Abstract
Induction of phase 2 enzymes and elevations of glutathione are major and sufficient strategies for protecting mammals and their cells against the toxic and carcinogenic effects of electrophiles and reactive forms of oxygen. Inducers belong to nine chemical classes and have few common properties except for their ability to modify sulfhydryl groups by oxidation, reduction, or alkylation. Much evidence suggests that the cellular “sensor” molecule that recognizes the inducers and signals the enhanced transcription of phase 2 genes does so by virtue of unique and highly reactive sulfhydryl functions that recognize and covalently react with the inducers. Benzylidene-alkanones and -cycloalkanones are Michael reaction acceptors whose inducer potency is profoundly increased by the presence of ortho- (but not other) hydroxyl substituent(s) on the aromatic ring(s). This enhancement correlates with more rapid reactivity of the ortho-hydroxylated derivatives with model sulfhydryl compounds. Proton NMR spectroscopy provides no evidence for increased electrophilicity of the β-vinyl carbons (the presumed site of nucleophilic attack) on the hydroxylated inducers. Surprisingly, these ortho-hydroxyl groups display a propensity for extensive intermolecular hydrogen bond formation, which may raise the reactivity and facilitate addition of mercaptans, thereby raising inducer potencies.
Mammalian cells have developed remarkably efficient protective mechanisms against both acute and chronic toxicities of electrophiles and reactive oxygen species that are the major causes of malignancy. The two primary lines of defense are (i) a family of phase 2 enzymes that detoxify electrophiles and serve as indirect antioxidants; and (ii) glutathione (GSH), the most abundant cellular nonprotein thiol. Phase 2 enzyme activities and GSH levels do not normally operate at their maximal capacity, but they can be transcriptionally induced by a wide variety of natural and synthetic chemical agents, thereby achieving efficient protection against carcinogenesis (1–3). The family of inducible phase 2 proteins is enormously diverse, and each member plays a distinct role in cellular protection. In addition to inducing the “classical” phase 2 drug-metabolizing enzymes, such as glutathione S-transferases (GST) and UDP-glucuronosyltransferase (4, 5) that conjugate xenobiotics with endogenous ligands, this group of inducible proteins now includes NAD(P)H:quinone reductase (NQO1) (6), epoxide hydrolase (7), heme oxygenase 1 (8, 9), ferritin (9), γ-glutamylcysteine synthetase (10–12), aflatoxin aldehyde reductase (13, 14), catalase and superoxide dismutase (12), dihydrodiol dehydrogenase (15), leukotriene B4 dehydrogenase (16), and glutathione S-conjugate efflux pumps (see ref. 17 for a review).
The genes for many of these proteins contain 5′-upstream antioxidant (electrophile) responsive elements (ARE/EpRE; consensus sequence TGACNNNGC), which regulate both their basal and inducible expression. The identities of the transcription factors that interact with the ARE elements are under active study (see ref. 18 for review), but clearly Nrf2, a member of the basic leucine zipper family of transcription factors, plays a central role in phase 2 enzyme expression (19, 20). The recent findings that nrf2-deficient mice are resistant to enzyme induction, display increased susceptibility to carcinogenesis, and are not protected by a phase 2 enzyme inducer provide major support for this conclusion (21). Nrf2 is normally localized mainly in the cytosol bound to a chaperone, Keap1 (19, 20). Exposure of cells to inducers disrupts the Keap1–Nrf2 complex, and Nrf2 migrates to the nucleus, where it binds (in heterodimeric forms with other transcription factors) to the ARE enhancer regions of phase 2 genes and stimulates their transcription. Keap1, a 624-amino acid protein, contains 25 cysteine residues, 9 of which are expected to have highly reactive sulfhydryl groups (low pKa values) because they are flanked by one or more basic amino acid residues (22). Because all inducers react with sulfhydryl groups (see below), the Keap1–Nrf2 complex is a plausible candidate for the cytoplasmic sensor system that recognizes and reacts with inducers.
To monitor inducer activity, we have measured NQO1, which is induced to high levels in many animal tissues and cells by a variety of dietary and synthetic agents (2, 6, 23, 24). A simple and rapid system for measuring the specific activity of NQO1 in murine hepatoma cells grown in 96-well plates (25, 26) has been invaluable for determining inducer potency. The concentration of a compound that is required to double NQO1 specific activity (CD value) provides a quantitative measure of inducer potency. Nine distinct chemical classes of monofunctional inducers have been identified (2, 23, 27, 28): (i) oxidizable diphenols and quinones; (ii) Michael reaction acceptors (olefins or acetylenes conjugated to electron-withdrawing groups); (iii) isothiocyanates; (iv) hydroperoxides; (v) trivalent arsenic derivatives; (vi) divalent heavy metal cations (Hg2+, Cd2+); (vii) vicinal dithiols; (viii) 1,2-dithiole-3-thiones; and (ix) carotenoids and other conjugated polyenes. The chemistry of these seemingly unrelated inducers provides little immediate insight into the nature of the cellular sensor target with which they might react: some are oxidants, others are antioxidants, and a third group (e.g., isothiocyanates) are neither. Nevertheless, although architecturally dissimilar, inducers share several common properties: (i) all are chemically reactive; (ii) all are electrophiles except for vicinal dithiols; (iii) most are substrates for GST (29); and (iv) all can covalently modify sulfhydryl groups by alkylation, oxidation, or reduction.
