MCT3, a specific marker of differentiated RPE, is downregulated after wounding. This report demonstrates for the first time a role for cell-cell contacts in restoring MCT3 expression after injury.
Abstract
Purpose.
MCT3 is a proton-coupled monocarboxylate transporter preferentially expressed in the basolateral membrane of the retinal pigment epithelium (RPE) and has been shown to play an important role in regulating pH and lactate concentrations in the outer retina. Decreased expression of MCT3 in response to trauma or disease could contribute to pathologic changes in the retina. The present study followed the expression of MCT3 after wounding and re-epithelialization of chick RPE explant and human fetal (hf) RPE cultures.
Methods.
Immunofluorescence microscopy and immunoblotting were performed to determine changes in MCT expression after scratch wounding and re-epithelialization of chick RPE/choroid explant cultures and hfRPE cell monolayers.
Results.
MCT3 expression and basolateral polarity were maintained in chick RPE/choroid explant cultures and hfRPE monolayers. Wounding resulted in loss of MCT3 and the upregulation of MCT4 expression in migrating cells at the edge of the wound. On re-epithelialization, MCT3 was detected in chick and hfRPE cells when cells became hexagonally packed and pigmented. However, in hfRPE cells, MCT4 was consistently expressed throughout the epithelial monolayer. RPE cells at the edges of chick explants and hfRPE cultures with a free edge expressed MCT4 but not MCT3.
Conclusions.
Wounding of RPE monolayers resulted in dedifferentiation of the cells at the edge of the wound, as evidenced by a loss of MCT3 and increased MCT4 expression. Collectively, these findings suggest that both cell-cell and cell-substrate interactions are essential in directing and maintaining differentiation of the RPE and expression of MCT3.
The retina is one of the most metabolically active tissues in the body and produces large quantities of lactate by the metabolism of glucose through aerobic glycolysis.1,2 Lactate produced by Müller cells is used by photoreceptors to fuel oxidative phosphorylation, whereas excess lactate is transported out of the retina to the choroidal blood supply by the retinal pigment epithelium (RPE).3 In this capacity, the RPE serves as the gatekeeper of the outer retina, regulating the flux of ions, metabolites, and fluid between the outer retina and the choroidal blood supply. Transepithelial transport of lactate is facilitated by monocarboxylate transporters (MCTs) in the apical and basolateral membranes of the RPE.4
The MCTs are members of the SLC16A gene family of transporters. Both the avian eye and the mammalian eye express a variety of these transporters, including MCT1 and MCT3 in the RPE and MCT4 in the retina.5 Although MCT3 is expressed preferentially in the basolateral membrane of the RPE, MCT1 is widely expressed in tissues throughout the body, and MCT4 is expressed in cells and tissues that are highly glycolytic.6
MCT1, MCT3, and MCT4 form a heterodimeric complex with CD147,6–9 a type I glycoprotein and member of the immunoglobulin superfamily.10 CD147 was first characterized as an extracellular matrix metalloproteinase inducer and a blood-brain barrier antigen.11–13 Assembly of the MCT/CD147 complex in the endoplasmic reticulum is required for the efficient trafficking of the mature transporter to the plasma membrane because in the absence of one subunit the other is targeted for degradation.8,9
The importance of MCTs in maintaining normal vision has been demonstrated in mice with targeted deletion of either CD147 (Bsg) or MCT3 (Slc16a8) genes. In Bsg−/− mice there was a decrease in the amplitudes of scotopic and photopic electroretinograms (ERGs) before degeneration of photoreceptor cells.14 Work from our laboratory suggested that the change in visual function observed in the Bsg−/− mice was caused by the loss of expression of MCT1, MCT3, and MCT4 in the RPE and retina and the disruption of metabolic coupling between Müller cells and photoreceptor cells.5 This hypothesis was supported by our recent study demonstrating that mice with a targeted deletion of the Slc16a8 (MCT3) gene exhibited decreased amplitudes of the light-stimulated ERG, whereas lactate levels in the retina were increased.15
The expression of RPE-specific genes can be altered in response to disease and trauma, thereby contributing to pathologic changes in this tissue.16 Removing RPE cells from eyes and placing them in culture also results in changes in gene expression that can vary with the time in culture and culture conditions.17–22 Our laboratory previously reported that MCT3 mRNA and protein were not detected in chick RPE cells when cultured on plastic or transwell inserts.23 These findings suggested that cell-cell, cell-substrate, or RPE-photoreceptor interactions are essential to regulate the expression of RPE specific genes. To obtain further insight into the regulation of MCT3 in RPE cells, we examined the expression of MCT3 in chick RPE/choroid explant cultures and hfRPE cells after the integrity of the epithelial monolayer was disrupted by mechanical wounding. Our findings show that disruption of cell-cell interactions leads to a downregulation of MCT3 and an up-regulation of MCT4. In addition, we reported that MCT3 was expressed after re-epithelialization but that the rapidity of MCT3 reexpression may be reliant on factors from the basal lamina. Taken together, these results indicate that the integrity of the RPE monolayer is essential for maintaining expression of MCT3 and that the basal lamina may contribute to the modulation of MCT expression in the RPE.
