Abstract
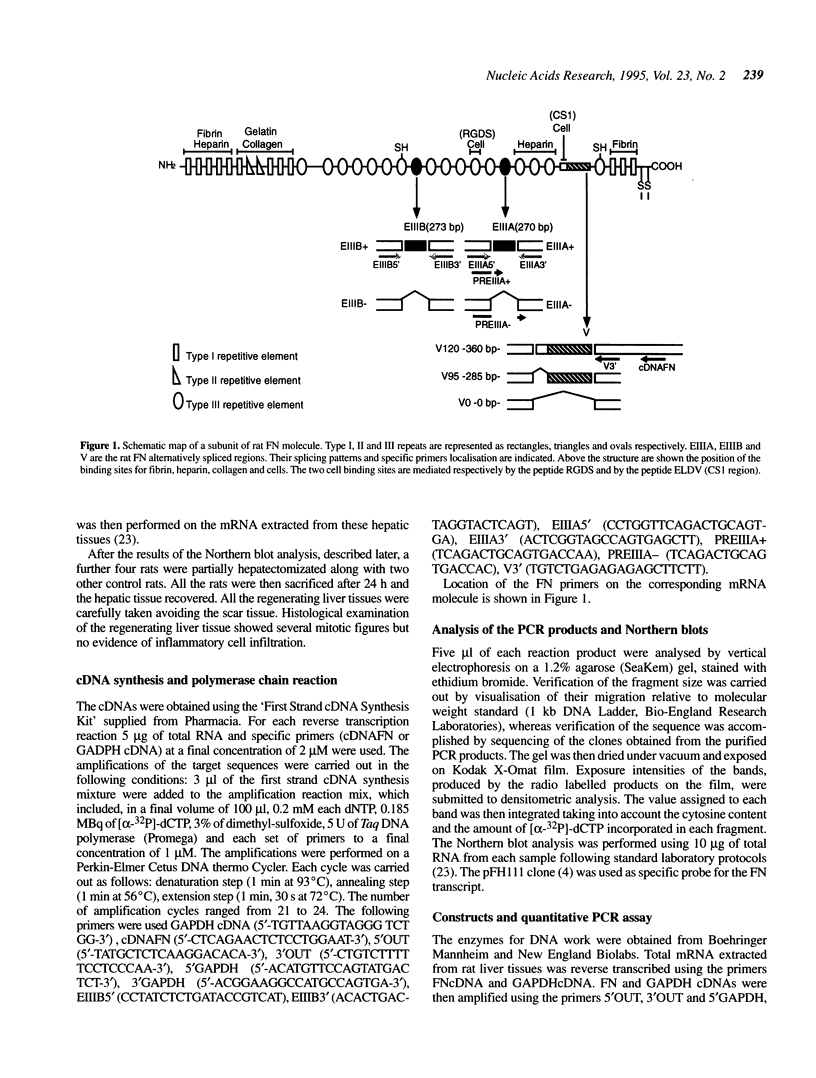
Fibronectin (FN) expression displays a complex regulation that results in precisely defined isoform patterns during different developmental stages, ageing and injury. The qualitative and quantitative changes that are observed derive from modulation of the rate of transcription of the single FN pre-mRNA and its specific differential processing in the EIIIA, EIIIB and V regions of rat FN. The liver is the major source of plasma FN which is characterised by the absence of the EIIIA and EIIIB exons. Here we show that in the rat regenerating liver there is a significant reprogramming of the splicing machinery that results in the synthesis by the liver of up to 17% of EIIIA+ FN linked with all the three V forms. On the other hand the EIIIB+ form is totally absent both in normal and regenerating liver. Furthermore there is a variation of the V pattern observed in the regenerating tissue, the V120 form (linked to both EIIIA+ and EIIIA- messengers) increases from 11 to 32%. The quantitative RT-PCR method was used to estimate the FN transcription rate, before and after partial hepatectomy. We have shown a 3-fold increase in FN mRNA in liver that is specifically linked to the regeneration process and not to the surgical stress.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barone M. V., Henchcliffe C., Baralle F. E., Paolella G. Cell type specific trans-acting factors are involved in alternative splicing of human fibronectin pre-mRNA. EMBO J. 1989 Apr;8(4):1079–1085. doi: 10.1002/j.1460-2075.1989.tb03476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernath V. A., Muro A. F., Vitullo A. D., Bley M. A., Barañao J. L., Kornblihtt A. R. Cyclic AMP inhibits fibronectin gene expression in a newly developed granulosa cell line by a mechanism that suppresses cAMP-responsive element-dependent transcriptional activation. J Biol Chem. 1990 Oct 25;265(30):18219–18226. [PubMed] [Google Scholar]
- Caputi M., Casari G., Guenzi S., Tagliabue R., Sidoli A., Melo C. A., Baralle F. E. A novel bipartite splicing enhancer modulates the differential processing of the human fibronectin EDA exon. Nucleic Acids Res. 1994 Mar 25;22(6):1018–1022. doi: 10.1093/nar/22.6.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dean D. C., Newby R. F., Bourgeois S. Regulation of fibronectin biosynthesis by dexamethasone, transforming growth factor beta, and cAMP in human cell lines. J Cell Biol. 1988 Jun;106(6):2159–2170. doi: 10.1083/jcb.106.6.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ffrench-Constant C., Hynes R. O. Patterns of fibronectin gene expression and splicing during cell migration in chicken embryos. Development. 1988 Nov;104(3):369–382. doi: 10.1242/dev.104.3.369. [DOI] [PubMed] [Google Scholar]