The prevailing view—for which direct evidence is lacking—is that the primary cellular sensor with which inducers must react to initiate the signaling of transcriptional activation is a protein endowed with highly reactive sulfhydryl group(s) that is chemically modified by the inducers. This view is consistent with the following properties: (i) the inducer potencies of Michael reaction acceptors parallel their reactivity with nucleophilic donors such as mercaptans (29, 30); (ii) many inducers are classical sulfhydryl reagents—e.g., diethyl maleate, 1-chloro-2,4-dinitrobenzene; (iii) the most potent metal inducers (Hg2+ > Cd2+ > Zn2+) have the highest affinities for mercaptans (27); (iv) the inducer potencies of a series of isothiocyanates are closely related to their second-order nonenzymatic rate constants of reaction with GSH (31); and (v) many inducers are substrates for GST (29). The idea that the primary cellular sensor protein may indeed contain two reactive vicinal thiol groups is consistent with the extremely high inducer potency of trivalent arsenicals such as phenylarsenoxide (CD = 57 nM), which can readily form very stable five-membered cyclic thioarsenites.
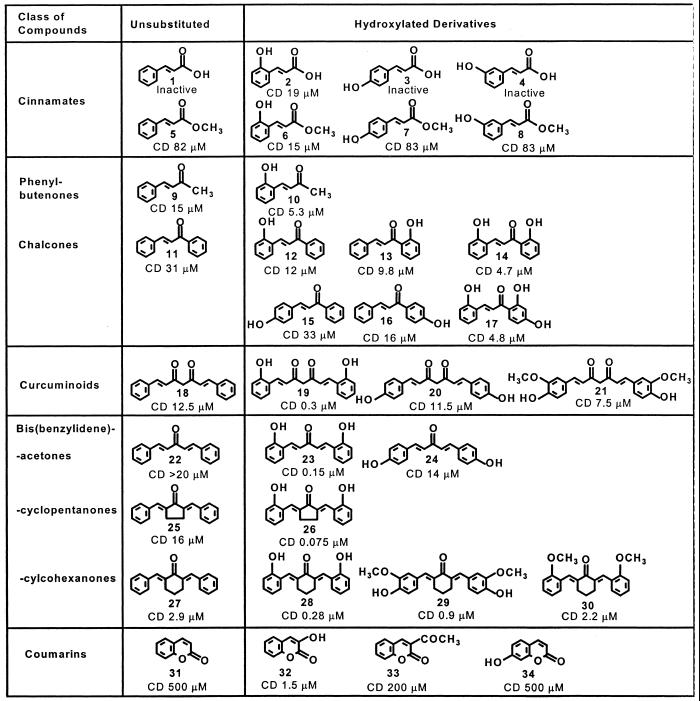
To gain further insight into the mechanisms of phase 2 enzyme induction and the role of sulfhydryl reactivity, we have analyzed a group of Michael reaction acceptor-containing plant cinnamates, coumarins, chalcones, curcuminoids, and the related synthetic bis(benzylidene)cycloalkanones, some of which are very potent inducers of NQO1 (Table 1). Remarkably, the presence of hydroxyl group(s) at the ortho position(s) on the aromatic ring(s) enhanced dramatically their inducer potencies (32, 33).
Table 1.
Structures and quinone reductase inducer potencies of several classes of Michael reaction acceptors
The concentrations (CD values) are shown that double the quinone reductase specific activity in Hepa 1c1c7 cells.
This paper focuses on the relation between structure and inducer potencies of a series of conjugated benzylidene ketone Michael reaction acceptors of plant and synthetic origins. We show that the potencies for phase 2 enzyme induction, for raising GSH levels in several cell types, and for reaction with model sulfhydryl compounds are closely related. The aforementioned dramatic and coordinate enhancements of potencies for these three processes by ortho-hydroxyl substituents on the aromatic rings of benzylidene groups is universal. This suggests that reaction with reactive sulfhydryl group(s) on the “sensor” protein that recognizes and reacts with inducers, possibly Keap1, is a critical component of the transcriptional activation of phase 2 proteins. We propose a mechanism whereby the ortho-hydroxyl substituents can enhance the nucleophilic reactivity of sulfhydryl groups.
Materials and Methods
Materials.