Materials and Methods
Animals
White Leghorn chick embryos used in these studies were procured from a local supplier and incubated in a forced draft incubator at 38.0°C to 38.5°C. C57Bl/6 mice used in these studies were purchased from Jackson Laboratories (Bar Harbor, ME) and were maintained on a 12-hour light/12-hour dark cycle. The animals were euthanatized during the light period of the cycle. All animal procedures were performed in compliance with National Institutes of Health guidelines as approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University (Philadelphia, PA). Procedures were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Chick Explant Culture
Eyes were enucleated from embryonic day (E)9 or E12 chick embryos, the anterior portion and neural retina were removed, and the remaining posterior eyecup was incubated in HEPES-buffered saline with 5 mM glucose (HBSG, pH 7.4) for 10 minutes at room temperature before the cup was cut into two to four sections. Using fine forceps, the RPE and choroid layers were dissected from the sclera and placed choroid side down onto nitrocellulose-coated two-well glass chamber slides containing minimal essential medium (MEM) with 10% fetal bovine serum (FBS). Explants were incubated overnight. The following day, three to four triangular wounds were made by brushing RPE cells off the basement membrane with a single piece of human hair. Explants were fixed in cold methanol at 1, 3, and 5 days after wounding.
Primary Cultures of Chick and hfRPE Cells
Chick RPE cells were isolated from E9 chick embryos as previously described.23 The eyecup was incubated in PBS containing 15 mM EDTA for 30 to 40 minutes before the RPE was gently peeled off the basement membrane. Cells were placed in MEM with 10% FBS, triturated, and plated on 35-mm tissue culture dishes before incubation at 37°C in 5% CO2 and air.
hfRPE cells were obtained from Sheldon Miller (National Eye Institute, Bethesda, MD). The research followed the tenets of the Declaration of Helsinki and the National Institutes of Health institutional review board. On arrival, cells were trypsinized and seeded onto clear transwell inserts (Transwell; cat. 3460; Corning Costar, Corning, NY) as described previously.18 When the cells were confluent, evenly pigmented, and had a transepithelial resistance greater than 800 Ω/ cm2, they were used for experiments. For wounding experiments, monolayers were scratch-wounded using a 200 μL pipet tip. Resultant wounds were approximately 1-mm wide.
Antibodies and Microscopy
Chicken MCT3 (Mab3C4) antibody used in these studies was produced and characterized in our laboratory as previously described.23 A polyclonal antibody to chicken MCT4 was generated against a 20-mer synthetic oligopeptide corresponding to the carboxyl terminal amino acids (FLKDEPEKNGEVVTNPETCV) of chicken MCT4 (Research Genetics, Huntsville, AL). Rabbits were boosted with the peptide at weeks 2, 6, and 8 and bled at weeks 0, 4, 8, and 10. The 8- and 10-week bleeds were combined and purified using immunoaffinity columns prepared with peptide antigen linked to agarose.
Human anti-peptide MCT3 and MCT4 were generated by Yenzyme and characterized in our laboratory as previously described.24 An anti–laminin antibody (3H11), developed by Willi Halfter,25 was obtained by Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biological Sciences (Iowa City, IA). Additional antibodies used for these studies were anti–Connexin 43 (Cx43; Invitrogen, Carlsbad, CA) and anti–β-actin (Sigma, St. Louis, MO). AlexaFluor-tagged secondary antibodies and AlexaFluor488-tagged phalloidin were purchased from Molecular Probes (Eugene, OR). A fluorescent dye (To-Pro-3 Iodide-633; Invitrogen) was used to visualize nuclei in mouse eye sections. Horseradish peroxidase (HRP)–conjugated secondary antibodies were purchased from Thermo Scientific (Pierce, Rockford, IL).
Immunofluorescence and Immunohistochemistry
For histologic assessment during development, eyes were excised from chick embryos at different stages of development (E3, E8) and fixed by immersion in 4% paraformaldehyde in PBS containing 3% sucrose for 5 minutes at room temperature followed by 30 minutes on ice. For E3 embryos, the whole embryos were fixed. Samples were washed with PBS and then equilibrated in PBS containing increasing concentrations of sucrose (10%, 20%, 30%). Tissues were then placed in tissue freezing medium (Triangle Biomedical Sciences, Durham, NC) and frozen in liquid nitrogen. Cryosections (8–12 μm) were collected on glass slides and labeled with antibodies as described.5 For histologic assessment after wounding, chick RPE/choroid explants were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA), 2.5% glutaraldehyde (Sigma, St. Louis, MO) in 0.1 M sodium cacodylate buffer (pH 7.4) with 8 mM CaCl2 for 2 hours on ice and post-fixed with 1% osmium tetroxide for 1 hour on ice. After dehydration in a graded ethanol series followed by propylene oxide, the explants were infiltrated and embedded in epoxy resin (Epon; Electron Microscopy Sciences). Sections (1 μm) were stained with methylene blue/azure blue, then examined and photographed using a microscope (E800; Nikon, Tokyo, Japan) equipped with a digital camera (Optronics, Goleta, CA).
For immunofluorescence labeling, chick/RPE explant cultures and hfRPE cell cultures were fixed in 4% paraformaldehyde in PBS for 5 minutes at room temperature and then 25 minutes on ice. Cultures were washed in PBS, permeabilized with methanol at −20°C for 3 minutes or 0.3% Triton at room temperature for 5 minutes. Mouse eyes were fixed and processed for cryosections as previously described.14 Cells and tissue sections were blocked using 5% BSA in PBS with 0.1% Tween 20 (PBST) and incubated with primary antibody overnight at 4°C. The next day, cultures were washed with PBST and incubated in secondary antibody for 30 minutes, washed, and mounted with mounting medium (Airvol 205; Air Products and Chemicals, Inc., Allentown, PA). Images were obtained on a laser scanning confocal microscope (LSM510; Carl Zeiss, Oberkochen, Germany) with a 20× objective. High-power images were taken with a 63× oil objective. Images (see Figs. 4, 5) were prepared from projections (2–10 slices) of Z-stack images taken with a 63× objective with or without the 2× zoom (see figure legends for specific magnifications). Images were processed (LSM510 [Carl Zeiss]; Photoshop 7.0 [Adobe, San Jose, CA]), with adjustments made only to brightness and contrast.