- Gutman A., Kornblihtt A. R. Identification of a third region of cell-specific alternative splicing in human fibronectin mRNA. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7179–7182. doi: 10.1073/pnas.84.20.7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh G. S., Hynes R. O. Elements regulating an alternatively spliced exon of the rat fibronectin gene. Mol Cell Biol. 1993 Sep;13(9):5301–5314. doi: 10.1128/mcb.13.9.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. Molecular biology of fibronectin. Annu Rev Cell Biol. 1985;1:67–90. doi: 10.1146/annurev.cb.01.110185.000435. [DOI] [PubMed] [Google Scholar]
- Ingber D. E., Prusty D., Frangioni J. V., Cragoe E. J., Jr, Lechene C., Schwartz M. A. Control of intracellular pH and growth by fibronectin in capillary endothelial cells. J Cell Biol. 1990 May;110(5):1803–1811. doi: 10.1083/jcb.110.5.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton A. A., Connelly C. M., Romo G. M., Mamuya W., Apstein C. S., Brecher P. Rapid expression of fibronectin in the rabbit heart after myocardial infarction with and without reperfusion. J Clin Invest. 1992 Apr;89(4):1060–1068. doi: 10.1172/JCI115685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt A. R., Gutman A. Molecular biology of the extracellular matrix proteins. Biol Rev Camb Philos Soc. 1988 Nov;63(4):465–507. doi: 10.1111/j.1469-185x.1988.tb00668.x. [DOI] [PubMed] [Google Scholar]
- Kornblihtt A. R., Umezawa K., Vibe-Pedersen K., Baralle F. E. Primary structure of human fibronectin: differential splicing may generate at least 10 polypeptides from a single gene. EMBO J. 1985 Jul;4(7):1755–1759. doi: 10.1002/j.1460-2075.1985.tb03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt A. R., Vibe-Pedersen K., Baralle F. E. Isolation and characterization of cDNA clones for human and bovine fibronectins. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3218–3222. doi: 10.1073/pnas.80.11.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood C. J., Senyei A. E., Dische M. R., Casal D., Shah K. D., Thung S. N., Jones L., Deligdisch L., Garite T. J. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N Engl J Med. 1991 Sep 5;325(10):669–674. doi: 10.1056/NEJM199109053251001. [DOI] [PubMed] [Google Scholar]
- Magnuson V. L., Young M., Schattenberg D. G., Mancini M. A., Chen D. L., Steffensen B., Klebe R. J. The alternative splicing of fibronectin pre-mRNA is altered during aging and in response to growth factors. J Biol Chem. 1991 Aug 5;266(22):14654–14662. [PubMed] [Google Scholar]
- Mamuya W. S., Brecher P. Fibronectin expression in the normal and hypertrophic rat heart. J Clin Invest. 1992 Feb;89(2):392–401. doi: 10.1172/JCI115598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzo S., Bagnarelli P., Giacca M., Manzin A., Varaldo P. E., Clementi M. Absolute quantitation of viremia in human immunodeficiency virus infection by competitive reverse transcription and polymerase chain reaction. J Clin Microbiol. 1992 Jul;30(7):1752–1757. doi: 10.1128/jcm.30.7.1752-1757.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