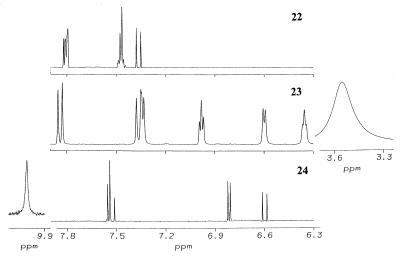
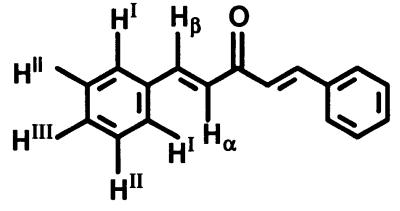
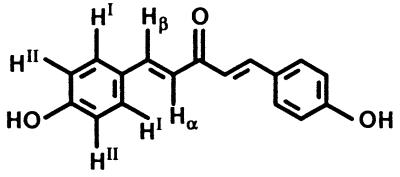
All structures are shown in Table 1. Chalcones 11, 15, and 16, bis(benzylidene)acetone 22, 2,6-bis(2-methoxybenzylidene)cyclohexanone 30, 1-chloro-2,4-dinitrobenzene, GSH, metaphosphoric acid, and 5,5′-dithio-bis(2-nitrobenzoic acid) were obtained from Sigma–Aldrich (Milwaukee, WI); chalcones 12, 13, 14, and 17 were from Indofine (Somerville, NJ). All other bis(benzylidene)alkanones were synthesized by condensation of the corresponding benzaldehyde with acetone or appropriate cycloalkanone (32, 34). The identities of the bisbenzylidene derivatives were established by 1H NMR spectroscopy (600 MHz, DMSO-d6, 25°C). Bis(benzylidene)acetone 22 δ (ppm): 7.80 (HI + Hβ), 7.47 (HII + HIII), 7.37 (Hα). Bis(2-hydroxybenzylidene)acetone 23 δ (ppm): 7.83 (Hβ), 7.35 (HI + Hα), 6.98 (HIII), 6.60 (HIV), 6.36 (HII), 3.57 (OH). Bis(4-hydroxybenzylidene)acetone 24 δ (ppm): 10.05 (OH), 7.55 (HI + Hβ), 6.81 (HII), 6.60 (Hα). The spectra are shown in Fig. 3 and the designation of the protons is given in Table 6. All benzylidene derivatives have E–E geometry as determined from coupling constants (acetones) or nuclear Overhauser enhancements (cycloalkanones).
Figure 3.
Proton NMR spectra (600-MHz, DMSO-d6) of bis(benzylidene)acetone (22), bis(2-hydroxybenzylidene)acetone (23), and bis(4-hydroxybenzylidene)acetone (24). The amplitudes of the hydroxyl resonances for 23 (3.57 ppm) and for 24 (10.05 ppm) are scaled with respect to the other resonances at 0.67-fold and 15-fold.
Table 6.
13C NMR spectral assignments for bis(benzylidene)acetones 22, 23, and 24
Cell Cultures.
All cells were grown at 37°C in a 5% CO2/95% air atmosphere. Hepa 1c1c7 murine hepatoma cells were cultured in α minimum essential medium (α-MEM), supplemented with 10% heat- and charcoal-treated FBS (1 g of charcoal per 100 ml of serum; 90 min at 55°C); MCF 7 human breast cancer cells (a gift from Alan J. Townsend, Wake Forest Univ. School of Medicine, Winston-Salem, NC) were cultured in Dulbecco's modified Eagle's medium (DMEM) with 5% FBS; and PE murine keratinocytes (a gift from Stuart H. Yuspa, National Cancer Institute, Bethesda, MD) and HaCaT human keratinocytes (a gift from G. Tim Bowden, Arizona Cancer Center, Tucson) were cultured in Eagle's minimum essential medium (EMEM) with 8% FBS, treated with Chelex resin (Bio-Rad) to remove Ca2+.
Enzyme Activity Assays.
All enzyme assays were at 25°C.
GSTs.
Cells (106) were grown in 10-cm plastic Petri dishes for 24 h, induced for 48 h, and lysed by sonication. Cell lysate supernatant fractions were then assayed for GST activities spectrophotometrically with 1-chloro-2,4-dinitrobenzene and GSH as substrates (35).
Quinone reductase.
Activity of NQO1 in each cell line was determined by the Prochaska test (25, 26). Cells were grown for 24 h in 96-well plates (10,000 per well for Hepa 1c1c7 and MCF 7 cells; 20,000 per well for PE cells) and then exposed to serial dilutions of inducers for 48 h. The concentration required to double the specific activity of NQO1 (CD value) was used as a measure of inducer potency.
Determination of Cellular GSH.
Cells were grown for 24 h in 96-well plates (10,000 per well for Hepa 1c1c7 and MCF 7 cells; 30,000 per well for PE cells), exposed to serial dilutions of inducers for 24 h, and finally lysed in 50 μl of 0.08% digitonin. One half of the wells were used for protein determination. The other half received 50 μl of ice-cold metaphosphoric acid (50 g/liter) in 2 mM EDTA to precipitate cellular protein. After 10 min at 4°C, plates were centrifuged at 1,500 × g for 15 min and 50 μl of the resulting supernatant fractions was transferred to the corresponding wells of a parallel plate. To each of these wells, 50 μl of 200 mM sodium phosphate buffer, pH 7.5, containing 10 mM EDTA, was added and total cellular GSH was determined by rate measurements in a recycling assay (36, 37).
Determination of Rate of Reaction with Sulfhydryl Groups.
Each compound was incubated with the corresponding sulfhydryl reagent in a mixture of equal volumes of acetonitrile and 100 mM Tris⋅HCl, pH 7.4, at 25°C, and the decrease in absorbance at the following wavelength maxima was monitored with time: 22, 330 nm; 23, 370 nm; 24, 320 nm; 25, 367 nm; and 26, 390 nm. The extinction coefficients were 35,000–38,000 M−1⋅cm−1.
NMR Spectroscopy. One-dimensional proton, as well as two-dimensional COSY, NOESY, HSQC, and HMBC NMR¶ spectra were obtained on a Varian Unity Plus 600-MHz spectrometer equipped with a 5-mm triple-resonance probe and gradient capabilities.
Northern Blot Analysis.