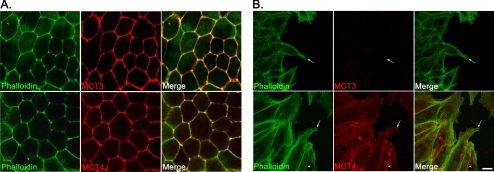
Figure 4.
Alteration in protein expression in wounded hfRPE cells. (A) Confocal immunofluorescence microscopy revealed that MCT3 (top) and MCT4 (bottom) co-localized with actin filaments at the lateral borders of hfRPE cells, indicative of basolateral polarity. (B) MCT3 expression was not detected at the leading edge of the wound (top, arrows). However, MCT4 expression (bottom) was observed both in cells at the leading edge of the wound (arrow) and at the lateral cell-cell borders (arrowhead). The cytoskeleton was visualized with phalloidin-488 labeling. Cells were fixed for immunofluorescence 48 hours after wounding. Scale bar, (A, B) 10 μm.
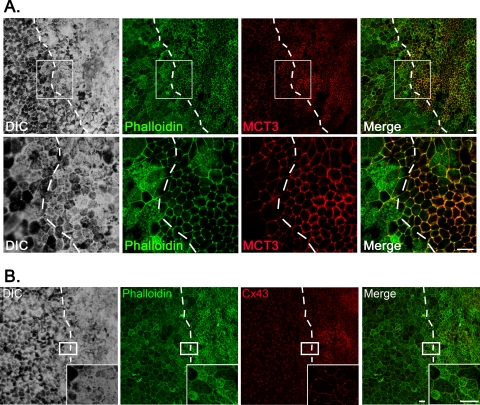
Figure 5.
hfRPE can differentiate after wounding and re-epithelialization. (A) Immunofluorescence confocal microscopy revealed that 16 days after wounding, MCT3 was detected in the re-epithelialized wound. Dotted lines: original wound edge. Lower: magnified images of the regions denoted by the boxed areas. Scale bar, 20 μm. (B) Confocal immunofluorescence microscopy showed expression of Connexin-43 (Cx43) at cell-cell borders in the healed wound. Insets: magnified images of the regions denoted by the boxed areas. Dotted lines: original wound edge. Scale bar, 20 μm.
Immunoblot Analysis
The RPE was dissected from the embryonic chick eye, and detergent-soluble cell lysates were prepared as previously described.15 For hfRPE, cells were harvested from the monolayer and the side of the transwell insert for extraction (see Fig. 3B). Before scraping, cells were washed with PBS and lysed with ice-cold lysis buffer (25 mM HEPES buffer, pH 7.4, 150 mM NaCl, 5 mM MgCl2, 1% Triton X-100, 60 mM N-octyl-β-glucopyranoside) containing protease inhibitors (protease inhibitor cocktail; Sigma) for 30 minutes, then centrifuged at 14,000g at 4°C for 30 minutes. The protein concentration of the cleared lysates was determined (BCA Reagent; Thermo Scientific, Pierce). Lysates were diluted in 4× LDS sample buffer (Invitrogen, Carlsbad, CA), and equal amounts of protein were loaded onto 4% to 12% Bis-Tris gels (NuPAGE; Invitrogen). Separated proteins were transferred electrophoretically from gels to membranes (Immobilon-P; Millipore, Bedford, MA). Membranes were incubated for 1 hour at room temperature in blocking buffer (20 mM Tris, 137 mM NaCl, pH 7.5, 5% dry skim milk) followed by 1-hour incubation with primary antibodies and 30-minute incubation with HRP-conjugated secondary antibodies diluted 1:5000. Reactive bands were visualized with enhanced chemiluminescent Western blotting detection reagents (Thermo Scientific, Pierce).
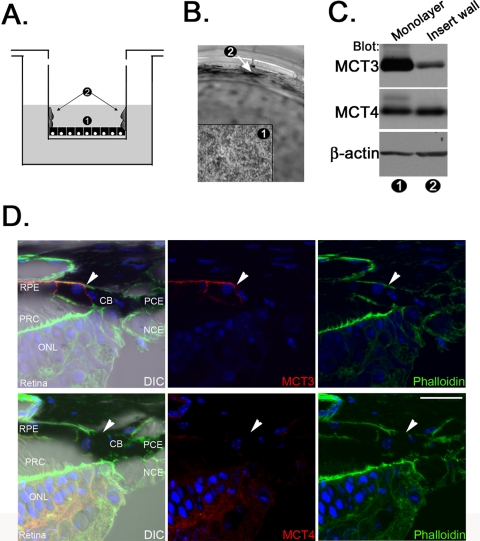
Figure 3.
