Abstract
Background
The US diet is high in salt, with the majority coming from processed foods. Reducing dietary salt is an important potential public health target.
Methods
We used the Coronary Heart Disease (CHD) Policy Model to quantify the benefits of potentially achievable population-wide reductions in dietary salt of up to 3 gm/day (1200 mg/day of sodium). We estimated cardiovascular disease rates and costs in age, sex, and race subgroups, compared salt reduction with other interventions to reduce cardiovascular risk, and determined the cost-effectiveness of salt reduction compared with drug treatment of hypertension.
Results
Reducing salt by 3 gm/day is projected to result in 60,000–120,000 fewer new CHD cases, 32,000–66,000 fewer new strokes, 54,000–99,000 fewer myocardial infarctions, and 44,000–92,000 fewer deaths from any cause annually. All segments of the population would benefit, with blacks benefiting proportionately more, women benefiting particularly from stroke reduction, older adults from reductions in CHD events, and younger adults from lower mortality rates. The cardiovascular benefits from lower salt are on par with benefits from reducing tobacco, obesity, or cholesterol. A regulatory intervention designed to achieve 3 gm/day salt reduction would save 194,000–392,000 quality-adjusted life-years and $10–24 billion in healthcare costs annually. Such an intervention would be cost-saving even if only a modest 1 gm/day reduction were achieved gradually over the decade from 2010–2019 and would be more cost-effective than treating all hypertensive individuals with medications.
Conclusions
Modest reduction in dietary salt could substantially reduce cardiovascular events and medical costs and should be a public health target.
Introduction
The US diet is high in salt. The Departments of Agriculture and Health and Human Services recommend daily intake of less than 6 grams of salt (2300 mg of sodium), with a lower target of 3.7 gm/day of salt for most adults (persons over age 40, blacks, and persons with hypertension.1 Despite these guidelines, in 2005-6 the average adult man in the US is estimated to have consumed 10.4 gm/day and the average woman 7.3 gm/day, amounts higher than preceding years.2
Reducing dietary salt lowers blood pressure and cardiovascular risk.3, 4 Lowering salt intake is challenging, in part because 75–80% of the salt in the US diet comes from processed foods, not from salt added during food preparation or consumption.5, 6 Many countries, including Japan, the United Kingdom, Finland, and Portugal, have reduced population-wide salt intake through a combination of regulations on the salt content in processed foods, labeling of processed and prepared foods, public education, and engagement with the food industry.7 To explore the potential impact of a modest reduction in dietary salt on population health, we used the Coronary Heart Disease (CHD) Policy Model, a computer simulation of heart disease in US adults ages 35–84, and an extension that assesses stroke. We estimated the effects in different segments of the US population, compared these projections to the health benefits expected from a range of other public health and clinical interventions aimed at reducing cardiovascular disease, and analyzed the relative cost-effectiveness of salt reduction compared with treatment of hypertensive individuals with medications.
Methods
Structure of the Model
The CHD Policy Model (see Appendix) is a computer-simulation, state-transition (Markov cohort) model of CHD incidence, prevalence, mortality, and costs in the US population over age 35 that has been used to describe CHD trends, and the effects of interventions to treat CHD risk factors.8, 9The Model has three sub-models: the demographic-epidemiologic sub-model predicts CHD incidence and non-CHD mortality among the population without CHD, stratified in these simulations by age, sex, and six risk factors; systolic blood pressure (SBP), use of anti-hypertensive medications, smoking, high density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and diabetes mellitus. After CHD develops, the bridge sub-model characterizes the initial CHD event and its sequelae for 30 days. Then, the disease-history sub-model predicts subsequent CHD events and CHD and non-CHD mortality among simulated subjects with CHD, stratified by age, sex, and history of events. Model inputs are derived from national datasets and calibrated to national event rate estimates.
In addition to the standard Model of the entire US population, we also created race-specific versions of the Model for the black and non-black population in the US. We derived race-specific distribution of risk factors for CHD from NHANES. The same Framingham-derived beta coefficients were used for all three versions of the Model, but the average incidence rate (alpha or intercept) was specific for each population.10 The average incidence rates for the black and non-black sub-populations from the Model were validated with national data.11 In sensitivity analyses we also examined black-specific beta coefficients. We did not assign a coefficient to use of anti-hypertensive medications; rather we used the SBP value or use of anti-hypertensive medications to define the hypertensive population that might be more responsive to a salt reduction. Finally, we extended the Model to estimate incident stroke using beta coefficients derived from Framingham and published rates of incident stroke.12, 13
Modeling approach and underlying assumptions
We modeled the linear effect of reducing daily salt intake by 0–3 grams/day14 using a lower estimate for the effect of salt reduction on SBP based on a large meta analysis3, 15 and a higher estimate based on clinical trial data.16, 17 We modeled an accentuated response to salt reduction among blacks, persons with hypertension, and persons 65 years or older (Table 1).16, 18–21 We compared reductions in events for salt restriction with other interventions aimed at reducing cardiovascular risk by modeling a 50% reduction in smoking and environmental tobacco exposure,22 a 5% reduction in body mass index among obese adults,8 treatment of low and intermediate risk individuals with statins in accordance with the guidelines outlined in the Adult Treatment Panel III,9 and treatment of hypertension as described in the ALLHAT trial.23, 24
Table 1.
