Trachoma is the leading cause of preventable blindness today, yet very little is known about the pathogenesis of the disease. This study showed that mucosal immune responses to CPAF are likely to be both a marker and a risk factor for inflammatory trachoma and severe trachomatous disease.
Abstract
Purpose.
Chlamydia trachomatis (Ct) remains the leading global cause of preventable blindness. There are limited data on humoral immune responses in trachoma. Evaluating these responses is important for understanding host–pathogen interactions and informing vaccine design. Antibodies to chlamydial heat shock protein 60 (cHSP60) have been associated with infertility and trachomatous scarring. Other proteins, including chlamydial protease-associated factor (CPAF) and a hypothetical protein unique to the family Chlamydiaceae, CT795, elicit strong immune responses in urogenital infections, but their role in trachomatous disease is unknown.
Methods.
This study was conducted to expand on previous cHSP60 findings and evaluate the association of CPAF and CT795 antibodies with ocular Ct infection and disease. Clinical trachoma grading was performed, and conjunctival samples were obtained from individuals with trachomatous trichiasis (TT; one or more inturned eyelashes) or inflammatory trachoma without trichiasis and control subjects without disease, all of whom resided in trachoma-endemic regions of Nepal. Ct infection was determined using commercial PCR. IgG and IgA tear antibodies against cHSP60, CT795, and CPAF fusion proteins were measured by quantitative ELISA.
Results.
Significantly higher IgG antibody levels were found against cHSP60, CPAF, and CT795 in the inflammatory cases compared with levels in the controls (P < 0.005 for all three). Ct infection was independently associated with IgG antibodies against all three immunogens in the inflammatory cases but not in the controls (P = 0.025, P = 0.03 and P = 0.017, respectively). Only IgG antibodies against CPAF were significantly elevated among the TT cases (P = 0.013).
Conclusions.
Among individuals with trachoma, IgG antibody responses to CPAF are likely to be both a marker and risk factor for inflammatory trachoma and severe trachomatous disease.
Trachoma, a disease that spans millennia, continues to be the leading cause of preventable blindness worldwide. Global estimates of blindness have ranged from ∼2 to 9 million people,1 although the actual rates are likely to be much higher, given the lack of screening programs in many countries. Chlamydia trachomatis (Ct), an obligate intracellular bacterium, is a known etiologic agent in trachoma.2 Other species of Chlamydiaceae are also likely to play a role in trachoma.2 The disease is endemic in rural areas of developing countries where people live in close proximity with one another and with livestock. In addition, a shortage of clean water impedes advances in health education that would improve facial cleanliness and thereby decrease hand-to-eye-to-hand transmission of Chlamydia.
The excessive global prevalence of trachoma prompted the World Health Organization (WHO) to establish the Alliance for the Global Elimination of Blinding Trachoma by 2020 and implement the SAFE program (Surgery, Antibiotics, Facial cleanliness, and Environment improvements). However, the recurrence of disease 6 to 24 months after surgery for trachomatous trichiasis (TT; more than one eyelash touching the globe of the eye) and after treatment of Ct infection with antibiotics3–6 is indicative of the complexity of controlling trachoma and its sequelae.
Humoral and cellular immune pathways are involved in the prevention and clearance of genital7–10 and ocular Ct infections.11–13 However, the immune correlates associated with disease or disease progression remain ill defined. Murine and nonhuman primate studies have provided data that characterize the cell-mediated immune response, suggesting that CD4+ T helper (Th)1 cells participate in primary and secondary urogenital Ct infection,8,14–16 whereas CD8+ Th1 cells are associated with scarring and upper reproductive tract infections.17,18
Although there is no murine model for trachoma, studies among humans and nonhuman primates have shown evidence of cellular infiltrates during active trachoma characterized by polymorphonuclear cells (PMNs), macrophages, T lymphocytes, and dendritic cells within the epithelial and plasma cells, specifically the IgA isotype, in the subepithelium.12,19 Furthermore, significantly decreased levels of the Th1 cytokines interleukin (IL)-12 and interferon (IFN)γ have been documented in trachoma patients with trachomatous scarring (TS).13,20 Recently, we expanded on cytokine and chemokine profiling for trachoma and demonstrated that the Th2 cytokines IL-4 and -13 are important in eliciting protective immunity, whereas the Th3/Tr1 cytokines IL-10 and -15 are risk factors for TS. IL-1b is also a significant risk factor for scarring, but its antagonist, IL-1Ra, is inversely associated with scarring, suggesting an immunoprotective effect.13
Elucidation of the humoral immune response in patients with urogenital and ocular Ct infections and disease has focused on antibody responses elicited against whole elementary bodies (EBs), the major outer membrane protein (MOMP),21,22 Chlamydia heat shock protein 60 (cHSP60),2,22–24 polymorphic membrane proteins (Pmps),25,26 and the plasmid protein pgp3.27 Neutralizing antibodies have been demonstrated against MOMP, indicating the potential importance of this protein in protective immune responses.28,29 In studies of trachoma populations, minimal differences have been observed in serum or tear antibodies against MOMP in individuals with and those without trachomatous disease.21,22 Conversely, elevated serum IgG antibody titers to cHSP60 have been associated with pelvic inflammatory disease, infertility, perihepatitis, and cervical cancer30–34 and also with follicular trachomatous inflammation (TF), intense trachomatous inflammation (TI), and TS, compared with titers in individuals without disease.2,22,24 However, there are no studies of tear or serum antibody responses to cHSP60 in TT cases.
Although Pmps and Pgp23 are important immunogens, we have been interested in characterizing the humoral immune response to chlamydial protease-associated factor (CPAF) and hypothetical protein CT795 in trachoma patients because of the emerging role of these proteins in immunity. CPAF has been shown to degrade host proteins,35 and has shown promise in eliciting a protective immune response as a vaccine construct in a mouse urogenital tract model.36,37 In addition, data have recently shown that urogenital infections in humans stimulate neutralizing antibodies against CPAF protease activity.38 CT795 is a hypothetical protein that is unique to the family Chlamydiaceae and elicits strong systemic antibody responses in women with chlamydial urogenital tract infections,39 although it is not clear whether this protein plays a role in the immunopathogenesis of disease.