The RPE requires continuous cell-cell contact to maintain MCT3 expression. (A) Schematic demonstrating migration of hfRPE cells in culture. 1, hfRPE monolayer; 2, hfRPE cells that migrated up the walls of the transwell insert. (B) Light micrograph demonstrating the movement of hfRPE up the wall of the transwell insert. Numbers correspond to those in (B). Arrow: area of migration up the wall of the insert. (C) Immunoblot analysis showing that MCT3 was expressed at high levels in lysates of hfRPE monolayers and at lower levels in lysates of hfRPE cells migrating up the sides of the transwell inserts. MCT4 expression was observed in each preparation. β-Actin, internal loading control. Numbers correspond to those in (B). (D) MCT3 expression was clearly limited to the RPE in the mouse eye. MCT4 was not expressed in this region. Arrow: border between the RPE and the ciliary epithelium. Blue: cells were stained to visualize nuclei. CB, ciliary body; PCE, pigmented ciliary epithelium; NCE, nonpigmented ciliary epithelium; PRC, photoreceptor cell; ONL, outer nuclear layer. Scale bar, 20 μm. Original magnification, 63× with a 2× zoom.
Results
Expression Patterns of MCT3 in Chick RPE
In the adult eye, the RPE is known to express high levels of lactate transporters, which regulate the transepithelial transport of excess lactate from the outer retina to the choroidal vessels. Two MCTs in particular are expressed in distinct membrane domains of the RPE—MCT1 in the apical and MCT3 in the basolateral membrane—so that their coordinated activities can facilitate a net flux of lactate out of the retina. MCT4 expression is restricted to the neural retina in the mature adult eye and, therefore, does not typically play a role in RPE function. In the current studies, we examined the expression of MCT3 and MCT4 in the chick eye during development. Using immunofluorescence confocal microscopy, MCT4 was detected in the neuro-ectoderm and in the inner and outer layers of the optic cup. Specifically, at E3, both the primitive RPE and the neural retina expressed MCT4, but MCT3 was not detected (Supplementary Fig. S1, top panels, http://www.iovs.org/cgi/content/full/51/10/5343/DC1). As differentiation proceeded, there was a loss of expression of MCT4 and an increase in expression of MCT3 in the RPE, as shown in sections of E8 eyes (Supplementary Fig. S1, bottom panels). Previous studies from our laboratory also showed that when chick RPE cells were grown in primary culture, they exhibited a loss of MCT3 mRNA and protein expression. To confirm these findings and to determine whether there was any alteration in expression of MCT4 in cultured cells, we examined the expression of these MCTs in freshly isolated E9 RPE cells and confluent cultures of RPE cells prepared from E9 chick embryos. Western blot analysis showed that in lysates from primary chick RPE cultures, there was a downregulation of MCT3 and an upregulation of MCT4 expression (Fig. 1A). In contrast, RPE cells isolated from chick RPE/choroid explant cultures maintained expression of MCT3; only very low levels of MCT4 were detected (Fig. 1B).
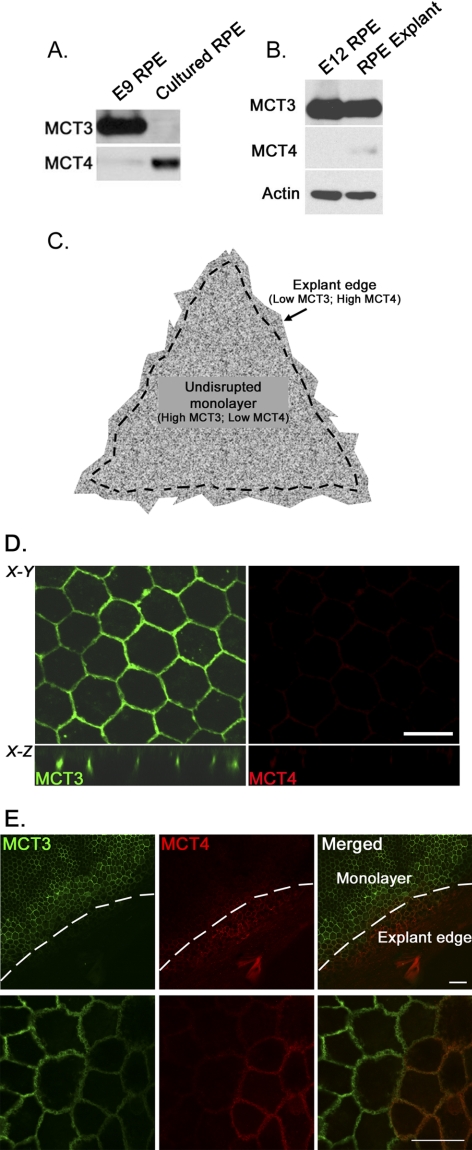
Figure 1.
Differential expression of MCTs in quiescent cells and dedifferentiated chick RPE cells. (A) Immunoblot analysis demonstrating that MCT3 was expressed in RPE harvested from E9 chick embryos. MCT3 was not detected when these cells were placed in culture (cultured RPE). MCT4 was not detected in E9 RPE but was found in cultured RPE lysates. (B) MCT3 expression was maintained in explant cultures of chick RPE, in which the basement membrane was not disrupted. MCT4 expression was not upregulated in these cultures. Actin served as an internal loading control. (C) Schematic representation of RPE/choroid culture model. Dotted line: regions of interest. (D) Confocal immunofluorescence microscopy showed that MCT3 was expressed basolaterally in chick RPE explant cultures, whereas MCT4 was detected at only very low levels in the intact RPE monolayers of these cultures. Scale bar, 20 μm. X-Y, en face view of RPE cultures. Scale bar, 10 μm. X-Z, x-z plane of the mid-region displayed in upper panels. (E) MCT3 was not detected at the edges of chick RPE explant cultures, where the monolayer was disrupted but MCT4 was detected in this region. Merged image: demarcation of expression of each of these isoforms. Lower panels are magnified images (63×) of cells in the upper panels (20×). Scale bars: 20 μm (top); 10 μm (bottom).