Change in systolic blood pressure associated with reductions in dietary salt, by sub-population.
|
Degree of reduction in dietary salt and corresponding change in systolic blood pressure (SBP in mmHg) |
Reference | |||||
|---|---|---|---|---|---|---|
| 1 gm/day salt reduction | 3 gm/day salt reduction | |||||
| Low SBP estimate |
High SBP estimate |
Low SBP estimate |
High SBP estimate |
|||
| Entire US population and non-black population sub-group | ||||||
| Hypertensive individuals* | 1.20 | 1.87 | 3.60 | 5.61 | 3, 14 | |
| Age ≥65 years | 1.20 | 1.87 | 3.60 | 5.61 | 16, 18, 19 20, 21 | |
| All others | 0.60 | 1.17 | 1.80 | 3.51 | 3, 14 | |
| Black population sub-group | ||||||
| Hypertensive individuals* | 1.80 | 3.03 | 5.40 | 9.10 | 3, 16, 18, 19, 21 | |
| Age ≥65 years | 1.20 | 1.87 | 3.60 | 5.61 | 16, 18, 19 | |
| All others | 1.20 | 1.87 | 3.60 | 5.61 | 16, 18, 19, 21 | |
based on systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or use of antihypertensive medications
We conducted simulations in the entire US population and among black and non-black subgroups and estimated annual reductions in incident CHD, total MI, incident stroke, and death from any cause as a result of reductions in dietary salt for the entire population and separately by age, sex and race. We projected healthcare costs saved and quality-adjusted life years (QALY) gained annually, overall and in the Medicare population, from a population-wide intervention to reduce salt, using the World Health Organization estimates for the cost of such a national effort of $1 per person annually,25 and from treatment of hypertension with antihypertensive medications.24 We also reported cumulative costs and effectiveness over the decade from 2010–2019 if the effects of an intervention were phased in gradually over time.
Sensitivity Analyses
We used Monte Carlo simulations to estimate the uncertainty of our projections for both the high and low estimates for the effects of salt reduction on SBP. Beta coefficients for the association of SBP, LDL and HDL cholesterol, and diabetes with both CHD events and deaths not associated with CHD were assumed to have a normal probability distribution, with standard errors derived from the fitted regression. We generated covariance matrices for each of these beta coefficients. On the basis of evidence for minimal correlation between factors, we assumed effects to be independent. For each simulation, we report the mean (±SE) for 1000 simulations. We conducted sensitivity analyses varying the impact of salt reduction on changes in cardiovascular risk based on estimates suggesting that treating blood pressure through salt reduction or medication use does not lower cardiovascular risk to the same level as native blood pressure.26
Results
A population-wide reduction in dietary salt of 3 gm/day (1200 mg/day of sodium) is projected to result in 60,000–120,000 fewer new cases of CHD, 54,000–99,000 fewer new and recurrent MIs, 32,000–66,000 fewer new strokes, and 44,000–92,000 fewer deaths from any cause annually compared with current levels of salt consumption. Since the relationship between reductions in salt and the projected reductions in event rates is linear over the range examined, even a more modest 1 gm/day reduction is projected to result in large reductions in annual cardiovascular events and deaths (20,000–40,000 fewer new cases of CHD, 18,000–35,000 fewer new and recurrent MIs, 11,000–23,000 fewer new strokes, and 15,000–32,000 fewer deaths from any cause).