The purpose of our study was to expand on previous cHSP60 antibody findings in trachoma populations and, given the lack of ocular immune studies for CPAF and CT795, assess their immunogenicity and potential role in trachoma pathogenesis. We determined the tear IgG and IgA antibody titers and frequencies for these three proteins and correlated the results with TF/TI and TT (a sequela of TS) cases compared with age-matched controls who resided in a trachoma-endemic region of Nepal. To our knowledge, this is the first study to focus on antibody responses in TT. In addition, we evaluated the influence of Ct infection, as determined by commercial PCR, on antibody production against each protein for each trachoma grade. Finally, because of the different methods used for detecting antibodies that are not standardized and that can result in false positives, we developed a new quantitative ELISA that eliminates the background in each sample, to ensure that the antibody titers and frequencies are precisely determined.
Methods
Patient Sample Collection
Verbal informed consent was obtained from all study subjects after approval of the protocol by the institutional review board of Children's Hospital and Research Center (Oakland, CA) and Netra Jhoti Shang (Nepal) and in accordance with the Declaration of Helsinki. Each patient's age, sex, and grade of trachoma were recorded, followed by conjunctival and tear sample collection, as we have described elsewhere.13 Grading of the upper tarsus of each study subject was performed according to the modified trachoma grading scale of Thylefors et al.40 Briefly, the grades were as follows: T0, no evidence of trachoma characterized by fewer than five follicles on the lower two thirds of the upper eyelid; TF/TI, follicular and/or intense trachomatous inflammation; and TT, trachomatous trichiasis.
Detection of Ct in Conjunctival Swabs
Each conjunctival swab sample was screened for the presence of Ct DNA (Amplicor-PCR assay; Roche Diagnostics, Branchburg, NJ), according to the manufacturer's instructions and a published method,22 after the total genomic DNA yield was determined (Nanodrop 2000c; Thermo-Fisher Scientific, Wilmington, DE), according to the manufacturer's instructions. Samples were defined as positive at OD450nm > 0.8, negative at OD450nm < 0.2, and equivocal at 0.2 < OD450nm < 0.8. Equivocal samples were resolved as negative or positive by PCR analysis that amplifies the ompA gene, as described elsewhere.5
Tear Sample Preparation
Tears were extracted from conjunctival sponges according to published methods.13 Briefly, sterile sponges (Weck-cel; Edward Weck, Inc., Research Triangle Park, NC) were placed in the inner canthus of each eye and allowed to saturate, as we have described previously.2,22 The sponges were immediately placed in a liquid nitrogen transport container and stored in the laboratory in a −80°C freezer. Tear extraction was performed by incubating swabs with 100 μL of resuspension fluid (50 mM Tris, 0.15 M NaCl, 10 mM CaCl2, and serine and cysteine protease inhibitor [Protease Inhibitor Cocktail tablets, Roche Diagnostics, Indianapolis, IN], adjusted to pH 7.5) and allowing them to sit for 30 minutes at 4°C. Fluid was collected via centrifugation at 16,000g for 20 minutes at 4°C and quantified by BCA protein assay (Thermo-Fisher Scientific Inc.). The background against Escherichia coli and glutathione S-transferase (GST) proteins was minimized by preabsorbing, 18 μg of tear fluid with 50 μL of GST lysate and 450 μL of 10% fetal bovine serum (FBS) in phosphate-buffered saline (PBS) overnight at 4°C. The supernatants were collected after centrifugation at 12,000g for 10 minutes.
Preparation of Recombinant cHSP60, CT795, and CPAF Fusion Proteins
Opening reading frames (ORFs) of cHSP60, CT795, and the mature form of CPAF were amplified by using the following primers specific for reference strain Ct serovar E cDNA with engineered restriction recognition sites at 5′-ends: cHSP60, forward 5′-GTCGACATGGTCGCTAAAAACAT-3′, reverse 5′-CTCGAGTTAATAGTCCATTCCTGCG-3′; CT795, forward 5′-GGATCCATGAGATTCTTGTTAG-3′, reverse 5′-GAATTCCTACTCAACAAATTCAGG-3′; and CPAF, forward 5′-GGATCCATACATTCTCCTGTAC-3′, reverse 5′-GTCGACTTAAAAACTACCATCTTC-3′. The chlamydial genes were cloned into the pGEX 6p-2 vector system (GE Healthcare, Piscataway, NJ), which encodes a GST fusion protein on the N terminus, according to the manufacturer's instructions. Nucleotide sequence analysis of each chlamydial gene insert was performed using GST sequencing primers (GE Healthcare) that flanked the insert, to verify proper orientation of the complete chlamydial gene. The E. coli BL21 (DE3) strain was transformed with each plasmid and induced with 0.1 to 0.2 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) at a cell density of OD600nm 0.5 to 1.0 for 2 to 4 hours. Cell pellets were resuspended in 1× PBS with serine and cysteine protease inhibitors (Protease Inhibitor Cocktail tablets; Roche Diagnostics). Soluble lysates were obtained via sonication at 4°C followed by incubation with 1% Triton X-100 for 30 minutes. Soluble and insoluble fractions were separated via centrifugation at 12,000g for 10 minutes at 4°C. Recombinant soluble and insoluble fractions were analyzed using SDS-PAGE on 4% to 20% Tris-glycine gels (NuGel, Austell, GA) where GST-cHSP60 and GST-CT795 were present in soluble lysates, whereas GST-CPAF remained in insoluble inclusion bodies. Solubilization of inclusion bodies was performed by resuspending the insoluble pellet in a denaturing buffer (8 M urea, 100 mM NaH2PO4, 10 mM Tris-Cl [pH 8.0]) and adding it dropwise to 1× PBS, resulting in a final urea concentration of 0.8 M. The soluble fraction was then obtained after centrifugation at 12,000g, where the supernatant demonstrated the mature form of CPAF with GST fusion tag (92 kDa) on an SDS-PAGE gel.
