Sodium bicarbonate cotransporter (NBC) expression was reduced in corneal endothelium by lentiviral delivery of shRNA. Corneal thickness in these eyes showed greater sensitivity to the topical carbonic anhydrase inhibitor brinzolamide, indicating that NBC works in conjunction with carbonic anhydrases as a component of the corneal endothelial pump.
Abstract
Purpose.
To evaluate the role of the sodium bicarbonate cotransporter (NBCe1) as a component of the corneal endothelial pump in the in vivo rabbit eye.
Methods.
Lentiviruses with NBCe1 shRNA and GFP expression cassettes were injected intracamerally. Knockdown efficacy was determined 1 week to 4 weeks later by immunofluorescence, Western blot analysis, and PCR. Functional effects were monitored by corneal thickness (CT) and brinzolamide sensitivity.
Results.
Within 24 hours there was a modest anterior chamber inflammation that resolved within 48 hours. At 4 × 106 IFU, more than 95% of the corneal endothelial surface showed GFP fluorescence above background within 7 days. At 14 to 21 days, signs of anterior chamber inflammation reemerged, and endothelial cell GFP fluorescence disappeared within 40 days after injection. The second phase of inflammation could be avoided by using GFP-less viruses. There was no significant difference in CT between scrambled sequence and NBCe1 shRNA–injected eyes over 3 weeks. Two drops of 1% brinzolamide produced 7.85% ± 3.3% corneal swelling within 5 hours of topical instillation. However, in corneas showing more than 25% NBCe1 knockdown (30 of 42 rabbits; 59% ± 15% knockdown), corneal swelling was significantly higher (10.1% ± 2.9%) relative to control eyes.
Conclusions.
FIV-based lentiviral vectors can transfect CE with shRNA in rabbits. The response to GFP is consistent, with previous studies showing the production of anti-GFP antibodies. Partial knockdown of NBCe1 did not affect baseline CT, which is consistent with the corneal endothelium having a substantial functional reserve. Provocative testing using, brinzolamide, however, revealed an underlying deficiency, confirming the importance of NBCe1 bicarbonate transport and demonstrating the concerted action between NBCe1 and carbonic anhydrases.
Maintenance of corneal hydration and transparency is dependent on the active ion transport properties of the corneal endothelium. Negatively charged stromal glycosaminoglycans provide passive swelling pressure that causes fluid imbibition (leak) into the stroma, which, when offset by the endothelial pump, produces steady state corneal hydration. This has been termed the pump-leak hypothesis.1,2 The details of the endothelial pump mechanism are incomplete, but numerous studies using ex vivo chamber-mounted rabbit corneas have shown that the endothelial pump is inhibited by the Na+/K+-ATPase inhibitor ouabain,3–5 dependent on the presence of bicarbonate,6–8 and slowed by carbonic anhydrase inhibitors.6–10 This has led to the working hypothesis that the endothelial pump is an active transport–dependent bicarbonate secretory mechanism.
In vitro physiological studies have shown that the basolateral (stromal side) corneal endothelium has substantial Na+-dependent and DIDS (4,4-diisothiocyanatostilbenedisulfonic acid)–sensitive bicarbonate permeability.11,12 This is attributed to an electrogenic 1 Na+/2 HCO3− cotransporter that is responsible for NaHCO3− uptake.13 This cotransporter has been identified as NBCe1, and siRNA knockdown in vitro has confirmed that it is responsible for bicarbonate entry into the endothelial cells from the basolateral side.14 Bovine corneal endothelial cells cultured on permeable substrates can produce a small (0.09 U) transendothelial (apical side alkaline) pH gradient that is reduced by NBCe1-specific siRNA,14 indicating that the basolateral loading of endothelial cells with bicarbonate by NBCe1 is required for apical efflux. Previous studies with ex vivo mounted–superfused rabbit corneas showed that the anion transport inhibitor DIDS slows the endothelial pump.3,15 DIDS inhibits NBCe1, but it is not specific and blocks other bicarbonate-dependent transporters and anion channels, including the Cl−/HCO3− exchanger AE2, which is expressed in corneal endothelium.16 Taken together, the in vitro studies suggest that NBCe1 is an integral component of the corneal endothelial pump; however, direct specific demonstration of the role of NBCe1 in the endothelial pump in vivo is lacking.
Corneal endothelial cells express at least three carbonic anhydrases, cytosolic CA II, apical membrane CA IV,17–20 and basolateral CA X (Bonanno et al., unpublished data). When applied directly to the endothelial surface in ex vivo chamber-mounted rabbit corneas, carbonic anhydrase inhibitors cause corneal edema.6–10 In addition to slowing the endothelial pump, carbonic anhydrase inhibitors slow NBCe1-dependent intracellular pH recovery, reduce cell-buffering capacity,11,21 and reduce apical compartment alkalinization,14 suggesting that carbonic anhydrases function in conjunction with bicarbonate transporters. Molecular and functional linkage between carbonic anhydrases and bicarbonate transporters, such as NBCe1, has been demonstrated for several bicarbonate transporters and carbonic anhydrases and has been termed a transport metabolon.22
In the present study, we explored knocking down expression of NBCe1 using an RNAi approach delivered by lentiviral vectors in the New Zealand White rabbit in vivo. The rabbit was chosen because it is the standard for corneal physiology studies, and measurements of corneal thickness and IOP are amenable using standard instrumentation. Lentiviral vectors containing GFP expression cassettes have been shown to transfect corneal endothelium in vivo23–26 and to provide long-term transduction; however, shRNA-mediated knockdown in vivo has not been reported. We found that shRNA-mediated NBCe1 knockdown in vivo was effective, and, in conjunction with inhibiting carbonic anhydrase activity, physiological effects could be elicited.
Methods
Pseudolentivirus Production
Feline immunodeficiency virus (FIV)–based pseudolentivirus construction reagents were obtained from System Biosciences (SBI; Mountain View, CA). Five different lentiviruses were used. The first was Lenti-GFP, which expresses copepod GFP driven by the human cytomegalovirus (CMV) promoter, obtained directly from SBI. Copepod GFP is similar to regular EGFP but has a brighter color. The second was Lenti-GFP-NBCe1-shRNA, which expresses H1 promoter-driven NBCe1-shRNA with CMV promoter-driven GFP as a reporter gene. The third was Lenti-NBCe1-shRNA, which expresses only H1 promoter-driven NBCe1-shRNA. The fourth was Lenti-GFP-scrambled-shRNA, which expresses H1 promoter-driven scrambled shRNA with CMV-driven GFP as a reporter gene. The fifth was Lenti-scrambled-shRNA, which expresses only H1 promoter-driven scrambled shRNA.