MCF 7 cells (106) were grown for 24 h in 10-cm Petri dishes and exposed to 5 μM 2,5-bis(2-hydroxybenzylidene)cyclopentanone (26) for a further 24 h. RNA (10 μg) was electrophoretically separated and transferred to Nytran membranes (38, 39). The cDNA for human NQO1 (a gift from David Ross, Univ. of Colorado, Boulder) was labeled with [α-32P]dCTP (40), heat-denatured, hybridized to the blot (18 h at 42°C), and washed. Radioactivities corresponding to NQO1 transcripts were quantified by phosphorimaging (Bio-Image analyzer BAS 2500, Fujifilm) and autoradiography at −80°C.
Results and Discussion
Potencies of Michael Reaction Acceptors as Inducers of NQO1 in Murine Hepatoma Cells.
Structure–activity analyses of a number of plant-derived natural and synthetic phenylpropenoid Michael reaction acceptors—i.e., cinnamates, chalcones, coumarins, curcuminoids, and bis(benzylidene)cycloalkanones established that the presence of unsubstituted hydroxyl group(s) at the ortho, but not para or meta, positions of the aromatic ring(s), increased the NQO1 inducer potency dramatically (Table 1). This conclusion is based on a large number of analogues belonging to several chemical classes (32, 33). Thus, compare 2,5-bis(benzylidene)cyclopentanone (25; CD 16 μM) and 2,5-bis(2-hydroxybenzylidene)cyclopentanone (26; CD 0.075 μM); and 2,6-bis(benzylidene)cyclohexanone (27; CD 2.9 μM) and 2,6-bis(2-hydroxybenzylidene)cyclohexanone (28; CD 0.28 μM). The enhancement of inducer potency ranges from 3-fold for the ketones to more than 300-fold for the coumarins. The double Michael reaction acceptor 26 was identified as the most potent inducer of this series, with a CD value of 0.075 μM and was capable of inducing NQO1 more than 10-fold without any detectable cytotoxicity.
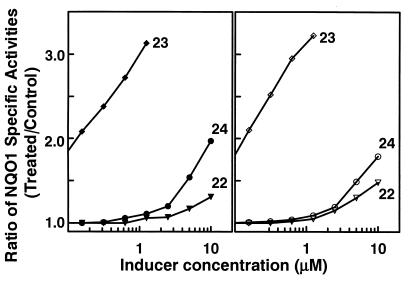
To extend the scope of these observations and to assess the importance of the central cycloalkanone ring, we now compare the inducer potencies of the acyclic bis(benzylidene)acetone, 22, and its ortho- and para-hydroxylated analogues, 23 and 24, respectively. The nonsubstituted 22 (CD > 20 μM) and its para-hydroxylated analogue 24 (CD 14 μM) were both weak inducers (Fig. 1). In sharp contrast, the ortho-hydroxylated derivative 23 was a very potent inducer of NQO1 in both murine hepatoma (Hepa 1c1c7) and murine papilloma (PE) cells, with CD values of 0.15 μM and 0.10 μM, respectively. Although the presence of a cycloalkanone ring is not required for inducer activity, hydroxyl groups at ortho positions on the aryl rings are also critical for high inducer potency of the acetone derivatives. Understanding the mechanism of this phenomenon is crucial for designing chemoprotective inducers.
Figure 1.
Induction of specific activities of NQO1 in Hepa 1c1c7 murine hepatoma cells (Left) and PE murine papilloma cells (Right) upon exposure for 48 h to bis(benzylidene)acetone, 22; bis(2-hydroxybenzylidene)acetone, 23; or bis(4-hydroxybenzylidene)acetone, 24.
Induction of Quinone Reductase 1 and GSTs and Elevation of GSH by Natural and Synthetic Michael Reaction Acceptors.
To establish that the observed elevation in the activities of phase 2 detoxification enzymes upon exposure to such compounds is restricted neither to NQO1 nor to the particular cell line (Hepa 1c1c7), we compared the GSH levels and the activities of GST and NQO1 in PE murine papilloma and MCF 7 human breast cancer cells with those in Hepa1c1c7 murine hepatoma cells. All three endpoints were significantly and in some instances dramatically increased when all cell types were exposed to 10 μM 2,5-bis(2-hydroxybenzylidene)cyclopentanone (26) for 48 h (Table 2).
Table 2.
Induction of NQO1 and GST and elevation of GSH by 10 μM 2,5-bis(2-hydroxybenzylidene)cyclopentanone (26) in three cell types
| Cell type | NQO1, nmol/min per
mg
|
GST, nmol/min per mg
|
GSH, nmol per mg
protein
|
|||
|---|---|---|---|---|---|---|
| Control | Treated | Control | Treated | Control | Treated | |
| Hepa | 477 | 4,000 | 305 | 490 | 16.9 | 38.1 |
| PE | 174 | 783 | 57.2 | 139 | 10.1 | 70.7 |
| MCF 7 | 1,398 | 4,200 | 2.2 | 3.8 | 26.4 | 83.1 |
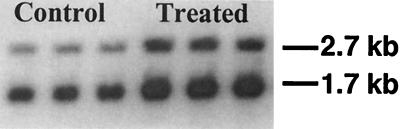
Northern blots (Fig. 2) demonstrated that exposure of human MCF 7 cells to 5 μM 2,5-bis(2-hydroxybenzylidene)cyclopentanone (26) for 24 h increased NQO1 mRNA levels. Upon hybridization to radiolabeled full-length NQO1 cDNA, two transcripts (1.7 and 2.7 kb) were easily detected. These correspond to the use of two alternative polyadenylation sites in the 3′-untranslated region of human NQO1 mRNA (41). The two transcripts were induced 3.0- and 3.9-fold, respectively.
