Abstract
Sickle cell disease (SCD) is one of the commonest severe inherited disorders, but specific treatments are lacking and the pathophysiology remains unclear. Affected individuals account for well over 250,000 births yearly, mostly in the Tropics, the USA, and the Caribbean, also in Northern Europe as well. Incidence in the UK amounts to around 12–15,000 individuals and is increasing, with approximately 300 SCD babies born each year as well as with arrival of new immigrants. About two thirds of SCD patients are homozygous HbSS individuals. Patients heterozygous for HbS and HbC (HbSC) constitute about a third of SCD cases, making this the second most common form of SCD, with approximately 80,000 births per year worldwide. Disease in these patients shows differences from that in homozygous HbSS individuals. Their red blood cells (RBCs), containing approximately equal amounts of HbS and HbC, are also likely to show differences in properties which may contribute to disease outcome. Nevertheless, little is known about the behaviour of RBCs from HbSC heterozygotes. This paper reviews what is known about SCD in HbSC individuals and will compare the properties of their RBCs with those from homozygous HbSS patients. Important areas of similarity and potential differences will be emphasised.
1. Introduction
Like homozygous HbSS individuals, individuals heterozygous for HbS and HbC (HbSCs) suffer from sickle cell disease (SCD) [1–6]. The condition in HbSC patients (here called HbSC disease cf. HbSS disease in homozygotes) not only has some overlap with that seen in HbSS patients, but also has distinctive laboratory and clinical features identifying it as a separate entity [6–8]. Although HbSC disease is one of the commonest significant genetic diseases worldwide, it is comparatively neglected with very few laboratory or clinical studies addressing the condition directly. Thus, whilst extensive research has been carried out on understanding SCD in HbSS patients, little relates specifically to the pathogenesis in HbSC patients. In clinical trials of potential novel therapies for SCD, HbSC patients are often specifically excluded. Furthermore, most clinical and laboratory features of HbSC disease have been inferred from studies of HbSS, which may not be appropriate. This paper addresses the pathophysiological differences shown by SCD in HbSS and HbSC patients and the diversity in their clinical complications. Particular reference is paid to the transport abnormalities of the RBC membrane.
2. Genotypic Variants of SCD
All SCD patients have the abnormal haemoglobin HbS in their red cells instead of the normal adult HbA [9–12]. HbS results from a single base mutation in codon 6 of the β-globin gene which causes a single amino acid substitution in position β6 (glutamic acid → valine, with net loss of one negative charge). Homozygous HbSS patients have two copies of the altered gene. The mutation arose in West Africa, where the high prevalence of HbS appears to be due to selection pressure conferred by a relative resistance to malaria. Malaria resistance has also increased the prevalence of a second abnormal Hb, HbC, which like HbS represents one of the most prevalent forms of abnormal human Hb. HbC also has a single mutation/amino acid change at the same position in β-Hb, but with lysine replacing glutamic acid (hence net loss of two negative charges). These changes in protein charge may alter how the different Hbs interact and modulate transporter function at the RBC membrane [13]. The charge differences are also used for electrophoretic tests for abnormal Hb, although care must be exercised to exclude certain non-SCD haemoglobinopathies which may mimic HbSC. Homozygous HbCC individuals show few disease symptoms apart from a mild haemolytic anaemia [6]. Heterozygotes of HbA with either HbS or HbC are also largely asymptomatic. Coinheritance of HbS and HbC to produce HbSC heterozygotes, however, results in a clinically significant disease similar, but not identical, to that in HbSS individuals [6–8]. Although globally HbSC heterozygotes represent about a third of SCD cases, their distribution is by no means uniform. HbC appears to have originated in Burkina Faso [6] where HbSC cases may outnumber those of HbSS. In other areas, such as the Middle East and India, HbSC cases are rare. In this context, it is worth pointing out that estimates of the frequency of different haemoglobinopathies are likely to be inexact, relying on outdated or incorrect information [14].
3. HbSC Disease as a Unique Clinical Entity
All cases of SCD, including those of HbSC disease, are characterised by shortened red cell life span and chronic anaemia, together with recurrent episodes of more acute vaso-occlusion, tissue ischaemia, and increased mortality [12]. Affected individuals have a poor quality of life with numerous complications, for example, pain, cerebrovascular disease (strokes), renal and pulmonary damage, leg ulcers, and priapism [2]. An important feature of SCD is that the clinical scenario is notably heterogeneous—patients may present with mild forms of the disease which rarely require medical intervention or alternatively with more severe complications warranting frequent hospitalisation and aggressive management. Presumably modifier genes and/or environmental factors are significant, but although this area is now receiving considerable attention, it remains poorly understood at present [15–17].
In most cases, HbSC disease is clinically milder than HbSS disease and the various complications of SCD usually occur less often or later in life [8]. For example, leg ulcers and other chronic vascular manifestations occur infrequently. Loss of splenic function is relatively delayed, preserving red cell scavenging and thereby possibly affecting disease complications. Nevertheless, HbSC disease still has a significant impact on patients who show haemolytic anaemia, organ failures (stroke, renal failure, chronic lung disease.), and increased mortality (with a median survival of 60 years for males in USA) [15, 18]. Pregnant women sometimes develop complications having been hitherto asymptomatic [19]. Complications also occur in children with the risk of stroke in childhood being about 100 times greater than that in the general population [18]. Furthermore, in HbSC heterozygotes, some of the serious complications of SCD (such as osteonecrosis) are as common as for HbSS patients and some (e.g., proliferative sickle retinopathy and possibly acute chest syndrome) occur more frequently [8]. This is also apparent for some central auditory and vestibular problems [19].