A previous study14 evaluated several siRNAs and found that the siRNA against the target 5′-AAAAAGAAGGAGGATGAGAAG-3′ (GenBank accession no. AF119816, 2902–2922) was the most efficient for knockdown of NBCe1 expression. Based on this siRNA sequence, an shRNA against NBCe1 was designed in accordance with the manufacturer's instructions (5′-GATTCAAAAAGAAGGAGGATGAGAAGCTTCCTGTCAGACTTCTCATCCTCCTTCTTTTTTTTTTG-3′ and 5′-AATTCAAAAAAAAAAGAAGGAGGATGAGAAGTCTGACAGGAAGCTTCTCATCCTCCTTCTTTTTG-3′). An shRNA targeting the wild-type firefly Luciferase gene was used as a scrambled control (5′-GATCCGTGCGTTGTTAGTACTAATCCTATTTGTGAAGCAGATGAAATAGGGTTGGTACTAGCAACGCACTTTTTG-3′ and 5′AATTCAAAAAGTGCGTTGCTAGTACCAACCCTATTTCATCTGCTTCACAAATAGGATTAGTACTAACAACGCACG-3′). ShNBCe1 and shLuciferase template oligonucleotides were synthesized (Integrated DNA Technologies, Coralville, IA) and cloned into FIV-based pSIF-H1-CopGFP and pSIF-H1-Puro shRNA vectors (SBI), respectively. Lentiviral constructs pSIF-H1-CopGFP-shNBCe1, pSIF-H1-Puro-shNBCe1, pSIF-H1-CopGFP-shLuciferase, and pSIF-H1-Puro-shLuciferase were individually packaged into lentiviral-transducing particles, which were pseudotyped with vesicular stomatitis virus G (VSV-G) protein (pPACK lentivector packaging kit; SBI). Briefly, lentivirus vector was combined with the mixture of pFIV-34N and pVSV-G plasmids and then was transfected into 293TN HEK cells by using liposome reagent (Invitrogen, Carlsbad, CA). Pseudovirus-containing medium was collected 48 and 72 hours after transfection. Pseudolentiviral particles were purified with PEG-it virus precipitation solution (SBI) overnight at 4°C and then centrifuged at 1500g for 30 minutes at 4°C. Residual PEG-it solution was removed by another centrifugation at 1500g for 5 minutes. Viral particles were washed and resuspended in cold, sterile PBS. Virus stocks were divided into aliquots and stored at −80°C. Virus titer was determined by flow cytometry or PCR. Serial dilutions of virus stocks were inoculated to 293TN HEK cells. Seventy-two hours after viral transduction, cells were collected for flow cytometry (FACSCalibur; BD Biosciences, Franklin Lakes, NJ) or a real-time PCR-based titration (UltraRapid Lentiviral Titer Kit; SBI). Using the FACS method, the relative titer was measured based on the fraction of fluorescent cells. Using real-time PCR-based titration, virus titer was measured based on the amplification of a small fragment from the lentivector-specific WPRE (Woodchuck hepatitis virus Post transcriptional Regulation Element) that is integrated into the genome of transduced cells.
Experimental Animals
Young New Zealand White rabbits (age range, 8–10 weeks; weight range, 1.8–2.2 kg) were used as the animal model. All animal procedures were performed in accordance with the Declaration of Helsinki, the Indiana University Animal Care and Use Committee, and the ARVO Statement for the Use Animals in Ophthalmic and Vision Research. For anterior chamber injections, slit lamp biomicroscopy, and confocal scanning laser ophthalmoscopy, rabbits were sedated with ketamine HCl (30 mg/kg) and xylazine HCl (5 mg/kg, intramuscularly). Topical anesthesia (0.5% proparacaine HCl, 2 drops; Akorn, Lake Forest, IL) was used for anterior chamber injections, central corneal thickness (CT), and intraocular pressure (IOP) measurements by ultrasound pachymetry (DGH Technology, Exton, PA) and tonometry (Tono-PEN XL; Reichert, Depew, NY), respectively. Rabbits were sedated and then euthanatized by injection of 2.5 mL sodium pentobarbital euthanatization solution (Sleepaway; Fort Dodge Animal Health, Fort Dodge, IA) intracardially.
Viral Injection
After the rabbit was sedated, one drop of 1% cyclopentolate HCl (Ocusoft, Rosenberg, TX) and two drops of 0.5% Proparacaine HCl were topically applied to the eye. With the aid of an ophthalmic surgical microscope, 30 to 110 μL, depending on the virus titer and dose, of the viral suspension mixed with 5 μg hexadimethrine bromide (Polybrene; Sigma, St. Louis, MO) was injected into the anterior chamber using a 30-gauge needle on a Hamilton glass syringe. The needle was inserted perpendicularly to the corneal surface 1 to 2 mm from the limbus and then, while the tip was still in the stroma, was turned and pointed toward the anterior-center of the anterior chamber. With the needle bevel facing the endothelium, the viral suspension was slowly injected over 30 seconds. To minimize leaking, the needle was left in place for 1 minute and was removed gradually. The eyes were treated prophylactically with antibiotic ointment (AK-Poly-Bac; Akorn Pharmaceutical) immediately after injection.
Corneal Thickness, IOP, and Brinzolamide Provocative Test
Baseline corneal thickness (ultrasound pachymetry) and IOP (Tono-Pen; Reichert) were measured in that order the day before eye injection and then approximately weekly thereafter. These measurements were performed at approximately 10 AM. Two days before eye injection and then approximately weekly thereafter, a brinzolamide provocative test was performed. CT was measured in each eye and then two drops of 1% brinzolamide (Azopt; Alcon, Fort Worth, TX), a carbonic anhydrase inhibitor, was applied topically. CT was then measured at 1, 3, 5, and 7 hours after brinzolamide application to obtain the peak level of corneal swelling.