Detection of Tear IgG and IgA Antibodies against cHSP60, CT795, and CPAF
Custom glutathione-coated plates were produced according to a published protocol.41 GST-fusion protein plates were developed by washing and coating plates with soluble lysates as described elsewhere.39 Briefly, GST alone, cHSP60, CT795, or CPAF were dispensed into wells at saturating dilutions of 1:10 (concentrations of >200 μg/mL of GST, cHSP60, and CT795, and >50 μg/mL of CPAF) and allowed to incubate overnight at 4°C. The plates were washed five times with PBS with 0.5% Tween-20 (PBST), and 50 μL of preabsorbed tear samples diluted in 10% FBS in PBS were added to the wells and incubated for 2 hours at room temperature. A set of standards was developed by adding to each plate 1:3-fold serial dilutions of a previously tested patient serum sample that demonstrated high antibody levels to cHSP60. This standard serum sample was obtained by preabsorbing at a 1:150 dilution in GST lysate with 10% FBS in PBS overnight. Samples were centrifuged, aliquotted to avoid multiple freeze thaws, and stored at −20°C. Plates were washed with PBST before horseradish peroxidase (HRP)–conjugated goat anti-human IgG was added, diluted 1:5000 or IgA diluted 1:20,000 (Jackson ImmunoResearch, West Grove, PA) and incubated for 1 hour. After a series of washes, the wells were incubated with 80 μL of substrate (Ultra TMB; Thermo-Fisher Scientific, Inc.) for 30 minutes for colorimetric development. The reaction was stopped with 2 N H2SO4, and the absorbances were recorded at 450 nm minus the 570-nm background.
Statistical Analysis
Associations between age, sex, and Ct infection for the TF/TI and TT cases versus the controls were initially performed with univariate logistic regression. A standard curve for the patient tear sample discussed earlier was used for all microtiter plates, to quantify antibody titers. Patient tear antibody titers against the chlamydial fusion proteins cHSP60, CT795, or CPAF were determined by analyzing the ratio of the mean of the anti-chlamydial antibody results to the mean of GST antibody results. Significant differences of tear antibody titers and frequency variations (defined as the percentage positive with ratios >2, demonstrating at least 3 SD above background GST control values) between trachoma grade and the age-matched control were performed by using multiple logistic regression adjusted for sex and Ct infection. The influence of Ct infection on the frequency of antichlamydial antibody production against each immunogen (see Tables 2–4) for each trachoma grade and age-matched control group was determined by multiple logistic regression adjusted for sex. Fisher's exact two-tailed tests were used to analyze tear IgA and IgG antibody frequencies of each chlamydial protein for each trachoma grade and age-matched control. A χ2 test for trend was performed on the data for TF/TI and TT outcomes with the respective control, for antibody responses to each chlamydial fusion protein by titer and frequency. Differences at P < 0.05 were statistically significant (Stata. ver. 9.0; Stata Corp, College Station, TX).
Table 2.
The Effect of C. trachomatis Infection, as Determined by PCR, on the Frequency of Tear IgG and IgA Antibodies to cHSP60
| IgG+ Tears n (%) |
OR (95% CI) | P | IgA+ Tears n (%) |
OR (95% CI) | P | |||
|---|---|---|---|---|---|---|---|---|
| Ct PCR− | Ct PCR+ | Ct PCR− | Ct PCR+ | |||||
| Controls | 4/49 (8.2) | 0/13 (0.0) | NA | NS | 1/49 (2.0) | 0/13 (0.0) | NA | NS |
| TF/TI | 10/45 (22) | 11/20 (55) | 3.9 (1.2–13.1) | 0.025 | 7/45 (16) | 3/20 (15) | 1.8 (0.4–9.8) | NS |
| Controls | 4/41 (9.8) | 2/16 (13) | 1.3 (0.2–8.5) | NS | 4/41 (9.8) | 2/16 (13) | 1.4 (0.2–9.3) | NS |
| TT | 11/47 (23) | 1/12 (8.3) | 0.2 (0.0–2.2) | NS | 8/47 (17) | 1/12 (8.3) | 0.3 (0.0–2.7) | NS |
C. trachomatis PCR data were unavailable for three patients in non-TF/TI and for two patients in the TT group. Statistical analysis was performed by logistic regression with adjustment for age and sex. Only significant differences (P < 0.05) between uninfected and C. trachomatis-infected individuals were reported.
Table 3.
The Effect of C. trachomatis Infection, as Determined by PCR, on the Frequency of Tear IgG and IgA Antibodies to CT795
| IgG+ Tears n (%) |
OR (95% CI) | P | IgA+ Tears n (%) |
OR (95% CI) | P | |||
|---|---|---|---|---|---|---|---|---|
| Ct PCR− | Ct PCR+ | Ct PCR− | Ct PCR+ | |||||
| Controls | 8/49 (16) | 3/13 (23) | 1.8 (0.4–8.8) | NS | 0/41 (0.0) | 0/16 (0.0) | NA | NS |
| TF/TI | 15/45 (33) | 15/20 (75) | 4.1 (1.1–14.8) | 0.03 | 2/45 (4.4) | 3/20 (15) | 3.34 (0.5–23.5) | NS |
| Controls | 5/41 (12) | 3/16 (19) | 1.9 (0.4–9.9) | NS | 0/41 (0.0) | 0/16 (0.0) | NA | NS |
| TT | 12/47 (26) | 2/12 (17) | 0.9 (0.1–5.5) | NS | 0/47 (0.0) | 0/12 (0.0) | NA | NS |
The description of the PCR data and statistical analysis is the same as in Table 2.
Table 4.
The Effect of C. trachomatis Infection, as Determined by PCR, on the Frequency of Tear IgG and IgA antibodies to CPAF
| IgG+ Tears n (%) |
OR (95% CI) | P | IgA+ Tears n (%) |
OR (95% CI) | P | |||
|---|---|---|---|---|---|---|---|---|
| Ct PCR− | Ct PCR+ | Ct PCR− | Ct PCR+ | |||||
| Controls | 10/49 (20) | 1/13 (7.7) | 0.4 (0.0–3.5) | NS | 2/49 (4.1) | 0/13 (0.0) | NA | NS |
| TF/TI | 18 (45 (40) | 16 (20 (80) | 5.0 (1.3–18.5) | 0.017 | 3 (45 (6.7) | 2 (20 (10) | 4.26 (0.5–35.8) | NS |
| Controls | 5 (41 (12) | 2 (16 (13) | 0.9 (0.2–5.8) | NS | 3 (41 (7.3) | 2 (16 (13) | 1.6 (0.2–11.4) | NS |
| TT | 17 (47 (36) | 3 (12 (25) | 0.8 (0.2–4.0) | NS | 3 (47 (6.4) | 1 (12 (8.3) | 3.8 (0.3–53.2) | NS |
The description of the PCR data and statistical analysis is the same as in Table 2.