Additionally, HbSC is haematologically distinct from HbSS, with higher Hb levels (but lower levels of HbF), lower rates of haemolysis and lower white cell counts [8]. Some of these features are well illustrated in clinical and haematological observations on patients from our clinics (see [20, Table 1]). These distinctive features imply that individuals with HbSC disease should be treated as a discrete subset of SCD patients.
Currently there is very little specific information on the pathophysiology and management of HbSC disease, with much being inferred from studies of HbSS patients. Differences in pathogenesis between HbSC and HbSS disease are expected, however. Understanding them will be important in the management of HbSC patients and may also contribute to a better appreciation of the condition in homozygous individuals.
4. Pathogenesis of SCD
Although the underlying molecular defect of SCD is long established, how HbS results in the clinical complications remains poorly understood. The chronic anaemia and acute ischaemic episodes both are associated with altered rheology and increased adhesiveness of both RBCs and vascular endothelium [21]. RBCs are more fragile and more readily scavenged from the circulation, contributing to the chronic anaemia, whilst microvascular occlusion is also encouraged causing the acute ischaemic events characteristic of SCD. Intravascular haemolysis is observed and the consequent release of Hb to circulate freely in plasma contributes to the vasculopathy, probably by scavenging nitric oxide (NO) and causing a functional deficiency of that molecule [22, 23]. Some authors divide the disease complications of SCD into two broad categories, with sequelae caused either predominantly by altered RBC rheology and elevated blood viscosity (e.g., pain, osteonecrosis, and acute chest syndrome) or by intravascular haemolysis and NO scavenging (e.g., pulmonary hypertension, stroke, priapism, and leg ulceration) [24–26]. In any event, polymerisation of HbS on deoxygenation is central to anaemia, vaso-occlusion, and haemolysis—although complete deoxygenation may not be needed, especially in the case of hyperdense RBCs with high cell [Hb], such as some of those found in HbSC individuals. Formation of long rods of HbS distorts RBC shape, reduces deformability, and increases viscosity, thus compromising vascular red cell rheology [27]. Other key events in the pathogenesis have been identified. First, red cell volume is critically important [28]. The increased cation permeability of HbS-containing red cells results in solute loss with water following osmotically. Consequently, [HbS] increases. As the rate of HbS polymerisation upon deoxygenation is proportional to a very high power of [HbS], a small reduction in cell volume and hence increase in [HbS] markedly encourages HbS polymerisation [27]. Second, red cell stickiness is also increased [21, 29–31]. This results, at least in part, from exposure of phosphatidylserine (PS) on the outer bilayer of the membrane [32]. Exposed PS is prothrombotic and increases adherence of red cells to macrophages and endothelium, contributing to chronic anaemia, haemolysis, and vaso-occlusion [33]. Again, the cause of PS exposure is not clear, but sickling-induced Ca2+ entry may play an important role [34]. Third, SCD represents an inflammatory state with raised levels of cytokines, chronic elevation of leukocyte counts, shortened leukocyte half-life, and abnormal activation of granulocytes, monocytes, and endothelium [35, 36]. The resulting cytokine stimulation of endothelial cells increases their adhesiveness to sickle RBCs [35, 36]. How these various changes interact to produce the symptoms of SCD represents a major research challenge. In addition, the extent to which these various mechanisms are involved in disease pathogenesis would be expected to differ between HbSS homozygotes and HbSC heterozygotes. For example, reduced intravascular haemolysis in the latter may ameliorate NO scavenging.
5. Altered Membrane Transport in Homozygous HbSS Cells
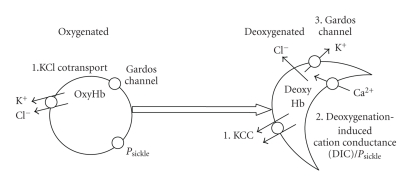
Increased membrane permeability of HbS-containing red cells contributes to SCD pathogenesis by promoting Ca2+ entry, KCl loss with water following osmotically, and hence RBC dehydration [28, 34, 37]. In HbSS cells, the involvement of three pathways has been proposed: the KCl cotransporter (KCC), the deoxygenation-induced cation conductance (or P sickle), and the Ca2+-activated K+ channel (or Gardos channel, KCNN4) [28]. These three systems are illustrated schematically in Figure 1.
Figure 1.
Schematic diagram of the main transport pathways activated in red blood cells (RBCs) from sickle cell patients. In RBCs from homozygous HbSS individuals, high cation permeability is accounted for by three main pathways [28, 37]. Under oxygenated conditions, the KCl cotransporter (KCC) is highly active. It is overexpressed in HbSS cells compared to HbAA ones and does not become quiescent as RBCs mature. It is stimulated further by low pH (reduction in extracelluar pH from 7.4 to 7). Under deoxygenated conditions, KCC remains active—again unlike the situation in HbAA RBCs [40]. In addition, two other pathways are observed. The deoxygenation-induced cation conductance (or P sickle) is activated as HbS polymerises. It mediates entry of Ca2+. Elevation in intracellular Ca2+ then leads to activation of the third pathway, the Ca2+-activated K+ channel, or Gardos channel. These three pathways result in solute loss, cell shrinkage and dehydration, and consequent increase in [HbS]. They thereby contribute to pathogenesis of sickle cell disease. They are also likely to be involved in solute loss from RBCs of patients heterozygous for HbS and HbC (HbSC genotype), though details are lacking and differences in their behaviour compared to that in HbSS cells are expected.