Immunofluorescence confocal microscopy was used to examine the expression of MCT3 and MCT4 in chick RPE/choroid explants. Figure 1C is a schematic representation of the RPE/choroid explant culture, indicating the regions of the monolayer shown in Figure 1D and the explant edge shown in Figure 1E. In the en face view of RPE/choroid explants (X-Y plane), MCT3 labeling was detected at cell-cell borders, characteristic of proteins polarized to the basolateral membrane (Fig. 1D, top left panel). MCT3 labeling was detected at the basolateral surface as seen in Figure 1D (X-Z plane, bottom left panel). Little MCT4 was detected in the RPE monolayer (Fig. 1D, right panels). However, at the borders of the explant cultures, where the cut edge resulted in the disruption of the basement membrane and in cell-cell contacts, we observed a loss of MCT3 and an increase in MCT4 expression (Fig. 1E). Taken together, these findings suggested that cell-cell and cell-matrix adhesion could contribute to maintaining the expression of MCT3.
Dedifferentiation of RPE Cells Accompanied by MCT Isoform Switching
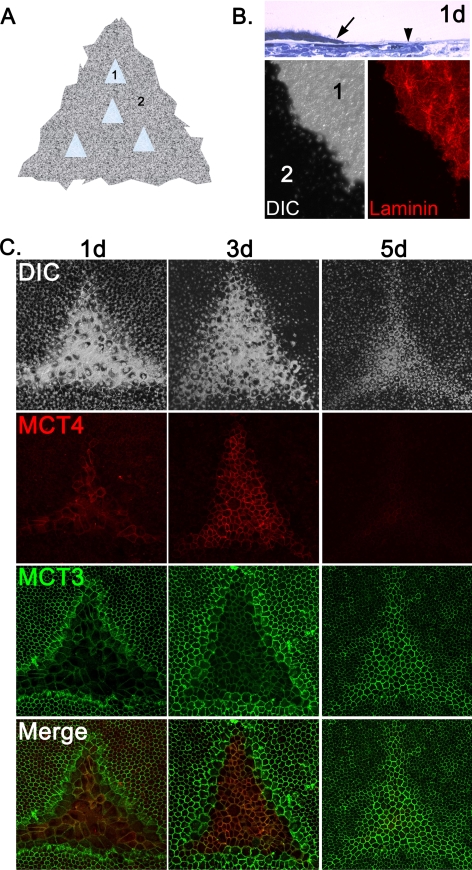
To address whether the switch in MCT isoform expression was caused by the disruption of cell-cell contacts, we performed scratch-wounding experiments on chick RPE/choroid explant cultures. The explant cultures were wounded by gently brushing off the RPE without disruption of the basal lamina (Fig. 2A). Explants were fixed and examined immediately after wounding, and thick plastic sections were examined to determine the integrity of the basement membrane after wounding (Fig. 2B, top panel). Wounded explants were also labeled with anti–laminin antibody and examined using immunofluorescence confocal microscopy. Laminin was detected in the wounded area, but in the non-wounded region, labeling was obscured by the overlying RPE (Fig. 2B, bottom panels). Overall, our histologic and immunohistochemical data indicated that the basal lamina remained intact after wounding of RPE/choroid explant cultures.
Figure 2.
Wounding of RPE explants cause MCT isoform switching. (A) Schematic representation of wounding paradigm for chick RPE/choroid explant cultures. 1, wound region; 2, intact monolayer. (B, top) Histologic section showing the region of wounding of the chick RPE explant culture at 1 day (1d) after wounding. Arrow: wound edge. Arrowhead: intact basement membrane. Bottom: wounds were labeled with anti–laminin antibody to demonstrate that the basement membrane remained intact after wounding. 1 and 2 correspond to numbering in (A). Left: differential interference contrast. Right: laminin labeling. (C) At 1 day after wounding, a switch in MCT isoform expression was detected in the leading edge of the wound, where MCT4 was turned on and MCT3 was turned off. MCT4 expression was further enhanced in proliferating and migrating cells inside the wound at 3 days after wounding, whereas MCT3 expression remained low in this region. By 5 days after wounding, the wound region had redifferentiated and MCT3 was re-expressed.
Chick RPE/choroid explants were cultured for 1, 3, and 5 days after wounding, then were fixed and colabeled with MCT3 and MCT4 antibodies and imaged using immunofluorescence confocal microscopy. After wounding, cells at the edge of the wound dedifferentiated and began to migrate into the wound area. At 1 day after wounding, MCT3 labeling decreased in cells at the edge of the wound, whereas MCT4 labeling increased in these cells (Fig. 2C, left panels). By 3 days after wounding, MCT4 was detected at high levels in the RPE cells that had resurfaced the wound but was not detected in the surrounding epithelia. In contrast, MCT3 was expressed at high levels in the undisturbed epithelium but was downregulated in the re-epithelialized wound (Fig. 2C, middle panels). By 5 days after wounding, RPE cells in the resurfaced wound were hexagonally packed, pigmented, and labeled with MCT3 antibody, whereas MCT4 labeling was no longer detected (Fig. 2C, right panels).