Results
Study Population Characteristics
Our study population consisted of 65 TF/TI and 59 TT cases and their respective age-matched controls. All cases were from the trachoma-endemic Kapilvastu District of Nepal. The TT cases and controls were significantly older than the TF/TI cases and controls (Table 1, P < 0.001). Tear IgG antibody titers against CT795 were inversely associated with age (odds ratio [OR], 0.978; 95% confidence interval [95% CI], 0.96–0.99, P = 0.008).
Table 1.
Demographics
| TF/TI |
TT |
|||
|---|---|---|---|---|
| Controls | Cases | Controls | Cases | |
| Age, y* (range) | 26.7 (10–70) | 26.5 (10–70) | 49.0 (13–75) | 49.7 (13–75) |
| Sex | ||||
| Male | 20 | 21 | 22 | 14 |
| Female | 45 | 44 | 37 | 45 |
| C. trachomatis PCR+ (%) | 13/62 (20) | 20/65 (31) | 16/57 (28) | 12/59 (20) |
The TF/TI cases and controls had a significantly lower age than did the TT cases and controls (P < 0.001).
Tear Antibody Responses against Chlamydial cHSP60, CT795, and CPAF Fusion Proteins for TF/TI Cases Compared with Controls
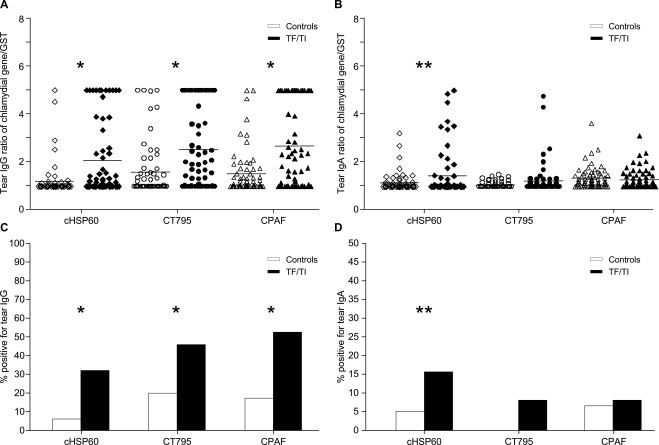
Figure 1 shows the results of the tear immune responses against cHSP60, CT795, and CPAF. There were significantly elevated ratios (chlamydial gene/GST alone) of tear IgG antibody titers against all three chlamydial immunogens for the TF/TI cases compared with the age-matched controls (Fig. 1A, mean titer of cHSP60: T0 = 1.21, TF/TI = 2.08, P < 0.005; mean titer of CT795: T0 = 1.60, TF/TI = 2.53, P < 0.005; and mean titer of CPAF: T0 = 1.53, TF/TI = 2.67, P < 0.005). These results remained significant when evaluating the frequency (percentage positive, ratio >2) of tear IgG antibodies against each chlamydial immunogen in the TF/TI cases and controls (Fig. 1C, mean frequencies of cHSP60: T0 = 0.06, TF/TI = 0.32; mean frequencies of CT795: T0 = 0.18, TF/TI = 0.46; mean frequencies of CPAF: T0 = 0.17, TF/TI = 0.52, all tests P < 0.005).
Figure 1.
Tear IgG and IgA antibodies against cHSP60, CT795, and CPAF fusion proteins in the TF/TI cases compared with levels in the age-matched controls. Preabsorbed tear samples were measured by ELISA for IgG (A) and IgA (B) antibody titers specific for the chlamydial proteins cHSP60, CT795, and CPAF. (A, B) Data represent antibody titers based on the chlamydial gene/GST ratio in the TF/TI cases (n = 65) or the age-matched controls (n = 65). Horizontal lines: geometric means. (C, D) Frequency of tear IgG and IgA antibodies, respectively, against cHSP60, CT795, and CPAF (ratios of chlamydial gene/GST ≥ 2) in the TF/TI cases compared with that in the age-matched controls. Significant differences between the TF/TI cases and controls were determined by multiple logistic regression adjusted for sex and Ct infection. *P < 0.005; **P < 0.05.
Although there was a trend toward elevated IgA antibody titers and frequency against CT795, statistical significance was evident only for cHSP60 (Fig. 1B, mean titer of cHSP60: T0 = 1.16, TF/TI = 1.45, P < 0.05; Fig. 1D, mean frequency of cHSP60: T0 = 0.05, TF/TI = 0.15, P < 0.05) between the TF/TI cases and controls. Tear IgG frequencies against CT795 and CPAF were significantly higher than tear IgA frequencies against the respective immunogens (mean frequency of CT795: IgG = 0.32, IgA = 0.04; mean frequency of CPAF: IgG = 0.35, IgA = 0.06, P < 0.001 for both), whereas there were minimal differences for cHSP60.
Tear Antibody Responses against Chlamydial cHSP60, CT795, and CPAF Fusion Proteins for TT Cases Compared with Controls
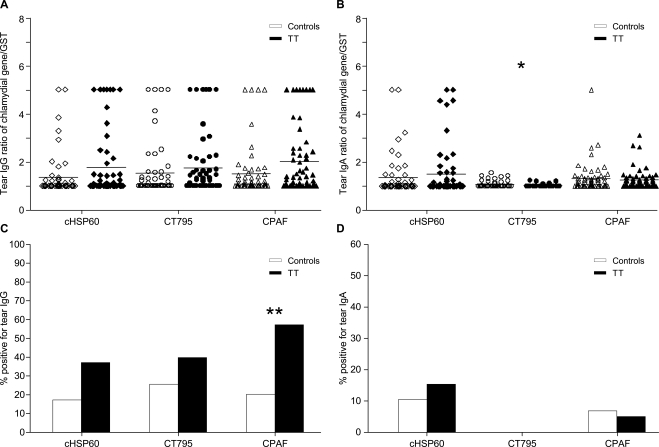
Figure 2 shows the results of the tear antibodies against cHSP60, CPAF, and CT795 fusion proteins for the patients with TT compared with the controls. Although modest increases in tear IgG antibody titers were found against all immunogens, only titers against CPAF (Fig. 2A) were significant (by χ2 analysis of trend [Fig. 2A]; mean values of CPAF: T0 = 1.49, TT = 2.00, P = 0.042). A significant difference in IgG antibody frequency was also observed against CPAF compared with that in age-matched controls (Fig. 2C, mean frequency of CPAF: T0 = 0.12, TT = 0.34, P = 0.013). Although there was a similar increase in antibodies for cHSP60 and CT795, these results were not significant for either titer or frequency.
Figure 2.