NBCe1 Western Blot Analysis
Eyeballs were enucleated after the rabbits were euthanatized. Corneas were dissected, and endothelium/Descemet's membrane was immediately peeled from the cornea using jeweler's forceps, placed into sterile PBS, and dissolved in 2% SDS sample buffer that contained 1% protease inhibitors (Sigma). The preparations were briefly sonicated (Sonifier 250; Branson, Danbury, CT) on ice and centrifuged at 10,000g for 5 minutes. An aliquot of the supernatant was taken for protein concentration measurement using the BCA protein assay (Bio-Rad, Hercules, CA). Samples (5 or 10 μg, not heated) were separated on SDS-PAGE and electroblotted to polyvinylidene difluoride (PVDF) membranes (Bio-Rad). Based on the prestained protein markers (Precision Plus protein standards; Bio-Rad), blots were horizontally cut into two parts just above the 50-kDa prestained marker. The upper part, with protein sizes greater than 50 kDa, was probed with NBCe1 polyclonal antibodies (mixture of AB-3212, 1:1000 and AB3204, 1:10,000; Chemicon, Temecula, CA). The lower part was probed with β-actin monoclonal antibody (Sigma) with a dilution of 1:10,000. Enhanced chemiluminescence (Thermo Scientific, Pittsburgh, PA) was used for detection. Films were scanned to produce digital images that were then subjected to densitometry analysis (UN-SCAN-IT gel 6.1). NBCe1 protein levels in test samples were normalized to β-actin. NBCe1 protein expression in the experimental eye was calculated relative to that in the control eye.
Immunofluorescence
Dissected corneas were washed with PBS and immediately fixed with PLP fixation buffer (2% paraformaldehyde, 75 mM lysine, 10 mM sodium periodate, 45 mM sodium phosphate [pH 7.4]) for 10 minutes. Corneas were rinsed with PBS, and endothelium/Descemet's strips were peeled off using jeweler's forceps and flattened onto microscope slides (Superfrost; Fisher Scientific). Strips were fixed again at room temperature for 20 minutes and washed with PBS. Slides were then kept for 5 minutes in PBS containing 1% SDS to unmask epitopes and were washed three times in PBS. Cells were blocked for 1 hour in PBS containing 0.2% bovine serum albumin (BSA), 5% goat serum, 0.01% saponin, and 50 mM NH4Cl. Rabbit polyclonal NBC-1 antibody diluted 1:100 in PBS/goat serum (1:1) was added to cells and incubated for 1 hour at room temperature. Slides were washed three times for 15 minutes in PBS containing 0.01% saponin. Then secondary antibody conjugated to Oregon Green (Molecular Probes) (1:1000) was applied for 1 hour at room temperature. Slides were washed, counterstained with DAPI, and mounted with anti-fade medium (Prolong; Molecular Probes) according to the manufacturer's instructions. Fluorescence was observed with an epifluorescence microscope (E600; Nikon, Tokyo, Japan) equipped with a cooled charge-coupled device camera (Princeton Instruments, Princeton, NY).
RNA Extraction and Real-Time PCR
Total RNA was extracted from dissected corneal endothelium (RNeasy Micro Kit; Qiagen, Valencia, CA). Total RNA (50 or 100 ng) was used for cDNA synthesis in a total volume of 20.5 μL with reverse transcriptase according to the manufacturer's instructions (QuantiMir TR Kit; SBI). Real-time PCR was used to quantify mRNA levels of NBCe1 (primer pair, 5′-CTATCCCTGCTCTGCTGGTC-3′ and 5′-AGCTGTCGATGTGAGCAATG-3′). GAPDH (primer pairs, 5′CTGGTCACCAGGGCTGCTTT-3′ and 5′-CACCAGCATCACCCCACTTG-3′) was quantified as an internal control. The expected NBCe1 and GAPDH products were 226 bp and 209 bp, respectively. Real-time PCR was performed using master mix (Brilliant II SYBR Green QPCR; Stratagene, La Jolla, CA) in a thermal cycling instrument (Stratagene Mx3005P) with the following cycle conditions: 50°C for 2 minutes, 95°C for 10 minutes, 40 cycles of 95°C for 30 seconds, 54°C for 1 minute, and 72°C for 30 seconds. For each rabbit, NBCe1 mRNA levels in test samples were expressed as fold changes relative to GAPDH (i.e., normalized to GAPDH using the formula 2−(Ct NBCe1 − Ct GAPDH), where Ct is the threshold cycle number for amplicon detection by real-time PCR. NBCe1 mRNA expression level in the experimental eye was expressed as a percentage of that in the paired control eye.
Percentage Area of Corneal Endothelium Transfected
GFP fluorescence was imaged in vivo using a scanning confocal microscope (HRA2; Heidelberg Engineering,Vista, CA). Images were analyzed using bioanalytical software (Metamorph; Universal Imaging, West Chester, PA). A 9-mm central diameter circle was used to determine pixel intensity (0–255) in control (PBS injected) and experimental eyes. Background fluorescence intensity distributions in control eyes were used to set the fluorescence threshold, which we chose to be 99% of the pixel fluorescence distribution. This value was typically between 15 and 20. In the paired experimental eye, any pixel with fluorescence intensity above the threshold value was counted as GFP positive. From this analysis, the percentage of the experimental corneal area above threshold and the average fluorescence intensity of this area were determined.
Statistical Analysis
Paired t-tests (OS-OD) with Bonferroni's correction for multiple comparisons were used to test statistical significance at P < 0.05.
Results
Figures 1A–D show that the FIV-based lentiviral GFP vector could efficiently transfect corneal endothelial cells in the rabbit in vivo. There was an initial anterior chamber inflammation within 24 hours of injection that self-resolved within 48 hours. Fluorescence appeared at around 5 days and was typically maximal by 14 days. Slit-lamp images (Fig. 1C) indicated that fluorescence was limited to the endothelium in the cornea. Similarly, Figure 1D shows that direct GFP fluorescence could be seen in Descemet's/endothelial peelings at 3 weeks after injection. As expected, all anterior chamber structures were transfected, including the iris (Fig. 1E) and the trabeculum (not shown). Figure 1F shows that GFP transgene expression in the endothelium was dose dependent, with maximal expression apparent between 12 and 14 days. Figure 1G summarizes these data showing that 4 × 106 IFU of Lenti-GFP, which represents a multiplicity of infection (MOI) of approximately 10, was sufficient to transfect more than 95% of the corneal endothelial surface, as measured by GFP fluorescence.
Figure 1.
