Abstract
Bitter taste receptors (TAS2Rs) of the tongue likely evolved to evoke signals for avoiding ingestion of plant toxins. We found expression of TAS2Rs on human airway smooth muscle (ASM) and considered these to be avoidance receptors for inhalants, leading to ASM contraction and bronchospasm. TAS2R agonists such as saccharin, chloroquine and denatonium evoked increased ASM [Ca2+]i in a Gβγ, PLCβ and IP3-receptor dependent manner which would be expected (like acetylcholine) to evoke contraction. Paradoxically, bitter tastants caused relaxation of isolated ASM, and dilation of airways that was 3-fold greater than β-agonists. Relaxation by TAS2Rs is from a localized [Ca2+]i response at the cell membrane which opens BKCa channels leading to ASM membrane hyperpolarization. Inhaled bitter tastants decreased airway obstruction in an asthma mouse model. Given the need for efficacious bronchodilators for treating obstructive lung diseases, this pathway can be exploited for therapy with the thousands of known synthetic and naturally occurring bitter tastants.
Asthma and chronic obstructive pulmonary disease (COPD) together affect 300 million individuals worldwide. The major source of morbidity and mortality from both diseases is airway obstruction, which often is due to actively constricted smooth muscle of the bronchi1. Although airway resistance in COPD has variable degrees of reversibility due to structural changes from smoking, therapies for COPD and asthma both include antagonists directed to bronchoconstrictive receptors, and agonists directed to receptors that relax airway smooth muscle (ASM)2,3. The major receptor signaling family of ASM that regulates contraction and relaxation are G-protein coupled receptors (GPCRs)3. There is an ongoing effort to identify GPCR pathways leading to regulation of airway tone, thereby providing for new treatment strategies for asthma and COPD. This is particularly relevant since the incidence of both diseases is increasing, and at least one-half of all patients are not well controlled with currently available agents4,5.
Unexpectedly, we recently found expression of several bitter taste receptors in isolated human ASM as part of a pan-GPCR screening effort6. The cognate G-protein for bitter taste receptors, gustducin, is also expressed in human ASM7,8. Receptors for bitter tastes on the tongue are thought to have evolved for avoidance of plant-based toxins9,10. These GPCRs consist of at least 25 receptor subtypes, with each recognizing a repertoire of agonists that usually overlaps with other bitter taste receptors, creating a redundant, broadly-tuned, avoidance and rejection network9,11–13. The finding of bitter taste receptors on ASM led to our original hypothesis that certain bronchospastic disorders, such as occupational asthma14, might be caused by environmental inhalants acting at these airway receptors leading to contraction and bronchoconstriction. This notion was based on the fact that bitter taste receptors couple to increases in [Ca2+]i in specialized taste cells of the tongue, and this signal is also found with known bronchoconstrictive GPCRs such as those for histamine, acetylcholine and bradykinin in ASM cells2. Using various approaches, we found that bitter tastants also increase [Ca2+]i in ASM cells, but unexpectedly found that bitter taste receptor agonists are bronchodilators, with significantly greater efficacy than any known therapeutic agent. These receptors transduce this relaxation response in ASM by a novel mechanism involving receptor generated Gβγ activation of phospholipase C (PLC) and a partitioned [Ca2+]i transient that opens cell surface K+ channels resulting in membrane hyperpolarization.
RESULTS
Human ASM express bitter taste receptors coupled to [Ca2+]i
Initial studies found that several known bitter taste receptor agonists (such as chloroquine, saccharin, and denatonium) evoked increased [Ca2+]i in cultured human ASM cells (Fig. 1a,b,c). The [Ca2+]i responses in ASM cells to these bitter tastants were found to be similar in magnitude to those for known bronchoconstrictive GPCR agonists such as histamine and bradykinin (Fig. 1c). These results prompted quantitative RT-PCR studies with primers for 25 TAS2R genes. Of note, the numerical designations of the TAS2Rs have very recently changed and here we utilize this new nomenclature (see http://www.genenames.org). Multiple TAS2R transcripts were found to be expressed in human ASM, with the TAS2R10, TAS2R14 and TAS2R31 subtypes being the most highly expressed (Table 1). Further screens with additional bitter tastants revealed [Ca2+]i responses to aristocholic acid, strychnine, quinine, colchicine, and yohimbine (Fig. 1c). We found a relatively low response in ASM to colchicine which activates TAS2R4 (a mid-level ASM expressor by RT-PCR) and no response to salicin which exclusively activates TAS2R1610 (which was not detected in ASM by RT-PCR). The robust response to strychnine (activates TAS2R10 and −46) is also consistent with TAS2R10 having high expression in ASM. Thus in ASM, the [Ca2+]i response to bitter tastants is concordant with a rank-order based on agonist specificity and the bitter taste receptor subtype expression in these cells. Immunofluorescence microscopy of human ASM cells using polyclonal antisera directed against four receptors found to have mRNA expressed by RT-PCR (TAS2R10, −14, −31, −19) and three receptors whose mRNAs were not detected (TAS2R7, −38, −43), revealed cell surface expression of the former four receptors but not for the latter three (Supplementary Fig. 1). These studies also revealed expression of the α subunit of gustducin in these cells (Supplementary Fig. 1). No [Ca2+]i response to the sweet receptor agonists sucralose and SC45647 was found, and the saccharin response was not blocked by the sweet receptor antagonist lactisole (Supplementary Fig. 2).
Figure 1.
Bitter tastants of diverse structures evoke increases in [Ca2+]i in human airway smooth muscle cells. Studies were performed with cultured primary ASM cells loaded with Fluo-4 AM. (a, b) [Ca2+]i transients and dose response curves to saccharin and chloroquine from 5−6 experiments. (c) Maximal [Ca2+]i responses to 1.0 mM of the bitter tastants aristocholic acid, chloroquine, colchicine, denatonium, quinine, saccharin, salicin, strychnine and yohimbine, and the bronchoconstrictive Gq-coupled agonists histamine (0.1 mM) and bradykinin (0.01 mM). Results are from 4−6 experiments. *, P < 0.01 vs. basal; #, P < 0.05 vs. denatonium. (d) The [Ca2+]i response to bitter tastant is ablated by the PLC inhibitor U73122 and the βγ antagonist gallein, and attenuated by the IP3 receptor antagonist 2APB. These studies were performed in the absence of extracellular calcium. Results shown are from a single representative experiment of at least three performed.
