper, 2007; Potok et al., 2008; Rizzoti et al., 2004; Zhao et al., 2009). At e10.5 Bmp2 begins its expression on ventral side of Rathke’s pouch. A gradient of ventral to dorsal BMP and dorsal to ventral FGF at e10.5 may specify the rostral tip thyrotropes on the ventral side, while the progenitors for the remaining cell types of the anterior and intermediate lobes remain unspecified in dorsal locations. At e11.5 the somatotropes, thyrotropes, lactotropes, corticotropes, and gonadotropes begin to exit the cell cycle. As cells exit the cycle they become specified, possibly by intrinsic signals such as NOTCH signaling, which is initially established by Prop1 (Raetzman et al., 2004), and potentially BMP2 and WNT signaling as well (Potok et al., 2008). At e13.5 this zone of specification is marked by p57Kip2 and CYCLIN E in a domain just ventral to the lumen (Bilodeau et al., 2009). Once cell specification occurs these cells sort themselves into functional networks by migrating to specific locations throughout the anterior lobe (Bonnefont et al., 2005). The sorting of cells in the anterior lobe rearranges the cells such that cells that exit the cell cycle concurrently are not necessarily located next to each other. The apparent stratification of the anterior lobe at e17.5 is, therefore, not a product of an ordered exit from the cell cycle, but of active movements of cells to preferred locations necessary for generating cell type specific networks.
Figure 11.
Proposed model for pituitary gland development. (A) Spatial representation of extrinsic and intrinsic factors that influence development of Rathke’s pouch (Bilodeau et al. observed the transition zone of p57+ cells at e13.5, unpublished observations indicate that this transition zone is present at e14.5 as well (M. Brinkmeier personal communication). (B) Balance of extrinsic factors at e10.5 necessary for Rathke’s pouch development and known transcription factors that drive differentiation of anterior lobe cell types at e12.5 and e14.5. (C) Enrichment of cell types in the pituitary gland at e17.5 that results from the sorting of cells into networks following cell specification.
Supplementary Material
Acknowledgments
This project has received contributions from a many people for which we are very grateful. Hoonkyo Suh, MinChul Cho, and Donna Martin initiated experiments that were further developed for this project. Cheryl Jacobs and Mary Anne Potok provided technical expertise. Lingling Zhang of the University of Michigan Center for Statistical Consultation and Research provided statistical consultation. Medical illustration for figure 11 was provided by Shayne Davidson, CMI. Michele Brinkmeier and Donna Martin provided critical reading of the manuscript. Funding was provided by NIH R01-HD34283 awarded to SAC.
Contributor Information
Amanda H. Mortensen, Email: avesper@umich.edu.
Sally A. Camper, Email: scamper@umich.edu.
References
- Alba M, Salvatori R. A mouse with targeted ablation of the growth hormone-releasing hormone gene: a new model of isolated growth hormone deficiency. Endocrinology. 2004;145:4134–4143. doi: 10.1210/en.2004-0119. [DOI] [PubMed] [Google Scholar]
- Baker BL, Gross DS. Cytology and distribution of secretory cell types in the mouse hypophysis as demonstrated with immunocytochemistry. Am J Anat. 1978;153:193–215. doi: 10.1002/aja.1001530203. [DOI] [PubMed] [Google Scholar]
- Barnhart KM, Mellon PL. The orphan nuclear receptor, steroidogenic factor-1, regulates the glycoprotein hormone alpha-subunit gene in pituitary gonadotropes. Mol Endocrinol. 1994;8:878–885. doi: 10.1210/mend.8.7.7527122. [DOI] [PubMed] [Google Scholar]