The first of these, KCC (likely KCC1 and KCC3 isoforms), is more active and abnormally regulated in HbSS cells [38–40]. Mean activity is enhanced >10-fold in unstimulated cells with several stimuli increasing activity further. In normal RBCs, cell swelling is an important trigger of KCC activity [41]. For HbSS cells, however, intracellular pH is probably the most important stimulus in vivo, with KCC activity reaching a peak at about pH 7 [38, 42]. The transporter also responds to O2 tension [43]. In normal red cells, high levels of O2 are required for KCC activity, with the transporter becoming inactivated at low O2. By contrast, in HbSS cells, the transporter remains active during full deoxygenation, thereby allowing it to respond to low pH in hypoxic areas (like active muscle beds) [40] (Figures 1 and 2). KCC is regulated by phosphorylation, through cascades of conjugate protein kinases and phosphatases [44], with differences apparent in HbSS cells compared with HbAA ones, but at present these are poorly defined. The relative deficiency of intracellular Mg2+ in HbSS cells [45, 46] probably acts to increase KCC activity by altering the activity of these regulatory enzymes.
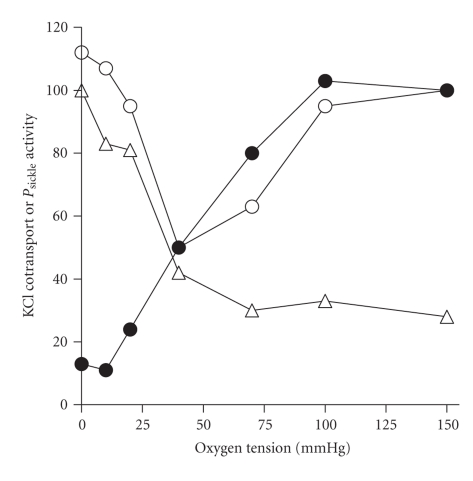
Figure 2.
Effect of oxygen tension on the activity of KCl cotransport (KCC) or P sickle in red blood cells (RBCs) from normal individuals or patients with sickle cell disease. The activity of each transport pathway is normalised—to the value in oxygenated cells (150 mmHg O2) for KCC activity and for that in deoxygenated RBCs (0 mmHg) in the case of P sickle—and given as a percentage. Solid circles give KCC activity in RBCs from normal HbAA individuals; open symbols give KCC activity (open circles) or P sickle activity (open triangles) in RBCs from sickle cell patients (HbSS homozygotes). In these experiments, total magnitude of KCC activity was about 10-fold greater in RBCs from HbSS individuals compared with HbAA ones. Note how the deoxygenation-induced KCC activity and activation of P sickle follow a similar dependence on O2 tension. Data taken from [67].
The second pathway, P sickle, is apparently unique to HbS-containing red cells [28, 34]. It is activated to a variable extent by deoxygenation, HbS polymerisation, and shape change [47, 48] (Figures 1 and 2). P sickle has the characteristics of a nonspecific cation channel [34]. An anion permeability is controversial, whilst, more recently, it has been proposed as permeable under certain conditions to nonelectrolytes [49]. The main effect of P sickle is probably the increased Ca2+ entry [49, 50] and possibly the Mg2+ loss [45]. Raised intracellular Ca2+ has several roles which include phospholipid scrambling [51]. It will also activate the third pathway responsible for HbS cell dehydration, the Gardos channel [52] (Figures 1 and 3). The Gardos channel is then capable of mediating very rapid efflux of K+ with Cl− following for electroneutrality and water osmotically.
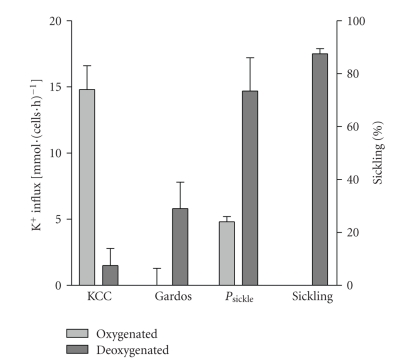
Figure 3.
Components of K+ transport pathways and sickling in red blood cells (RBCs) from sickle cell disease patients heterozygous for HbS and HbC (HbSC genotype). K+ influxes are given as flux units [mmol·(l cells·h)−1] measured at 5 mM [K+]o and numbers of sickled cells as a percentage of total RBCs in fully oxygenated (150 mmHg O2) or deoxygenated (0 mmHg) conditions. Although this technique measures a K+ influx, because of the high K+ content of RBCs, net solute movement through the transport systems will be outwards. KCl cotransport activity was calculated as the Cl−-dependent K+ influx, Gardos channel activity as the clotrimazole (5 μM)-sensitive K+ influx, and P sickle as the Cl−-independent K+ influx (Cl− substituted with NO3 −). Sickling, P sickle, and Gardos channel activation occurs in deoxygenated conditions—as for HbSS RBCs—but KCC activity is low when O2 is removed (as in RBCs from HbAA cells). Data taken from [64].