Table 1.
mRNA Expression of Bitter Taste Receptors in Human ASM Cells
| Receptor | Ratio ADRB2 |
|---|---|
| TAS2R10 | 3.96 ± .893 |
| TAS2R14 | 3.51 ± .397 |
| TAS2R31 | 3.41 ± .498 |
| TAS2R5 | 1.76 ± .190 |
| TAS2R4 | 1.45 ± .271 |
| TAS2R19 | 1.37 ± .249 |
| TAS2R3 | 0.83 ± .079 |
| TAS2R20 | 0.71 ± .202 |
| TAS2R45 | 0.70 ± .118 |
| TAS2R50 | 0.48 ± .033 |
| TAS2R30 | 0.31 ± .060 |
| TAS2R9 | 0.31 ± .034 |
| TAS2R13 | 0.26 ± .037 |
| TAS2R42 | 0.26 ± .009 |
| TAS2R46 | 0.25 ± .041 |
| TAS2R1 | 0.17 ± .027 |
| TAS2R8 | 0.15 ± .007 |
| TAS2R39 | ND |
| TAS2R43 | ND |
| TAS2R7 | ND |
| TAS2R40 | ND |
| TAS2R16 | ND |
| TAS2R38 | ND |
| TAS2R41 | ND |
| TAS2R60 | ND |
| TAS1R1 | ND |
| TAS1R2 | ND |
| ADRB2 | 1.0 (reference) |
| ADORA1 | 2.43 ± .446 |
| LTB4R | 0.29 ± .056 |
Results are normalized to expression of the ADRB2. ADORA1 and LTB4R represent high- and low-expressing GPCRs as positive controls, respectively. ND, not detected. Results are from 4–6 experiments.
Transfection of ASM cells with siRNA directed against TAS2R10 decreased TAS2R10 mRNA by 36 ± 1.8% compared to the scrambled siRNA control, which was accompanied by a 26 ± 2.0% decrease in [Ca2+]i stimulation by the TAS2R10 agonist strychnine (P < 0.05 vs. control). In additional studies, ASM cells were incubated with media alone or polyclonal antisera directed against TAS2R10, TAS2R7 or isotype-specific IgG and then strychnine-promoted [Ca2+]i determined. TAS2R10 antisera decreased strychnine-promoted [Ca2+]i responses in a dose-dependent manner (maximal inhibition of ~77%, Supplementary Fig. 3) consistent with the RT-PCR and immunofluorescence results showing expression of this bitter taste receptor. As a control for nonspecific effects, antisera against TAS2R7 (which is not expressed in ASM) at the same titers had no significant effect on [Ca2+]i stimulation, nor did IgG. Taken together, the above studies confirm expression of bitter taste receptors on ASM cells and link expression to bitter tastant-mediated [Ca2+]i signaling. The increase in [Ca2+]i in human ASM cells elicited by bitter tastants was not dependent on the presence of extracellular Ca2+, and the response was ablated by the Gβγ inhibitor gallein, the PLCβ inhibitor U73122, and partially inhibited by the inositol-3-phosphate (IP3) receptor antagonist 2APB (Fig. 1d). These results in ASM cells are consistent with the signal transduction for bitter taste receptors in specialized taste cells of the tongue, where the Ggust-associated βγ activates PLCβ resulting in IP3 generation which activates the IP3 receptor on the sarco(endo)plasmic reticulum, releasing Ca2+ from this intracellular store9.
Bitter taste receptors evoke airway relaxation
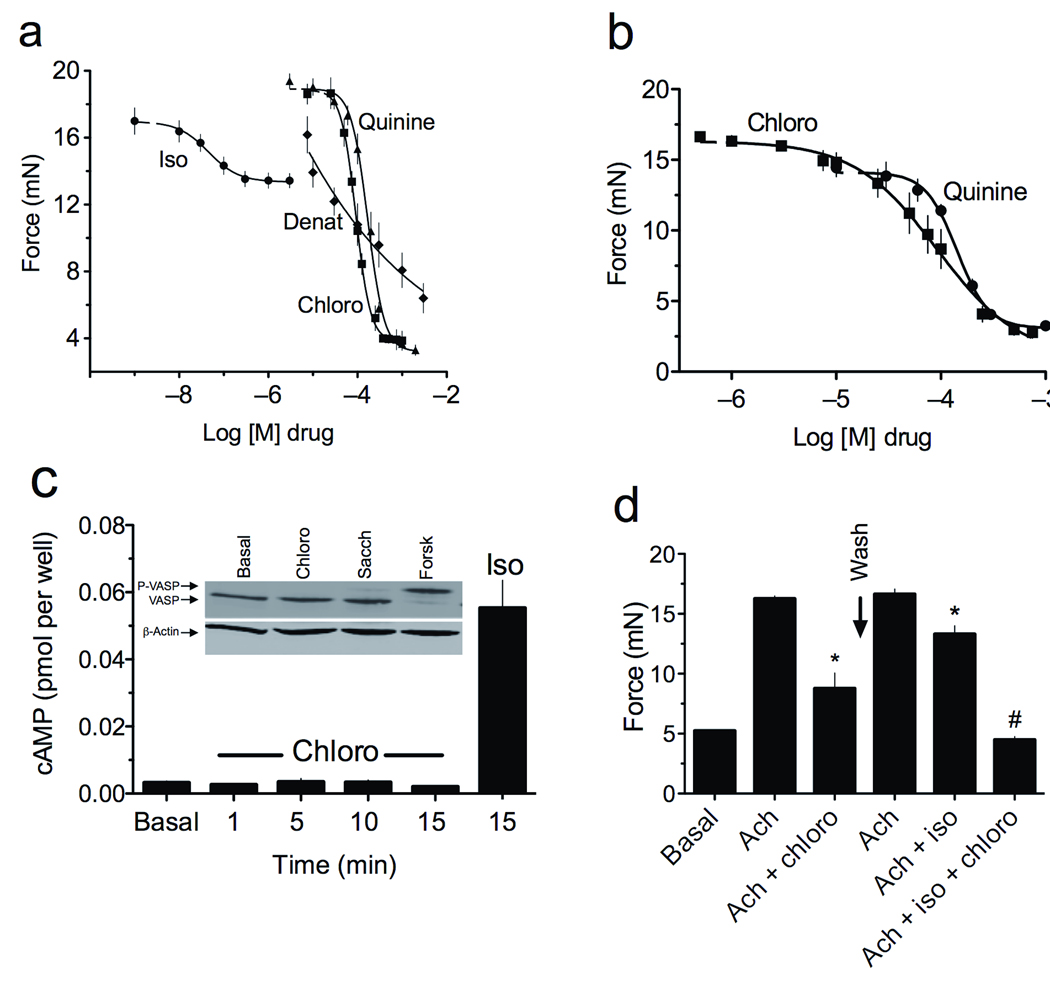
Given that the increase in [Ca2+]i promoted by some of the bitter tastants in human ASM was similar in magnitude to that of ligands acting on bronchoconstrictive GPCRs, we initially assumed that bitter taste receptors evoked ASM contraction. However, in isolated intact mouse airways, chloroquine, denatonium and quinine caused a dose-dependent relaxation, with a maximal response of >90% loss of the active contraction evoked by acetylcholine or serotonin (Fig. 2a,b). The maximal relaxation response to the full β2AR agonist isoproterenol under these same experimental conditions was a 30 ± 9.2% reduction in active tension (Fig. 2a). Bitter tastants were also found to relax baseline tracheal ring tension (Supplementary Fig. 4). The relaxation response to bitter tastants was fully reversible, as washing tracheal rings while maintaining the presence of the contractile agent alone resulted in a return to contraction that was equivalent in magnitude to the control condition (Supplementary Fig. 5). In a limited number of studies in fourth order bronchi obtained from non-diseased portions of human lung tissues, we also observed relaxation from 50−80% from chloroquine or saccharin on acetylcholine contracted rings (Supplementary Fig. 6). Bitter tastant-mediated airway relaxation was not altered by the cyclooxygenase inhibitor indomethacin or the nitric oxide synthase inhibitor L-NAME (data not shown), suggesting a direct activation of ASM receptors rather than a secondary response generated from bronchoactive ligands generated from airway epithelial cells. Airway relaxation observed with β-agonists is due to β2AR coupling to an increase in cAMP with subsequent PKA activation2. However, we found no evidence for chloroquine-promoted increases in cAMP or PKA activation in intact cultured ASM cells, as assessed by a sensitive radioimmunoassay or by the PKA-mediated phosphorylation of vasodilator-stimulated phosphoprotein (VASP), respectively (Fig. 2c). In a set of serial dosing and washout experiments with intact airways and a submaximal dose of chloroquine, we found that exposure to both chloroquine and isoproterenol resulted in relaxation that was greater than that found with either compound alone (Fig. 2d). These data indicate that bitter tastants evoke marked airway relaxation which is reversible, is not due to cell injury since ASM functional contraction and relaxation are not impaired after washout, is non-cAMP dependent, and is additive to that of a β-agonist.
Figure 2.
Bitter tastants evoke bronchodilatation in a non-cAMP dependent manner. (a) Dose-response curves of relaxation for the β-agonist isoproterenol (iso) and the bitter taste receptor agonists chloroquine (chloro), denatonium (denat), and quinine, derived from intact mouse tracheas contracted with 1.0 mM acetylcholine (n = 7 experiments). (b) Chloroquine and quinine relax intact mouse airway tracheas contracted by 1.0 mM serotonin (n = 4 experiments). (c) Cultured human ASM cells were incubated with 1.0 mM chloroquine for the indicated times, or for 15 min with 30 µM isoproterenol, and cAMP measured by radioimmunoassay. There was no evidence for chloroquine-promoted cAMP accumulation (n = 3 experiments). Inset: Cultured human ASM cells were exposed to 1.0 mM chloroquine or saccharin (sacc), or 10 µM forskolin (forsk), and cell extracts were immunoblotted to ascertain PKA-mediated VASP phosphorylation (upper band), a cAMP promoted event. Forskolin, which stimulates cAMP production, resulted in phosphorylation of VASP as indicated by the upper band. Neither chloroquine nor saccharin promoted VASP phosphorylation, consistent with the cAMP measurements. (d) The airway relaxation response to isoproterenol and chloroquine are additive. Intact mouse tracheas were contracted with 1.0 mM acetylcholine (ach) which was maintained in the bath when chloroquine (200 µM) or isoproterenol (30 µM), or both drugs, were added. After chloroquine exposure, the rings were washed and then rechallenged with the same dose of acetylcholine. *, P < 0.05 vs. acetylcholine alone; #, P < 0.01 vs. acetylcholine + isoproterenol, or chloroquine alone. Results are from four experiments.