Tear IgG and IgA antibodies against cHSP60, CT795, and CPAF fusion proteins in the TT cases compared with levels in age-matched controls. Preabsorbed tear samples were measured by an ELISA for IgG (A) and IgA (B) antibody titers specific for the chlamydial proteins cHSP60, CT795, and CPAF. (A, B) Data represent antibody titers based on the chlamydial gene/GST ratio in the TT cases (n = 59) or the age-matched controls (n = 65). Horizontal lines: geometric means. (C, D) Frequency of tear IgG and IgA antibodies, respectively, against cHSP60, CT795, and CPAF (ratios of chlamydial gene/GST ≥ 2) for the TT cases compared with the age-matched controls. Significant differences between TT and control groups were determined by using multiple logistic regression adjusted for sex and Ct infection. *P < 0.05; **P = 0.013.
The overall frequency of tear IgA level was significantly lower than that of IgG for both CT795 and CPAF, independent of disease status, although comparable levels were observed for cHSP60 (mean frequency of CT795: IgG = 0.19, IgA = 0.00; CPAF: IgG = 0.23, IgA = 0.08, P < 0.001 and P = 0.007, respectively). Of novel interest was the significant elevation of tear anti-CT795 IgA levels (Fig. 2B, mean frequency of CT795: T0 = 1.06, TT = 1.02, P < 0.05) among the controls compared with the TT cases.
Effect of Ct Infections on Tear IgG and IgA Antibody Responses against Chlamydial cHSP60, CT795, and CPAF Fusion Proteins
To determine whether a Ct infection biases the humoral immune response, we analyzed the effect of infection on these IgG and IgA antibody responses (ratios > 2) in the TF/TI or TT cases and in the respective controls. Table 2 shows the results of these studies. There was a significant increase in the frequency of tear IgG antibodies to cHSP60 in the TF/TI cases during Ct infection compared with that in the uninfected TF/TI cases (mean frequency of cHSP60 in TF/TI group: Ct negative = 0.22, Ct positive = 0.55, OR, 3.94 [95% CI, 1.19–13.10]; P = 0.025). Antibody levels against CT795 and CPAF showed patterns comparable to those of cHSP60; Ct infections were associated with a significant increase in IgG antibodies against CT795 (Table 3, mean frequency of CT795 in the TF/TI group: Ct negative = 0.33, Ct positive = 0.75, P = 0.03; OR, 4.1 [1.1–14.8]) and CPAF (Table 4, mean frequency of CPAF in the TF/TI group: Ct negative = 0.40, Ct positive = 0.80, P = 0.017; OR, 5.0 [95% CI, 1.3–18.5]) in the TF/TI group.
Between the TT cases and respective controls, there were minimal differences in the association of Ct infection and antibody levels against chlamydial recombinant proteins (Tables 2–4).
Discussion
Both humoral and cellular pathways appear to be involved in the prevention and resolution of primary and recurrent Ct ocular and sexually transmitted infections.42 Our study expanded on the limited characterization of the humoral immune response in trachoma by analyzing tear antibody levels against three chlamydial proteins, cHSP60, CPAF, and CT795, that are all immunogens and either known or potential factors in chlamydial disease pathogenesis.31,39,43,44 To generate high-quality antibody data, we developed a robust quantitative ELISA that can become the standard assay in future antibody studies. The use of E. coli in the production of fusion proteins can result in contaminating bacterial proteins and, together with the diverse viscosity of biological samples, could result in a significant difference among results when not controlling for these factors. In this study, we combined previous protocols45,46 with additional controls and standard curves to quantify tear antibody levels. A standard curve derived from the same sample was used on every ELISA plate, as were similar tear protein concentrations for all samples. In addition, by controlling for GST background and preabsorbing all tear samples with FBS and GST lysates, we developed a standardized method for adjusting for background levels and quantifying antibody levels, which should assist in the comparison of results from different studies.
Our study focused on characterizing host immunoglobulins that are present at the foci of infection and disease, the conjunctiva. Although ocular immunoglobulins may be locally or systemically derived or represent a combination of both, our main focus was to characterize the protective or pathogenic events occurring in the conjunctiva in association with the levels and frequencies of the immunoglobulins present in the eye, regardless of their original origin. In our Nepali sample, the TF/TI cases had significantly more frequency of antibodies and higher tear IgG antibody titers against cHSP60, CPAF, and CT795 fusion proteins than did the individuals without trachoma. This effect was more pronounced for all three immunogens when there was concurrent Ct infection. For tear IgA, significantly elevated antibody titers and more frequency of antibodies were present against cHSP60 in the TF/TI but not the TT cases compared with the controls. In the TT cases, there was a significantly higher frequency of tear IgG antibodies against CPAF compared with the controls. Together, these data suggest that CPAF is both a marker and potential risk factor for active inflammatory disease and the severe sequelae of TT.
Several studies have identified host antibodies to cHSP60 in association with an array of chronic diseases, including salpingitis and tubal factor infertility,31,43,44,47 reactive arthritis,48 heart disease,49 and trachoma.2,22,24 Of note, women with Ct-associated tubal factor infertility are more likely than women with other causes of infertility to have antibodies to cHSP60-specific amino acids 201-300.50 The high degree of sequence homology between human HSP60 and cHSP60 (>70%) suggests that microbe-induced autoimmunity is a factor in some of these diseases.49 Further support for this idea comes from the identification of 100% amino acid homology of four defined epitopes shared by human HSP60 and cHSP6046 and documentation of human serum antibody crossreactivity to seven other epitopes also shared by human HSP60 and cHSP60.51 A monoclonal antibody specific for bacterial HSP60, including cHSP60, has been shown to recognize a unique 40-kDa human protein (CCP40) expressed by hematopoietic cell lines, including peripheral blood progenitor cells,52 although the importance of this protein is not understood.