These mechanisms cause solute loss and HbSS cell shrinkage. Episodes may be short lived and produce only modest degrees of solute loss. But they may occur repeatedly during the lifetime of the RBCs, often during deoxygenation-induced sickling events. Accordingly, HbSS cells show an increase in MCHC of a few percent compared to normal red cells (c.34 g·dL−1 cf. 33 in HbAA cells, density approx 1.085 g·mL−1), but importantly there is a large range about this mean with many dense cells (>1.095 g·ml−1, MCHC c.38 g·dl−1), some of which are exceedingly dense (1.125 g·ml−1, c.50 g·dl−1) [53]. A significant feature of HbSS RBCs is their marked heterogeneity, with certain subpopulations possibly more important in pathogenesis [28]. The densest HbSS cells are mainly older ones, presumably following repeated episodes of solute loss [54]. Reticulocytes are mostly low density (c.26 g·dl−1), as they are in normal individuals [55]. However, there is a small fraction of young, dense HbSS cells, the so-called fast-track reticulocytes, which become rapidly dehydrated on deoxygenation while still young [28, 56].
6. Altered Properties of HbSC Cells
RBCs from HbSC patients also show K+ loss, raised MCHC and haemolytic anaemia with reticulocytosis [5, 57–59]. The properties of HbSC RBCs, however, differ in important respects from those of HbSS cells. In HbSC cells, K+ loss and dehydration are markedly more pronounced [58, 59]. MCHC is particularly high, at about 37 g·dl−1 (cf. 33 g·dl−1 in HbAA individuals; 33-34 g·dl−1 for the reversibly sickled fraction of HbSS patients) [57, 58]. Whilst most reticulocytes from normal HbAA and HbSS are characteristically low density (26 g·dl−1), HbSC reticulocytes are mainly high density (MCHC c.34 g·dl−1) [5, 58]. Usually older RBCs are denser; however the monotonic decrease in reticulocyte count with increasing cell density observed for red cells from HbSS patients (as well as HbAA and HbAS individuals) does not occur in HbSC patients [5, 60]. Instead, HbSC reticulocytes are fairly evenly distributed across the different RBC densities [5], or perhaps even more concentrated in the denser fractions [60]. This has been taken as evidence that a significant proportion of young HbSC cells begin their lives with a high density [5], rather than undergoing a more gradual dehydration observed in HbSS cells upon repeat episodes of sickling. In this respect, perhaps the majority of HbSC reticulocytes behave like the “fast-track” reticulocytes of HbSS patients [56]—cells which dehydrate rapidly on leaving the bone marrow—but this remains to be established. It also raises the question as to what constitutes RBCs in the less dense HbSC fractions. Can shrunken HbSC cells regain lost solute and increase their volume? If so, what is the mechanism and what are transport systems involved?
As heterozygotes, HbSC cells contain both HbS and HbC, in approximately equal amounts (i.e., 50%). This contrasts with the lower HbS content (c.40%) found in sickle trait HbAS cells [5]. Crystals of HbC are sometimes present in oxygenated RBCs. In contrast to HbS polymers, these deposits are lost on deoxygenation [60]. Because of the high HbS content and polymerisation, HbSC cells also show a deoxygenation-induced sickling shape change. In this case, however, rather than the HbSS sickles and holly leaf forms, deoxygenated HbSC cells show multifolded shapes such as “pita breads” and “tricorns” [60], perhaps because of the high surface area to volume ratio subsequent to their more marked dehydration. How HbS and HbC interact has also received some attention. Using different Hb mixtures, a direct interaction between the two Hbs appears to only slightly enhance HbS polymerisation. Much more important in HbSC disease is RBC dehydration and consequently the high MCHC [5, 61, 62]. High MCHC and lower levels of HbF may have an effect on the extent and kinetics of HbS polymerisation whilst concurrent Hb mutations (such as β-thalassaemia) may also play a significant role.
It is therefore critical to understand fully the mechanisms by which these RBCs shrink, but our understanding of the mechanisms involved remains uncertain. Oxygenated HbSC cells have elevated KCC activity that is stimulated by low pH and swelling [13, 60]. Cytoplasmic protein concentration has been suggested as the “volume” sensor of RBCs [63]. It is therefore intriguing to speculate that high KCC activity in oxygenated HbSC cells may result from the presence of HbC crystals which would lower the total concentration of soluble Hb, as occurs in swollen RBCs. In effect, the cells “think” that they are swollen and so activate mechanisms to lose solutes and water, namely, KCC. On the other hand, deoxygenated HbSC cells also show increased K+ efflux, to an extent apparently greater than that observed in deoxygenated HbSS cells [58]. Which pathway mediates the flux in deoxygenated conditions, however, has not been established. If P sickle is involved, given the lower [HbS] of HbSC cells, it is not clear why it should be activated to a greater extent than in HbSS cells. KCC and the Gardos channel represent obvious alternative pathways.