Dose-response of lentivirus-GFP anterior chamber fluorescence in New Zealand White rabbits. (A–E) Intracameral injection of 4 × 106 IFU. (A) En face corneal endothelial GFP fluorescence image using a confocal microscope 7 days after injection. Scale bar, 4 mm. (B) Fourteen days after injection. (C) Slit-lamp image of endothelial fluorescence 14 days after injection. (D) GFP fluorescence from endothelial peeling 21 days after injection. Scale bar, 60 μm. (E) Iris fluorescence 14 days after injection. (F) Average (SD) fluorescence intensity over time of various viral doses (n = 3 for each dose). Average fluorescence intensity was calculated by integrating the fluorescence intensity over the area of measurement (9-mm diameter) after background subtraction. (G) Average fluorescence intensity and the percentage area of the measured corneal area that had fluorescence values above threshold for each viral dose.
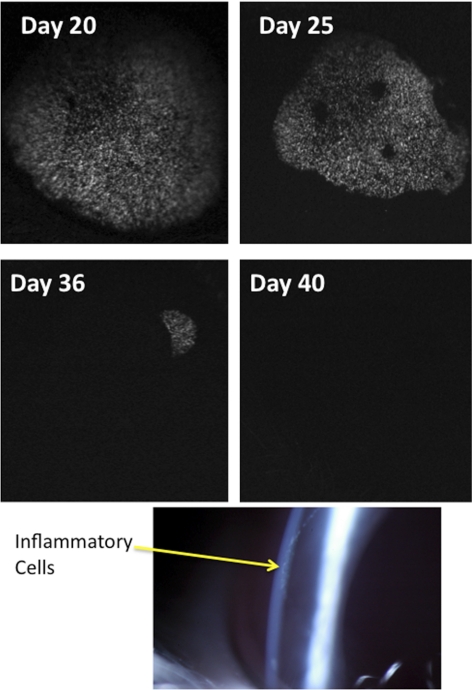
Figure 2 shows that between 14 and 21 days after injection, endothelial GFP fluorescence began to shrink circumferentially. This was concomitant with a moderate anterior chamber uveitis, with inflammatory cells apparent on the endothelial surface (keratic precipitates) in a circumlinear fashion (see Fig. 2). Coincident with the inflammation there was some corneal edema that could be eliminated with topical prednisolone. The GFP fluorescence progressively shrank and fell below threshold detection between 28 and 40 days after injection. This was concomitant with self-resolution of the anterior uveitis. Viral transfection with Lenti-GFP-NBCe1-shRNA produced a similar transient GFP expression.
Figure 2.
Loss of GFP fluorescence. Representative successive corneal endothelial fluorescence images from the same eye. Bottom: slit-lamp image showing keratic precipitates (arrow) in a circumferential pattern from an eye injected with 4 × 106 IFU Lenti-GFP 25 days after injection.
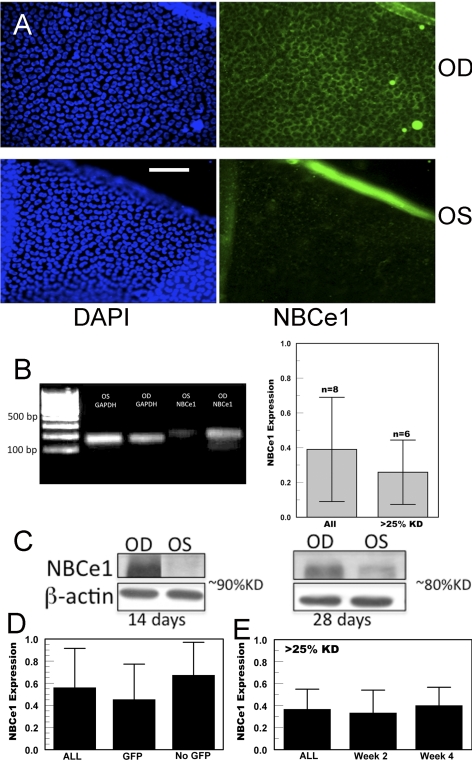
Without knowing whether the GFP rejection phenomenon would adversely affect shRNA expression, we also constructed a GFP-less NBCe1 shRNA lentivirus. Although we could not gauge the approximate transfection level in vivo by viewing fluorescence, we found that these viruses did not produce a secondary inflammation. Eighteen eyes were injected with Lenti-NBCe1-shRNA virus, and 24 eyes were injected with the Lenti-GFP-NBCe1-shRNA virus (total, 42 eyes). In both cases, the right eye was injected with a control virus (Lenti-Scrambled-shRNA or Lenti-GFP-Scrambled-shRNA), and the left eye was injected with the NBCe1-specific shRNA virus. Figure 3A shows NBCe1 immunofluorescence of endothelial peelings from a control and an experimental eye (both injected with GFP-less transgenes) at 4 weeks after injection. The control peeling shows the characteristic basolateral staining for NBCe1,13,27 whereas there is significant loss of staining in the NBCe1 shRNA-transfected cells. Additional evidence of effective knockdown is indicated in Figure 3B, which shows significantly reduced NBCe1 transcript levels, as measured by real-time PCR, in eight pairs of eyes at 2 to 3 weeks after injection. At the protein level, Figure 3C shows selected Western blot analysis at 2 (GFP+) and 4 (GFP−) weeks after injection, suggesting significant knockdown of NBCe1 is possible with either GFP or GFP-less expressing viral transgenes. Summary data (Fig. 3D) from all 42 rabbits injected with NBCe1 shRNA indicate that Lenti-GFP-NBCe1-shRNA was at least as effective as Lenti-NBCe1-shRNA. Of the Lenti-GFP-NBCe1-shRNA–injected eyes, 12 were analyzed at 14 days after injection (NBCe1 knockdown, 52% ± 36%), and 12 were analyzed after GFP fluorescence was not detectable (>28 days) (NBCe1 knockdown, 56% ± 27%), indicating that loss of GFP fluorescence or elimination of GFP expression did not adversely affect shRNA knockdown.
Figure 3.
Knockdown of NBCe1 expression. (A) Representative immunofluorescence images (blue, nuclear DAPI; green, NBCe1) of corneal endothelial peelings from the right (OD, Lenti-scrambled) and left (OS, Lenti-NBCe1-shRNA) corneas 4 weeks after injection. Scale bar, 100 μm. (B, left) Representative real-time PCR products. Right: summary results from eight rabbits and only those showing >25% knockdown. (C) Representative Western blot analysis 14 and 28 days after injection. (D) Summary of Western blot analysis (n = 34) and quantitative PCR (n = 8) data from all rabbits analyzed (n = 42) and subdivided into those coexpressing GFP (n = 24) and without GFP (n = 18). (E) Rabbits that had >25% knockdown of NBCe1 (n = 30) and subdivided at 2 weeks (n = 19) and 4 weeks (n = 11) after injection.