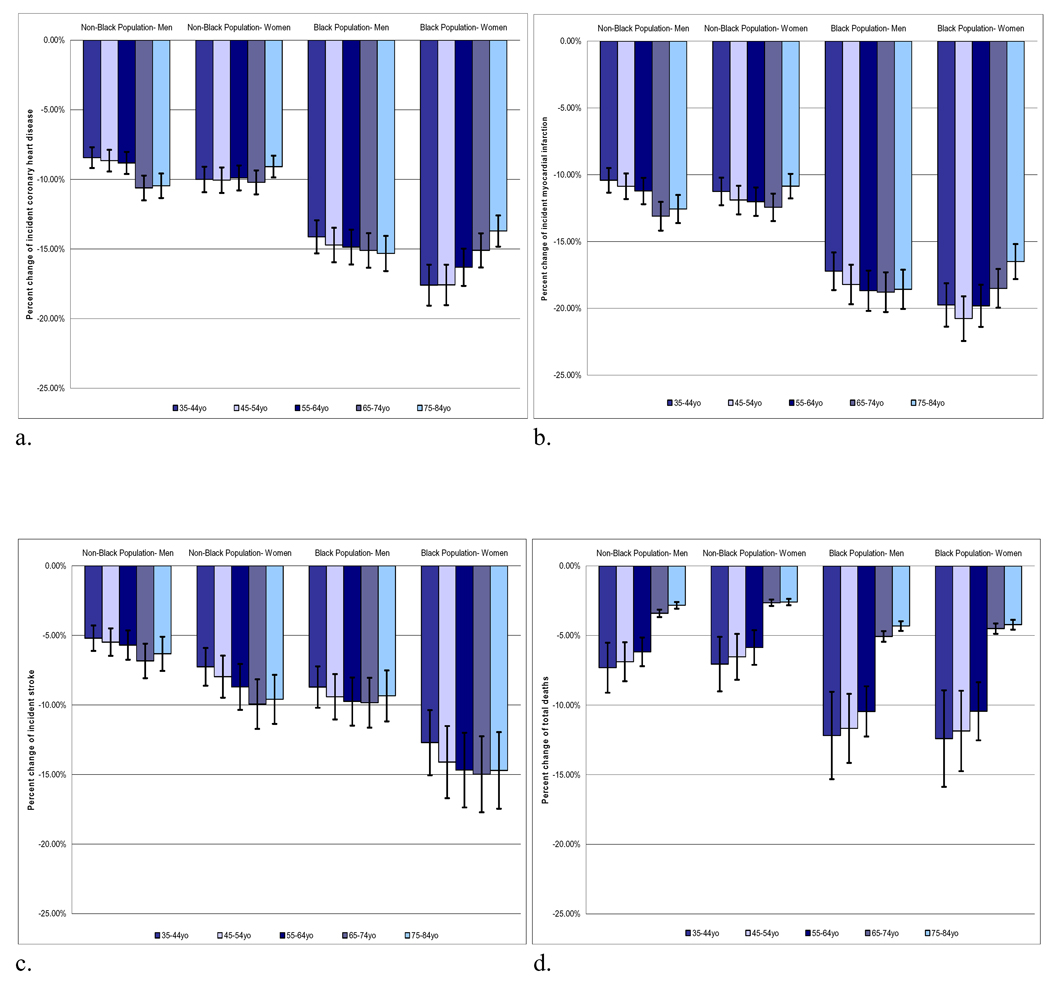
All adult age groups, both sexes, and blacks and non-blacks would all be expected to benefit from reductions in salt intake (Figure). The anticipated relative benefits in blacks were greater than those for non-blacks across all age and sex groups. Women were projected to have greater reductions in stroke than men, with rates decreasing by 9–15% among black women and by 5–9% among non-black women. All age groups would be expected to benefit, with middle-aged and older populations expected to experience large relative reductions in incident CHD and new and recurrent MI and stroke. Young and middle aged adults were projected to experience a large relative reduction in morality, with 7–11% lower mortality rates for blacks between 35–64 years and 3–6% lower mortality rates for non-blacks in this age range.
Figure.
Percent change in cardiovascular events with 3 gm/day reduction in dietary salt by US sub-populations. a) incident coronary heart disease, b) new and recurrent myocardial infarctions, c) incident stroke, d) death from any cause.
Sensitivity analyses
If a lower blood pressure level reached as a result of reduced salt intake is not as advantageous as the same native blood pressure level, the health benefit of salt reduction would be smaller (Table 2). If individuals 65 years of age or older have the same degree of salt sensitivity as those under 65 years of age, the estimated benefits of salt reductions are minimally changed. If blacks have no greater salt sensitivity than non-black populations, the magnitude of the anticipated effects on blacks would be reduced, but blacks would still have greater reductions in cardiovascular events and deaths because of their higher prevalence of hypertension.
Table 2.
Annual rate differences in cardiovascular rate reductions for 3 gm/day reduction in dietary salt by assumptions of differential salt sensitivity by age and race*
| Change in rate per 10,000 (SD)† (percent change) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Incident CHD | Total MI | Incident Stroke | All-Cause Mortality | |||||
| Low | High | Low | High | Low | High | Low | High | |
| Main Simulation | ||||||||
| US Population | −4.7 (0.4) −6.1 % |
−8.3 (0.8) −10.7% |
−3.7 (0.3) −7.7% |
−6.2 (0.6) −12.8% |
−2.4 (0.3) −5.2% |
−3.9 (0.5) −8.2% |
−3.3 (0.5) −2.7% |
−5.4 (0.8) −4.4% |
| Non-black population sub-group | −4.3 (0.4) −6.1% |
−7.0 (0.6) −9.8% |
−3.4 (0.3) −7.7% |
−5.3 (0.4) −12.0% |
−2.2 (0.3) −5.1% |
−3.4 (0.5) −8.0% |
−3.1 (0.4) −2.7% |
−4.9 (0.7) −4.3% |
| Black population sub-group | −7.9 (0.7) −9.8% |
−12.6 (1.0) −15.8% |
−5.8 (0.5) −11.8% |
−9.3 (0.7) −18.7% |
−4.2 (0.5) −7.7% |
−6.7 (0.9) −12.4% |
−5.3 (0.7) −4.4% |
−8.5 (1.2) −7.0% |
| Sensitivity analysis | ||||||||
| Diminished cardiovascular risk reversal with blood pressure lowering‡ | ||||||||
| US Population | −2.8 (0.3) −4.1% |
−4.6 (0.4) −6.6% |
−2.5 (0.2) −5.2% |
−4.0 (0.3) −8.3% |
−1.6 (0.2) −3.5% |
−2.6 (0.3) −5.5% |
−2.3 (0.3) −1.8% |
−3.6 (0.5) −2.9% |
| No increased salt sensitivity due to age | ||||||||
| US Population | −4.5 (0.4) −5.9% |
−7.3 (0.6) −9.6% |
−3.5 (0.3) −7.2% |
−5.6 (0.5) −11.5% |
−2.2 (0.3) −4.7% |
−3.6 (0.5) −7.6% |
−3.2 (0.4) −2.5% |
−5.2 (0.7) −4.1% |
| No increased salt sensitivity due to race | ||||||||
| Black population sub-group (no increase by race) | −5.2 (0.5) −6.5% |
−8.2 (0.7) −10.3% |
−4.0 (0.3) −8.0% |
−6.1 (0.5) −12.4% |
−2.8 (0.4) −5.2% |
−4.4 (0.6) −8.1% |
−3.5 (0.5) −2.9% |
−5.5 (0.8) −4.6% |
| Black-specific beta coefficients based on the ARIC Study§ | ||||||||
| Black population sub-group (alternate betas) | −6.4 (2.8) −8.0% |
−10.0 (4.2) −12.5% |
−4.6 (2.0) −9.0% |
−7.0 (2.9) −13.9% |
−4.2 (0.6) −7.7% |
−6.8 (0.9) −12.5% |
−4.7 (1.2) −3.8% |
−7.4 (1.8) −6.1% |
Results for the 1 gm/day reduction are in the online appendix
Rates are age-adjusted to the US population
Uses black-specific beta coefficients for all of the CHD risk factors based on a published analysis from the Atherosclerosis Risk In Communities (ARIC) study42.