A number of manoeuvres which reduce reticulocyte density may provide evidence for the transport pathways involved in their dehydration. Both Cl− removal and deoxygenation shift HbSC reticulocytes to lower densities, consistent with solute retention following inhibition of KCC [60]. Hypotonic swelling of HbSC cells also reduces the deoxygenation-induced K+ loss [58], perhaps through reduction in [HbS] removing a P sickle-like element of K+ flux.
In preliminary studies, we have observed high KCC activity in oxygenated unfractionated HbSC cells, which was almost completely inhibited on deoxygenation [64]. Thus, KCC in HbSC cells behaved like that in HbSS cells at high O2 tension and like that in HbAA cells when tension was reduced [64] (Figure 3). We also found activation of a deoxygenation-induced Cl−-independent K+ flux [64], a deoxygenation-induced nonelectrolyte permeability [65] and a deoxygenation-induced rise in K+ conductance in patch-clamp experiments [66] (Figure 4), namely, a P sickle-like permeability, together with activation of the Gardos channel. In this context, it is interesting that HbC has a higher affinity for the RBC membrane than either HbA or HbS [59] leading to an early suggestion that HbSC interaction is involved in modulating RBC permeability.
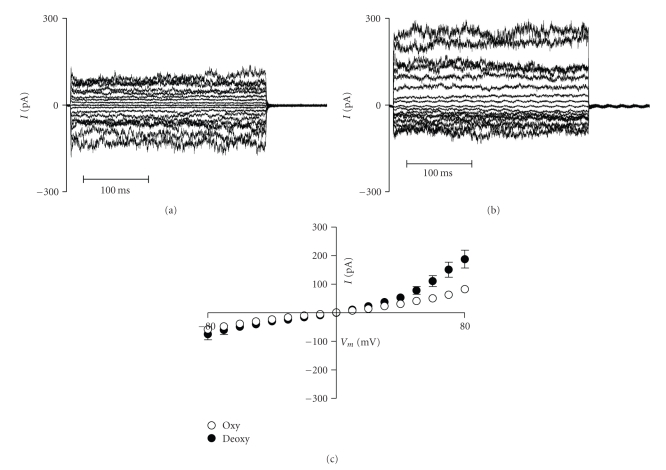
Figure 4.
Conductance of red blood cells (RBCs) from sickle cell patients heterozygous for HbS and HbC (HbSC genotype). (a), (b) Representative whole-cell recordings from (a) oxygenated and (b) deoxygenated RBCs. (c) Mean whole-cell currents + S.E.M., n = 5. Test potentials from −80 to +80 mV were applied for 300 ms in 10 mV increments from a holding potential of −10 mV. Measurements were made using Na+-containing bath and pipette solutions. Data taken from [20]. See [66] for experimental details. The conductance of RBCs from HbSC patients is high and increases further on deoxygenation.
It is apparent, however, that our understanding of the permeability of HbSC cells requires further investigation.
7. Conclusion: The Importance of Cell Dehydration in HbSC Disease
Understanding dehydration is particularly relevant for HbSC cells. The solubility of deoxygenated HbS is about 17 g·dl−1 compared to 70 g·dl−1 for HbA. As HbS represents only about half the total Hbs in HbSC cells, a relatively small decrease in MCHC (from an RBC total of 37 to 33 g·dl−1) will prevent HbS polymerisation [61] while retaining the functionally important discocyte morphology. As HbS constitutes about half the Hbs in these RBCs, this would mean a fall in [HbS] from 18.5 to 16.5. In comparison, in HbSS cells, a reduction of MCHC to <25 g·dl−1 is required, by which time RBCs will be spherocytic, and cell swelling per se will adversely affect rheology [58]. Notwithstanding their relevance to dehydration and sickling, the permeability of HbSC cells has not been well studied nor compared in detail with that of HbSS cells. Several areas require more careful investigation. The interaction between different Hbs, membrane target sites regulating permeability, the transport pathways involved, the role of cell density, oxygenation, volume, and pH presents a complex pattern of modalities controlling solute content and hence cell density and MCHC. Control by phosphorylation remains mainly unexplored. The challenge ahead lies to define the most important stimuli and how they interact to determine cell volume. A major therapeutic goal is the ability to prevent HbSC cell dehydration or to promote rehydration.
Abbreviations
Here we term red cells from HbSS patients as HbSS cells, those from HbSC individuals as HbSC cells.
Acknowledgment
The authors thank the Medical Research Council, the British Heart Foundation, and the Wellcome Trust for financial support.
References
- 1.Serjeant GR. Sickle-cell disease. The Lancet. 1997;350(9079):725–730. doi: 10.1016/S0140-6736(97)07330-3. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg MH. Management of sickle cell disease. The New England Journal of Medicine. 1999;340(13):1021–1030. doi: 10.1056/NEJM199904013401307. [DOI] [PubMed] [Google Scholar]
- 3.Hickman M, Modell B, Greengross P, et al. Mapping the prevalence of sickle cell and beta thalassaemia in England: estimating and validating ethnic-specific rates. British Journal of Haematology. 1999;104(4):860–867. doi: 10.1046/j.1365-2141.1999.01275.x. [DOI] [PubMed] [Google Scholar]
- 4. NHS sickle cell & thalassaemia screening programme, 2010.
