The OGF–OGFr system is a native biological regulator of cell proliferation in rabbit Tenon's capsule fibroblasts, and may offer a means to improve the success of glaucoma filtration surgery in a safe and nontoxic manner.
Abstract
Purpose.
Glaucoma filtration surgery often fails because of the fibrotic reaction from Tenon's capsule fibroblasts (TCFs). This study examined whether the interaction of the opioid growth factor (OGF) [Met5]-enkephalin with its receptor (OGFr) is a regulator of TCF proliferation.
Methods.
The presence of OGF and its receptor (OGFr) was determined in rabbit TCFs (RTCFs) by immunocytochemistry. The kinetics of OGFr were established in receptor binding assays. The ability of OGF to inhibit RTCF proliferation was assessed with dose–response, receptor mediation, and reversibility studies. Dependence on OGF and OGFr was ascertained by antibody neutralization and siRNA studies, respectively. The mechanism of action of the OGF–OGFr axis on survival (apoptosis, necrosis) and DNA synthesis of RTCFs was elucidated.
Results.
OGF and OGFr were detected in RTCF cells, and specific and saturable binding to OGFr was recorded. Exogenous OGF had a dose-dependent, reversible, and receptor-mediated inhibitory effect on cell proliferation. Endogenous OGF was found to be constitutively produced and tonically active in cell replication, with neutralization of this peptide causing acceleration of cell proliferation. The silencing of OGFr by using siRNA technology stimulated cell replication, validating OGFr's integral role. The mechanism of OGF–OGFr action was not related to cell survival, but rather to DNA synthesis—specifically, the cyclin-dependent kinase inhibitory pathway. Knockdown of p16 or p21 eliminated OGF's inhibitory effect on growth.
Conclusions.
The OGF–OGFr system is a native biological regulator of cell proliferation in RTCFs and may offer a means of improving the success of glaucoma filtration surgery in a safe and nontoxic manner.
Glaucoma affects more than 65 million people worldwide and is the leading cause of blindness.1 The major risk factor for glaucoma is elevated intraocular pressure, which is caused by inadequate drainage of aqueous humor from the anterior chamber of the eye and is responsible for optic nerve injury. When glaucoma fails to respond to pharmacologic therapy or laser treatment, glaucoma filtration surgery (trabeculectomy) is the most frequently used procedure for reducing intraocular pressure.2,3 The purpose of glaucoma filtration surgery is to create a scleral fistula that enables the drainage of aqueous humor from the anterior chamber to the subconjunctival space.4,5 This surgical wound is unusual, because the successful endpoint is a state of arrested healing. Complete healing results in occlusive fibrosis, which obliterates the subconjunctival space and therefore results in a failure to control intraocular pressure.6 Thus, the extent of postoperative Tenon's capsule fibroblast (TCF) proliferation and secondary fibrosis determine surgical success7,8 and result in a surgical failure rate ranging from 2% to 40%.7,8
Antimetabolites, including mitomycin C and 5-fluorouracil, are commonly used to inhibit TCF proliferation in an attempt to increase the success of glaucoma filtration surgery.8,9 However, these agents can produce significant adverse side effects and postoperative complications, such as ocular hypotony with secondary choroidal detachment and hypotony maculopathy and progressive thinning of the filtering bleb, resulting in blebitis or endophthalmitis.5,9–13 Therefore, these complications have prompted research into alternative treatments that may modulate the subconjunctival healing response after trabeculectomy in a more physiologic manner.
[Met5]-enkephalin is an opioid pentapeptide that serves as a tonically active inhibitory growth factor targeted to cell proliferation processes in neural and non-neural cells, including neoplasias.14 In view of this peptide's role in regulating growth-related processes, [Met5]-enkephalin has been termed the opioid growth factor (OGF).14 The action of OGF in regulating cellular proliferation is dependent on a nonclassic opioid receptor, OGFr, which has no homology with classic opioid receptors at the nucleotide or protein levels.14 Although the OGF–OGFr system has the pharmacologic attributes of opioid–receptor relationships (e.g., stereospecificity and naloxone reversibility), it utilizes signaling pathways that are entirely different from those related to classic opioid–receptor interactions.15,16 The OGF–OGFr axis has been identified and demonstrated to function in normal cellular renewal, wound healing, inflammation, and angiogenesis.14,17–20
OGF has been detected by immunohistochemistry in fibroblasts of rat dermis.21 The relationship of the OGF–OGFr axis to biological processes of fibroblasts, however, is unknown. In the present investigation, we explored the question of whether the OGF–OGFr axis is present and functional in TCFs and the mechanism(s) underlying these pathways. Because TCFs are important in the outcome of glaucoma filtration surgery, information derived from this study regarding the toxicity, efficacy, and mechanism of action of the OGF–OGFr system may be of importance in increasing the success of this surgical procedure.
Materials and Methods
Rabbit Tenon's Capsule Fibroblasts
Male New Zealand White (RSI:NZW) rabbits (∼1.5 kg) were purchased from RSI Farms (Mocksville, NC) and housed individually in stainless-steel cages under standard laboratory conditions; water and food were continuously available. All investigations conformed to the guidelines in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the regulations of the National Institutes of Health (NIH, Bethesda, MD), the U.S. Department of Agriculture, and the Department of Comparative Medicine of The Pennsylvania State University (Hershey, PA). Five rabbits were used in the studies.
The animals were euthanatized with sodium pentobarbital (100 mg/kg; IP), and Tenon's capsule was harvested. After they were dissected and washed, the tissue pieces were transferred to a 25-cm2 flask containing Dulbecco's modified Eagle's medium (DMEM) supplemented with 20% fetal bovine serum (FCS), 1.2% sodium bicarbonate, and antibiotics (5000 U/mL penicillin, 50 μg/mL streptomycin, and 100 μg/mL neomycin), and dispersed by pipette throughout the flask. The cells were maintained in a 37°C incubator with a 5% CO2/95% air mixture. Once the tissue had adhered to the flask, additional aliquots of DMEM with 20% FCS (1 mL) were added to the flask daily until a total volume of 5 mL was obtained. The medium was changed weekly until substantial outgrowth was observed. Once the outgrowth had reached approximately 50% confluence, the tissue was removed, and the rabbit TCFs (RTCFs) were subcultured in complete DMEM with 10% FCS.