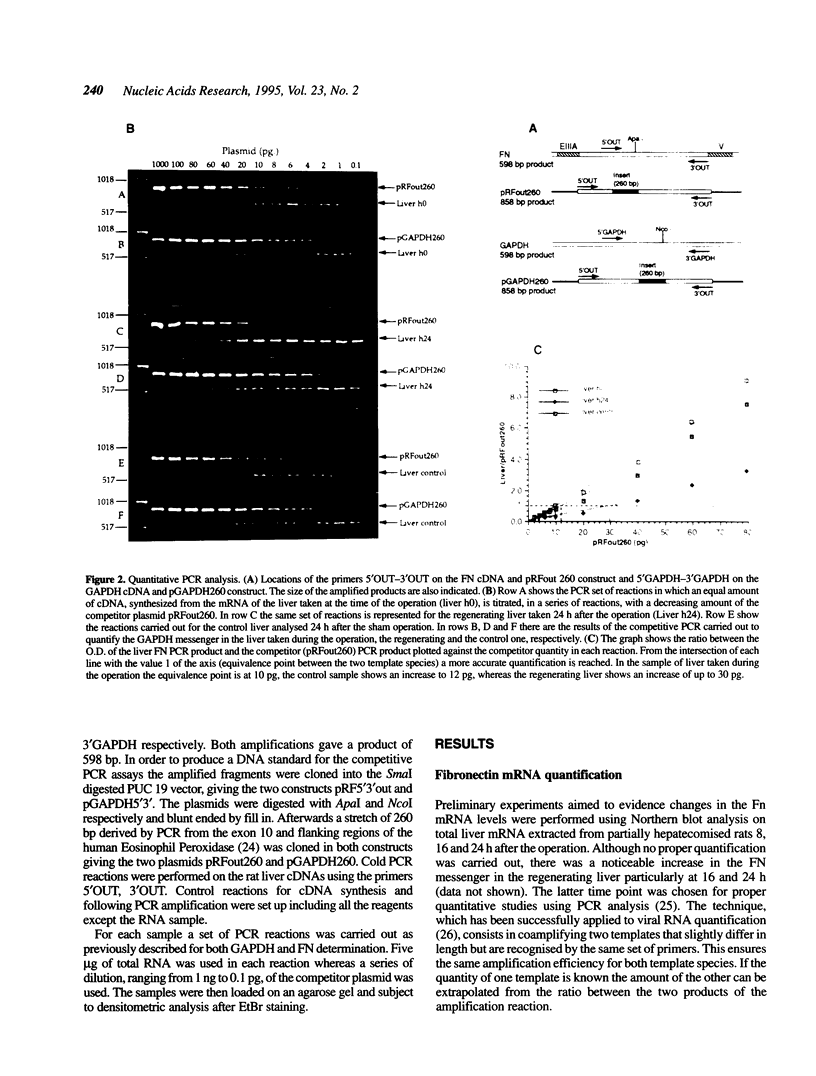
- Murata Y., Seo H., Sekiguchi K., Imai T., Lee J., Matsui N. Specific induction of fibronectin gene in rat liver by thyroid hormone. Mol Endocrinol. 1990 May;4(5):693–699. doi: 10.1210/mend-4-5-693. [DOI] [PubMed] [Google Scholar]
- Norton P. A., Hynes R. O. Alternative splicing of chicken fibronectin in embryos and in normal and transformed cells. Mol Cell Biol. 1987 Dec;7(12):4297–4307. doi: 10.1128/mcb.7.12.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odenthal M., Neubauer K., Baralle F. E., Peters H., Meyer zum Büschenfelde K. H., Ramadori G. Rat hepatocytes in primary culture synthesize and secrete cellular fibronectin. Exp Cell Res. 1992 Dec;203(2):289–296. doi: 10.1016/0014-4827(92)90001-o. [DOI] [PubMed] [Google Scholar]
- Oyama F., Murata Y., Suganuma N., Kimura T., Titani K., Sekiguchi K. Patterns of alternative splicing of fibronectin pre-mRNA in human adult and fetal tissues. Biochemistry. 1989 Feb 7;28(3):1428–1434. doi: 10.1021/bi00429a072. [DOI] [PubMed] [Google Scholar]
- Pagani F., Zagato L., Vergani C., Casari G., Sidoli A., Baralle F. E. Tissue-specific splicing pattern of fibronectin messenger RNA precursor during development and aging in rat. J Cell Biol. 1991 Jun;113(5):1223–1229. doi: 10.1083/jcb.113.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. S., Odermatt E., Schwarzbauer J. E., Hynes R. O. Organization of the fibronectin gene provides evidence for exon shuffling during evolution. EMBO J. 1987 Sep;6(9):2565–2572. doi: 10.1002/j.1460-2075.1987.tb02545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G., Knittel T., Odenthal M., Schwögler S., Neubauer K., Meyer zum Büschenfelde K. H. Synthesis of cellular fibronectin by rat liver fat-storing (Ito) cells: regulation by cytokines. Gastroenterology. 1992 Oct;103(4):1313–1321. doi: 10.1016/0016-5085(92)91522-6. [DOI] [PubMed] [Google Scholar]
- Sakamaki K., Tomonaga M., Tsukui K., Nagata S. Molecular cloning and characterization of a chromosomal gene for human eosinophil peroxidase. J Biol Chem. 1989 Oct 5;264(28):16828–16836. [PubMed] [Google Scholar]
- Schwartz M. A., Ingber D. E., Lawrence M., Springer T. A., Lechene C. Multiple integrins share the ability to induce elevation of intracellular pH. Exp Cell Res. 1991 Aug;195(2):533–535. doi: 10.1016/0014-4827(91)90407-l. [DOI] [PubMed] [Google Scholar]
- Zardi L., Carnemolla B., Siri A., Petersen T. E., Paolella G., Sebastio G., Baralle F. E. Transformed human cells produce a new fibronectin isoform by preferential alternative splicing of a previously unobserved exon. EMBO J. 1987 Aug;6(8):2337–2342. doi: 10.1002/j.1460-2075.1987.tb02509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]