Bitter taste receptors relax ASM by localized [Ca2+]i and BKCa channels
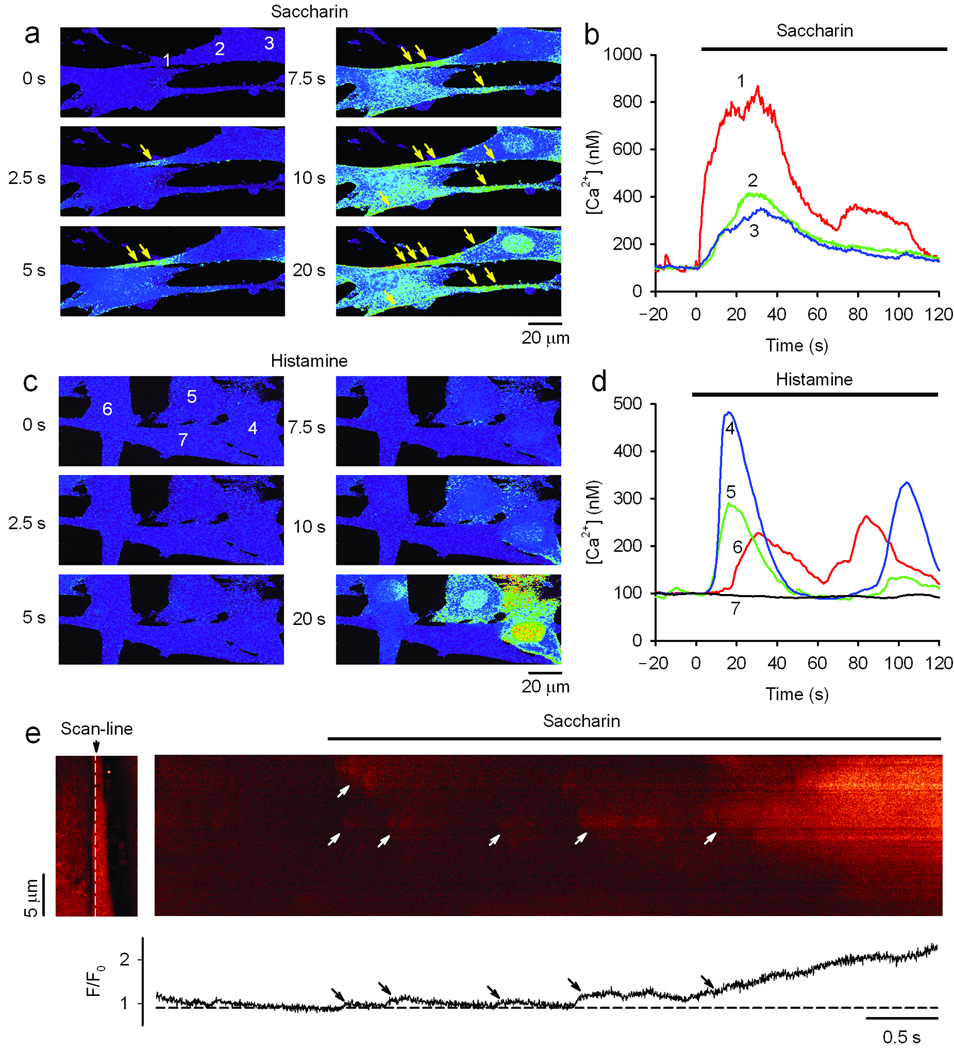
To further ascertain the mechanism by which bitter taste receptors evoke ASM relaxation, we used magnetic twisting cytometry15 to measure dynamic changes in stiffness of isolated human ASM cells (Fig. 3), thus removing any potential confoundment from unrecognized mechanisms present in intact tissue. In these experiments magnetic particles attached to the cell by a peptide linker provide a highly quantitative measurement of single-cell stiffness, with isoproterenol and histamine exposure resulting in the expected relaxation and contraction from baseline, respectively (Fig. 3a). Chloroquine and saccharin exposure (Fig. 3a) resulted in ASM relaxation at this single cell level, confirming that these bitter tastants act directly on smooth muscle cells, which is consistent with our findings in the coordinated relaxation response of intact airways. The relaxation response to saccharin was not blocked by the PKA inhibitor H89 (Fig. 3b) confirming results from cAMP and VASP phosphorylation measurements. Inhibition of PLC by U73122 eliminated the saccharin-promoted relaxation of isolated ASM (Fig. 3b). In light of our findings with PLC inhibition (as well as βγ inhibitors and IP3 receptor antagonists) on bitter tastant-promoted increases in [Ca2+]i (Fig. 1d), these results indicated that the relaxation response of these receptors in ASM is triggered by, or requires, intracellular Ca2+ release. Consistent with this concept, the chloroquine EC50 values for [Ca2+]i release in cultured ASM cells (70 ± 10 µM, Fig. 1a) and in the relaxation of intact airways (93 ± 4.3 µM, Fig. 2a) are virtually identical. The dependence of relaxation on SR Ca2+ release was further supported by results of studies with the SR Ca2+ re-uptake inhibitor thapsigargin, which depletes the SR of [Ca2+]i. Thapsigargin pre-incubation blocked chloroquine and other bitter tastant-mediated [Ca2+]i stimulation in ASM cells (Supplementary Fig. 7), and also chloroquine-mediated relaxation of intact airway rings (Supplementary Fig. 8). We considered a potential mechanism for Ca2+-mediated relaxation in ASM was via hyperpolarization due to stimulation of the large conductance Ca2+-activated K+-channel (BKCa). These channels are known to be expressed on human ASM16 (which we confirmed; Supplementary Fig. 1) and have been reported to regulate airway tone17. To test whether the observed relaxation is due to bitter tastant-triggered Ca2+ activation of BKCa, human ASM cells were pretreated with carrier, a Ca2+-dependent K+ channel antagonist charybdotoxin, or the specific BKCa channel antagonist iberiotoxin. As shown in Fig. 3b, both pretreatments ablated saccharin-mediated ASM relaxation as assessed in isolated cells. Similar results were found with chloroquine (data not shown). And finally, pretreatment with iberiotoxin also attenuated chloroquine-promoted relaxation in the isolated mouse airway (Fig. 3c). The relaxation of ASM from BKCa activation would be expected to be from membrane hyperpolarization18. ASM cells were loaded with a membrane potential-sensitive fluorescent dye19, and as shown in Fig. 3d, exposure to KCl and histamine resulted in the expected membrane depolarization. With exposure to the bitter tastants chloroquine and saccharin, membrane hyperpolarization was readily observed. Furthermore, bitter tastant-promoted membrane hyperpolarization was completely inhibited by iberiotoxin (Fig. 3e). Thus the highly efficacious bronchodilator response from ASM bitter taste receptors appears to be due to [Ca2+]i-dependent activation of BKCa, which is distinct from histamine promoted increases in [Ca2+]i which causes contraction. This suggested that the intracellular distribution of the Ca2+ responses to histamine and bitter tastants are different, and indeed, it is established that a high concentration of localized [Ca2+]i is associated with BKCa activation20. To define the characteristics of saccharin-promoted [Ca2+]i increases in ASM cells, real-time confocal imaging was performed in Fluo-3 loaded cells (Fig. 4a,c). As shown, localized [Ca2+]i signals were detected at the slender ends and sarcolemmal regions of ASM. This response was rapid (e.g., observed within 2.5 sec in the cross-sectional studies, Fig. 4a) and the magnitude was greater than at the central region of the myocytes (Fig. 4b). When using the line-scan mode at regions within 1 µm and parallel to the cell membrane of ASM cells, spatially and temporally discernible [Ca2+]i events were detected very early after the application of saccharin, prior to the subsequent sustained localized rise in [Ca2+]i (Fig. 4e). These results confirm the notion that saccharin promotes localized [Ca2+]i signals in ASM cells. In contrast, the response to histamine in ASM cells caused a rapid rise in [Ca2+]i throughout the cell (Fig. 4c,d), without the localized features observed with saccharin.