In some experiments, we found that there was little or no knockdown of NBCe1. Using a success criterion of 25% or greater knockdown of NBCe1, 71% of the experiments were successful (30 of 42 eye injections). Figure 3E shows that the average knockdown of this subgroup was 63% ± 18% and that this was sustained over several weeks. The longest postinjection knockdown analysis was performed in two rabbits at 10 weeks (52% and 56% NBCe1 knockdown).
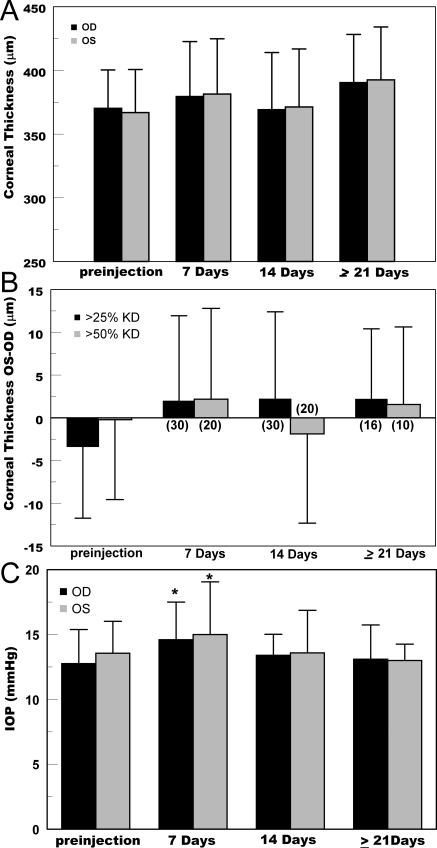
Corneal endothelial function was gauged as change in baseline corneal thickness or differential brinzolamide-induced corneal swelling relative to the paired control Lenti-scrambled-shRNA injected eye. Functional data were not collected during any periods of anterior chamber inflammation (e.g., in GFP-expressing eyes approximately14–30 days after injection). Figure 4A shows absolute corneal thickness in control and experimental eyes over time for all rabbits, suggesting a small difference between control eyes (OD) and NBCe1-shRNA injected eyes (OS). There was also a small increase in corneal thickness in both eyes over 4 weeks, which reflected normal growth effects in these young animals.28 Using only those rabbits in which the experimental eye showed ≥25% knockdown, analysis of pairwise differences (OS-OD) in corneal thickness (Fig. 4B) showed a small increase in corneal thickness in the experimental eye compared with control, but these differences were not statistically significant. Figure 4B also shows that even when we considered a subgroup that showed more than 50% knockdown (n = 20), no significant differences in baseline corneal thickness were found.
Figure 4.
Corneal thickness and IOP after injection of Lenti-scrambled (OD) and Lenti-NBCel-shRNA (OS). (A) Average (SD) central corneal thickness of all rabbits. (B) Average of paired differences (OS-OD) in corneal thickness for only rabbits with >25% NBCe1 knockdown or >50% knockdown (P > 0.05). Number of rabbits in each category is shown in parentheses. (C) Average (SD) of IOP values from all rabbits showing >25% knockdown of NBCe1. *Significantly different from preinjection (P < 0.005).
Corneal thickness can be secondarily affected by IOP. Increased IOP will reduce stromal imbibition pressure and thereby reduce corneal thickness.1,29 However, IOP must increase by at least 6 mm Hg to produce a 10-μm (3.5%) change in rabbit corneal thickness. Figure 4C shows that at 7 days there was a small, but significant (P < 0.05), increase in IOP in both control (+1.9 mm Hg) and experimental (+1.5 mm Hg) eyes compared with before injection. However, at later time points, there were no significant differences from preinjection values, and there were no significant differences between control and experimental eyes at any time point, indicating that IOP is not a secondary factor influencing analysis of differences in corneal thickness in these experiments.
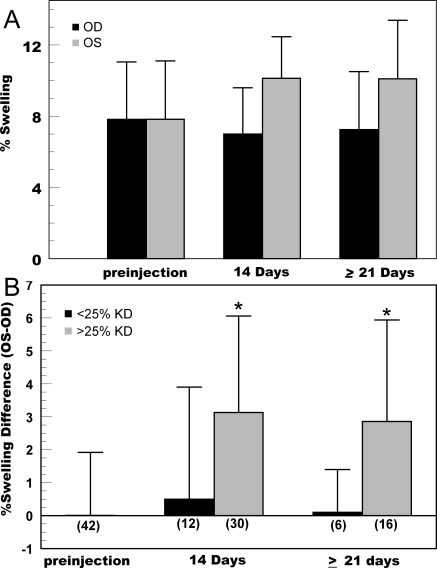
Because bicarbonate transporters work in conjunction with carbonic anhydrase activity, we examined the possibility that the sum of NBCe1 knockdown together with carbonic anhydrase inhibition could elicit a functional effect. Although the use of topical carbonic anhydrase inhibitors does not affect corneal thickness in the normal human cornea,30–32 Figure 5A shows that two drops of 1% brinzolamide in the New Zealand White rabbit produced 7.85% ± 3.3% corneal swelling between 3 and 5 hours. In addition, brinzolamide-induced swelling in the NBCe1-shRNA injected eyes (>25% knockdown) increased to 10.1% ± 2.9%. Figure 5B shows that analysis of pairwise differences (OS-OD) in corneal swelling is highly significant (P < 0.005). Moreover, pairwise differences in brinzolamide-induced swelling of the 11 rabbits with less than 25% knockdown of NBCe1 were not significant (differences of 0.5% ± 3.4% swelling at 2 weeks and 0.1% ± 1.3% swelling at 3 weeks).
Figure 5.
Brinzolamide provocative test results in rabbits with NBCe1knockdown. (A) Average (SD) of peak changes in corneal thickness 3 to 5 hours after topical application of two drops of 1% brinzolamide to both eyes. OD, control (injected with scrambled sequence). OS, >25% knockdown of NBCe1-shRNA. (B) Average (SD) of paired differences (OS-OD) in peak brinzolamide-induced corneal swelling measured at 2 and 3 weeks after injection in rabbits demonstrating <25% and >25% knockdown of NBCe1. *Significantly different from 0 (P < 0.005). Number of rabbits in each category is shown in parentheses.