- Bilodeau S, Roussel-Gervais A, Drouin J. Distinct developmental roles of cell cycle inhibitors p57Kip2 and p27Kip1 distinguish pituitary progenitor cell cycle exit from cell cycle reentry of differentiated cells. Mol Cell Biol. 2009;29:1895–1908. doi: 10.1128/MCB.01885-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefont X, Lacampagne A, Sanchez-Hormigo A, Fino E, Creff A, Mathieu MN, Smallwood S, Carmignac D, Fontanaud P, Travo P, Alonso G, Courtois-Coutry N, Pincus SM, Robinson IC, Mollard P. Revealing the large-scale network organization of growth hormone-secreting cells. Proc Natl Acad Sci U S A. 2005;102:16880–16885. doi: 10.1073/pnas.0508202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmeier ML, Potok MA, Davis SW, Camper SA. TCF4 deficiency expands ventral diencephalon signaling and increases induction of pituitary progenitors. Dev Biol. 2007;311:396–407. doi: 10.1016/j.ydbio.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R, Lunyak V, Rosenfeld M. Signaling and transcriptional control of pituitary development. Curr Opin Genet Dev. 2002;12:534–539. doi: 10.1016/s0959-437x(02)00337-4. [DOI] [PubMed] [Google Scholar]
- Carbajo-Perez E, Motegi M, Watanabe YG. Cell-Proliferation in the anterior pituitary of mice during growth. Biomedical Research-Tokyo. 1989;10:275–281. [Google Scholar]
- Carbajo-Perez E, Watanabe Y. Cellular proliferation in the anterior pituitary of the rat during the postnatal period. Cell Tissue Res. 1990;261:333–338. doi: 10.1007/BF00318674. [DOI] [PubMed] [Google Scholar]
- Chen J, Gremeaux L, Fu Q, Liekens D, Van Laere S, Vankelecom H. Pituitary progenitor cells tracked down by side population dissection. Stem Cells. 2009;27:1182–1195. doi: 10.1002/stem.51. [DOI] [PubMed] [Google Scholar]
- Davis S, Camper S. Noggin regulates Bmp4 activity during pituitary induction. Dev Biol. 2007;305:145–160. doi: 10.1016/j.ydbio.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moerlooze L, Spencer-Dene B, Revest J, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127:483–492. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- Dutta S, Dietrich JE, Westerfield M, Varga ZM. Notch signaling regulates endocrine cell specification in the zebrafish anterior pituitary. Dev Biol. 2008;319:248–257. doi: 10.1016/j.ydbio.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson J, Norlin S, Jessell TM, Edlund T. Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development. 1998;125:1005–1015. doi: 10.1242/dev.125.6.1005. [DOI] [PubMed] [Google Scholar]
- Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci U S A. 2008;105:2907–2912. doi: 10.1073/pnas.0707886105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Lavandeira M, Quereda V, Flores I, Saez C, Diaz-Rodriguez E, Japon MA, Ryan AK, Blasco MA, Dieguez C, Malumbres M, Alvarez CV. A GRFa2/Prop1/stem (GPS) cell niche in the pituitary. PLoS One. 2009;4:e4815. doi: 10.1371/journal.pone.0004815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacey E. Manipulating the mouse embryo: a laboratory manual. Cold Spring Harbor Laboratory Press; Plainview, New York: 1994. [Google Scholar]
- Holley SJ, Hall SB, Mellon PL. Complementary expression of IGF-II and IGFBP-5 during anterior pituitary development. Dev Biol. 2002;244:319–328. doi: 10.1006/dbio.2002.0608. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Yoshimoto T. Developmental changes in proliferative activity of cells of the murine Rathke’s pouch. Cell Tissue Res. 1991;263:41–47. doi: 10.1007/BF00318398. [DOI] [PubMed] [Google Scholar]
- Japon MA, Rubinstein M, Low MJ. In situ hybridization analysis of anterior pituitary hormone gene expression during fetal mouse development. J Histo Cyto. 1994;42:1117–1125. doi: 10.1177/42.8.8027530. [DOI] [PubMed] [Google Scholar]