MCT3 and MCT4 Expression in hfRPE Cell Cultures
hfRPE cells grown on transwell filters, though not cultured on a native matrix substrate such as Bruch's membrane, are pigmented, hexagonally packed, and express genes characteristic of and uniquely expressed by the RPE in situ. However, cells at the periphery of the culture have a free edge and continue to proliferate and extend up the wall of the transwell insert (Figs. 3A, 3B). We examined MCT3 and MCT4 expression in the detergent soluble lysates prepared from these different populations of hfRPE cells. Immunoblot analysis revealed that hfRPE cells in the monolayer expressed high levels of MCT3, whereas cells along the wall of the insert expressed decreased levels of MCT3, indicative of a dedifferentiated and migratory phenotype (Fig. 3C). Interestingly, hfRPE cells also expressed MCT4 at both stages of differentiation (Fig. 3C).
The finding from our studies with chick RPE/choroid explants showed that if wounded RPE cells reestablished cell-cell junctions, cells could redifferentiate and express MCT3. However, cells at the periphery of the explant culture that had a free edge remained undifferentiated and expressed MCT4 rather than MCT3 (Fig. 1E). In the eye, the RPE is derived from the outer layer of the optic cup and is continuous with the pigmented layer of the ciliary epithelium (PCE). Thus, even at the perimeter of the retina in vivo, the RPE does not have a free edge. Although the RPE and PCE form junctional complexes, MCT3 was detected only in the basolateral membrane of the RPE (Fig. 3D). Consistent with our previous studies, MCT4 was detected only in the neural retina and not in the RPE or CE (Fig. 3D).
Decrease of MCT3 Expression at the Leading Edge of Wounded hfRPE
Given that hfRPE cells express both MCT3 and MCT4, we next wanted to determine the localization of these transporters in the epithelium. Using confocal microscopy, we found that, as in other RPE model systems, MCT3 staining was detected at the lateral borders of polarized hfRPE monolayers, indicative of basolateral localization of the protein (Fig. 4A, top panels). MCT4 was also polarized to the lateral borders of these cells, consistent with basolateral distribution of the transporter (Fig. 4A, bottom panels).
To determine whether the expression of MCT3 was dependent on cell-cell adhesion, we performed scratch-wound assays on hfRPE cells grown on transwell filters. Up to 72 hours after wounding, we observed with immunofluorescence confocal microscopy a loss of MCT3 expression in the wounded area (Fig. 4B, top panels). MCT4 was expressed at the wound edge and was detected not only at the cell-cell borders (arrowhead) but also in the leading lamellipodia (Fig. 4B, bottom panels, arrow). Wounds were sealed by 3 days after wounding; however, MCT3 was not detected in cells of the healed wound until 10 days after wounding (data not shown) and was not uniformly expressed until 16 days after wounding (Fig. 5A). The reexpression of MCT3 correlated with the formation of a tightly packed epithelial monolayer, increased pigmentation, and expression of connexin-43 (Cx43) at the lateral borders (Fig. 5B). These changes in expression are consistent with the more differentiated phenotype observed on re-epithelialization.
Discussion
In the eye, the barrier and transport properties of the RPE are essential for maintaining the health and functional activity of the photoreceptor cells. The vectorial transport of nutrients and metabolites into and out of the retina depends on the specific expression and polarized distribution of metabolic transporters in the apical and basolateral plasma membranes of the RPE. Recent work from our laboratory has demonstrated the importance of MCT3, an RPE-specific lactate transporter, in regulating light-stimulated rod responses and lactate levels in the outer retina. The present studies showed that disruption of the RPE monolayer resulted in the downregulation of MCT3 at the edge of the wound and that MCT3 was reexpressed in cells in the wound area only after re-epithelialization and re-establishment of cell-cell contact.
In the embryonic chick eye, we observed a switch in the expression of MCT isoforms in the RPE during development. MCT4, a lactate transporter primarily expressed in glycolytic tissues, was detected in the neuro-ectoderm, the optic vesicle, and the optic cup in developing chick embryos (Supplementary Fig. S1, top panels). As the RPE cells of the outer layer of the optic cup began to pigment and differentiate into the mature RPE, there was a decrease in MCT4 and an increase in MCT3 labeling (Supplementary Fig. S1, bottom panels). This switch correlated temporally with development of the choroidal vasculature and retinal differentiation. Interestingly, our current studies also revealed that there was a switch in MCT expression when E9 to E12 RPE cells were isolated and grown in culture; there was a downregulation of MCT3 and an upregulation of MCT4 were observed. This finding suggested that factors from the neural retina or the basement membrane, or maintenance of cell-cell contacts, may be essential for directing and maintaining the differentiated properties of the RPE.23,24
Two RPE culture models were used to examine the regulation of MCT3: chick RPE/choroid explant cultures and hfRPE cells. The chick RPE/choroid explants were cultured while attached to their native basement membrane, whereas the hfRPE cells were plated on filters coated with human extracellular matrix. As observed in RPE in situ, both the chick RPE/choroid explants and the hfRPE cell cultures expressed MCT3 in the basolateral membrane. Scratch-wounding of chick RPE/choroid explants and hfRPE monolayers led to a loss of MCT3 expression in cells at the leading edges of the wounds. After re-epithelialization of the monolayers, the cells redifferentiated, as indicated by hexagonal packing of cells, pigmentation, and expression of MCT3. Although both chick RPE/explant cultures and hfRPE cell cultures were re-epithelialized by 3 days after wounding, the time course for redifferentiation of these RPE cultures was different. MCT3 was detected in re-epithelialized wounds after 5 days after wounding in chick RPE/choroid explant cultures but not until 16 days after wounding in the hfRPE cells. These results are consistent with other studies showing that loss of cell-cell contact led to epithelial-mesenchymal transition and increased migration of RPE cells.26 In addition, the more rapid differentiation of the chick RPE cells suggests that specific components of the basal lamina may be responsible for directing the differentiation of RPE. Indeed, many studies have demonstrated the importance of the basement membrane in modulating RPE differentiation.19,27 Overall, these findings suggest that re-expression of MCT3 in RPE cells after wounding was dependent on both the reestablishment of cell-cell contacts and the composition of the basement membrane.