Purity of the RTCF Population
The purity of the harvested RTCFs was assessed by examination of cells based on morphology and staining with phalloidin. The RTCFs were seeded onto 22-mm coverslips (100,000 cells per well) and allowed to adhere for 24 hours before they were stained with hematoxylin and eosin or phalloidin. Human umbilical vein endothelial cells (HUVECs) were used as the positive control for phalloidin staining. Purity was determined with six random grids/coverslip; three coverslips per treatment were evaluated. The number of fibroblasts per total number of cells provided the purity of the cultures.
Immunocytochemistry
Log-phase RTCFs were plated onto 22-mm diameter coverslips and, 72 hours later, were fixed and stained with anti-OGF and -OGFr antibodies, according to published procedures.18 Rabbit polyclonal antibodies to OGF and OGFr were generated in the laboratory and have been fully characterized.22 All cells were stained with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen, Carlsbad, CA), to visualize cell nuclei. Control experiments included cells incubated only with secondary antibodies (goat-anti rabbit IgG, TRITC, Invitrogen). At least three coverslips were examined by using differential interference contrast (DIC) or epifluorescence microscopy, with at least 10 fields examined per coverslip.
OGFr Binding Assays
Receptor binding assays for OGFr were performed using log-phase cells and custom synthesized [3H]-[Met5]-enkephalin (52.7 Ci/mmol; Perkin Elmer-New England Nuclear, Boston, MA).18 Four independent assays were performed in duplicate.
Cell Growth
Cells were plated in 24-well plates and counted 24 hours later (time 0), to determine seeding efficiency. OGF or other compounds were added at time 0, and media and compounds were replaced daily. All drugs were prepared in sterile water, and dilutions represent final concentrations of the compounds. An equivalent volume of sterile water (vehicle) was added to control wells. At designated times, the cells were harvested, stained with trypan blue, and counted with a hemacytometer. At least two aliquots per well of at least two wells per treatment per time point were sampled. Three to five independent assays were performed for each of the experiments (i.e., dose–response, growth curves, receptor mediation, and reversibility).
To determine the specific opioid peptide involved in growth regulation, we added a variety of endogenous peptides and synthetic compounds (10−6 M) to the RTCFs for 72 hours, including [DAla2, MePhe4, Glycol5]-enkephalin (DAMGO), morphine, [D-Pen2,5]-enkephalin (DPDPE), [D-Ala2, D-Leu5]-enkephalin (DADLE), dynorphin A, endomorphin-1, U-69593, and β-endorphin, as well as the opioid antagonist naltrexone (NTX). An equivalent volume of sterile water served as the control. Drug or vehicle and medium were changed daily. Cell proliferation was then evaluated (Promega Cell Titer 96 Aqueous One Solution Cell Proliferation Assay; Promega Corp., Madison, WI). Absorbance was recorded at 290 nm with a 750-nm filter on a plate reader (Bio-Rad, Hercules, CA). The plate consisted of 10 wells per treatment and the mean of those absorbencies was plotted. Three independent experiments were performed.
Specificity of Endogenous OGF
The specificity of endogenous OGF for cell growth was determined by treating the cells with a polyclonal antibody to OGF (1:50; CO172); preimmune rabbit serum (1:50) served as the control. Serum and medium were changed daily, and the cells were counted after 72 hours of treatment. Cell viability was determined with at least two aliquots per well, and at least three wells per treatment were evaluated. Three independent experiments were performed.
Specificity of OGFr: Knockdown with OGFr-siRNA
The OGFr-targeted siRNA (antisense, 5′-uagaaacucagguuuggcg-3′; sense, 5′-cgccaaaccugaguuucua-3′) was designed and obtained as a ready-annealed, purified duplex probe from Ambion (Austin, TX). Cells (5 × 104 well) were seeded in 24-well plates containing 1 mL of medium without antibiotics. The cells were transfected with either 20 nM OGFr siRNA or scrambled siRNA (Ambion) solutions with an oligo-complexing transfection reagent (Oligofectamine; Invitrogen), in serum- and antibiotic-free medium. The cells were incubated for 4 hours at 37°C before the addition of 10−6 M OGF or NTX. The cultures were incubated for an additional 20 hours, and fresh complete medium (2 mL) either lacking or containing OGF or NTX was added. At 72 hours, the cells were collected and either counted or harvested for RNA for Northern blot analysis. Three independent experiments were conducted.
Total RNA was extracted (Paris Kit; Ambion), separated on an agarose gel, and transferred to a nylon membrane (Immobilon; Bio-Rad). Membranes were probed with 32P-dCTP-OGFr cDNA. Control for equal loading was achieved by stripping and reprobing the blots with radiolabeled GAPDH; the optical density of each band was then determined and analyzed (QuickOne; Bio-Rad). Each value was normalized to GAPDH from the same blot. Three independent experiments were conducted.
The level of OGFr protein knockdown was determined by using semiquantitative immunocytochemistry on a subset of cultures that were seeded and transfected with siRNAs on round coverslips. For quantification of OGFr protein levels, images were captured at the same exposure time with special care taken to avoid photobleaching samples. The mean intensity of staining was determined for at least 100 cells per group from at least two coverslips per group.
The level of OGFr protein knockdown was further evaluated by performing Western blot analysis on a subset of cultures that were transfected with siRNAs. At 72 hours after the start of transfection, cells were collected for Western blot analysis, to determine the level of OGFr knockdown. Three independent experiments were conducted.