- Kieny M, Mauger A, Sengel P. Early regionalization of somitic mesoderm as studied by the development of axial skeleton of the chick embryo. Dev Biol. 1972;28:142–161. doi: 10.1016/0012-1606(72)90133-9. [DOI] [PubMed] [Google Scholar]
- Kita A, Imayoshi I, Hojo M, Kitagawa M, Kokubu H, Ohsawa R, Ohtsuka T, Kageyama R, Hashimoto N. Hes1 and Hes5 control the progenitor pool, intermediate lobe specification, and posterior lobe formation in the pituitary development. Mol Endocrinol. 2007;21:1458–1466. doi: 10.1210/me.2007-0039. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Shatz CJ. Neurogenesis of the cat’s primary visual cortex. J Comp Neurol. 1985;242:611–631. doi: 10.1002/cne.902420409. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Ishii S. Atlas of endocrine organs: vertebrates and invertebrates. Springer-Verlag; Berlin; New York: 1992. [Google Scholar]
- Muglia LJ, Jacobson L, Dikkes P, Majzoub JA. Corticotropin-releasing hormone deficiency reveals fetal but not adult glucocorticoid need. Nature. 1995;373:427–432. doi: 10.1038/373427a0. [DOI] [PubMed] [Google Scholar]
- Navratil AM, Knoll JG, Whitesell JD, Tobet SA, Clay CM. Neuroendocrine plasticity in the anterior pituitary: gonadotropin-releasing hormone-mediated movement in vitro and in vivo. Endocrinology. 2007;148:1736–1744. doi: 10.1210/en.2006-1153. [DOI] [PubMed] [Google Scholar]
- Nowakowski RS, Lewin SB, Miller MW. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J Neurocytol. 1989;18:311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- Nowicki JL, Burke AC. Hox genes and morphological identity: axial versus lateral patterning in the vertebrate mesoderm. Development. 2000;127:4265–4275. doi: 10.1242/dev.127.19.4265. [DOI] [PubMed] [Google Scholar]
- Ogasawara K, Nogami H, Tsuda MC, Gustafsson JA, Korach KS, Ogawa S, Harigaya T, Hisano S. Hormonal regulation of prolactin cell development in the fetal pituitary gland of the mouse. Endocrinology. 2009;150:1061–1068. doi: 10.1210/en.2008-1151. [DOI] [PubMed] [Google Scholar]
- Potok MA, Cha KB, Hunt A, Brinkmeier ML, Leitges M, Kispert A, Camper SA. WNT signaling affects gene expression in the ventral diencephalon and pituitary gland growth. Dev Dyn. 2008;237:1006–1020. doi: 10.1002/dvdy.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulichino A-M, Vallette-Kasic S, Tsai JP-Y, Couture C, Gauthier Y, Drouin J. TPIT determines alternate fates during pituitary cell differentiation. Genes & Development. 2003 doi: 10.1101/gad.1065703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetzman LT, Cai JX, Camper SA. Hes1 is required for pituitary growth and melanotrope specification. Dev Biol. 2007;304:455–466. doi: 10.1016/j.ydbio.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetzman LT, Ross SA, Cook S, Dunwoodie SL, Camper SA, Thomas PQ. Developmental regulation of Notch signaling genes in the embryonic pituitary: Prop1 deficiency affects Notch2 expression. Dev Biol. 2004;265:329–340. doi: 10.1016/j.ydbio.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Rizzoti K, Brunelli S, Carmignac D, Thomas PQ, Robinson IC, Lovell-Badge R. SOX3 is required during the formation of the hypothalamo-pituitary axis. Nat Genet. 2004;36:247–255. doi: 10.1038/ng1309. [DOI] [PubMed] [Google Scholar]
- Senovilla L, Nunez L, Villalobos C, Garcia-Sancho J. Rapid changes in anterior pituitary cell phenotypes in male and female mice after acute cold stress. Endocrinology. 2008;149:2159–2167. doi: 10.1210/en.2007-1030. [DOI] [PubMed] [Google Scholar]