Discussion
Previous studies have shown that the corneal endothelium can be transfected after anterior chamber injection of various viral vectors and can produce GFP expression in vivo.23–26 Several studies have demonstrated effective protein knockdown in the eye using viral vectors expressing shRNA or ribozymes, but only in the retina.33–38 This is the first report of shRNA-mediated in vivo knockdown in the corneal endothelium and anterior chamber.
Lentiviral vectors were chosen because of their long-term expression and low immunogenicity. We found that NBCe1 knockdown was sustained at least 10 weeks after injection, and other studies have shown that significantly longer transgene expression can be expected.23,25,26 Initial experiments used GFP expression to determine appropriate viral dosage. This indicated that 4 × 106 IFU was sufficient to transfect the corneal endothelial surface. However, we found that transfection efficiency, as judged by GFP fluorescence (Fig. 1) or by NBCe1 knockdown (Fig. 4), was highly variable. In pilot experiments, higher doses of GFP-less virus were tried, but knockdown was not noticeably better and inflammatory responses were more prominent. The transfection variability was probably caused by the relatively large volume of the anterior chamber, where the target cells represent less than half the surface area of this space. The dose used represents an MOI of approximately 10. However, because of the geometry of the anterior chamber and the presence of aqueous flow, the effective MOI must be considerably lower, yet the data (>95% area above fluorescence threshold) suggest that the effective MOI is at least ≥1. In contrast to the transfection of retinal structures after subretinal injection or transfection of the trabecular meshwork, by which most of the intracamerally injected virus will come in contact with that target, corneal endothelial transfection is bound to be more variable. Corneal endothelial transfection in vivo could possibly be enhanced and transfection of other structures could be reduced by using virus-linked magnetic nanoparticles or other forms of tropism.
Anterior chamber inflammatory responses were present in almost all eyes in the first 48 hours after virus injection. These responses were relatively mild and resolved spontaneously. However, as mentioned, the injection of high doses (>107 IFU) led to larger and longer lasting initial inflammation. More interesting was the observation of a second bout of anterior uveitis in GFP-expressing eyes that coincided with a visible gradual loss of GFP fluorescence (Fig. 2). Previous studies that report anterior chamber GFP expression in rats, mice, or monkeys25,26,35,38,39 did not report this phenomenon. However, a recent report found inflammation and anti-GFP antibodies in one monkey 70 days after GFP transfection.40 On the other hand, GFP expression in the Dutch Belted Rabbit retina consistently demonstrated inflammation and loss of GFP41 and the generation of anti–GFP antibodies.42 The loss of GFP expression in the rabbit could be inhibited by using a 30-day immunosuppression regimen.42 Therefore, we can conclude that this secondary inflammation and loss of GFP in our experiments is a species-specific immune response.
During the GFP-dependent inflammation, we noted a moderate anterior chamber response and the formation of keratic precipitates. As expected, corneal thickness increased during the inflammation but was not severe, as evidenced by our ability to obtain clear endothelial images. Furthermore, the increased corneal thickness could be eliminated with topical steroid treatment. Nevertheless, functional evaluation of the endothelium would have been complicated during inflammation-induced edema and, hence, was not performed in knockdown experiments during this period. To avoid this inflammation, we used the Lenti-NBCe1-shRNA virus. Our data show that either GFP or GFP-less NBCe1-shRNA constructs efficiently reduced NBCe1 expression. Moreover, shRNA knockdown of NBCe1 after the inflammation resolved and GFP disappeared was similar to that before the secondary inflammation or to that in GFP-less transfected cells, suggesting that the immune response resulted in very specific silencing of the GFP transgene. This is consistent with reports that showed that inflammation secondary to lentiviral transfection was specific to the transgene product and not to lentiviral epitopes.43,44 In conclusion, the use of GFP expression is convenient for estimating transfection in the rabbit corneal endothelium; however, it produces a 12- to 20-day period of inflammation during which fluorescence disappears. This had no effect on shRNA expression, which may be consistent with CMV promoter-specific silencing.43
Previously, we had tested several siRNA sequences for knockdown of NBCe1 expression in cultured corneal endothelial cells.14 Knockdown in vitro was 80% to 90%, which is considerably greater than what we achieved in vivo using the same RNAi sequence that gave the best knockdown in vitro. Functional consequences of NBCe1 knockdown in vitro were reduced basolateral bicarbonate permeability, reduced basolateral bicarbonate flux, and reduced apical compartment alkalinization.14 Given the dependence of the endothelial pump on bicarbonate and the sensitivity to the anion transport inhibitor DIDS, our expectation was that NBCe1 knockdown would slow the pump and result in an increase in corneal thickness. Knockdown was complete at 2 weeks. Data at 7, 14, and ≥21 days suggest that there was a small increase in baseline thickness in the experimental eyes, but this was not statistically significant. Using only those eyes with greater than 50% knockdown also failed to show significant differences in baseline thickness. This suggests that partial reduction (∼65% knockdown) of NBCe1 expression by itself is insufficient to affect endothelial function. Upregulation of other transport mechanisms that could potentially substitute for NBCe1 activity (e.g., Na+/H+ exchange) in response to reduced NBCe1 expression are possible, but one would expect a period of increased corneal thickness followed by a return to baseline if this were the case. Another approach is to knock out NBCe1 expression.45 However, NBCe1 knockouts have significant developmental and systemic abnormalities and die shortly after birth, limiting their usefulness in analyzing the role of NBCe1 in the cornea.
Taking into consideration that NBCe1 expression is partially reduced and that bicarbonate transport is facilitated by carbonic anhydrase activity, we tested whether the carbonic anhydrase inhibitor brinzolamide could uncover a functional difference. We found that brinzolamide-induced corneal swelling was significantly greater in the cornea with reduced NBCe1 expression relative to the control. Use of topical carbonic anhydrase inhibitors in humans generally does not affect corneal thickness.30–32 Therefore, we were surprised that brinzolamide caused an increase in corneal thickness in the normal rabbit cornea. We also confirmed that the preservative (0.01% benzylalkonium chloride) in brinzolamide did not affect rabbit corneal thickness. Presumably, this brinzolamide sensitivity in the rabbit may be attributed to a species difference in total carbonic anhydrase activity. There are several clinical reports of significant corneal edema in humans using carbonic anhydrase inhibitors in which it was found that corneal endothelial function was marginal.46–50 Our results are analogous to this clinical scenario, i.e., NBCe1 knock down creates a marginally functional corneal endothelium that, with the inhibition of carbonic anhydrases, pushes functional activity below a tipping point that yields increased corneal edema. To produce a definitive effect on baseline corneal thickness, it is likely that NBCe1 knockdown must be greater than 80% to 90%.