Mechanisms of OGF-Modulated Growth Inhibition: DNA Synthesis, Apoptosis, and Necrosis
The effect of OGF on DNA synthesis (BrdU incorporation), apoptosis (TUNEL), and necrosis (trypan blue) of RTCFs was evaluated. Cells were seeded onto 22-mm diameter coverslips, placed in six-well plates (5 × 104 cells/coverslip), and treated with OGF or NTX for 72 hours; media and drugs were replaced daily. Three hours before the cells were fixed, 30 μM BrdU (Sigma-Aldrich, St. Louis, MO) was added to a subset of cultures. The cells were fixed in 10% neutral buffered formalin for 10 minutes and either stained with antibodies to BrdU (anti–BrdU-BOD, Invitrogen), to assess DNA synthesis, or processed for TUNEL, to assess apoptosis and necrosis. Three independent experiments were performed.
siRNA Knockdown of p16 and p21
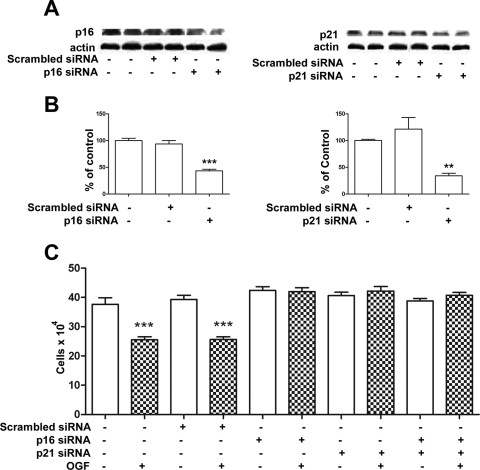
Log phase cells were transfected with 20-nM concentrations of p16 and/or p21 siRNAs (Santa Cruz Biotechnology, Santa Cruz, CA) or scrambled siRNAs (Ambion) with a transfection reagent (Oligofectamine; Invitrogen) in serum- and antibiotic-free medium for 4 hours at 37°C before the addition of OGF (10−6 M). The cultures were incubated an additional 20 hours, and then 2 mL fresh complete medium lacking or containing OGF was added. OGF and medium were changed daily. At 72 hours after the start of transfection, the cells were collected for growth curves or for Western blot to determine the level of p16 and p21 knockdown. Three independent experiments were performed, with at least two wells per treatment per experiment evaluated.
Western Immunoblot Analysis
To determine the level of protein knockdown resulting from siRNA transfections, we collected the cells and solubilized them in 200 μL RIPA buffer (1× PBS, 10 μM IGEPAL, 1 mg/mL SDE, and 5 mg/mL deoxycholic acid), containing a cocktail of protease and phosphatase inhibitors (Roche, Boulder, CO). Total protein concentrations were measured with a DC protein assay kit (Bio-Rad).
Equal amounts of protein (60 μg) were subjected to 15% SDS-PAGE followed by transfer of proteins to nitrocellulose using standard protocols. Antibodies to p16 (F-12, mouse IgG; Santa Cruz Biotechnology), p21 (B6B, mouse IgG; BD PharMingen, San Diego, CA), and β-actin (AC-15, mouse IgG; Sigma-Aldrich), were purchased from commercial sources, whereas rabbit anti-OGFr was made in our laboratory.22 Membranes were probed with primary antibodies (1:200), followed by appropriate horseradish peroxidase–conjugated secondary antibodies, and developed with a chemiluminescence Western blot detection system (Amersham ECL; GE Health Care, Piscataway, NJ). Equal loading of total protein was ensured by stripping the blots with stripping buffer (62.5 mM Tris-HCl, 100 mM β-mercaptoethanol, and 2% SDS [pH 6.7]) at 50°C and reprobing with anti–β-actin (1:5000).
The optical density of each band was determined by densitometry (Quick One; Bio-Rad), and each value was normalized to β-actin from the same blot. The percentage of protein knockdown was calculated by dividing the normalized value of the transfected samples by the normalized value of the nontransfected samples. Means ± SE were determined from the results of three independent Western blot analyses.
Chemicals
The following compounds were obtained from the indicated sources: [Met5]-enkephalin, NTX, naloxone (NAL), DAMGO, morphine, DPDPE, DADLE, dynorphin A, endomorphin-1, and β-endorphin from Sigma-Aldrich and U69583 from Upjohn Diagnostics (Kalamazoo, MI).
Statistics
Data on cell counts, number of dead cells, and presence of BrdU-positive cells were analyzed by one-way analysis of variance and Newman-Keuls tests. Nonlinear regression analysis was performed to establish the doubling time of RTCFs. Results were significant at P < 0.05.
Results
Purity of RTCFs
Bright-field and fluorescence microscopy revealed that RTCF cultures were 99.2% pure (Fig. 1A). There was a distinct difference in the distribution of fluorescent phalloidin in RTCFs compared with HUVECs, indicating that isolated cultures of RTCFs were relatively devoid of cell types derived from blood vessels (data not shown).
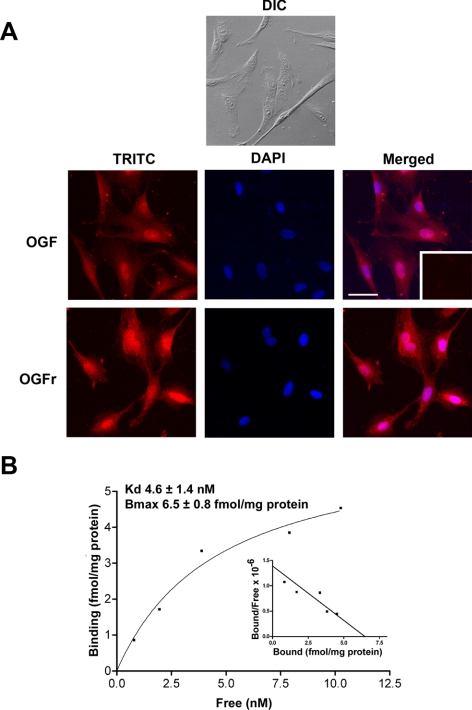
Figure 1.