After scratch wounding of the RPE, we also found that MCT4 was expressed in the migrating cells at the leading edge of the wound. MCT4 was not detected after re-differentiation of the RPE monolayer in the chick explant cultures, mimicking the coordinated regulation of MCT3 and MCT4 expression observed during embryonic development (Supplementary Fig. S1). In the hfRPE cultures, we found MCT4 expression in the migrating cells at the edge of the wound; however, MCT4 was also expressed at the basolateral membrane of polarized hfRPE monolayers. These data support our speculation that factors from the basement membrane are also required to modulate MCT expression in the RPE.
The increased expression of MCT4 in cells at the edge of the wound is interesting, because previous reports from our laboratory have shown a role for MCT4 in cell motility in the human RPE cell line ARPE-19. These studies showed that MCT4 interacted with the adhesion receptor β1-integrin and that silencing of MCT4 slowed cell migration.28 The observation that MCT4 is also increased in chick RPE cells after wounding and is localized to the leading edges of migrating hfRPE cells would indicate a role for MCT4 in RPE cell motility in these models.
Similar to the wounded edges of the RPE cultures, we found that MCT4, but not MCT3, was detected in RPE cells in the periphery of the chick RPE explants and hfRPE cultures, where cells had a free edge. Cells at the free edges in both these culture are like “wounds that cannot heal” and, in that capacity, provide a model for studying RPE cell migration after injury or disease. In vivo, the RPE does not have a free edge but is continuous with the pigmented epithelium of the ciliary body, forming an uninterrupted epithelium around the eye. Therefore, the models of RPE used in this study, though not exact mimics of RPE in vivo, provide interesting insights about RPE biology during trauma or disease, namely, aberrant migration and proliferation. Our previous observations highlighting a role for MCT4 in cell motility support this hypothesis, and understanding whether this transporter plays a role in RPE migration in ocular diseases characterized by aberrant wound healing, such as proliferative vitreoretinopathy, warrants further study.
Along with changes in MCT3 expression after wounding, downregulation of the gap junction protein Cx43 was also observed in hfRPE cells during wound healing (data not shown) but was detected at the lateral cell borders when the wound had redifferentiated. Recently, it was reported that Cx43 contributes to the differentiation of RPE through cAMP signaling.29 Specifically, it was reported that differentiated RPE cells exhibit increased levels of cAMP, which then increase the expression of Cx43 in the epithelium. RPE cells fail to differentiate without the cAMP-induced expression of Cx43, highlighting the importance of this gap junction protein in regulating the differentiation state of the RPE. The temporal correlation in expression of Cx43 and MCT3 suggests that Cx43 may provide an additional level of regulation that contributes to the differentiation of RPE cells after wounding and re-epithelialization.
Taken together, these studies demonstrated that MCT3 is a specific marker for differentiated RPE and that expression of MCT3 in the RPE is dependent on the maintenance of cell-cell junctions. Our data also highlight the importance of the basement membrane in modulating the speed of differentiation and the ability to turn off expression of MCT4 after redifferentiation of wounded RPE. Understanding the exquisite control of MCT expression in quiescent and migratory RPE is critical for the understanding of RPE biology during diseases such as proliferative vitreoretinopathy and may be useful in designing stem cell and transplant therapies to repair diseased or damaged RPE.
Supplementary Material
Footnotes
Supported by National Eye Institute Grants EY012042 (NJP) and EY06658 (GBG); and National Institutes on Alcohol Abuse and Alcoholism Training Grant AA07463 (SG-C).