Comparison with other interventions to prevent cardiovascular disease
Even modest population reductions in dietary salt would be expected to provide comparable reductions in cardiovascular events as are projected from public health interventions targeting tobacco, obesity, primary prevention with statins, and drug treatment of hypertension based on simulations for the same time frame and underlying population (Table 3). For example, achieving a 3 gm/day reduction in dietary salt would have approximately the same impact on CHD events as a 50% reduction in tobacco use, a 5% reduction in body mass index among obese adults, or treatment of low and intermediate risk individuals with statins. Salt reduction would have a far greater benefit on stroke prevention than these comparison interventions. The 3 gm/day salt reduction has about the same projected mortality benefit compared with the medical treatment of all hypertensive individuals.
Table 3.
Comparative impact of various population interventions on annual reductions in cardiovascular events
| Change in absolute number of events (SD) and percent change from expected | ||||
|---|---|---|---|---|
| Incident CHD | Total myocardial infarction |
Incident Stroke | All-cause mortality | |
| Salt reduction | ||||
| 1 gm/day - Low | −22,000 (2,000) −2.0% |
−20,000 (1,800) −2.6% |
−13,000 (1,800) −1.7% |
−17,000 (2,400) −0.9% |
| High | −37,000 (3,300) −3.3% |
−32,000 (2,900) −4.2% |
−20,000 (2,900) −2.7% |
−28,000 (3,800) −1.4% |
| 2 gm/day – Low | −44,000 (4,000) −4.0% |
−39,000 (3,500) −5.1% |
−25,000 (3,500) −3.4% |
−34,000 (4,600) −1.7% |
| High | −71,500 (6,300) −6.4 % |
−62,500 (5,400) −8.1% |
−40,000 (5,400) −5.3% |
−55,000 (7,500) −2.8% |
| 3 gm/day - Low | −66,000 (5,800) −5.9% |
−58,000 (5,100) −7.6% |
−37,000 (5,100) −5.0% |
−51,000 (7,100) −2.6% |
| High | −110,000 (9,200) −9.6% |
−92,000 (7,800) −12.0% |
−59,000 (8,100) −7.8% |
−81,000 (11,000) −4.1% |
| Smoking cessation* | −41,000 (10,000) −3.7% |
−92,000 (14,000) −11.9% |
−32,000 (13,000) −4.4% |
−84,000 (9,300) −4.3% |
| Weight loss† | −59,000 (3,500) −5.3% |
−61,000 (3,200) −8.0% |
−5,600 (600) −0.7% |
−36,000 (2,000) −2.0% |
| Cholesterol treatment for primary prevention ‡ | −52,000 (5,600) −5.3% |
−17,000 (1,800) −2.9% |
−6,600 (200) −0.9% |
−5.400 (540) −0.3% |
| Blood pressure treatment with medications among hypertensive individuals§ | −100,000 (11,000) −9.3% |
−100,000 (9,700) −13.1% |
−69,000 (11,000) −9.3% |
−80,000 (10,000) −4.1% |
Elimination of 50% tobacco use/exposure
5 percent reduction in BMI among obese adults
Full adherence to ATPIII guidelines in people with 10 Yr CHD risk <20%
Systolic blood pressure reduction in hypertensive individuals based on ALLHAT
Cost-Effectiveness
A national effort to decrease salt consumption by 3 gm/day would result in an estimated annual gain of 194,000–392,000 quality-adjusted life-years (QALYs) and $10–24 billion in saved healthcare costs. Even if salt targets were achieved gradually over the decade (Table 4), decreases in salt consumption to the target reduction of only a more modest 1 gm/day were projected to be cost-savings. Salt reduction strategies are projected to compare favorably to the treatment of all hypertensive individuals with antihypertensive medications -- a strategy that would result in more QALYs gained, but at a cost of $6,000–26,000 per QALY gained. Even if the federal government bore the entire cost of a regulatory program to reduce salt consumption, the federal government would also be expected to realize reduced healthcare costs within Medicare, saving $6–12 in healthcare expenditures for each dollar spent on the regulation of salt. Of note is that antihypertensive medications retain a cost-effective benefit when added to a successful population-wide reduction of salt intake, but the number of individuals requiring treatment with medications would be markedly reduced; a 3 gm/day reduction in salt would result in 16–24% fewer women and 22–34% fewer men with hypertension and an additional savings of $3–6 billion annually in hypertension treatment avoided.