The presence and distribution of OGF and OGFr in rabbit Tenon's capsule fibroblasts. (A) Photomicrographs of log-phase RTCFs visualized with differential interference (DIC) microscopy or immunocytochemistry of samples stained with antibodies (1:100) to [Met5]-enkephalin (OGF) or OGFr. Nuclei were visualized with DAPI. Rhodamine-conjugated IgG (1:1000) served as the secondary antibody (inset). Immunoreactivity was associated with the cytoplasm and the nucleus; staining was not observed in cell preparations incubated with secondary antibodies only (inset). Scale bar, 15 μm. (B) Representative saturation isotherm of specific binding of [3H]-[Met5]-enkephalin to nuclear homogenates of RTCFs. Mean ± SE binding affinity (Kd) and maximum binding capacity (Bmax) from four independent assays performed in duplicate. Representative Scatchard plot (inset) of specific binding of radiolabeled [Met5]-enkephalin to RTCF nuclear proteins revealed a one-site model of binding.
OGF and OGFr in RTCFs
Immunocytochemical staining of the RTCFs with antibodies specific for OGF or OGFr demonstrated the presence of both peptide and receptor in the cytoplasm and the nucleus (Fig. 1A).
Receptor binding assays detected specific and saturable binding of radiolabeled OGF in the nuclear fraction of RTCFs. The binding data suggested a one-site model of binding, with a binding capacity (Bmax) of 6.5 ± 0.8 femtomoles/mg protein and a binding affinity (Kd) of 4.6 ± 1.4 nM (Fig. 1B).
Effect of OGF on Growth of RTCFs
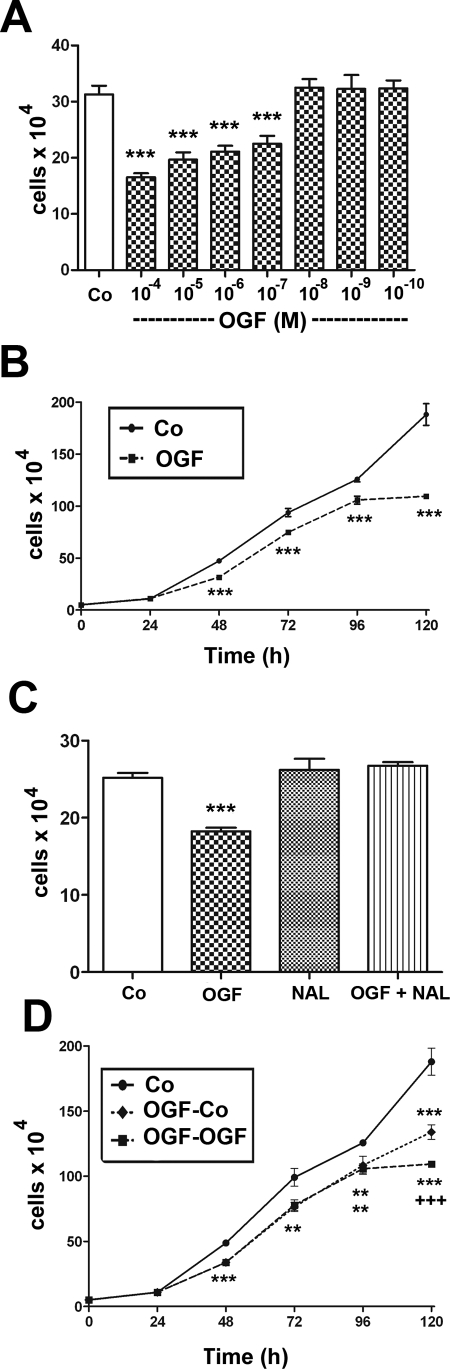
OGF in dosages of 10−4, 10−5, 10−6, and 10−7 M depressed the number of cells by 47%, 37%, 32%, and 28%, respectively, from the levels in control cultures, when measured at 72 hours (Fig. 2A). However, dosages of 10−8, 10−9, and 10−10 M OGF had the same number of cells as were in the control cultures. Log phase cultures treated with 10−6 M OGF had 22%, 20%, 16%, and 41% fewer cells than did sterile-water–treated cultures at 48, 72, 96, and 120 hours, respectively (Fig. 2B). Nonlinear regression analysis revealed that the doubling times for the control and OGF groups were 35.0 ± 0.4 and 43.8 ± 0.5 hours, respectively (P < 0.01). Control cultures were confluent by 120 hours, and the experiment was concluded.
Figure 2.
OGF inhibited growth of RTCFs in a dose-related, temporal, receptor-mediated, and reversible manner. The cells were seeded at 50,000/well; media and OGF and sterile water (Co) were replaced daily. (A) Growth of RTCFs subjected to various concentrations of OGF for 72 hours. (B) Growth of RTCFs treated with OGF (10−6 M) or an equivalent volume of sterile water for a 120-hour period. (C) Opioid receptor mediation of the growth inhibitory effects of OGF in RTCFs. Cell cultures were subjected to OGF (10−6 M), the opioid antagonist NAL (10−6 M), OGF and NAL, or sterile water (Co) for 72 hours. (D) Reversibility of the growth inhibitory effects on RTCFs treated with 10−6 M OGF or sterile water (Co). At 72 hours, one-half of the OGF-treated cultures continued to receive OGF (OGF–OGF) for an additional 48 hours, and one-half of the plates were treated with sterile water for an additional 48 hours (OGF–Co). Control (Co) cultures received sterile water throughout the 120 hours. Compounds and media were replaced daily. All experiments were repeated three to five times, and data represent mean ± SE of at least two aliquots/well from at least two wells/group. Significantly different from respective controls (A–D) at **P < 0.01, ***P < 0.001, and from the OGF–Co group at +++P < 0.001 (D).
To determine whether the effects of OGF were mediated by opioid receptors, we exposed RTCF cultures simultaneously to OGF and the short-acting opioid antagonist NAL at concentrations of 10−6 M. No change in the number of cells from control levels was recorded in preparations subjected to both OGF and NAL or NAL alone (Fig. 2C). However, RTCFs treated with 10−6 M OGF exhibited a 28% decrease in the number of cells compared with RTCFs treated with sterile water.