- Seuntjens E, Denef C. Progenitor cells in the embryonic anterior pituitary abruptly and concurrently depress mitotic rate before progressing to terminal differentiation. Mol Cell Endocrinol. 1999;150:57–63. doi: 10.1016/s0303-7207(99)00028-3. [DOI] [PubMed] [Google Scholar]
- Shibusawa N, Yamada M, Hirato J, Monden T, Satoh T, Mori M. Requirement of thyrotropin-releasing hormone for the postnatal functions of pituitary thyrotrophs: ontogeny study of congenital tertiary hypothyroidism in mice. Mol Endocrinol. 2000;14:137–146. doi: 10.1210/mend.14.1.0404. [DOI] [PubMed] [Google Scholar]
- Slabaugh MB, Lieberman ME, Rutledge JJ, Gorski J. Ontogeny of growth hormone and prolactin gene expression in mice. Endocrinology. 1982;110:1489–1497. doi: 10.1210/endo-110-5-1489. [DOI] [PubMed] [Google Scholar]
- Soji T, Mabuchi Y, Kurono C, Herbert DC. Folliculo-stellate cells and intercellular communication within the rat anterior pituitary gland. Microsc Res Tech. 1997;39:138–149. doi: 10.1002/(SICI)1097-0029(19971015)39:2<138::AID-JEMT5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Szabo K, Csanyi K. The vascular architecture of the developing pituitary-median eminence in the rat. Cell Tissue Res. 1982;224:563–577. doi: 10.1007/BF00213753. [DOI] [PubMed] [Google Scholar]
- Takuma N, Sheng HZ, Furuta Y, Ward JM, Sharma K, Hogan BLM, Pfaff SL, Westphal H, Kimura S, Mahon KA. Formation of Rathke’s pouch requires dual induction from the diencephalon. Development. 1998;125:4835–4840. doi: 10.1242/dev.125.23.4835. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Yasutaka S, Kominami R, Shinohara H. Proliferation and differentiation of pituitary somatotrophs and mammotrophs during late fetal and postnatal periods. Anat Embryol (Berl) 2001a;204:469–475. doi: 10.1007/s429-001-8003-x. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Yasutaka S, Kominami R, Shinohara H. Proliferation and differentiation of thyrotrophs in the pars distalis of the rat pituitary gland during the fetal and postnatal period. Anat Embryol (Berl) 2001b;203:249–253. doi: 10.1007/s004290100161. [DOI] [PubMed] [Google Scholar]
- Treier M, Gleiberman AS, O’Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. Multistep signaling requirements for pituitary organogenesis in vivo. Genes & Development. 1998;12:1691–1704. doi: 10.1101/gad.12.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J, Thomas P. Genetic determinants of mammalian pituitary morphogenesis. Front Biosci. 2007;12:125–134. doi: 10.2741/2053. [DOI] [PubMed] [Google Scholar]
- Ward RD, Raetzman LT, Suh H, Stone BM, Nasonkin IO, Camper SA. Role of PROP1 in pituitary gland growth. Mol Endocrinol. 2005;19:698–710. doi: 10.1210/me.2004-0341. [DOI] [PubMed] [Google Scholar]
- Ward RD, Stone BM, Raetzman LT, Camper SA. Cell proliferation and vascularization in mouse models of pituitary hormone deficiency. Mol Endocrinol. 2006;20:1378–1390. doi: 10.1210/me.2005-0409. [DOI] [PubMed] [Google Scholar]
- Wen S, Ai W, Alim Z, Boehm U. Embryonic gonadotropin-releasing hormone signaling is necessary for maturation of the male reproductive axis. Proc Natl Acad Sci U S A. 2010;107:16372–16377. doi: 10.1073/pnas.1000423107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki M, Miyake A, Tagashira S, Itoh N. Structure and expression of the rat mRNA encoding a novel member of the fibroblast growth factor family. J Biol Chem. 1996;271:15918–15921. doi: 10.1074/jbc.271.27.15918. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Mailloux CM, Hermesz E, Palkovits M, Westphal H. A role of the LIM-homeobox gene Lhx2 in the regulation of pituitary development. Dev Biol. 2009 doi: 10.1016/j.ydbio.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.