Table 4.
Cost and effectiveness of salt reduction and hypertension treatment annually and cumulatively from 2010–2019
| Costs and QALYs for the US population (SD) | Costs and QALYs for the MediCare population(SD) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost of intervention (billions) |
Change in healthcare cost (billions)1 |
Change in QALYs (thousands) |
Cost per QALY |
Healthcare cost saved per dollar spent on intervention |
Cost of intervention (billions) |
Change in healthcare cost (billions) |
Change in QALYs (thousands) |
Cost per QALY |
Healthcare cost saved per dollar spent on intervention |
|
| Population reduction in dietary salt | ||||||||||
| 1gm/day | ||||||||||
| Low | $0.32 | −$4.1 (0.8) | 74.9 (8.8) | cost savings | $15.4 (3.0) | $0.3 | −$0.7 (0.06) | 46 (4.1) | cost savings | $2.5 (0.2) |
| High | $0.32 | −$7.0 (1.4) | 120 (14.6) | cost savings | $26.1 (5.2) | $0.3 | −$1.0 (0.1) | 72 (6.4) | cost savings | $3.8 (0.4) |
| 3gms/day | ||||||||||
| Low | $0.32 | −$12.1 (2.4) | 220 (26) | cost savings | $45.2 (9.1) | $0.3 | −$2.0 (0.2) | 135.0 (12.2) | cost savings | $7.3 (0.7) |
| High | $0.32 | −$20.4 (4.1) | 350 (42) | cost savings | $76.0 (15.4) | $0.3 | −$3.0 (0.3) | 208.3 (18.6) | cost savings | $11.1 (1.1) |
| Blood pressure treatment with medications among hypertensive individuals 3 | ||||||||||
| $19.5 (0.07) |
−$14.2 (2.7) |
360 (42) | $15,800 (9,900) |
$0.7 (0.1) | $9.3 (0.03) |
−$3.4 (0.3) |
260 (24) |
$23,300 (3,600) |
$0.4 (0.04) | |
| Cumulative cost and effectiveness of gradually reducing dietary salt over the decade from 2010–20194 | ||||||||||
| 1gm/day | ||||||||||
| Low | $2.72 | −$18.9 (3.8) | 220 (27) | cost savings | $7.0 (1.4) | $2.7 | −$4.3 (0.4) | 220 (20) | cost savings | $1.6 (0.2) |
| High | $2.72 | −$31.6 (6.5) | 350 (43) | cost savings | $11.8 (2.4) | $2.7 | −$6.1 (0.6) | 240 (21) | cost savings | $2.3 (0.2) |
| 3gms/day | ||||||||||
| Low | $2.72 | −$56.9 (11.5) |
650 (78) | cost savings | $21.2 (4.3) | $2.7 | −$12.1 (1.2) |
420 (37) | cost savings | $4.5 (0.5) |
| High | $2.72 | −$95.6 (19.6) |
1,000 (127) | cost savings | $35.6 (7.3) | $2.7 | −$18. (1.9) |
665 (58) | cost savings | $6.9 (0.7) |
Healthcare costs for the US adults population age 35 years and older, discounted at 3% over the decade
$1 per person estimate per year for the cost of a population-wide regulatory approach to salt reduction based on the World Health Organization estimate, US population of 306,913,687 as of July 2009. http://www.census/gov, discounted at 3% over the decade
Treatment of all hypertensive individuals to the degree observed in clinical trials25.and cost effectiveness analysis24
Gradual reduction from 2010–2019, with one third of the total reduction achieved in 2012, a second 1/3 in 2015, and a third 1/3 in 2019
Discussion
Despite evidence linking salt intake to hypertension and cardiovascular disease, salt intake in the US diet is actually on the rise. These worsening trends have led to calls for population-wide interventions to reduce salt in the US diet,27 as have already been adopted in other countries.7 Our findings provide evidence to support these calls. Our postulated 3 gm/day reduction in dietary – a reduction in the range targeted by other developed countries -is projected to benefit the entire US population and yield substantial reductions in morbidity, mortality, and costs. The population-wide benefits from salt reduction are similar in magnitude to the health benefits that would accrue from other public health and clinical interventions and would be cost-savings, even if only a more modest 1 gm/day reduction is gradually achieved over time. Changes in behavior are notoriously difficult to achieve, and individual approaches to achieving lower dietary salt have largely proven ineffective. Nevertheless, cholesterol levels fell in the US prior to the widespread use of medications, and smoking rates have fallen substantially through a combination of regulatory, public health, and individual approaches to smoking cessation. The large and growing burden of hypertension despite improved medical therapies28 and the potential for lower dietary salt to aid in the prevention and treatment of hypertension reinforce the urgent need for this approach.