Disclosure: S. Gallagher-Colombo, None; A. Maminishkis, None; S. Tate, None; G.B. Grunwald, None; N.J. Philp, None
References
- 1. Wang L, Tornquist P, Bill A. Glucose metabolism in pig outer retina in light and darkness. Acta Physiol Scand. 1997;160:75–81 [DOI] [PubMed] [Google Scholar]
- 2. Winkler BS, Arnold MJ, Brassell MA, Sliter DR. Glucose dependence of glycolysis, hexose monophosphate shunt activity, energy status, and the polyol pathway in retinas isolated from normal (nondiabetic) rats. Invest Ophthalmol Vis Sci. 1997;38:62–71 [PubMed] [Google Scholar]
- 3. Poitry-Yamate CL, Poitry S, Tsacopoulos M. Lactate released by Muller glial cells is metabolized by photoreceptors from mammalian retina. J Neurosci. 1995;15:5179–5191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Philp NJ, Yoon H, Grollman EF. Monocarboxylate transporter MCT1 is located in the apical membrane and MCT3 in the basal membrane of rat RPE. Am J Physiol. 1998;274:R1824–R1828 [DOI] [PubMed] [Google Scholar]
- 5. Philp NJ, Ochrietor JD, Rudoy C, Muramatsu T, Linser PJ. Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin-null mouse. Invest Ophthalmol Vis Sci. 2003;44:1305–1311 [DOI] [PubMed] [Google Scholar]
- 6. Kirk P, Wilson MC, Heddle C, Brown MH, Barclay AN, Halestrap AP. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 2000;19:3896–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilson MC, Meredith D, Halestrap AP. Fluorescence resonance energy transfer studies on the interaction between the lactate transporter MCT1 and CD147 provide information on the topology and stoichiometry of the complex in situ. J Biol Chem. 2002;277:3666–3672 [DOI] [PubMed] [Google Scholar]
- 8. Deora AA, Philp N, Hu J, Bok D, Rodriguez-Boulan E. Mechanisms regulating tissue-specific polarity of monocarboxylate transporters and their chaperone CD147 in kidney and retinal epithelia. Proc Natl Acad Sci USA. 2005;102:16245–16250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gallagher SM, Castorino JJ, Wang D, Philp NJ. Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB-231. Cancer Res. 2007;67:4182–4189 [DOI] [PubMed] [Google Scholar]
- 10. Altruda F, Cervella P, Gaeta ML, et al. Cloning of cDNA for a novel mouse membrane glycoprotein (gp42): shared identity to histocompatibility antigens, immunoglobulins and neural-cell adhesion molecules. Gene. 1989;85:445–451 [DOI] [PubMed] [Google Scholar]
- 11. Seulberger H, Unger CM, Risau W. HT7, Neurothelin, Basigin, gp42 and OX-47—many names for one developmentally regulated immuno-globulin-like surface glycoprotein on blood-brain barrier endothelium, epithelial tissue barriers and neurons. Neurosci Lett. 1992;140:93–97 [DOI] [PubMed] [Google Scholar]
- 12. Biswas C, Zhang Y, DeCastro R, et al. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55:434–439 [PubMed] [Google Scholar]
- 13. Fadool JM, Linser PJ. Differential glycosylation of the 5A11/HT7 antigen by neural retina and epithelial tissues in the chicken. J Neurochem. 1993;60:1354–1364 [DOI] [PubMed] [Google Scholar]
- 14. Hori K, Katayama N, Kachi S, et al. Retinal dysfunction in basigin deficiency. Invest Ophthalmol Vis Sci. 2000;41:3128–3133 [PubMed] [Google Scholar]
- 15. Daniele LL, Sauer B, Gallagher SM, Pugh EN, Jr, Philp NJ. Altered visual function in monocarboxylate transporter 3 (Slc16a8) knockout mice. Am J Physiol Cell Physiol. 2008;295:C451–C457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rattner A, Toulabi L, Williams J, Yu H, Nathans J. The genomic response of the retinal pigment epithelium to light damage and retinal detachment. J Neurosci. 2008;28:9880–9889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rizzolo LJ, Chen X, Weitzman M, Sun R, Zhang H. Analysis of the RPE transcriptome reveals dynamic changes during the development of the outer blood-retinal barrier. Mol Vis. 2007;13:1259–1273 [PubMed] [Google Scholar]
- 18. Maminishkis A, Chen S, Jalickee S, et al. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47:3612–3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gullapalli VK, Sugino IK, Van Patten Y, Shah S, Zarbin MA. Impaired RPE survival on aged submacular human Bruch's membrane. Exp Eye Res. 2005;80:235–248 [DOI] [PubMed] [Google Scholar]
- 20. Sonoda S, Spee C, Barron E, Ryan SJ, Kannan R, Hinton DR. A protocol for the culture and differentiation of highly polarized human retinal pigment epithelial cells. Nat Protoc. 2009;4:662–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pratt CH, Vadigepalli R, Chakravarthula P, Gonye GE, Philp NJ, Grunwald GB. Transcriptional regulatory network analysis during epithelial-mesenchymal transformation of retinal pigment epithelium. Mol Vis. 2008;14:1414–1428 [PMC free article] [PubMed] [Google Scholar]
- 22. Gong J, Sagiv O, Cai H, Tsang SH, Del Priore LV. Effects of extracellular matrix and neighboring cells on induction of human embryonic stem cells into retinal or retinal pigment epithelial progenitors. Exp Eye Res. 2008;86:957–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Philp N, Chu P, Pan TC, et al. Developmental expression and molecular cloning of REMP, a novel retinal epithelial membrane protein. Exp Cell Res. 1995;219:64–73 [DOI] [PubMed] [Google Scholar]
- 24. Philp NJ, Wang D, Yoon H, Hjelmeland LM. Polarized expression of monocarboxylate transporters in human retinal pigment epithelium and ARPE-19 cells. Invest Ophthalmol Vis Sci. 2003;44:1716–1721 [DOI] [PubMed] [Google Scholar]
- 25. Halfter W. A heparan sulfate proteoglycan in developing avian axonal tracts. J Neurosci. 1993;13:2863–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tamiya S, Liu L, Kaplan HJ. Epithelial-mesenchymal transition and proliferation of retinal pigment epithelial cells initiated upon loss of cell-cell contact. Invest Ophthalmol Vis Sci. 2010;51:2755–2763 [DOI] [PubMed] [Google Scholar]
- 27. Klimanskaya I. Retinal pigment epithelium. Methods Enzymol. 2006;418:169–194 [DOI] [PubMed] [Google Scholar]
- 28. Gallagher SM, Castorino JJ, Philp NJ. Interaction of monocarboxylate transporter 4 with beta1-integrin and its role in cell migration. Am J Physiol Cell Physiol. 2009;296:C414–C421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kojima A, Nakahama K, Ohno-Matsui K, et al. Connexin 43 contributes to differentiation of retinal pigment epithelial cells via cyclic AMP signaling. Biochem Biophys Res Commun. 2008;366:532–538 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.