Figure 3.
Isolated airway smooth muscle responses to bitter tastants as assessed by single cell mechanics and membrane potentials. (a) isoproterenol (iso), chloroquine (chloro) and saccharin (sacc) relax, while histamine (hist) contracts, isolated ASM cells. (b) The relaxation responses in isolated ASM cells to 1 mM saccharin are inhibited by the PLCβ inhibitor U73122 (1 µM), 10 nM of the BKCa antagonists iberiotoxin (IbTx) and charybdotoxin (ChTx), but are unaffected by 100 nM of the PKA inhibitor H89. (c) The relaxation response to 1 mM chloro in isolated mouse airway is inhibited by 100 nM IbTx. Results are representative of 5−8 experiments performed. (d) ASM cells loaded with a fluorescence-based membrane potential-sensitive dye reveal hyperpolarization in response to 1.0 mM chloroquine and saccharin (representative of four experiments performed). (e) Chloroquine and saccharin-promoted ASM hyperpolarization is ablated by preincubation with 100 nM IbTx. Results represent the peak responses from four experiments. *, P < 0.01 vs. vehicle control.
Figure 4.
Saccharin preferentially triggers localized [Ca2+]i responses in ASM cells. (a,c) Sequential confocal images of Fluo-3 loaded cells shows activation of localized [Ca2+]i increases in the cell periphery upon exposure of ASM cells to 0.3 mM saccharin, and a generalized increase in [Ca2+]i with exposure to 1.0 µM histamine. The images are Fluo-3 fluorescence after background subtraction and baseline normalized (F/F0) with intensity encoded by pseudo-color. The arrows highlight local [Ca2+]i “hot-spots”. (b,d) Local [Ca2+]i transients measured in regions of interest (ROI). Saccharin activated a rapid rise of Ca2+ in the peripheral end (ROI 1), but a smaller and gradual increase of [Ca2+]i in the central regions (ROI 2,3) of the cell. The histamine response (ROI 4–7) was asynchronous and was observed throughout the cells. (e) Confocal linescan imaging shows spatially and temporally resolved local [Ca2+]i events activated by saccharin in a peripheral site. The scan line (white dashed line) was placed within 1 µm parallel to the cell membrane at one end of an elongated ASM cell as shown in the left panel. Arrows indicate several local [Ca2+]i events that occur prior to the more defined increase within the isolated region. The bottom panel is the spatially averaged normalized fluorescence signal (F/F0) generated from the linescan. Results are from single experiments representative of five performed.
Bitter tastants counteract asthmatic bronchoconstriction
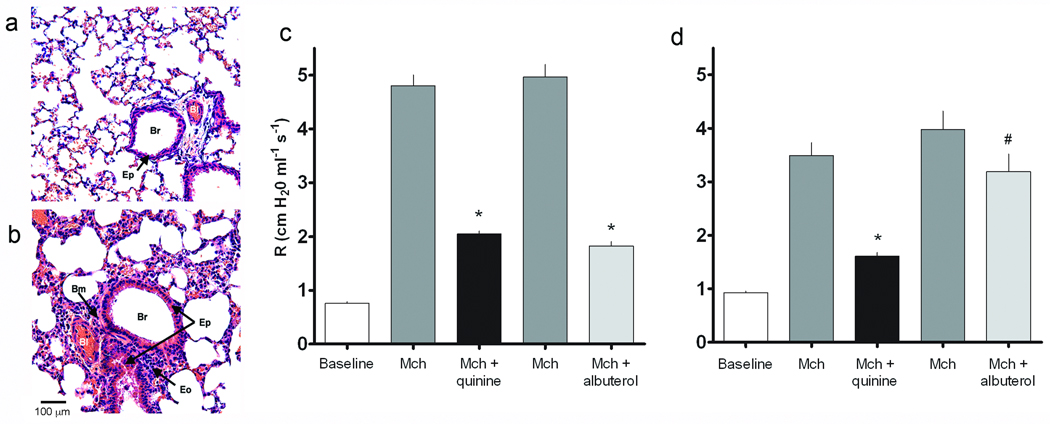
Collectively, the above results all pointed to a novel ASM relaxation pathway that might be utilized for treating reversible obstructive lung diseases such as asthma. To assess this potential, bitter tastants were administered by inhalation in the context of a mouse model of allergic airway inflammation and bronchial hyperresponsiveness. Mice were sensitized to ovalbumin and then repetitively challenged with inhaled ovalbumin, which resulted in acute airway inflammation (Fig. 5a,b). Airway resistance was measured in these intact, sedated, intubated mice at baseline, after inhalation of the bronchoconstrictor methacholine, and then after inhalation of bitter tastants during the bronchoconstrictive phase. The positive control for these studies was inhaled albuterol, a β-agonist that is the most commonly utilized bronchodilator in asthma therapy. In the ovalbumin-sensitized mice, the concentration of inhaled methacholine required to increase baseline airway resistance by 4–5 fold was 8 mg/ml, compared to ≥ 16 mg/ml for non-sensitized mice, confirming the airway hyperreactivity phenotype. Inhaled aerosolized quinine decreased airway resistance in normal and sensitized mice by 53 ± 2.96% and 50 ± 8%, respectively, indicating efficacy in a pathophysiologically relevant model of obstructive airway disease (Fig. 5c,d). Bronchodilatory effects were also found with denatonium, amounting to 44 ± 6% in the normal mice and 57 ± 4% in the sensitized mice. Albuterol showed a lower reduction of airway resistance compared to quinine in the ovalbumin-challenged mice (Fig. 5d), suggesting that bitter tastants may be more effective than β-agonists in an inflammatory hyperresponsive state such as asthma.