Considerable literature links higher salt intake with higher blood pressure and increased cardiovascular risk,15, 29 and randomized trials have demonstrated that a lower salt diet lowers blood pressure16, 30 and cardiovascular risk.31 Despite concerns about the accurate assessment of salt intake, adherence with low-salt interventions, and theoretical increased risks of very low salt diets, several large meta-analyses and reports from the Institute of Medicine3, 5, 15, 26, 32 concluded that reducing dietary salt would lower blood pressure and cardiovascular risk. Professional societies including the American Medical Association, the American Heart Association, the American Society of Hypertension, and the World Health Organization have all endorsed population-wide efforts to reduce salt intake.
Our results are similar to other analyses33, 34 and extend them in important ways. We incorporated updated prevalence distributions of cardiovascular risk factors, particularly hypertension, in the entire US population and in black and non-black subpopulations. We considered current levels of hypertension treatment, treatment and control of other cardiovascular risk factors, and competing and ongoing risks among persons in whom deaths were averted. Our comparisons of the cardiovascular benefits of salt reduction were similar to those anticipated for established public-health targets such as tobacco, obesity, and LDL cholesterol. Targeted interventions have very large per-person effects, but their benefits are restricted to the smaller numbers of higher-risk, affected individuals. Lowering salt in the US diet would result in small but measurable blood pressure reductions across the entire US population, thereby reducing cardiovascular disease in all adults at risk.
A national regulatory effort to lower dietary salt intake would be cost saving even if only modest salt reduction were achieved after a decade-long period. If the population-wide approach to lowering salt were a federal effort, the healthcare savings to the current major federally sponsored healthcare program – Medicare- would be greater than the cost of the regulatory intervention itself, even without incremental benefits afforded to younger, non-Medicare-covered persons. Some costs, such as those borne by the food industry in reformulating processed foods, are not considered in these analyses. However, as salt intake is reduced, individuals appear to prefer food with less salt,15 likely related to accommodation of taste receptors - a process that occurs over weeks to months.35 In the UK a 10% population-reduction in salt was achieved over 4 years36 without reduction in sales of the products included in the initial voluntary effort and without consumer complaints about taste. The magnitude of the health benefit suggests that salt should be a regulatory target of the Food and Drug Administration, which currently designates salt as a food additive that is “generally regarded as safe.”27
We projected that certain sub-populations may experience a proportionately greater benefit from similar levels of salt reduction. Blacks have high rates of hypertension and cardiovascular diseases that contribute to racial disparities in mortality;37 their benefits from salt reduction could potentially narrow these disparities. Women would also experience a proportionately greater benefit because of their higher risk for stroke.11 Young and middle-aged adults could benefit because of the relative importance of blood pressure elevations in younger adults without major risk factors. Blood pressure elevations in young adulthood accelerate atherosclerosis9 and morbidity by middle age,38 yet younger adults with hypertension are less likely to be on treatment or have their blood pressure controlled.39 The benefits of salt reduction could be even greater than we projected because hypertension may be completely prevented or its onset delayed by lowering salt intake even earlier during childhood and adolescence.40
Projections such as ours are limited by uncertainty in the modeling inputs. We modeled the effects of salt reduction on blood pressure based on published data and assumed that the health benefits of salt reduction were mediated through these blood pressure reductions. We did not account fully for possible effects of salt reductions unrelated to blood pressure, such as potential improvements in outcomes of the increasing numbers of patients with heart failure or prevention of other highly morbid conditions such as end-stage renal disease. Our estimates of differential effects of salt reduction by age and race were extrapolated from clinical trial data, and there is more uncertainty about these effects on the total population; however, sensitivity analyses suggest that our primary findings are not very dependent on variations in these assumptions. We modeled only linear effects of salt reduction on reductions in blood pressure. Others have suggested that these effects may be non-linear,16 with greater reductions in blood pressure at lower salt intake; such an assumption would result in larger reductions in cardiovascular disease than we present here.