In a study in The Gambia, the frequency of serum IgG antibody responses to cHSP60 was significantly associated with the TS cases compared with the controls; no other trachoma grades were evaluated.24 These systemic findings have not been supported by other studies in The Gambia21,23 or in our prior studies in different Nepali trachoma populations.2,22 We evaluated mucosal immune responses and found that villagers with tear IgG antibodies to cHSP60 were significantly more likely to have TI or TS than were those without any evidence of trachoma,2,22 but there were no differences in tear IgA responses. In the present study, we found a significant association of the frequency of both IgA and IgG tear antibody responses and titers against cHSP60 in the TF/TI cases compared with the controls. The frequency of IgG, but not IgA, antibodies was independently associated with Ct infection. The collective IgG findings are consistent with those in experiments in cynomolgus monkeys in which local ocular Chlamydia-specific IgG-secreting B cells were more numerous than those in distant inguinal lymph nodes and peripheral blood during Ct infection or after ocular challenge with cHSP60 in immune cynomolgus monkeys.53
Although there were no significant cHSP60 tear antibody frequency or titer associations with TT cases, our study is the first, to our knowledge, to evaluate individuals with TT. Our findings may reflect both the small sample size and the fact that, in end-stage disease, there can be scarring of lacrimal glands and other periocular tissues, resulting in insufficient production of antibodies or decreased antibody production simply from disease burn-out.54–56 TT is the more advanced stage of TS, and it occurs from repeated chlamydial infections. By the time it develops, the immune damage from cHSP60 has most likely already occurred.
IgG molecules play an immunologic role in neutralization, opsonization, and complement activation. Unlike the neutralization properties demonstrated with host antibodies against the PmpD and MOMP in vitro28 and in vivo7 or the complement-mediated lysis of Ct-infected cells from monoclonal antibodies directed against the chlamydial glycolipid exoantigen (GLXA),57 the pathogenic activity of cHSP60 is thought to be immunologically mediated. It is characterized by an inflammatory response involving B- and T-lymphocyte infiltration of the conjunctivae, including large numbers of antigen-specific lymphocytes58 and the subsequent development of scarring,59 which have the features of a delayed-type hypersensitivity (DTH) reaction. In experimental studies involving cynomolgus monkeys, inoculation of cHSP60 into the eyes of immune animals resulted in a DTH reaction.60 These findings are similar to those in the salpinx of monkeys that were challenged with cHSP60.61 Although these studies are important, the discovery of other Ct immunogens from genomic, proteomic, and antigen screening studies suggests the need to expand on the localized immune response to characterize other chlamydial epitopes and their immunobiologic role in trachoma.
Antibodies against the unique chlamydial protein CT795 were first identified in 2006 among women with urogenital Ct infections.39 In the same study, early and late protein production was identified within inclusion bodies of Ct-infected HeLa cells. The only other study of CT79562 has shown that antibodies to the protein (demonstrated by an ELISA) correlate with other Ct serologic tests. However, there are no data on serologic responses and the different disease states associated with Ct. In the present study, we found a significantly increased frequency of antibodies and elevated IgG tear antibody titer against CT795 in the TF/TI cases, compared with those in the controls, but no difference in tear IgA results. Ct infection was independently associated with a higher frequency of IgG but not IgA antibodies to CT795 in the TF/TI cases. Surprisingly, there was a significantly lower titer of tear IgA in the TT cases than in the controls. These data suggest either insufficient antibody responses or a possible immunoprotective role, although the biological significance has not been determined. The former is less likely, in that antibody frequency and titer of other immunogens did not differ between the TT cases and controls. Additional research is needed to determine the association of antibodies to CT795 in different trachoma and sexually transmitted disease populations, with or without documented disease.
CPAF has been well studied since its identification by Zhong et al.35 less than 10 years ago. CPAF is known from in vitro studies to be secreted into the host cytosol by 16 hours after infection,63 resulting in degradation of host transcription factor RFX5 (involved in major histocompatibility complex [MHC] I and II expression),35 CD1d,64 keratin 8,65 and proapoptotic BH3 proteins (i.e., Puma and Bik).66 These studies suggested that CPAF plays a role in chronic infection because of its late expression, cytosolic location, and downregulation of host antigen presentation machinery, including MHC-I and -II and CD1d, and prolongation of host cell survival from loss of BH3 proteins.
CPAF has been shown to elicit a robust systemic humoral immune response among women with Ct urogenital infections.63 A striking feature of this response was the higher antibody levels directed against CPAF, compared with MOMP and cHSP60,63 suggesting that this immunogen plays a role in the pathologic course of the disease or in protection. In a recent study of the murine model for urogenital chlamydial infections, intranasal immunization with CPAF plus CpG stimulated antigen-specific CD4+ T cells and IFNγ production that resulted in a protective response.67 Other murine vaccine studies demonstrated earlier resolution of genital C. muridarum infection and Chlamydia-specific IFNγ production compared with controls.36,37 However, an evaluation of CPAF in uninfected human cells found that cell death occurred via nonapoptotic pathways, which suggests that this protein is involved in inflammation and disease.68 In our study, we found a significantly increased frequency of tear antibodies and elevated titers against CPAF in the TF/TI cases and TF/TI cases with Ct infections. There was also a significant association of IgG antibodies against CPAF in the TT cases compared with that in the respective controls. Together, these findings suggest that the humoral IgG immune responses to CPAF may be a risk factor for persistent or chronic chlamydial disease, although further confirmatory studies in other populations and characterization of IgG subclasses and pathologic pathways are needed.
The humoral characterization of tear antibodies against cHSP60, CT795, and CPAF among a trachoma endemic population expands our understanding of microbe-induced immunopathogenesis. However, because tear volume in each individual is limited, it is difficult to perform large-scale immunoglobulin characterization of isotypes and subclasses specific to each chlamydial immunogen. Future studies are needed to more precisely decipher the immunobiologic associations of antibody responses to each immunogen as either pathologic or immunoprotective.
Footnotes
Supported in part by Public Health Service Grants R01 EY/AI 012219 and R01AI059647 (DD) from the National Institutes of Health.