- 5.Bunn HF, Noguchi CT, Hofrichter J, Schechter GP, Schechter AN, Eaton WA. Molecular and cellular pathogenesis of hemoglobin SC disease. Proceedings of the National Academy of Sciences of the United States of America. 1982;79(23):7527–7531. doi: 10.1073/pnas.79.23.7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagel RL, Steinberg MH, Hemoglobin SC. disease and HbC disorders. In: Steinberg MH, Forget BG, Higgs DR, Nagel RL, editors. Disorders of Hemoglobin. Cambridge, UK: Cambridge University Press; 2001. pp. 756–785. [Google Scholar]
- 7.Nagel RL, Lawrence C. The distinct pathobiology of sickle cell-hemoglobin C disease: therapeutic implications. Hematology/Oncology Clinics of North America. 1991;5(3):433–451. [PubMed] [Google Scholar]
- 8.Nagel RL, Fabry ME, Steinberg MH. The paradox of hemoglobin SC disease. Blood Reviews. 2003;17(3):167–178. doi: 10.1016/s0268-960x(03)00003-1. [DOI] [PubMed] [Google Scholar]
- 9.Pauling L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia, a molecular disease. Science. 1949;110(2865):543–548. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- 10.Ingram VM. Gene mutations in human haemoglobin: the chemical difference between normal and sickle cell haemoglobin. Nature. 1957;180(4581):326–328. doi: 10.1038/180326a0. [DOI] [PubMed] [Google Scholar]
- 11.Marotta CA, Wilson JT, Forget BG, Weissman SM. Human β globin messenger RNA. III. Nucleotide sequences derived from complementary DNA. The Journal of Biological Chemistry. 1977;252(14):5040–5053. [PubMed] [Google Scholar]
- 12.Bunn HF, Forget BG. Hemoglobin: Molecular, Genetic and Clinical Aspects. Philadelphia, Pa, USA: WB Saunders; 1986. [Google Scholar]
- 13.Olivieri O, Vitoux D, Galacteros F, et al. Hemoglobin variants and activity of the (K+Cl−) cotransport system in human erythrocytes. Blood. 1992;79(3):793–797. [PubMed] [Google Scholar]
- 14.Weatherall DJ. The inherited diseases of haemoglobin are an emerging global health burden. Blood. 2010;115:4331–4336. doi: 10.1182/blood-2010-01-251348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinberg MH. Genetic etiologies for phenotypic diversity in sickle cell anemia. TheScientificWorldJournal. 2009;9:46–67. doi: 10.1100/tsw.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sebastini P, Solovieff N, Hartley SW, et al. Genetic modifiers of the severity of sickle cell anemia idenitified through a genome-wide association study. American Journal of Hematology. 2010;85:29–35. doi: 10.1002/ajh.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thein SL, Menzel S. Discovering the genetics underlying foetal haemoglobin production in adults. British Journal of Haematology. 2009;145(4):455–467. doi: 10.1111/j.1365-2141.2009.07650.x. [DOI] [PubMed] [Google Scholar]
- 18.Powars D, Chan LS, Schroeder WA. The variable expression of sickle cell disease is genetically determined. Seminars in Hematology. 1990;27(4):360–376. [PubMed] [Google Scholar]
- 19.Ohene-Frempong K, Steinberg MH. Clinical aspects of sickle cell anemia in adults and children. In: Steinberg MH, Forget BG, Higgs DR, Nagel RL, editors. Disorders of Hemoglobin. Cambridge, UK: Cambridge University Press; 2001. pp. 611–670. [Google Scholar]
- 20.Dalibalta S, Ellory JC, Browning JA, Wilkins RJ, Rees DC, Gibson JS. Novel permeability characteristics of red blood cells from sickle cell patients. Blood Cells, Molecules, and Diseases. 2010;45(1):46–52. doi: 10.1016/j.bcmd.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Hebbel RP. Beyond hemoglobin polymerization: the red blood cell membrane and sickle disease pathophysiology. Blood. 1991;77(2):214–237. [PubMed] [Google Scholar]
- 22.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nature Medicine. 2002;8(12):1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 23.Kato GJ, McGowan V, Machado RF, et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107(6):2279–2285. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stuart MJ, Nagel RL. Sickle-cell disease. The Lancet. 2004;364(9442):1343–1360. doi: 10.1016/S0140-6736(04)17192-4. [DOI] [PubMed] [Google Scholar]
- 25.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. Journal of the American Medical Association. 2005;293(13):1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 26.Wood KC, Hsu LL, Gladwin MT. Sickle cell disease vasculopathy: a state of nitric oxide resistance. Free Radical Biology and Medicine. 2008;44(8):1506–1528. doi: 10.1016/j.freeradbiomed.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Eaton WA, Hofrichter J. Hemoglobin S gelation and sickle cell disease. Blood. 1987;70(5):1245–1266. [PubMed] [Google Scholar]