Reversibility experiments were performed to evaluate whether the inhibitory effects of OGF were permanent and toxic. After 72 hours of treatment with OGF, cell counts were 20% less than in control cultures (Fig. 2D). Cultures that no longer received OGF (OGF–Co) and were examined 48 hours later (i.e., 120 hours) had 29% fewer cells than in control cultures (Co), whereas the cell count was subnormal by 41% in preparations continuing to receiving OGF (OGF–OGF). The OGF–Co cultures had 18% more cells than did the OGF–OGF cultures, and the difference was statistically significant.
Inhibitory Effect of OGF on Cell Proliferation
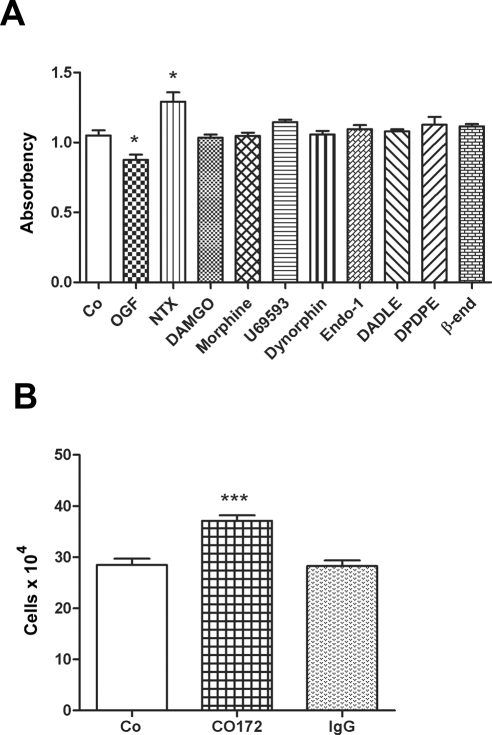
To determine whether opioids other than OGF influence the proliferation of RTCFs, we examined the effects of a variety of synthetic and natural opioids, recognizing μ, δ, and κ opioid receptors. After 72 hours of treatment, cell counts in these cultures subjected to other opioids was comparable with control counts (Fig. 3A). OGF-treated RTCF counts, however, were subnormal by 17%. Moreover, exposure to the opioid antagonist NTX revealed a 23% increase in cell counts compared with the control cultures.
Figure 3.
OGF was the specific opioid peptide involved in the growth inhibition of RTCFs. (A) The effects of various endogenous and exogenous opioids (10−6 M) on RTCF number for 72 hours. (B) RTCFs were treated with a polyclonal antibody specific for OGF (CO172), preimmune serum (IgG), or sterile water; antibodies and peptide were replaced daily. Cell counts were recorded at 72 hours. Data from the experiment in (A) represent the mean ± SE of at least 10 wells/group performed in triplicate. Data for the experiment in (B) represent the mean ± SE of at least two aliquots/well from at least three wells/group. Significantly different from respective controls at *P < 0.05 and ***P < 0.001.
To further understand the specificity of OGF in the repression of RTCFs, we treated the cultures daily with polyclonal antibody to OGF or preimmune serum and recorded the number of cells after 72 hours. Cell counts of RTCFs treated with OGF antibody were 30% greater than in sterile-water– and preimmune-serum–treated cultures (Fig. 3B), demonstrating that OGF neutralization by an antibody can increase cell proliferation. Cell counts in the control and preimmune serum groups were comparable.
Effect of Silencing of OGFr in RTCFs on the Inhibitory Action of Endogenous and Exogenous OGF and the Stimulatory Action of NTX
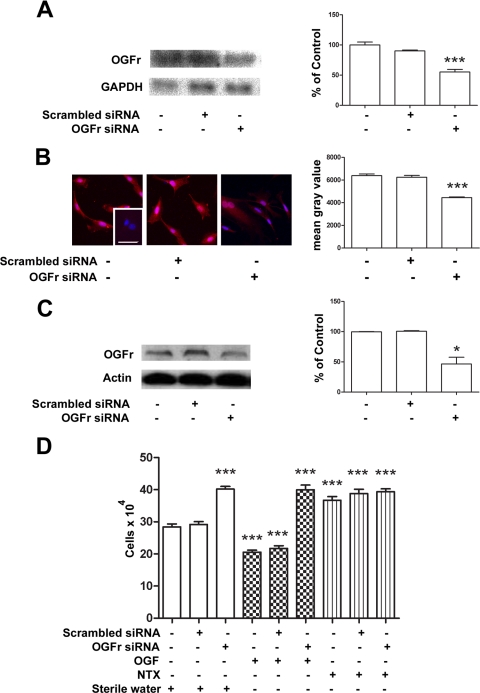
In an assessment of the requirement of OGFr for OGF's inhibitory action on cell proliferation, siRNA technology was used to silence OGFr expression. OGFr siRNA-transfected RTCF cells had a 51% reduction in OGFr mRNA levels relative to nontransfected cells (Fig. 4A). Cells exposed to scrambled siRNA had OGFr mRNA levels comparable to those in nontransfected cells. Semiquantitative immunocytochemistry revealed that OGFr siRNA transfected RTCFs had approximately 70% of OGFr immunoreactivity, as recorded for nontransfected cells (Fig. 4B). Western blot analysis demonstrated that OGFr siRNA transfected RTCFs had a 53% decrease in OGFr protein levels relative to nontransfected cells (Fig. 4C). RTCF cells transfected with OGFr siRNA had 41% more cells than cultures that were not transfected (Fig. 4D). The addition of exogenous OGF or NTX had no inhibitory or stimulatory effects, respectively, on cells transfected with OGFr siRNA, compared with cultures treated with OGFr siRNA and sterile water.
Figure 4.