In summary, we describe a new approach to studying the mechanisms of the corneal endothelial pump in vivo using RNAi. This approach can potentially be used singly or in combination to test various components of the pump. Our data are consistent with the sodium bicarbonate cotransporter, NBCe1, working synergistically with carbonic anhydrases as an integral component of the pump. Moreover, we illustrate the use of carbonic anhydrase inhibitors for uncovering endothelial dysfunction, which could potentially be developed as a clinical provocative test.
Footnotes
Supported by National Institutes of Health Grant EY008834.
Disclosure: C. Liu, None; Q. Cheng, None; T. Nguyen, None; J.A. Bonanno, None
References
- 1. Baum J, Maurice D, McCarey B. The active and passive transport of water across the corneal endothelium. Exp Eye Res. 1984;39:335–342 [DOI] [PubMed] [Google Scholar]
- 2. Maurice D. The location of the fluid pump in the cornea. J Physiol. 1972;221:43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Riley MV, Winkler BS, Peters MI, Czajkowski CA. Relationship between fluid transport and in situ inhibition of Na(+)-K+ adenosine triphosphatase in corneal endothelium. Invest Ophthalmol Vis Sci. 1994;35:560–567 [PubMed] [Google Scholar]
- 4. Anderson EI, Fischbarg J. Biphasic effects of insulin and ouabain on fluid transport across rabbit corneal endothelium. J Physiol. 1978;275:377–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wingham CG, Guggenheim JA, Hodson SA. Sodium movement into and out of corneal encothelium. Eur J Physiol. 1994;428:577–582 [DOI] [PubMed] [Google Scholar]
- 6. Hodson S, Miller F. The bicarbonate ion pump in the endothelium which regulates the hydration of rabbit cornea. J Physiol. 1976;263:563–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fischbarg J, Lim J. Role of cations, anions, and carbonic anhydrase in fluid transport across rabbit corneal endothelium. J Physiol. 1974;241:647–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Riley M, Winkler B, Czajkowski C, Peters M. The roles of bicarbonate and CO2 in transendothelial fluid movement and control of corneal thickness. Invest Ophthalmol Vis Sci. 1995;36:103–112 [PubMed] [Google Scholar]
- 9. Narula P, Xu M, Kuang K, Akiyama R, Fischbarg J. Fluid transport across cultured bovine corneal endothelial cell monolayers. Am J Physiol. 1992;262:C98–103 [DOI] [PubMed] [Google Scholar]
- 10. Kuang K, Xu M, Koniarek J, Fischbarg J. Effects of ambient bicarbonate, phosphate and carbonic anhydrase inhibitors on fluid transport across rabbit endothelium. Exp Eye Res. 1990;50:487–493 [DOI] [PubMed] [Google Scholar]
- 11. Bonanno JA, Giasson C. Intracellular pH regulation in fresh and cultured bovine corneal endothelium, II: Na/HCO3 cotransport and Cl/HCO3 exchange. Invest Ophthalmol Vis Sci. 1992;33:3068–3079 [PubMed] [Google Scholar]
- 12. Bonanno JA, Giasson C. Intracellular pH regulation in fresh and cultured bovine corneal endothelium, I: Na/H exchange in the absence and presence of HCO3−. Invest Ophthalmol Vis Sci. 1992;33:3058–3067 [PubMed] [Google Scholar]
- 13. Sun XC, Bonanno JA. Identification and cloning of the Na/HCO(3−) cotransporter (NBC) in human corneal endothelium. Exp Eye Res. 2003;77:287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li J, Sun XC, Bonanno JA. Role of NBC1 in apical and basolateral HCO3− permeabilities and transendothelial HCO3− fluxes in bovine corneal endothelium. Am J Physiol Cell Physiol. 2005;288:C739–C746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liebovitch L, Fischbarg J. Effects of inhibitors of passive Na+ and HCO3 fluxes on electrical potential and fluid transport across rabbit corneal endothelium. Curr Eye Res. 1982;2:183–186 [DOI] [PubMed] [Google Scholar]
- 16. Bonanno JA. Identity and regulation of ion transport mechanisms in the corneal endothelium. Prog Retin Eye Res. 2003;22:69–94 [DOI] [PubMed] [Google Scholar]
- 17. Sun XC, Li J, Cui M, Bonanno JA. Role of carbonic anhydrase IV in corneal endothelial HCO3− transport. Invest Ophthalmol Vis Sci. 2008;49:1048–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cui W, Liu G, Liang R. Expression of carbonic anhydrase IV in rabbit corneal endothelial cells. Chin Med J (Engl). 2002;115:1641–1644 [PubMed] [Google Scholar]
- 19. Gottsch JD, Seitzman GD, Margulies EH, et al. Gene expression in donor corneal endothelium. Arch Ophthalmol. 2003;121:252–258 [DOI] [PubMed] [Google Scholar]
- 20. Holthofer H, Siegal GJ, Tarkkanen A, Tervo T. Immunocytochemical localization of carbonic anhydrase, NaK-ATPase and the bicarbonate chloride exchanger in the anterior segment of the human eye. Acta Ophthalmol. 1991;69:149–154 [DOI] [PubMed] [Google Scholar]
- 21. Bonanno JA, Srinivas SP, Brown M. Effect of acetazolamide on intracellular pH and bicarbonate transport on bovine corneal endothelium. Exp Eye Res. 1995;60:425–434 [DOI] [PubMed] [Google Scholar]
- 22. McMurtrie HL, Cleary HJ, Alvarez BV, et al. The bicarbonate transport metabolon. J Enzyme Inhib Med Chem. 2004;19:231–236 [DOI] [PubMed] [Google Scholar]
- 23. Bainbridge JW, Stephens C, Parsley K, et al. In vivo gene transfer to the mouse eye using an HIV-based lentiviral vector; efficient long-term transduction of corneal endothelium and retinal pigment epithelium. Gene Ther. 2001;8:1665–1668 [DOI] [PubMed] [Google Scholar]
- 24. Borras T, Gabelt BT, Klintworth GK, Peterson JC, Kaufman PL. Non-invasive observation of repeated adenoviral GFP gene delivery to the anterior segment of the monkey eye in vivo. J Gene Med. 2001;3:437–449 [DOI] [PubMed] [Google Scholar]