Even with these limitations, our simulations suggest that modest reductions in dietary salt would yield substantial health benefits across the adult US population by lowering cardiovascular event rates, deaths, and medical costs. Our findings support the urgent need for action to achieve these readily attainable benefits to the cardiovascular health of the nation.
Supplementary Material
Acknowledgments
The Framingham Heart Study (FHS) and Framingham Offspring Study (FOS) are conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the FHS and FOS Investigators. This manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the FHS, the FOS or the NHLBI.
We gratefully acknowledge the work of Ms. Tekeshe Mekonnen, MS, who was invaluable in the preparation of the text and figures for this manuscript, and David Fairley, PhD, who developed the Monte Carlo simulation routine used in this work. This work was supported in part by a Grant-in-Aid from the American Heart Association Western States Affiliate (09GRNT2060096).
Literature cited
- 1.Application of lower sodium intake recommendations to adults--United States, 1999–2006. MMWR Morb Mortal Wkly Rep. 2009;58:281–283. [PubMed] [Google Scholar]
- 2.Service AR, editor. U.S. Department of Agriculture ARS, Beltsville Human Nutrition Research Center, Food Surveys Research Group (Beltsville, MD) and U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics (Hyattsville, MD) What We Eat in America, NHANES 2003–2004 Documentation: Dietary Interview - Individual Foods -- First Day (DR1IFF_C) 2006. [Google Scholar]
- 3.He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD004937. CD004937. [DOI] [PubMed] [Google Scholar]
- 4.Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, D.C: The National Academies Press; 2004. Panel on Dietary Reference Intakes for Electorlytes and Water SCotSEoDRI, Food and Nutrition Board, Institute of Medicine of the National Academies. [Google Scholar]
- 5.Hooper L, Bartlett C, Davey SG, Ebrahim S. Advice to reduce dietary salt for prevention of cardiovascular disease. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD003656.pub2. CD003656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattes RD, Donnelly D. Relative contributions of dietary sodium sources. J Am Coll Nutr. 1991;10:383–393. doi: 10.1080/07315724.1991.10718167. [DOI] [PubMed] [Google Scholar]
- 7.He FJ, Macgregor GA. A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J Hum Hypertens. 2008 doi: 10.1038/jhh.2008.144. [DOI] [PubMed] [Google Scholar]
- 8.Bibbins-Domingo K, Coxson P, Pletcher MJ, Lightwood J, Goldman L. Adolescent overweight and future adult coronary heart disease. N Engl J Med. 2007;357:2371–2379. doi: 10.1056/NEJMsa073166. [DOI] [PubMed] [Google Scholar]
- 9.Pletcher MJ, Lazar L, Bibbins-Domingo K, et al. Comparing impact and cost-effectiveness of primary prevention strategies for lipid-lowering. Ann Intern Med. 2009;150:243–254. doi: 10.7326/0003-4819-150-4-200902170-00005. [DOI] [PubMed] [Google Scholar]
- 10.D'Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart Disease and Stroke Statistics--2009 Update. A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 12.Williams GR, Jiang JG, Matchar DB, Samsa GP. Incidence and occurrence of total (first-ever and recurrent) stroke. Stroke; a journal of cerebral circulation. 1999;30:2523–2528. doi: 10.1161/01.str.30.12.2523. [DOI] [PubMed] [Google Scholar]
- 13.Kissela B, Schneider A, Kleindorfer D, et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke; a journal of cerebral circulation. 2004;35:426–431. doi: 10.1161/01.STR.0000110982.74967.39. [DOI] [PubMed] [Google Scholar]
- 14.He FJ, MacGregor GA. How far should salt intake be reduced? Hypertension. 2003;42:1093–1099. doi: 10.1161/01.HYP.0000102864.05174.E8. [DOI] [PubMed] [Google Scholar]
- 15.He FJ, MacGregor GA. Effect of modest salt reduction on blood pressure: a meta-analysis of randomized trials. Implications for public health. J Hum Hypertens. 2002;16:761–770. doi: 10.1038/sj.jhh.1001459. [DOI] [PubMed] [Google Scholar]
- 16.Sacks FM, Svetkey LP, Vollmer WM, et al. DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 17.MacGregor GA, Markandu ND, Sagnella GA, Singer DR, Cappuccio FP. Double-blind study of three sodium intakes and long-term effects of sodium restriction in essential hypertension. Lancet. 1989;2:1244–1247. doi: 10.1016/s0140-6736(89)91852-7. [DOI] [PubMed] [Google Scholar]
- 18.Bray GA, Vollmer WM, Sacks FM, Obarzanek E, Svetkey LP, Appel LJ. A further subgroup analysis of the effects of the DASH diet and three dietary sodium levels on blood pressure: results of the DASH-Sodium Trial. Am J Cardiol. 2004;94:222–227. doi: 10.1016/j.amjcard.2004.03.070. [DOI] [PubMed] [Google Scholar]