OGFr was necessary for OGF's inhibitory action on growth. (A) Northern blot analysis, (B) semiquantitative densitometry, and (C) Western blot analysis demonstrating the specificity and level of OGFr knockdown in RTCFs. Log-phase cells were transfected for 24 hours with either scrambled siRNA or OGFr siRNA. Forty-eight hours after transfection, the cells were harvested and the RNA isolated. Data (percent of OGFr/GAPDH ratio) represent the mean ± SE of two blots from independent experiments. ***Significantly different from nontransfected cultures at P < 0.001. (B) Photomicrographs of log-phase RTCFs stained with a polyclonal antibody to OGFr, demonstrating the extent of OGFr protein knockdown. The cells were transfected for 24 hours with OGFr siRNA or scrambled siRNA and incubated in medium for an additional 48 hours. Photomicrographs of cells stained with OGFr were taken with the same exposure time. Semiquantitative measurement of OGFr immunocytochemistry demonstrated the level of protein knockdown in the RTCFs. Decreased OGFr staining intensity (mean gray value) is indicative of decreased OGFr protein expression. Data represent the mean ± SE. ***Significantly different from nontransfected cells at P < 0.001. (C) Western blot analysis and quantitative densitometry demonstrating the level of OGFr knockdown. The cells were transfected with OGFr siRNA or scrambled siRNA. Seventy-two hours after the start of transfection, the cells were harvested and the protein isolated. Data (% of OGFr/actin) represent the mean ± SE of three independent blots. *Significant difference between nontransfected and scrambled-siRNA–transfected cultures at P < 0.05. (D) Growth of RTCFs cultures transfected with OGFr siRNA or scrambled siRNA for 24 hours and treated with OGF (10−6 M), NTX (10−6 M), or an equivalent volume of sterile water for 72 hours; compounds and media were changed daily. Values represent the mean ± SE cell counts of at least two aliquots/well and least two wells/treatment. ***Significantly different at P < 0.001 from cultures that were not transfected.
Effect of OGF on DNA Synthesis but Not on Apoptosis or Necrosis
To investigate whether OGF decreases cell proliferation by altering DNA synthesis, we assessed the amount of incorporated BrdU. After 72 hours of treatment, the percentage of BrdU-positive cells in the OGF group was 40% lower than in the control cells (Fig. 5). NTX-treated RTCFs had a labeling index that was 35% greater than control levels. Programmed cell death was assessed by TUNEL staining, and necrosis was monitored by trypan blue staining. Apoptosis and necrosis were negligible with all treatments, with RTCF cultures subjected to vehicle, NTX, or OGF, each having less than 1 apoptotic/necrotic cell per well (data not shown).
Figure 5.
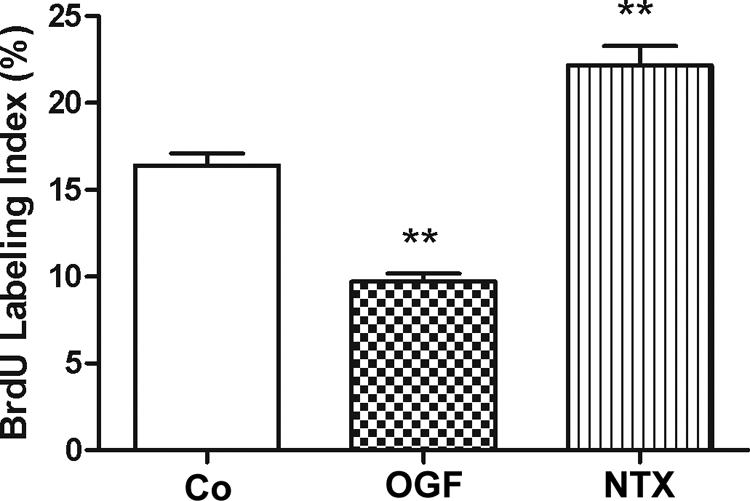
OGF's inhibitory effects on DNA synthesis. RTCFs were seeded on 22-mm coverslips and treated for 72 hours with 10−6 M concentrations of OGF, NTX, or an equivalent volume of sterile water. BrdU (30 μM) was incorporated into RTCFs for 3 hours before staining. Data represent the percent BrdU-positive cells (mean ± SE). **Significantly different from control cells at P < 0.01.
Role of p16 and p21 in OGF-Induced Growth Inhibition
To test the role of p16 and/or p21 in OGF-induced inhibitory action on RTCF cell growth, we treated the cells with scrambled siRNA, p16 siRNA, p21 siRNA, or both p16 siRNA and p21 siRNA. Cells transfected with p16 or p21 siRNA had significantly reduced levels of p16 (56%) or p21 (66%) protein compared with that in nontransfected cells after 72 hours (Figs. 6A, 6B). Growth analysis of RTCF cells transfected with p16 or p21 siRNAs revealed that both p16 and p21 induction are necessary for OGF inhibitory action (Fig. 6C). RTCF cells that were not transfected or were transfected with scrambled siRNA and treated with OGF (10−6 M) for 72 hours had reductions in growth of 32%.
Figure 6.
The OGF–OGFr axis in RTCFs inhibits DNA synthesis and requires the presence of both p16 and p21 pathways. (A) RTCFs were transfected for 24 hours with p16, p21, or scrambled siRNA, and total proteins were isolated 48 hours after transfection, separated by SDS-PAGE, and probed with antibodies specific to p16 or p21 to demonstrate level of protein knockdown. (B) Densitometric analysis of three Western blot analyses from independent experiments was performed, and p16 and p21 expression (% p16/actin and p21/actin ratio) is expressed relative to nontransfected cultures. (C) p16 and p21 pathways are necessary for OGF's inhibitory action on cell proliferation. RTCFs were transfected with p16, p21, p16 and p21, or scrambled siRNA for 24 hours and subsequently treated with 10−6 M OGF or an equivalent volume of sterile water for 72 hours. Data represent the mean ± SE cell counts for at least two aliquots/well and at least two wells/treatment. Significantly different from wild-type cultures as well as those transfected with scrambled siRNA at **P < 0.01 and ***P < 0.001.