- 28.Lew VL, Bookchin RM. Ion transport pathology in the mechanism of sickle cell dehydration. Physiological Reviews. 2005;85(1):179–200. doi: 10.1152/physrev.00052.2003. [DOI] [PubMed] [Google Scholar]
- 29.Hoover R, Rubin R, Wise G, Warren R. Adhesion of normal and sickle erythrocytes to endothelial monolayer cultures. Blood. 1979;54(4):872–876. [PubMed] [Google Scholar]
- 30.Hebbel RP, Boogaerts MAB, Eaton JW, Steinberg MH. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. The New England Journal of Medicine. 1980;302(18):992–995. doi: 10.1056/NEJM198005013021803. [DOI] [PubMed] [Google Scholar]
- 31.Kaul DK, Liu X-D, Zhang X, et al. Peptides based on αV-binding domains of erythrocyte ICAM-4 inhibit sickle red cell-endothelial interactions and vaso-occlusion in the microcirculation. American The Journal of Physiology. 2006;291(5):C922–C930. doi: 10.1152/ajpcell.00639.2005. [DOI] [PubMed] [Google Scholar]
- 32.Yamaja Setty BN, Kulkarni S, Stuart MJ. Role of erythrocyte phosphatidylserine in sickle red cell-endothelial adhesion. Blood. 2002;99(5):1564–1571. doi: 10.1182/blood.v99.5.1564. [DOI] [PubMed] [Google Scholar]
- 33.Kuypers FA. Red cell membrane lipids in hemoglobinopathies. Current Molecular Medicine. 2008;8(7):633–638. doi: 10.2174/156652408786241429. [DOI] [PubMed] [Google Scholar]
- 34.Joiner CH. Cation transport and volume regulation in sickle red blood cells. American The Journal of Physiology. 1993;264(2):C251–C270. doi: 10.1152/ajpcell.1993.264.2.C251. [DOI] [PubMed] [Google Scholar]
- 35.Yamaja Setty BN, Betal SG. Microvascular endothelial cells express a phosphatidylserine receptor: a functionally active receptor for phosphatidylserine-positive erythrocytes. Blood. 2008;111(2):905–914. doi: 10.1182/blood-2007-07-099465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Setty BNY, Betal SG, Zhang J, Stuart MJ. Heme induces endothelial tissue factor expression: potential role in hemostatic activation in patients with hemolytic anemia. Journal of Thrombosis and Haemostasis. 2008;6(12):2202–2209. doi: 10.1111/j.1538-7836.2008.03177.x. [DOI] [PubMed] [Google Scholar]
- 37.Gibson JS, Ellory JC. Membrane transport in sickle cell disease. Blood Cells, Molecules, and Diseases. 2002;28(3):303–314. doi: 10.1006/bcmd.2002.0515. [DOI] [PubMed] [Google Scholar]
- 38.Brugnara C, Bunn HF, Tosteson DC. Regulation of erythrocyte cation and water content in sickle cell anemia. Science. 1986;232(4748):388–390. doi: 10.1126/science.3961486. [DOI] [PubMed] [Google Scholar]
- 39.Canessa M, Spalvins A, Nagel RL. Volume-dependent and NEM-stimulated K+, Cl− transport is elevated in oxygenated SS, SC and CC human red cells. FEBS Letters. 1986;200(1):197–202. doi: 10.1016/0014-5793(86)80538-5. [DOI] [PubMed] [Google Scholar]
- 40.Gibson JS, Speake PF, Ellory JC. Differential oxygen sensitivity of the K+-Cl− cotransporter in normal and sickle human red blood cells. The Journal of Physiology. 1998;511(1):225–234. doi: 10.1111/j.1469-7793.1998.225bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hall AC, Ellory JC. Evidence for the presence of volume-sensitive KCl transport in ’young’ human red cells. Biochimica et Biophysica Acta. 1986;858(2):317–320. doi: 10.1016/0005-2736(86)90338-x. [DOI] [PubMed] [Google Scholar]
- 42.Ellory JC, Hall AC, Ody SA. Is acid a more potent activator of KCl co-transport than hypotonicity in human red cells? The Journal of Physiology. 1990;420:p. 149. [Google Scholar]
- 43.Gibson JS, Cossins AR, Ellory JC. Oxygen-sensitive membrane transporters in vertebrate red cells. Journal of Experimental Biology. 2000;203(9):1395–1407. doi: 10.1242/jeb.203.9.1395. [DOI] [PubMed] [Google Scholar]
- 44.Gibson JS, Ellory JC. K+-Cl− cotransport in vertebrate red cells. In: Bernhardt I, Ellory JC, editors. Red Cell Membrane Transport in Health and Disease. New York, NY, USA: Springer; 2004. pp. 197–220. [Google Scholar]
- 45.Ortiz OE, Lew VL, Bookchin RM. Deoxygenation permeabilizes sickle cell anaemia red cells to magnesium and reverses its gradient in the dense cells. The Journal of Physiology. 1990;427:211–226. doi: 10.1113/jphysiol.1990.sp018168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willcocks JP, Mulquiney PJ, Ellory JC, Veech RL, Radda GK, Clarke K. Simultaneous determination of low free Mg2+ and pH in human sickle cells using 31P NMR spectroscopy. The Journal of Biological Chemistry. 2002;277(51):49911–49920. doi: 10.1074/jbc.M207551200. [DOI] [PubMed] [Google Scholar]
- 47.Mohandas N, Rossi ME, Clark MR. Association between morphologic distortion of sickle cells and deoxygenation-induced cation permeability increase. Blood. 1986;68(2):450–454. [PubMed] [Google Scholar]