Figure 2.
Northern blot analysis of NQO1 mRNA isolated from MCF 7 cells. Lanes 1–3 (from left), controls; lanes 4–6, treated with 5 μM 2,5-bis(2-hydroxybenzylidene)cyclopentanone (26) for 24 h.
The induction patterns of NQO1, GST, and GSH were similar in all three cell lines, but the increases were most dramatic in PE murine keratinocytes—e.g., exposure of these cells to 2,5-bis(2-hydroxybenzylidene)cyclopentanone (26) raised the concentration of GSH from 4 mM to nearly 30 mM. We therefore used this cell line as a model to correlate the potencies of such compounds to induce NQO1 and elevate GSH. Table 3 shows the correlation of the rank orders of inducer potencies of the bis(benzylidene)alkanones for both assays. Again, the most potent compounds bear ortho-hydroxyl substitutions on the aryl rings—i.e., bis(2-hydroxybenzylidene)acetone (23) and 2,6-bis(2-hydroxybenzylidene)cyclohexanone (28). Both compounds induced NQO1 and elevated GSH at comparable concentrations: with CD values of 0.14 μM (compound 23) and 0.16 μM (compound 28), and concentrations that elevated GSH by 50% of 0.4 μM (compound 23) and 0.3 μM (compound 28), respectively.
Table 3.
Comparison of potencies of bis(benzylidene)alkanones in inducing NQO1 and elevating GSH in PE murine papilloma cells
| Compound | Conc. that doubles NQO1 activity,* μM | Conc. that elevates GSH by 50%,* μM |
|---|---|---|
| Acetone derivatives | ||
| Bis(benzylidene)- (22) | >20 (6) | 5.3 (6) |
| Bis(2-hydroxybenzylidene)- (23) | 0.14 (1) | 0.4 (2) |
| Bis(4-hydroxybenzylidene)- (24) | >10 (5) | >10 (7) |
| Cyclohexanone derivatives | ||
| 2,6-Bisbenzylidene- (27) | 2.5 (4) | 2.5 (4) |
| 2,6-Bis(2-hydroxybenzylidene)- (28) | 0.16 (2) | 0.3 (1) |
| 2,6-Bis(2-methoxy-4-hydroxybenzylidene)- (29) | 0.6 (3) | 0.9 (3) |
| 2,6-Bis(2-methoxybenzylidene)- (30) | 2.5 (4) | 3.7 (5) |
The potency order rank is shown in parentheses.
Examination of a series of chalcones confirmed these findings. The chalcones are a diverse group of naturally occurring plant metabolites that can be regarded as open-chain flavonoids, in which the two aromatic rings are bridged by an α,β-unsaturated carbonyl (Michael reaction acceptor) moiety. We have previously reported a detailed structure–activity study for their ability to induce NQO1 in Hepa 1c1c7 cells (32, 33) (see Table 1). The potencies of these compounds in raising GSH levels correlated with those that elevate NQO1. The nonsubstituted chalcone 11 was the least effective, whereas the presence of phenolic hydroxyl(s) on either aryl ring(s) increased the inducer potency (data not shown). The simultaneous presence of three hydroxyl groups has a profound effect, as represented by 2,2′,4′-trihydroxychalcone (17), which was the most potent inducer in this series, capable of elevating NQO1 and GSH more than 6- and 3-fold, respectively.
The seemingly lower sensitivity of the GSH response is not surprising, considering that (i) this tripeptide is normally already present in the cell at millimolar concentrations, and (ii) the enzyme catalyzing the rate-limiting step in its synthesis, γ-glutamylcysteine synthetase, is subject to feedback inhibition by the final product, GSH (42). The mechanisms involved in the transcriptional regulation of this enzyme as part of the phase 2 detoxification enzyme cascade have been recently reviewed (43). The finding that the cellular levels of GSH could be further increased severalfold by components of the human diet (e.g., chalcones), or by very low concentrations of their synthetic analogues [e.g., bis(benzylidene)alkanones], was especially intriguing and could be of critical importance under certain pathological conditions, in which the GSH status is compromised. Notably, coordinate induction of GSH together with several phase 2 proteins can be achieved by dietary means in vivo and has been observed repeatedly in rodent tissues (17, 44).
Relation of Inducer Potency of Bis(benzylidene) Ketone Michael Reaction Acceptors to Their Rates of Reaction with Sulfhydryl Groups.