Discussion
This study is the first to show that the OGF–OGFr axis is present and functions in TCFs. In a tissue culture model of RTCFs, both OGF and OGFr were detected by immunocytochemistry, and receptor binding studies revealed that the OGF receptor was capable of recognizing OGF. Cell proliferation assays ascertained that OGF depressed cell counts in a dose-dependent fashion. OGF inhibitory activity was rapid and persistent and did not exhibit tolerance when tested as early as 24 hours after peptide administration and extending for 5 days thereafter. The effects of OGF on RTCFs were receptor mediated (blocked by NAL in the absence of an effect of NAL alone) and reversible (indicating a cytostatic but not toxic action). Cells subjected to a wide variety of synthetic and natural opioids, including those specific for μ (DAMGO, endomorphin 1), δ (DPDPE, DADLE), and κ (U69593, dynorphin) opioid receptors, showed that none of these compounds had any effect on growth at a concentration of 10−6 M at which OGF markedly depressed cell proliferation. Thus, these results reveal a native OGF-mediated biological pathway that regulates cell proliferation in RTCFs.
Even though OGF was the only opioid peptide to inhibit RTCF proliferation, and OGF was detected in these cells by immunocytochemistry, the question can be raised as to whether endogenous OGF is a functional native peptide related to growth. Two pieces of evidence support this conclusion. First, the growth of cells exposed to the general opioid antagonist NTX was accelerated, indicating that the opioid related to cell proliferation is constitutively expressed and tonically active. Second, and more specific to OGF, neutralization of endogenous OGF by an antibody to this peptide stimulated cell replication. Thus, depletion of endogenous OGF removed the inhibitory influence on these cells, thereby interrupting homeostatic equilibrium in cell proliferation and upsetting the delicate balance in regulating the cell count by peptide activity.
An extensive body of literature shows that OGF interacts with OGFr to regulate cell proliferation, implying that the effects on controlling cell number by endogenous OGF, as well as exogenously administered peptide, are mediated by this receptor.14–20 OGFr was detected in RTCFs by immunocytochemistry and receptor binding technology; however, OGF is [Met5]-enkephalin, and this opioid peptide is known to bind to classic opioid receptors such as μ, δ, and κ as well.23 To examine the specificity of OGF for OGFr with respect to regulation of cell proliferation, we determined the effects of knocking down OGFr with siRNA technology. Cells treated with OGFr siRNA were observed to have an increase in the number of cells, suggesting that attenuating OGFr compromises the action of endogenous OGF. Moreover, exogenously administered OGF, which depressed the cell counts in log-phase cultures, did not have any effect when the cells were transfected with OGFr siRNA. It is interesting to note that although OGFr siRNA knocked down OGFr transcriptional activity by approximately 50%, this level of activity was sufficient to increase the number of RTCFs by 41%, yet the addition of NTX to these cultures did not increase cell proliferation further. These results suggest that the magnitude of the knockdown of OGFr in these cells is capable of interfering with the depressive interaction of OGF and OGFr that reduces cell counts. Of course, OGF–OGFr interactions and function are dependent on a host of other factors, such as the requirement of nucleocytoplasmic transport by way of nuclear localization signals, carrier proteins, and guidance by nucleoporins and presumably DNA binding proteins.24 Moreover, in modulating cell proliferation, it is well known that OGF–OGFr signaling is targeted to the cyclin-dependent inhibitory kinase pathway (i.e., p16, p21). Deciphering these complex relationships in the context of the present experiments will necessitate further study. However, all the data, together with the knowledge that OGF is the peptide involved with modulating the cell count of RTCFs, clearly indicate that proliferation of these fibroblasts is dependent on the OGF–OGFr axis.
The decrease in cell count resulting from treatment with OGF could be due to a decrease in cell survival because of programmed cell death, necrosis, and/or a reduction in DNA synthesis and subsequent cell replication. Our data showed neither apoptosis nor necrosis in RTCFs, a result consistent with previous experiments involving tissue culture.18 However, DNA synthesis in cultures treated with OGF exhibited a marked decrease from control levels. Consistent with previous studies,14–18,20 these data indicate that the role of OGF involves the regulation of cell proliferation.
With respect to the mechanism of OGF activity in inhibiting DNA synthesis, studies in normal cells, including human dermal fibroblasts, have shown that regulation requires both p16 and p21,15 suggesting that cyclin-dependent inhibitory kinase pathways are a target of OGF. Given these results, we raised the question of whether OGF targets the p16 and/or p21 pathways in RTCFs. Transfection of RTCFs with p16 and/or p21 siRNAs and treatment with OGF, showed that both p16 and p21 are necessary for OGF inhibitory action.
Many adjunctive therapies having been studied in an attempt to increase the success of glaucoma filtration surgery; however, most have harmful side effects (e.g., infection, loss of vision) that can nullify any beneficial attributes of treatment.5,8–13 It is necessary to examine other therapies that can have results similar to those of traditional adjunctive therapies (e.g., 5-fluorouracil and mitomycin C) without actively killing healthy cells located in and around the surgical area. OGF is a nontoxic, native, and safe biotherapy that does not invoke apoptosis or necrosis, but instead works through cyclin-dependent inhibitory kinase pathways to inhibit proliferation of normal cells. Given the effectiveness of OGF in the cell cultures documented herein, studies with OGF in an in vivo model of glaucoma filtration surgery are warranted.
Footnotes
Supported in part by National Institutes of Health Grant EY01666.