- 48.Lew VL, Ortiz OE, Bookchin RM. Stochastic nature and red cell population distribution of the sickling- induced Ca2+ permeability. The Journal of Clinical Investigation. 1997;99(11):2727–2735. doi: 10.1172/JCI119462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Browning JA, Robinson HC, Ellory JC, Gibson JS. Deoxygenation-induced non-electrolyte pathway in red cells from sickle cell patients. Cellular Physiology and Biochemistry. 2007;19(1–4):165–174. doi: 10.1159/000099204. [DOI] [PubMed] [Google Scholar]
- 50.Rhoda MD, Apovo M, Beuzard Y, Giraud F. Ca2+ permeability in deoxygenated sickle cells. Blood. 1990;75(12):2453–2458. [PubMed] [Google Scholar]
- 51.Woon LA, Holland JW, Kable EPW, Roufogalis BD. Ca2+ sensitivity of phospholipid scrambling in human red cell ghosts. Cell Calcium. 1999;25(4):313–320. doi: 10.1054/ceca.1999.0029. [DOI] [PubMed] [Google Scholar]
- 52.Gárdos G. The function of calcium in the potassium permeability of human erythrocytes. Biochimica et Biophysica Acta. 1958;30(3):653–654. doi: 10.1016/0006-3002(58)90124-0. [DOI] [PubMed] [Google Scholar]
- 53.Nagel RL, Platt OS. General pathophysiology of sickle cell anemia. In: Steinberg MH, Forget BG, Higgs DR, Nagel RL, editors. Disorders of Hemoglobin. Cambridge, UK: Cambridge University Press; 2001. pp. 494–526. [Google Scholar]
- 54.Franco RS, Palascak M, Thompson H, Joiner CH. KCl cotransport activity in light versus dense transferrin receptor- positive sickle reticulocytes. The Journal of Clinical Investigation. 1995;95(6):2573–2580. doi: 10.1172/JCI117958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franco RS, Palascak M, Thompson H, Rucknagel DL, Joiner CH. Dehydration of transferrin receptor-positive sickle reticulocytes during continuous or cyclic deoxygenation: role of KCl cotransport and extracellular calcium. Blood. 1996;88(11):4359–4365. [PubMed] [Google Scholar]
- 56.Bookchin RM, Ortiz OE, Lew VL. Evidence for a direct reticulocyte origin of dense red cells in sickle cell anemia. The Journal of Clinical Investigation. 1991;87(1):113–124. doi: 10.1172/JCI114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Serjeant GR, Serjeant BE. A comparison of erythrocyte characteristics in sickle cell syndromes in Jamaica. British Journal of Haematology. 1972;23(2):205–213. doi: 10.1111/j.1365-2141.1972.tb03473.x. [DOI] [PubMed] [Google Scholar]
- 58.Fabry ME, Kaul DK, Raventos-Suarez C. SC erythrocytes have an abnormally high intracellular hemoglobin concentration. Pathophysiological consequences. The Journal of Clinical Investigation. 1982;70(6):1315–1319. doi: 10.1172/JCI110732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ballas SK, Larner J, Smith ED, Surrey S, Schwartz E, Rappaport EF. The xerocytosis of HbSC disease. Blood. 1987;69:124–130. [PubMed] [Google Scholar]
- 60.Lawrence C, Fabry ME, Nagel RL. The unique red cell heterogeneity of SC disease: crystal formation, dense reticulocytes, and unusual morphology. Blood. 1991;78(8):2104–2112. [PubMed] [Google Scholar]
- 61.Fabry ME, Harrington J, Chang H, Nagel RL. Critical contribution of cell density to the pathophysiology of SC cells. Clinical Research. 1982;30, article 559a [Google Scholar]
- 62.Bookchin RM, Balazs T. Ionic strength dependence of the polymer solubilities of deoxyhemoglobin S + C and S + A mixtures. Blood. 1986;67(4):887–892. [PubMed] [Google Scholar]
- 63.Minton AP. Influence of macromolecular crowding on intracellular association reactions: possible role in volume regulation. In: Strange K, editor. Cellular and Molecular Physiology of Cell Volume Regulation. Boca Raton, Fla, USA: CRC Press; 1994. pp. 181–190. [Google Scholar]
- 64.Gibson JS, Muzyamba MC, Ball SE, Ellory JC. K+ transport in HbSC-containing human red blood cells. The Journal of Physiology. 2001;535, article S008:p. 27. [Google Scholar]
- 65.Ellory JC, Sequeira R, Constantine A, Wilkins RJ, Gibson JS. Non-electrolyte permeability of deoxygenated sickle cells compared. Blood Cells, Molecules, and Diseases. 2008;41(1):44–49. doi: 10.1016/j.bcmd.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 66.Browning JA, Staines HM, Robinson HC, Powell T, Ellory JC, Gibson JS. The effect of deoxygenation on whole-cell conductance of red blood cells from healthy individuals and patients with sickle cell disease. Blood. 2007;109(6):2622–2629. doi: 10.1182/blood-2006-03-001404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gibson JS, Khan A, Speake PF, Ellory JC. O2 dependence of K+ transport in sickle cells: effect of different cell populations and the substituted benzaldehyde 12C79. The FASEB Journal. 2001;15(3):823–832. doi: 10.1096/fj.00-0177com. [DOI] [PubMed] [Google Scholar]