Disclosure: T. Skwor, None; R.P. Kandel, None; S. Basravi, None; A. Khan, None; B. Sharma, None; D. Dean, None
References
- 1. Resnikoff S, Pascolini D, Etya'ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851 [PMC free article] [PubMed] [Google Scholar]
- 2. Dean D, Kandel RP, Adhikari HK, Hessel T. Multiple Chlamydiaceae species in trachoma: implications for disease pathogenesis and control. PLoS Med. 2008;5:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Melese M, Chidambaram JD, Alemayehu W, et al. Feasibility of eliminating ocular Chlamydia trachomatis with repeat mass antibiotic treatments. JAMA. 2004;292:721–725 [DOI] [PubMed] [Google Scholar]
- 4. Atik B, Thanh TT, Luong VQ, Lagree S, Dean D. Impact of annual targeted treatment on infectious trachoma and susceptibility to reinfection. JAMA. 2006;296:1488–1497 [DOI] [PubMed] [Google Scholar]
- 5. Zhang H, Kandel RP, Sharma B, Dean D. Risk factors for recurrence of postoperative trichiasis: implications for trachoma blindness prevention. Arch Ophthalmol. 2004;122:511–516 [DOI] [PubMed] [Google Scholar]
- 6. West ES, Mkocha H, Munoz B, et al. Risk factors for postsurgical trichiasis recurrence in a trachoma-endemic area. Invest Ophthalmol Vis Sci. 2005;46:447–453 [DOI] [PubMed] [Google Scholar]
- 7. Cotter TW, Meng Q, Shen ZL, Zhang YX, Su H, Caldwell HD. Protective efficacy of major outer membrane protein-specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4704–4714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morrison SG, Su H, Caldwell HD, Morrison RP. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4(+) T cells but not CD8(+) T cells. Infect Immun. 2000;68:6979–6987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moore T, Ananaba GA, Bolier J, et al. Fc receptor regulation of protective immunity against Chlamydia trachomatis. Immunology. 2002;105:213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ito JI, Lyons JM. Role of gamma interferon in controlling murine chlamydial genital tract infection. Infect Immun. 1999;67:5518–5521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Young E, Taylor HR. Immune mechanisms in chlamydial eye infection: cellular immune responses in chronic and acute disease. J Infect Dis. 1984;150:745–751 [DOI] [PubMed] [Google Scholar]
- 12. el-Asrar AM, Van den Oord JJ, Geboes K, Missotten L, Emarah MH, Desmet V. Immunopathology of trachomatous conjunctivitis. Br J Ophthalmol. 1989;73:276–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Skwor TA, Atik B, Kandel RP, Adhikari HK, Sharma B, Dean D. Role of secreted conjunctival mucosal cytokine and chemokine proteins in different stages of trachomatous disease. PLoS Negl Trop Dis. 2008;2:e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rank RG, Soderberg LS, Barron AL. Chronic chlamydial genital infection in congenitally athymic nude mice. Infect Immun. 1985;48:847–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramsey KH, Rank RG. Resolution of chlamydial genital infection with antigen-specific T-lymphocyte lines. Infect Immun. 1991;59:925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hawkins RA, Rank RG, Kelly KA. A Chlamydia trachomatis-specific Th2 clone does not provide protection against a genital infection and displays reduced trafficking to the infected genital mucosa. Infect Immun. 2002;70:5132–5139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van Voorhis WC, Barrett LK, Sweeney YT, Kuo CC, Patton DL. Repeated Chlamydia trachomatis infection of Macaca nemestrina fallopian tubes produces a Th1-like cytokine response associated with fibrosis and scarring. Infect Immun. 1997;65:2175–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Voorhis WC, Barrett LK, Sweeney YT, Kuo CC, Patton DL. Analysis of lymphocyte phenotype and cytokine activity in the inflammatory infiltrates of the upper genital tract of female macaques infected with Chlamydia trachomatis. J Infect Dis. 1996;174:647–650 [DOI] [PubMed] [Google Scholar]
- 19. Patton DL, Taylor HR. The histopathology of experimental trachoma: ultrastructural changes in the conjunctival epithelium. J Infect Dis. 1986;153:870–878 [DOI] [PubMed] [Google Scholar]
- 20. Bobo L, Novak N, Mkocha H, Vitale S, West S, Quinn TC. Evidence for a predominant proinflammatory conjunctival cytokine response in individuals with trachoma. Infect Immun. 1996;64:3273–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ghaem-Maghami S, Bailey RL, Mabey DC, et al. Characterization of B-cell responses to Chlamydia trachomatis antigens in humans with trachoma. Infect Immun. 1997;65:4958–4964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hessel T, Dhital SP, Plank R, Dean D. Immune response to chlamydial 60-kilodalton heat shock protein in tears from Nepali trachoma patients. Infect Immun. 2001;69:4996–5000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holland MJ, Bailey RL, Hayes LJ, Whittle HC, Mabey DC. Conjunctival scarring in trachoma is associated with depressed cell-mediated immune responses to chlamydial antigens. J Infect Dis. 1993;168:1528–1531 [DOI] [PubMed] [Google Scholar]
- 24. Peeling RW, Bailey RL, Conway DJ, et al. Antibody response to the 60-kDa chlamydial heat-shock protein is associated with scarring trachoma. J Infect Dis. 1998;177:256–259 [DOI] [PubMed] [Google Scholar]
- 25. Tan C, Hsia RC, Shou H, et al. Chlamydia trachomatis-infected patients display variable antibody profiles against the nine-member polymorphic membrane protein family. Infect Immun. 2009;77:3218–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gomes JP, Hsia RC, Mead S, Borrego MJ, Dean D. Immunoreactivity and differential developmental expression of known and putative Chlamydia trachomatis membrane proteins for biologically variant serovars representing distinct disease groups. Microbes Infect. 2005;7:410–420 [DOI] [PubMed] [Google Scholar]
- 27. Comanducci M, Manetti R, Bini L, et al. Humoral immune response to plasmid protein pgp3 in patients with Chlamydia trachomatis infection. Infect Immun. 1994;62:5491–5497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crane DD, Carlson JH, Fischer ER, et al. Chlamydia trachomatis polymorphic membrane protein D is a species-common pan-neutralizing antigen. Proc Natl Acad Sci U S A. 2006;103:1894–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Byrne GI, Stephens RS, Ada G, et al. Workshop on in vitro neutralization of Chlamydia trachomatis: summary of proceedings. J Infect Dis. 1993;168:415–420 [DOI] [PubMed] [Google Scholar]