Figure 5.
Bitter taste receptor agonists relieve bronchoconstriction in a mouse model of asthma. (a,b) Photomicrographs from sections of control and ovalbumin challenged mouse lungs shows eosinophilic inflammation of the airway, epithelial hyperplasia and basement membrane thickening in ovalbumin challenged airways (hematoxylin and eosin stain). Br, bronchus; Bm, basement membrane; Eo, eosinophil; Ep, epithelium; Bl, blood vessel. Airway resistance in control (c) and ovalbumin challenged mice (d) was measured at baseline, and in response to aerosolized methacholine (mch), and to quinine or the β-agonist albuterol given during the bronchoconstrictive phase (n = 5 experiments). The studies were carried out with a dose of methacholine which resulted in a 4−5-fold increase in airway resistance over baseline (≥ 16 mg/ml in control mice and 8 mg/ml in ovalbumin-challenged mice). *, P < 0.01 vs. methacholine; #, P < 0.05 vs. methacholine.
DISCUSSION
GPCRs expressed on ASM represent the major family of signaling receptors that regulate airway tone and caliber. Within the airway milieu in asthma, multiple locally generated ligands act on these receptors ultimately leading to bronchoconstriction. The pro-bronchoconstrictive GPCRs couple to Gαq, increase [Ca2+]i, and trigger ASM contraction. In contrast, GPCRs coupled to Gαs increase cAMP, relax ASM and bronchodilate, with the β2-adrenergic receptor being the target for β-agonists, the most commonly utilized therapeutic for bronchospasm. Identification of novel ASM receptors that lead to bronchoconstriction and dilation further refines our understanding of the signaling network at play in asthma, leading to potential new therapeutic approaches. Here we unexpectedly found expression of several GPCRs belonging to the bitter taste receptor family on human ASM. Given that bitter signaling has been thought to serve an aversion response, we assumed that ASM bitter taste receptors would act to contract the muscle leading to bronchoconstriction, with shortness of breath as the cue to escape from a noxious environment. A recent report21 has also identified bitter taste receptors on motile cilia of airway epithelial cells that increase beat frequency, considered a mechanical defense against noxious inhalants. These receptors are also found in the anterior nasal cavity where they promote sneezing and regulate respiratory rate22, again repulsion-like responses to noxious stimuli. Bitter tastants signaled to increased [Ca2+]i in ASM via a Gβγ-, PLCβ- and IP3 receptor-dependent manner. The increased [Ca2+]i would be expected to cause ASM constriction, such as that observed with Gαq-coupled receptor activation, but we observed marked relaxation in intact airways of mice and humans as well as isolated human ASM cells.
The dose-response curves for [Ca2+]i stimulation and ASM relaxation by a given bitter tastant showed equivalent EC50 values, suggestive of a connection between this intracellular response and the physiological response. And indeed, depletion of SR Ca2+ resulted in the loss of bitter tastant-mediated increases in ASM cell [Ca2+]i as well as the relaxation effect observed in intact airway rings. Confocal fluorescence imaging of [Ca2+]i showed localized increases due to bitter tastants, which included early calcium events occurring within a distance of 1 µm to the cell surface. We considered bitter taste receptor-mediated, [Ca2+]i-dependent, opening of BKCa because of this tight relationship we found between [Ca2+]i and relaxation, the known expression of BKCa on ASM, and this channel’s requirement for membrane-associated [Ca2+]i for activation. And indeed, blockade of BKCa ablated single cell relaxation, intact airway relaxation, and membrane hyperpolarization from bitter tastants. The basis for the restricted [Ca2+]i response from bitter tastants remains to be defined. Interestingly, a small degree of depolarization was observed from bitter tastants in the context of BKCa blockade, suggesting that the two calcium pools (histamine receptor-promoted vs. TAS2R-promoted) may have some overlap. Nevertheless, the net effect of histamine-mediated [Ca2+]i increase is depolarization and contraction while for bitter tastants is hyperpolarization and relaxation.
Finally, aerosolized administration of bitter tastants relaxed the airways in a mouse model of allergic inflammation, indicating that the pathway has therapeutic relevance in a diseased state. The evolutionary basis for bronchodilating bitter taste receptors on ASM remains speculative. They may act in a compensatory manner to acyl-homoserine lactones which are agonists at these receptors23 and are secreted by Gram-negative bacteria24 during bronchitis or pneumonia. This action may provide protection against bronchospasm or airway closure which might otherwise lead to worsening disease.
There is an unmet need for additional therapeutic options in the treatment of obstructive airway diseases such as asthma. Here we show that agents which bind bitter taste receptors cause marked bronchodilation of intact airways which was ~3-fold greater than that promoted by β-agonist in intact airways. Furthermore, the effect of bitter taste receptors appears to be additive to that of β-agonists, consistent with different mechanisms of action, and thus combination therapy could be considered. The choice of compounds that could be developed for this purpose is extensive, with marked structural diversity. Indeed, there are many known synthetic agents, developed for other purposes, which activate bitter taste receptors10 and are non-toxic. And, there are thousands of plant-derived bitter tastants and their metabolites that could have favorable therapeutic profiles10.