- 19.Vollmer WM, Sacks FM, Ard J, et al. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH-sodium trial. Ann Intern Med. 2001;135:1019–1028. doi: 10.7326/0003-4819-135-12-200112180-00005. [DOI] [PubMed] [Google Scholar]
- 20.Cappuccio FP, Markandu ND, Carney C, Sagnella GA, MacGregor GA. Double-blind randomised trial of modest salt restriction in older people. Lancet. 1997;350:850–854. doi: 10.1016/S0140-6736(97)02264-2. [DOI] [PubMed] [Google Scholar]
- 21.Swift PA, Markandu ND, Sagnella GA, He FJ, MacGregor GA. Modest salt reduction reduces blood pressure and urine protein excretion in black hypertensives: a randomized control trial. Hypertension. 2005;46:308–312. doi: 10.1161/01.HYP.0000172662.12480.7f. [DOI] [PubMed] [Google Scholar]
- 22.Lightwood JM, Coxson PG, Bibbins-Domingo K, Williams LW, Goldman L. Coronary heart disease attributable to passive smoking: CHD Policy Model. Am J Prev Med. 2009;36:13–20. doi: 10.1016/j.amepre.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 24.Heidenreich P, Davis B, Cutler J, et al. Cost-effectiveness of Chlorthalidone, Amlodipine, and Lisinopril as First-step Treatment for Patients with Hypertension: An Analysis of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Journal of General Internal Medicine. 2008;23:509–516. doi: 10.1007/s11606-008-0515-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Organization WH. Paris, France. Geneva: World Health Organization; Reducing salt intake in populations: Report of a WHO forum and technical meeting. 5–7 October 2006. 2007
- 26.Law MR, Frost CD, Wald NJ. By how much does dietary salt reduction lower blood pressure? III--Analysis of data from trials of salt reduction. BMJ. 1991;302:819–824. doi: 10.1136/bmj.302.6780.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Havas S, Dickinson BD, Wilson M. The urgent need to reduce sodium consumption. JAMA. 2007;298:1439–1441. doi: 10.1001/jama.298.12.1439. [DOI] [PubMed] [Google Scholar]
- 28.Chobanian AV. Shattuck Lecture. The hypertension paradox--more uncontrolled disease despite improved therapy. New England Journal of Medicine. 2009;361:878–887. doi: 10.1056/NEJMsa0903829. [DOI] [PubMed] [Google Scholar]
- 29.Stamler J, Rose G, Stamler R, Elliott P, Dyer A, Marmot M. INTERSALT study findings. Public health and medical care implications. Hypertension. 1989;14:570–577. doi: 10.1161/01.hyp.14.5.570. [DOI] [PubMed] [Google Scholar]
- 30.Maruthur NM, Wang NY, Appel LJ. Lifestyle interventions reduce coronary heart disease risk: results from the PREMIER Trial. Circulation. 2009;119:2026–2031. doi: 10.1161/CIRCULATIONAHA.108.809491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook NR, Cutler JA, Obarzanek E, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP) BMJ. 2007;334:885–888. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otten JJHJ, Meyers LD. The dietary reference intakes: The essential guide to nutrient requirements. Washington: Food and Nutrition Board (FNB) and Institute of Medicine (IOM); 2006. [Google Scholar]
- 33.Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000058. e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palar K, Sturm R. Potential societal savings from reduced sodium consumption in the U.S. adult population. Am J Health Promot. 2009;24:49–57. doi: 10.4278/ajhp.080826-QUAN-164. [DOI] [PubMed] [Google Scholar]
- 35.Blais CA, Pangborn RM, Borhani NO, Ferrell MF, Prineas RJ, Laing B. Effect of dietary sodium restriction on taste responses to sodium chloride: a longitudinal study. Am J Clin Nutr. 1986;44:232–243. doi: 10.1093/ajcn/44.2.232. [DOI] [PubMed] [Google Scholar]
- 36. [Accessed August 4, 2008];Dietary sodium levels surveys. 2008 at http://www.food.gov.uk/science/dietarysurveys/urinary.
- 37.Wong MD, Shapiro MF, Boscardin WJ, Ettner SL. Contribution of major diseases to disparities in mortality. N Engl J Med. 2002;347:1585–1592. doi: 10.1056/NEJMsa012979. [DOI] [PubMed] [Google Scholar]
- 38.Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360:1179–1190. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 40.He FJ, MacGregor GA. Importance of salt in determining blood pressure in children: meta-analysis of controlled trials. Hypertension. 2006;48:861–869. doi: 10.1161/01.HYP.0000245672.27270.4a. [DOI] [PubMed] [Google Scholar]
- 41.Asaria P, Chisholm D, Mathers C, Ezzati M, Beaglehole R. Chronic disease prevention: health effects and financial costs of strategies to reduce salt intake and control tobacco use. Lancet. 2007;370:2044–2053. doi: 10.1016/S0140-6736(07)61698-5. [DOI] [PubMed] [Google Scholar]
- 42.Chambless LE, Heiss G, Shahar E, Earp MJ, Toole J. Prediction of Ischemic Stroke Risk in the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2004;160:259–269. doi: 10.1093/aje/kwh189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.