The only known universal property of all phase 2 inducers is their reactivity with sulfhydryl groups, which has been proposed as responsible for the initial “sensing” of inducers. Does the introduction of ortho-hydroxyl group(s) in benzylidene alkanones, which dramatically increases their inducer potencies, also increase the reactivity of such compounds with mercaptans? Comparison of the second-order reaction rate constants (k2) of 2,5-bis(benzylidene)cyclopentanone (25; CD 16 μM) and 2,5-bis(2-hydroxybenzylidene)cyclopentanone (26; CD 0.075 μM) with a variety of monothiols and dithiols revealed consistently higher rates (1.4- to 12.7-fold) for the hydroxylated species (Table 4). The impact of the hydroxyl groups on the reactivity with GSH, dithiothreitol, and dithioerythritol was especially large (≈10-fold). This correlation was fully supported by the reactivities of the unsubstituted (22; CD >20 μM) and ortho-hydroxylated (23; CD 0.15 μM) bis(benzylidene)acetone derivatives with dithiothreitol and GSH (Table 5). This rate enhancement was especially impressive with GSH (35-fold). In contrast, para-hydroxyl substitution decreased the reaction rate enormously, consistent with the much lower electrophilicity of the β-carbon of the olefinic group, which is the presumed site of nucleophilic attack (Table 6). Most importantly, the rates of sulfhydryl addition to these compounds correlated with their ability to elevate cellular GSH in three different cell lines, in agreement with the previous observations that the potency of many phase 2 enzyme inducers paralleled their reactivity in the Michael reaction (29, 30).
Table 4.
Second-order rate constants (k2) for the reactions of 2,5-bis(benzylidene)cyclopentanone (25) and 2,5-bis(2-hydroxybenzylidene)cyclopentanone (26) with sulfhydryl reagents
| Sulfhydryl reagent |
k2 × 103,
M−1⋅sec−1
|
Ratio | |
|---|---|---|---|
| 25 | 26 | ||
| 2-Mercaptoethanol | 13.5 | 97.2 | 7.2 |
| 2,3-Dimercaptopropanol | 35.4 | 48.2 | 1.4 |
| 1,2-Butanedithiol | 9.04 | 18.1 | 2.0 |
| 1,4-Butanedithiol | 3.08 | 11.8 | 3.8 |
| 2,3-Butanedithiol | 3.23 | 4.57 | 1.4 |
| 1,4-Dithiothreitol | 3.11 | 35.7 | 11.7 |
| 1,4-Dithioerythritol | 2.30 | 29.3 | 12.7 |
| GSH | 9.26 | 87.3 | 9.4 |
Table 5.
Comparison of reactivities with sulfhydryl groups and potencies in elevating GSH of bis(benzylidene)acetone derivatives in three cell types
| Acetone derivatives |
k2 for reaction,
M−1⋅sec−1
|
Conc. that
elevates GSH by 50%, μM
|
|||
|---|---|---|---|---|---|
| With DTT | With GSH | Hepa 1c1c | PE | HaCaT | |
| Bis(benzylidene)- (22) | 0.417 | 0.145 | 3.7 | 5.3 | 1.5 |
| Bis(2-hydroxybenzylidene)- (23) | 2.50 | 5.0 | 0.075 | 0.4 | 0.13 |
| Bis(4-hydroxybenzylidene)- (24) | 0.014 | 0.001 | 6.0 | >10 | 2.1 |
DTT, dithiothreitol.
NMR Spectroscopic Characteristics of Bis(benzylidene)acetone Michael Reaction Acceptors: Implications for Sulfhydryl Group Reactivity and Inducer Potency.
The correlation of inducer activity and sulfhydryl group reactivity prompted us to examine the electron density at the vinyl carbons (the presumed site of Michael addition). A series of one- and two-dimensional NMR spectroscopic experiments [i.e., dqfCOSY, NOESY, HMBC, and HMQC]¶ were performed to assign unambiguously the proton and carbon resonances of compounds 22, 23, and 24. All compounds were dissolved in DMSO-d6; additional analyses in benzene-d6 were required for 22 (45).
As expected, the presence of a powerful electron-withdrawing para-hydroxyl group in 24 resulted in a substantial downfield shift (by 15.8 ppm) of the adjacent carbons (CII), indicating a significant decrease in electron density. Surprisingly, this modification caused a substantial upfield shift (by 16.5 ppm) for the β-vinyl carbons (C—Hβ), indicating an increased electron density at this carbon in 24, in agreement with the observed decreased reactivity with dithiothreitol and GSH at the vinyl site (Table 5).
Surprisingly, the introduction of ortho-hydroxyl groups did not have a significant effect on the carbon chemical shifts (compare compounds 22 and 23). Moreover, the slight (and probably insignificant) shifts of the vinyl carbon resonances were upfield, indicating slightly increased electron density. Although contradictory to the increased reactivity with nucleophiles, these findings are entirely consistent with our previous observations on substituted cinnamates (29) and hydroxylated bis(benzylidene)cycloalkanones (32). The conclusion is therefore inescapable that the large inducer potency increases resulting from ortho-hydroxyl groups cannot be ascribed to increased electrophilicity of the β−vinyl carbon atoms of the Michael acceptor groups, and must depend on some other properties of these systems.
Notably, in the 1H NMR spectrum of 24, the chemical shift for the para-hydroxyl appears at 10.05 ppm, as expected for a phenolic hydroxyl (Fig. 3). In sharp contrast, no resonance at ≈10 ppm (or indeed up to >50 ppm) was observed in the spectrum of the ortho-hydroxylated 23. However, a very broad upfield resonance (linewidth = 35 Hz compared with 4 Hz for Hβ) at 3.57 ppm was observed at high concentrations (10–20 mM in DMSO-d6). Moreover, it shifted to 3.38 ppm upon lowering the concentration to 1 mM, while all other resonances remained unchanged. This resonance, distinct from the residual H2O in DMSO, completely disappeared in D2O. Taken together, the unusual upfield position and line broadening of this chemical shift and its concentration dependence strongly indicate that the phenolic hydroxyl of bis(2-hydroxybenzylidene)acetone (23) is involved in extensive intermolecular hydrogen bonding [as observed for neat methanol (46)]. Thus, the potential to participate in hydrogen bond formation may be responsible for the observed enhanced reactivity with model sulfhydryl groups (and higher inducer potency).