METHODS
[Ca2+]i, cAMP and membrane potential measurements
Primary human ASM cells were obtained from a commercial source (Clonetics) and maintained as described25, with experiments performed using cells at passages 3−8. For detecting changes in [Ca2+]i, attached cells (80,000 cells/well) were loaded with Fluo-4 AM (BD Biosciences) with probenecid for one hour, agonists added by an automated pipetting system in triplicate, and the 525 nm signals were generated by excitation at 485 nm using a Flex Station II (Molecular Devices). Unless otherwise stated, the media for these experiments contained 1.5 mM calcium. In some studies, ASM were transfected with TAS2R10 siRNA (Invitrogen) or a scrambled-sequence siRNA control by electroporation (Nucleofector, Lonza) using 4 µg of siRNA. cAMP was measured by a 125I-cAMP based radioimmunoassay26. The effects of bitter tastants on membrane potential of whole ASM cells was measured using a membrane potential-sensitive fluorescent dye (Molecular Devices) as described19 in a 96 well plate format.
Ex vivo intact airway physiology
All mouse studies were approved by the Animal Care and Use Committee, and human tissue studies were approved by the Institutional Review Board, of the University of Maryland, Baltimore. Five mm sections of trachea from FVB/N mice were excised and studied in an isometric myograph system (Radnoti) as previously described27. A passive tension of 5 mN was applied for each ring for a baseline. For relaxation studies, rings were contracted with agents such as acetylcholine using a fixed concentration, which was maintained during addition of multiple doses of isoproterenol or bitter tastants. Fourth-order bronchi from freshly obtained human tissue from surgical specimens were dissected from regions without gross pathology, rings prepared, and studied in a similar manner.
Magnetic twisting cytometry
Dynamic changes in baseline cell stiffness were measured as an indicator of contraction and relaxation of isolated human ASM cells using magnetic twisting cytometry as described in detail previously15. For each individual ASM cell, baseline stiffness was measured for the first 60 s, and after drug addition stiffness was measured continuously for the next 540 s. In some experiments, cells were pre-exposed to vehicle or inhibitors for 10 min prior to addition of GPCR agonists.
Confocal imaging of regional and local [Ca2+]i signals
Regional and local [Ca2+]i signals were visualized as previously described28,29 using the membrane permeable [Ca2+]i -sensitive fluorescent dye Fluo-3 acetoxymethyl ester (Fluo-3 AM). Cultured human ASM were loaded with 5 µM Fluo-3 AM for 30−45 min at ~22 °C. Cells were washed with Tyrode solution containing extracellular Fluo-3 AM and incubated for 15−30 min to allow complete de-esterification of cytosolic dye. Excitation was at 488 nm and fluorescence was measured at >505 nm. Two dimension images were scanned at 0.22 µm/pixel, 512 pixels/line, 256 lines/image once every 0.5 s. Linescan images were collected at 0.075 µm/pixel, 512 pixels/line at 2 ms intervals for 10,000 lines/image. Amplitude of [Ca2+]i signal were calibrated to absolute [Ca2+]i by a pseudo-ratio method30.
Bitter taste receptor expression
Total RNA was extracted from human ASM cells and reverse transcription was carried out using 2 µg RNA and oligo dT primers. Real time PCR was carried out using methods previously described in detail31, with specific primers (Applied Biosystems) for the 25 TAS2Rs, GAPDH, and the other indicated genes using an Applied Biosystems 7300 Real Time PCR system. Data were analyzed using the ΔΔCT method with ADRB2 as the reference32. The PCR products derived from primers for TAS2R10, 14, 31, 5, 4, and 19 were sequenced and verified to be the indicated human bitter taste receptor. Immunofluorescence microscopy was carried out using fixed cells as described25.
Ovalbumin sensitization and pulmonary function testing
Sensitization of 6-week old BALB/c mice was carried out by i.p. injections of 100 µg ovalbumin in 200 µl alum, or alum alone (control) on days 0 and 14. Mice were then challenged with 1.0% aerosolized ovalbumin on days 19, 21 and 24. Twenty-four hours after the last challenge, mice were sedated and intubated, and ventilated, and measurements of airway resistance taken as previously described33. Mice were challenged with doubling doses of aerosolized methacholine (beginning with 2.0 mg/ml in the nebulizer) until the sustained airway resistance became ~4−5-fold greater than baseline. Three minutes after the last methacholine inhalation, the bitter tastants quinine (150 µg) or denatonium (200 µg), or the β-agonist albuterol (3.0 µg) were administered by aerosol over 10 s. Resistance measurements (Raw, cm H2O/ml−1/s−1) were taken every 30 s throughout the experiment.
Statistical analysis
Dose response curves for [Ca2+]i and ex vivo tracheal ring studies were analyzed by iterative non-linear (sigmoidal) least squares fitting. Results from all studies were compared using paired or unpaired two-way t-tests (depending on study design), with P < 0.05 considered significant. When multiple comparisons were sought, an ANOVA with post-hoc t-tests was utilized with a correction for multiple comparisons. Data are presented as mean ± standard error.
Supplementary Material
ACKNOWLEDGMENTS
Supported by US National Heart Lung and Blood Institute grants HL045967 and HL071609 (to S. Liggett), HL071835 and HL075134 (to J. Sham) and HL087560 (to D. Deshpande).
Footnotes
AUTHOR CONTRIBUTIONS
D.A.D., single cell mechanics and imaging, data analysis and manuscript preparation; W.C.H.W., expression studies, gene knockdown, airway physiology, data analysis, manuscript preparation; E.L.M., calcium signaling and data analysis; K.S.R., intact airway studies, expression studies, data analysis, manuscript preparation; R.M.S., airway physiology; S.S.A., single cell mechanics, data analysis, manuscript preparation; J.S.K.S., confocal calcium imaging, data analysis, manuscript preparation; S.B.L. directed all studies, data analysis and interpretation, and is the primary author of the manuscript.