Disclosure: M.S. Klocek, None; J.W. Sassani, None; R.N. Donahue, None; P.J. McLaughlin, None; I.S. Zagon, None
References
- 1. Sherwood MB. A sequential, multiple-treatment, targeted approach to reduce wound healing and failure of glaucoma filtration surgery in a rabbit model (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2006;104:478–482 [PMC free article] [PubMed] [Google Scholar]
- 2. Atreides S-PA. Wound healing modulation in glaucoma filtering surgery. Int Ophthalmol Clinic. 2004;44:61–106 [DOI] [PubMed] [Google Scholar]
- 3. Lee DA, Higginbotham EJ. Glaucoma and its treatment: a review. Am J Health Syst Pharm. 2005;62:691–699 [DOI] [PubMed] [Google Scholar]
- 4. Chang MR, Cheng Q, Lee DA. Basic science and clinical aspects of wound healing in glaucoma filtering surgery. J Ocul Pharmacol Ther. 1998;14:75–95 [DOI] [PubMed] [Google Scholar]
- 5. Kim TH, Kim SW, Woo JM, et al. Co-treatment of suberoylanilide hydroxamic acid and mitomycin-C induces the apoptosis of rabbit Tenon's capsule fibroblast and improves the outcome of glaucoma filtration surgery. Curr Eye Res. 2008;33:237–245 [DOI] [PubMed] [Google Scholar]
- 6. Cordeiro MF, Bhattacharya SS, Schultz GS, Khaw PT. TGF-β1, -β2, -β3 in vitro: biphasic effects on Tenon's fibroblast contraction, proliferation, and migration. Invest Ophthalmol Vis Sci. 2000;41:756–763 [PubMed] [Google Scholar]
- 7. Skuta GL, Parrish RK. Wound healing in glaucoma filtering surgery. Surv Ophthalmol. 1987;32:149–170 [DOI] [PubMed] [Google Scholar]
- 8. Khaw PT, Occleston NL, Schultz G, Grierson I, Sherwood MB, Larkin G. Activation and suppression of fibroblast function. Eye. 1994;8:11–915 [DOI] [PubMed] [Google Scholar]
- 9. Lama PJ, Fechtner RD. Antifibrotics and wound healing in glaucoma surgery. Surv Ophthalmol. 2003;48:314–346 [DOI] [PubMed] [Google Scholar]
- 10. Mietz H, Welsandt G, Hueber A, Esser C, Krieglstein GK. Synergistic effects of combined cytotoxic and apoptosis-inducing drugs on Tenon's capsule fibroblasts in vitro and in vivo. Graefes Arch Clin Ex Ophthalmol. 2997;245:1367–1375 [DOI] [PubMed] [Google Scholar]
- 11. Naruse S, Yamada J, Hamuro J, Kobayashi T, Mori K, Kinoshita S. AC0576 decreases production of pro-inflammatory chemokine and extracellular matrix by human Tenon's capsule fibroblasts. Exp Eye Res. 2004;79:223–230 [DOI] [PubMed] [Google Scholar]
- 12. Crowston JG, Wang XY, Khaw PT, Zoellner, Healey PR. Human serum reduces mitomycin-C toxicity in human Tenon's fibroblasts. Invest Ophthalmol Vis Sci. 2006;47:946–952 [DOI] [PubMed] [Google Scholar]
- 13. Seong GJ, Park C, Kim CY, et al. Mitomycin-C induces the apoptosis of human Tenon's capsule fibroblasts by activation of c-Jun N-terminal kinase 1 and caspase-3 protease. Invest Ophthalmol Vis Sci. 2005;46:3545–3552 [DOI] [PubMed] [Google Scholar]
- 14. Zagon IS, Verderame MF, McLaughlin PJ. The biology of the opioid growth factor receptor (OGFr). Brain Res Rev. 2002;38:351–376 [DOI] [PubMed] [Google Scholar]
- 15. Cheng F, McLaughlin PJ, Verderame MF, Zagon IS. The OGF–OGFr axis utilizes the p16INK4a and p21WAF1/CIP1 pathways to restrict normal cell proliferation. Mol Bio Cell. 2009;20:319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheng F, McLaughlin PJ, Verderame MF, Zagon IS. Dependence on nuclear localization signals of the opioid growth factor receptor (OGFr) in the regulation of cell proliferation. Exp Biol Med. 2009;234:532–541 [DOI] [PubMed] [Google Scholar]
- 17. Blebea J, Mazo JE, Kihara TK, et al. Opioid growth factor modulates angiogenesis. J Vasc Surg. 2000;32:364–373 [DOI] [PubMed] [Google Scholar]
- 18. Donahue RN, McLaughlin PJ, Zagon IS. Cell proliferation of human ovarian cancer is regulated by the opioid growth factor-opioid growth factor receptor axis. Am J Physiol. 2009;296:R1716–R1725 [DOI] [PubMed] [Google Scholar]
- 19. Zagon IS, Rahn KA, Bonneau RH, Turel AP, McLaughlin PJ. Opioid growth factor suppresses expression of experimental autoimmune encephalomyelitis. Brain Res. 2010;1310:154–161 [DOI] [PubMed] [Google Scholar]
- 20. McLaughlin PJ, Park SS, Conway A, Donahue RN, Zagon IS, Goldenberg D. Growth inhibition of thyroid follicular cell-derived cancers by the opioid growth factor (OGF): opioid growth factor receptor (OGFr) axis. BMC Cancer. 2009;9:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zagon IS, Rhodes RE, McLaughlin PJ. Localization of enkephalin immunoreactivity in diverse tissues and cells of the developing and adult rat. Cell Tissue Res. 1986;246:561–565 [DOI] [PubMed] [Google Scholar]
- 22. Zagon IS, McLaughlin PJ. Production and characterization of polyclonal and monoclonal antibodies to zeta (ζ) opioid receptor. Brain Res. 1993;630:295–302 [DOI] [PubMed] [Google Scholar]
- 23. Leslie FM. Methods used for the study of opioid receptors. Pharmacol Rev. 1987;39:197–249 [PubMed] [Google Scholar]
- 24. Zagon IS, Ruth TB, McLaughlin PJ. Nucleocytoplasmic distribution of opioid growth factor (OGF) and its receptor (OGFr) in tongue epithelium. Anat Rec A Discov Mol Cell Evol Biol. 2005;282:24–37 [DOI] [PubMed] [Google Scholar]