Although the exact mechanism(s) by which the ortho-hydroxyl group increases phase 2 enzyme inducer potency and enhances sulfhydryl group reactivity remains unknown, its large impact is consistently observed among all compounds examined in this study. Our findings recall those of Kupchan and his colleagues (47, 48), who observed an enhancement in the rate of cysteine addition to a number of sesquiterpene lactones (α-methylene-γ-lactones) when an aliphatic hydroxyl group was present adjacent to the methylene functionality. The authors postulated that “this rate enhancement presumably arises from a neighboring group facilitation of the addition of S− anion, or proton transfer at some intermediate stage in the addition.”
We suggest that the powerful accelerating effect of
ortho-hydroxyl groups on the reactivity of
benzylidenealkanones with sulfhydryl groups, and the associated
increases in inducer potency for protective phase 2 proteins, may be
explained by the enhanced reactivity (lowering of the
pKa) of the SH group through inductive/hydrogen
bonding of the neighboring phenolic hydroxyl group(s), as
follows:
![]()
The validation of this suggestion requires identification of the proposed highly reactive sulfhydryl group(s) of the biological sensor molecule (possibly the Nrf2-Keap1 complex) and elucidation of the chemical details of its reactivity with inducers.
Acknowledgments
We acknowledge with gratitude enlightened and helpful discussions with our colleagues Philip A. Cole, Yoshitaka Ichikawa, and Thomas W. Kensler. This work was supported by National Institutes of Health Grants CA 44530 and DK 28616 (to Albert S. Mildvan) and by generous gifts from the Lewis and Dorothy Cullman Foundation, New York.
Abbreviations
- CD value
concentration required to double the specific activity of NQO1
- GSH
glutathione
- GST
glutathione S-transferase
- monofunctional inducers
those that cause transcriptional activation of phase 2 enzymes without significant effects on the induction of phase 1 enzymes (cytochromes P450)
- NQO1
NAD(P)H:(quinone-acceptor) oxidoreductase, EC 1.6.99.2, also known as quinone reductase 1 and DT diaphorase
Footnotes
See commentary on page 2941.
dqfCOSY, double quantum-filtered correlated spectroscopy; HMBC, heteronuclear multiple bond correlation; HMQC, heteronuclear multiple quantum correlation; NOESY, nuclear Overhauser effect spectroscopy.
References
- 1.Kensler T W. Environ Health Perspect. 1997;105 (Suppl. 4):965–970. doi: 10.1289/ehp.97105s4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talalay P, Fahey J W, Holtzclaw W D, Prestera T, Zhang Y. Toxicol Lett. 1995;82/83:173–179. doi: 10.1016/0378-4274(95)03553-2. [DOI] [PubMed] [Google Scholar]
- 3.Talalay P. Proc Am Phil Soc. 1999;143:52–72. [Google Scholar]
- 4.Benson A M, Batzinger R P, Ou S-Y L, Bueding E, Cha Y-N, Talalay P. Cancer Res. 1978;38:4486–4495. [PubMed] [Google Scholar]
- 5.Cha Y-N, Bueding E. Biochem Pharmacol. 1979;28:1917–1921. doi: 10.1016/0006-2952(79)90645-2. [DOI] [PubMed] [Google Scholar]
- 6.Benson A M, Hunkeler M J, Talalay P. Proc Natl Acad Sci USA. 1980;77:5216–5220. doi: 10.1073/pnas.77.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benson A M, Cha Y N, Bueding E, Heine H S, Talalay P. Cancer Res. 1979;39:2971–2977. [PubMed] [Google Scholar]
- 8.Prestera T, Talalay P, Alam J, Ahn Y I, Lee P J, Choi A M. Mol Med. 1995;1:827–837. [PMC free article] [PubMed] [Google Scholar]
- 9.Primiano T, Kensler T W, Kuppusamy P, Zweier J L, Sutter T R. Carcinogenesis. 1996;17:2291–2296. doi: 10.1093/carcin/17.11.2291. [DOI] [PubMed] [Google Scholar]
- 10.Mulcahy R T, Wartman M A, Bailey H H, Gipp J J. J Biol Chem. 1997;272:7445–7454. doi: 10.1074/jbc.272.11.7445. [DOI] [PubMed] [Google Scholar]
- 11.Moinova H R, Mulcahy R T. J Biol Chem. 1998;273:14683–14689. doi: 10.1074/jbc.273.24.14683. [DOI] [PubMed] [Google Scholar]
- 12.Otieno M A, Kensler T W, Guyton K Z. Free Radical Biol Med. 2000;28:944–952. doi: 10.1016/s0891-5849(00)00175-1. [DOI] [PubMed] [Google Scholar]
- 13.Ellis E M, Judah D J, Neal G E, O'Connor T, Hayes J D. Cancer Res. 1996;56:2758–2766. [PubMed] [Google Scholar]