- 25. Challa P, Luna C, Liton PB, et al. Lentiviral mediated gene delivery to the anterior chamber of rodent eyes. Mol Vis. 2005;11:425–430 [PMC free article] [PubMed] [Google Scholar]
- 26. Barraza RA, Rasmussen CA, Loewen N, et al. Prolonged transgene expression with lentiviral vectors in the aqueous humor outflow pathway of nonhuman primates. Hum Gene Ther. 2009;20:191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun XC, Bonanno JA, Jelamskii S, Xie Q. Expression and localization of NaHCO3 cotransporter in bovine corneal endothelium. Am J Physiol Cell Physiol. 2000;279:C1648–C1655 [DOI] [PubMed] [Google Scholar]
- 28. Hedbys B, Mishima S. The thickness-hydration relationship of the cornea. Exp Eye Res. 1966;5:221–228 [DOI] [PubMed] [Google Scholar]
- 29. Fatt I, Weissman B. Physiology of the Eye: An Introduction to the Vegetative Functions. Oxford: Butterwort-Heinemann; 1992 [Google Scholar]
- 30. Egan CA, Hodge DO, McLaren JW, Bourne WM. Effect of dorzolamide on corneal endothelial function in normal human eyes. Invest Ophthalmol Vis Sci. 1998;39:23–29 [PubMed] [Google Scholar]
- 31. Giasson CJ, Nguyen TQ, Boisjoly HM, Lesk MR, Amyot M, Charest M. Dorzolamide and corneal recovery from edema in patients with glaucoma or ocular hypertension. Am J Ophthalmol. 2000;129:144–150 [DOI] [PubMed] [Google Scholar]
- 32. Kaminski S, Hommer A, Koyuncu D, Biowski R, Barisani T, Baumgartner I. Influence of dorzolamide on corneal thickness, endothelial cell count and corneal sensibility. Acta Ophthalmol Scand. 1998;76:78–79 [DOI] [PubMed] [Google Scholar]
- 33. Georgiadis A, Tschernutter M, Bainbridge JW, et al. AAV-mediated knockdown of Peripherin-2 in vivo using miRNA-based hairpins. Gene Ther. 2010;17:486–493 [DOI] [PubMed] [Google Scholar]
- 34. O'Reilly M, Palfi A, Chadderton N, et al. RNA interference-mediated suppression and replacement of human rhodopsin in vivo. Am J Hum Genet. 2007;81:127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paskowitz DM, Greenberg KP, Yasumura D, et al. Rapid and stable knockdown of an endogenous gene in retinal pigment epithelium. Hum Gene Ther. 2007;18:871–880 [DOI] [PubMed] [Google Scholar]
- 36. Tam LC, Kiang AS, Kennan A, et al. Therapeutic benefit derived from RNAi-mediated ablation of IMPDH1 transcripts in a murine model of autosomal dominant retinitis pigmentosa (RP10). Hum Mol Genet. 2008;17:2084–2100 [DOI] [PubMed] [Google Scholar]
- 37. Justilien V, Pang JJ, Renganathan K, et al. SOD2 knockdown mouse model of early AMD. Invest Ophthalmol Vis Sci. 2007;48:4407–4420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gorbatyuk M, Justilien V, Liu J, Hauswirth WW, Lewin AS. Suppression of mouse rhodopsin expression in vivo by AAV mediated siRNA delivery. Vision Res. 2007;47:1202–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Loewen N, Fautsch MP, Teo WL, Bahler CK, Johnson DH, Poeschla EM. Long-term, targeted genetic modification of the aqueous humor outflow tract coupled with noninvasive imaging of gene expression in vivo. Invest Ophthalmol Vis Sci. 2004;45:3091–3098 [DOI] [PubMed] [Google Scholar]
- 40. Buie LK, Rasmussen CA, Porterfield EC, et al. Self-complementary AAV virus (scAAV) safe and long-term gene transfer in the trabecular meshwork of living rats and monkeys. Invest Ophthalmol Vis Sci. 51:236–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Doi K, Hargitai J, Kong J, et al. Lentiviral transduction of green fluorescent protein in retinal epithelium: evidence of rejection. Vision Res. 2002;42:551–558 [DOI] [PubMed] [Google Scholar]
- 42. Doi K, Kong J, Hargitai J, Goff SP, Gouras P. Transient immunosuppression stops rejection of virus-transduced enhanced green fluorescent protein in rabbit retina. J Virol. 2004;78:11327–11333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brooks AR, Harkins RN, Wang P, Qian HS, Liu P, Rubanyi GM. Transcriptional silencing is associated with extensive methylation of the CMV promoter following adenoviral gene delivery to muscle. J Gene Med. 2004;6:395–404 [DOI] [PubMed] [Google Scholar]
- 44. Abordo-Adesida E, Follenzi A, Barcia C, et al. Stability of lentiviral vector-mediated transgene expression in the brain in the presence of systemic antivector immune responses. Hum Gene Ther. 2005;16:741–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gawenis LR, Bradford EM, Prasad V, et al. Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/HCO3− cotransporter. J Biol Chem. 2007;282:9042–9052 [DOI] [PubMed] [Google Scholar]
- 46. Domingo Gordo B, Urcelay Segura JL, Conejero Arroyo J, Balado Vazquez P, Rodriguez Ausin P. [Corneal descompensation in patients with endothelial compromise treated with topical dorzolamide]. Arch Soc Esp Oftalmol. 2002;77:139–144 [PubMed] [Google Scholar]
- 47. Epstein RJ, Brown SV, Konowal A. Endothelial changes associated with topical dorzolamide do appear to be significant (Letter). Arch Ophthalmol. 2004;122:1089, author reply 1090. [DOI] [PubMed] [Google Scholar]
- 48. Konowal A, Morrison JC, Brown SV, et al. Irreversible corneal decompensation in patients treated with topical dorzolamide. Am J Ophthalmol. 1999;127:403–406 [DOI] [PubMed] [Google Scholar]
- 49. Tanimura H, Minamoto A, Narai A, Hirayama T, Suzuki M, Mishima HK. Corneal edema in glaucoma patients after the addition of brinzolamide 1% ophthalmic suspension. Jpn J Ophthalmol. 2005;49:332–333 [DOI] [PubMed] [Google Scholar]
- 50. Wirtitsch MG, Findl O, Kiss B, Petternel V, Heinzl H, Drexler W. Short-term effect of dorzolamide hydrochloride on central corneal thickness in humans with cornea guttata. Arch Ophthalmol. 2003;121:621–625 [DOI] [PubMed] [Google Scholar]