References
- 1.Vignola AM, Bonsignore G, Chanez P, Bousquet J. Fatal Asthma. New York: Marcel Dekker, Inc.; 1998. pp. 139–155. [Google Scholar]
- 2.Billington CK, Penn RB. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir. Res. 2003;4:2–24. [PMC free article] [PubMed] [Google Scholar]
- 3.Green SA, Liggett SB. The Genetics of Asthma. New York: Marcel Dekker, Inc.; 1996. pp. 67–90. [Google Scholar]
- 4.Drazen JM, Silverman EK, Lee TH. Heterogeneity of therapeutic responses in asthma. Br. Med. Bull. 2000;56:1054–1070. doi: 10.1258/0007142001903535. [DOI] [PubMed] [Google Scholar]
- 5.Malmstrom K, et al. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Montelukast/Beclomethasone Study Group. Ann. Intern. Med. 1999;130:487–495. doi: 10.7326/0003-4819-130-6-199903160-00005. [DOI] [PubMed] [Google Scholar]
- 6.Einstein R, et al. Alternative splicing of the G protein-coupled receptor superfamily in human airway smooth muscle diversifies the complement of receptors. Proc. Natl. Acad. Sci. 2008;105:5230–5235. doi: 10.1073/pnas.0801319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Misior AM, et al. Glucocorticoid- and protein kinase A-dependent transcriptome regulation in airway smooth muscle. Am. J Respir. Cell Mol. Biol. 2009;41:24–39. doi: 10.1165/rcmb.2008-0266OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosse Y, Maghni K, Hudson TJ. 1alpha,25-dihydroxy-vitamin D3 stimulation of bronchial smooth muscle cells induces autocrine, contractility, and remodeling processes. Physiol. Genomics. 2007;29:161–168. doi: 10.1152/physiolgenomics.00134.2006. [DOI] [PubMed] [Google Scholar]
- 9.Meyerhof W. Elucidation of mammalian bitter taste. Rev. Physiol. Biochem. Pharmacol. 2005;154:37–72. doi: 10.1007/s10254-005-0041-0. [DOI] [PubMed] [Google Scholar]
- 10.Meyerhof W, et al. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses. 2010;35:157–170. doi: 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]
- 11.Chandrashekar J, et al. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 12.Adler E, et al. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 13.Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 14.Lombardo LJ, Balmes JR. Occupational asthma: a review. Environ. Health Perspect. 2000;108(Suppl 4):697–704. doi: 10.1289/ehp.00108s4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.An SS, Fabry B, Trepat X, Wang N, Fredberg JJ. Do biophysical properties of the airway smooth muscle in culture predict airway hyperresponsiveness? Am. J. Respir. Cell Mol. Biol. 2006;35:55–64. doi: 10.1165/rcmb.2005-0453OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin G, et al. Interleukin-4 activates large-conductance, calcium-activated potassium (BKCa) channels in human airway smooth muscle cells. Exp. Physiol. 2008;93:908–918. doi: 10.1113/expphysiol.2008.042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morin C, Sirois M, Echave V, Gomes MM, Rousseau E. Functional effects of 20-HETE on human bronchi: hyperpolarization and relaxation due to BKCa channel activation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L1037–L1044. doi: 10.1152/ajplung.00145.2007. [DOI] [PubMed] [Google Scholar]
- 18.Kotlikoff MI, Kamm KE. Molecular mechanisms of β-adrenergic relaxation of airway smooth muscle. Annu. Rev. Physiol. 1996;58:115–141. doi: 10.1146/annurev.ph.58.030196.000555. [DOI] [PubMed] [Google Scholar]
- 19.Baxter DF, et al. A novel membrane potential-sensitive fluorescent dye improves cell-based assays for ion channels. J. Biomol. Screen. 2002;7:79–85. doi: 10.1177/108705710200700110. [DOI] [PubMed] [Google Scholar]
- 20.Fakler B, Adelman JP. Control of K(Ca) channels by calcium nano/microdomains. Neuron. 2008;59:873–881. doi: 10.1016/j.neuron.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Shah AS, Ben Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finger TE, et al. Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc. Natl. Acad. Sci. U. S. A. 2003;100:8981–8986. doi: 10.1073/pnas.1531172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brockhoff A, Behrens M, Massarotti A, Appendino G, Meyerhof W. Broad tuning of the human bitter taste receptor hTAS2R46 to various sesquiterpene lactones, clerodane and labdane diterpenoids, strychnine, and denatonium. J. Agric. Food Chem. 2007;55:6236–6243. doi: 10.1021/jf070503p. [DOI] [PubMed] [Google Scholar]
- 24.Sbarbati A, et al. Acyl homoserine lactones induce early response in the airway. Anat. Rec. 2009;292:439–448. doi: 10.1002/ar.20866. [DOI] [PubMed] [Google Scholar]
- 25.Panebra A, Schwarb MR, Glinka CB, Liggett SB. Heterogeneity of transcription factor expression and regulation in human airway epithelial and smooth muscle cells. Am. J. Physiol. Lung. 2007;293:L453–L462. doi: 10.1152/ajplung.00084.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liggett SB, et al. Altered patterns of agonist-stimulated cAMP accumulation in cells expressing mutant β2-adrenergic receptors lacking phosphorylation sites. Mol. Pharmacol. 1989;36:641–646. [PubMed] [Google Scholar]
- 27.McGraw DW, et al. Airway smooth muscle prostaglandin-EP1 receptors directly modulate beta2-adrenergic receptors within a unique heterodimeric complex. J. Clin. Invest. 2006;116:1400–1409. doi: 10.1172/JCI25840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang XR, et al. Multiple ryanodine receptor subtypes and heterogeneous ryanodine receptor-gated Ca2+ stores in pulmonary arterial smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;289:L338–L348. doi: 10.1152/ajplung.00328.2004. [DOI] [PubMed] [Google Scholar]
- 29.Zhang WM, et al. ET-1 activates Ca2+ sparks in PASMC: local Ca2+ signaling between inositol trisphosphate and ryanodine receptors. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285:L680–L690. doi: 10.1152/ajplung.00067.2003. [DOI] [PubMed] [Google Scholar]
- 30.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 31.Small KM, et al. Polymorphisms of the cardiac presynaptic α2Cadrenergic receptors: diverse intragenic variability with haplotype-specific functional effects. Proc. Natl. Acad. Sci. 2004;101:13020–13025. doi: 10.1073/pnas.0405074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.McGraw DW, et al. Crosstalk between Gi and Gq/Gs pathways in airway smooth muscle regulates bronchial contractility and relaxation. J. Clin. Invest. 2007;117:1391–1398. doi: 10.1172/JCI30489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.