- 14.Kelly V P, Ellis E M, Manson M M, Chanas S A, Moffat G J, McLeod R, Judah D J, Neal G E, Hayes J D. Cancer Res. 2000;60:957–969. [PubMed] [Google Scholar]
- 15.Ciaccio P J, Jaiswal A K, Tew K D. J Biol Chem. 1994;269:15558–15562. [PubMed] [Google Scholar]
- 16.Primiano T, Kensler T W, Trush M A, Sutter T R. Adv Exp Med Biol. 1999;469:599–605. doi: 10.1007/978-1-4615-4793-8_87. [DOI] [PubMed] [Google Scholar]
- 17.Hayes J D, McLellan L I. Free Radical Res. 1999;31:273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- 18.Hayes J D, Ellis E M, Neal G E, Harrison D J, Manson M M. Biochem Soc Symp. 1999;64:141–168. [PubMed] [Google Scholar]
- 19.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 20.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel J D, Yamamoto M. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramos-Gomez M, Kwak M-K, Dolan P M, Itoh K, Yamamoto M, Talalay P, Kensler T W. Proc Natl Acad Sci USA. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snyder G H, Cennerazzo M J, Karalis A J, Field D. Biochemistry. 1981;20:6509–6519. doi: 10.1021/bi00526a001. [DOI] [PubMed] [Google Scholar]
- 23.Dinkova-Kostova A T, Talalay P. Free Radical Biol Med. 2000;29:231–240. doi: 10.1016/s0891-5849(00)00300-2. [DOI] [PubMed] [Google Scholar]
- 24.Long D J, II, Waikel R L, Wang X J, Perlaky L, Roop D R, Jaiswal A K. Cancer Res. 2000;60:5913–5915. [PubMed] [Google Scholar]
- 25.Prochaska H J, Santamaria A B. Anal Biochem. 1988;169:328–336. doi: 10.1016/0003-2697(88)90292-8. [DOI] [PubMed] [Google Scholar]
- 26.Fahey J W, Zhang Y, Talalay P. Proc Natl Acad Sci USA. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prestera T, Zhang Y, Spencer S R, Wilczak C A, Talalay P. Adv Enzyme Regul. 1993;33:281–296. doi: 10.1016/0065-2571(93)90024-8. [DOI] [PubMed] [Google Scholar]
- 28.Khachik F, Bertram J S, Huang M-T, Fahey J W, Talalay P. In: Proceedings of the International Symposium on Antioxidant Food Supplements in Human Health. Packer L, Hiramatsu M, Yoshikawa T, editors. San Diego: Academic; 1998. pp. 203–229. [Google Scholar]
- 29.Spencer S R, Xue L, Klenz E M, Talalay P. Biochem J. 1991;273:711–717. doi: 10.1042/bj2730711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talalay P, De Long M J, Prochaska H J. Proc Natl Acad Sci USA. 1988;85:8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang, Y. (2001) Carcinogenesis, in press.
- 32.Dinkova-Kostova A T, Abeygunawardana C, Talalay P. J Med Chem. 1998;41:5287–5296. doi: 10.1021/jm980424s. [DOI] [PubMed] [Google Scholar]
- 33.Dinkova-Kostova A T, Talalay P. Carcinogenesis. 1999;20:911–914. doi: 10.1093/carcin/20.5.911. [DOI] [PubMed] [Google Scholar]
- 34.Mora P T, Szeki T. J Am Chem Soc. 1950;72:3009–3013. [Google Scholar]
- 35.Habig W H, Pabst M J, Jakoby W B. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 36.Anderson M E. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- 37.Richie J P, Jr, Skowronski L, Abraham P, Leutzinger Y. Clin Chem. 1996;42:64–70. [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1996. pp. 7.35–7.39. [Google Scholar]
- 39.Hossain M A, Fielding K E, Trescher W H, Ho T, Wilson M A, Laterra J. Eur J Neurosci. 1998;10:2490–2499. [PubMed] [Google Scholar]
- 40.Feinberg A P, Vogelstein B. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 41.Jaiswal A K, McBride O W, Adesnik M, Nebert D W. J Biol Chem. 1988;263:13572–13578. [PubMed] [Google Scholar]
- 42.Richman P G, Meister A. J Biol Chem. 1975;250:1422–1426. [PubMed] [Google Scholar]
- 43.Wild A C, Mulcahy R T. Free Radical Res. 2000;32:281–301. doi: 10.1080/10715760000300291. [DOI] [PubMed] [Google Scholar]
- 44.Hayes J D, Pulford D J. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka H, Yamada K, Kawazura H. J Chem Soc Perkin Trans. 1978;2(3):231–235. [Google Scholar]
- 46.Breitmaier E. Structure Elucidation by NMR in Organic Chemistry. Chichester, U.K.: Wiley; 1993. p. 61. [Google Scholar]
- 47.Kupchan S M, Fessler D C, Eakin M A, Giacobbe T J. Science. 1970;168:376–378. doi: 10.1126/science.168.3929.376. [DOI] [PubMed] [Google Scholar]
- 48.Kupchan S M, Eakin M A, Thomas A M. J Med Chem. 1971;14:1147–1152. doi: 10.1021/jm00294a001. [DOI] [PubMed] [Google Scholar]