- 30. Eckert LO, Hawes SE, Wolner-Hanssen P, et al. Prevalence and correlates of antibody to chlamydial heat shock protein in women attending sexually transmitted disease clinics and women with confirmed pelvic inflammatory disease. J Infect Dis. 1997;175:1453–1458 [DOI] [PubMed] [Google Scholar]
- 31. Ness RB, Soper DE, Richter HE, et al. Chlamydia antibodies, chlamydia heat shock protein, and adverse sequelae after pelvic inflammatory disease: the PID Evaluation and Clinical Health (PEACH) Study. Sex Transm Dis. 2008;35:129–135 [DOI] [PubMed] [Google Scholar]
- 32. Money DM, Hawes SE, Eschenbach DA, et al. Antibodies to the chlamydial 60 kd heat-shock protein are associated with laparoscopically confirmed perihepatitis. Am J Obstet Gynecol. 1997;176:870–877 [DOI] [PubMed] [Google Scholar]
- 33. Peeling RW, Kimani J, Plummer F, et al. Antibody to chlamydial hsp60 predicts an increased risk for chlamydial pelvic inflammatory disease. J Infect Dis. 1997;175:1153–1158 [DOI] [PubMed] [Google Scholar]
- 34. Paavonen J, Lehtinen M. Interactions between human papillomavirus and other sexually transmitted agents in the etiology of cervical cancer. Curr Opin Infect Dis. 1999;12:67–71 [DOI] [PubMed] [Google Scholar]
- 35. Zhong G, Fan P, Ji H, Dong F, Huang Y. Identification of a chlamydial protease-like activity factor responsible for the degradation of host transcription factors. J Exp Med. 2001;193:935–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murthy AK, Cong Y, Murphey C, et al. Chlamydial protease-like activity factor induces protective immunity against genital chlamydial infection in transgenic mice that express the human HLA-DR4 allele. Infect Immun. 2006;74:6722–6729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murthy AK, Chambers JP, Meier PA, Zhong G, Arulanandam BP. Intranasal vaccination with a secreted chlamydial protein enhances resolution of genital Chlamydia muridarum infection, protects against oviduct pathology, and is highly dependent upon endogenous gamma interferon production. Infect Immun. 2007;75:666–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sharma J, Dong F, Pirbhai M, Zhong G. Inhibition of proteolytic activity of a chlamydial proteasome/protease-like activity factor by antibodies from humans infected with Chlamydia trachomatis. Infect Immun. 2005;73:4414–4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sharma J, Zhong Y, Dong F, Piper JM, Wang G, Zhong G. Profiling of human antibody responses to Chlamydia trachomatis urogenital tract infection using microplates arrayed with 156 chlamydial fusion proteins. Infect Immun. 2006;74:1490–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bull World Health Organ. 1987;65:477–483 [PMC free article] [PubMed] [Google Scholar]
- 41. Murray AM, Kelly CD, Nussey SS, Johnstone AP. Production of glutathione-coated microtitre plates for capturing recombinant glutathione S-transferase fusion proteins as antigens in immunoassays. J Immunol Methods. 1998;218:133–139 [DOI] [PubMed] [Google Scholar]
- 42. Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5:149–161 [DOI] [PubMed] [Google Scholar]
- 43. Bax CJ, Dorr PJ, Trimbos JB, et al. Chlamydia trachomatis heat shock protein 60 (cHSP60) antibodies in women without and with tubal pathology using a new commercially available assay. Sex Transm Infect. 2004;80:415–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sziller I, Fedorcsak P, Csapo Z, et al. Circulating antibodies to a conserved epitope of the Chlamydia trachomatis 60-kDa heat shock protein is associated with decreased spontaneous fertility rate in ectopic pregnant women treated by salpingectomy. Am J Reprod Immunol. 2008;59:99–104 [DOI] [PubMed] [Google Scholar]
- 45. Dean D, Powers VC. Persistent Chlamydia trachomatis infections resist apoptotic stimuli. Infect Immun. 2001;69:2442–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Campanella C, Marino Gammazza A, Mularoni L, Cappello F, Zummo G, Di Felice V. A comparative analysis of the products of GROEL-1 gene from Chlamydia trachomatis serovar D and the HSP60 var1 transcript from Homo sapiens suggests a possible autoimmune response. Int J Immunogenet. 2009;36:73–78 [DOI] [PubMed] [Google Scholar]
- 47. Tiitinen A, Surcel HM, Halttunen M, et al. Chlamydia trachomatis and chlamydial heat shock protein 60-specific antibody and cell-mediated responses predict tubal factor infertility. Hum Reprod. 2006;21:1533–1538 [DOI] [PubMed] [Google Scholar]
- 48. Kuipers JG, Sibilia J, Bas S, et al. Reactive and undifferentiated arthritis in North Africa: use of PCR for detection of Chlamydia trachomatis. Clin Rheumatol. 2009;28:11–16 [DOI] [PubMed] [Google Scholar]
- 49. Bachmaier K, Neu N, de la Maza LM, Pal S, Hessel A, Penninger JM. Chlamydia infections and heart disease linked through antigenic mimicry. Science. 1999;283:1335–1339 [DOI] [PubMed] [Google Scholar]
- 50. Arno JN, Yuan Y, Cleary RE, Morrison RP. Serologic responses of infertile women to the 60-kd chlamydial heat shock protein (hsp60). Fertil Steril. 1995;64:730–735 [DOI] [PubMed] [Google Scholar]
- 51. Yi Y, Zhong G, Brunham RC. Continuous B-cell epitopes in Chlamydia trachomatis heat shock protein 60. Infect Immun. 1993;61:1117–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rosenberg HF, Morrison RP, Li F. Identification of a 40-kDa human protein that cross-reacts with prokaryotic hsp60/chaperonins. Biochem Biophys Res Commun. 1995;208:697–703 [DOI] [PubMed] [Google Scholar]
- 53. Pal S, Taylor HR, Huneke RB, Prendergast RA, Whittum-Hudson JA. Frequency of antigen-specific B cells during experimental ocular Chlamydia trachomatis infection. Infect Immun. 1992;60:5294–5297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dean D. Pathogenesis of chlamydia ocular infections. In: Tasman W, Jaeger EA. eds. Duane's Foundations of Clinical Ophthalmology. Philadelphia: Lippincott Williams & Wilkins; 2010. In press [Google Scholar]
- 55. Tabbara KF, Bobb AA. Lacrimal system complications in trachoma. Ophthalmology. 1980;87:298–301 [DOI] [PubMed] [Google Scholar]
- 56. el-Asrar AM, Geboes K, al-Kharashi SA, et al. An immunohistochemical study of collagens in trachoma and vernal keratoconjunctivitis. Eye. 1998;12:1001–1006 [DOI] [PubMed] [Google Scholar]
- 57. Webley WC, Vora GJ, Stuart ES. Cell surface display of the chlamydial glycolipid exoantigen (GLXA) demonstrated by antibody-dependent complement-mediated cytotoxicity. Curr Microbiol. 2004;49:13–21 [DOI] [PubMed] [Google Scholar]
- 58. Pal S, Pu Z, Huneke RB, Taylor HR, Whittum-Hudson JA. Chlamydia-specific lymphocytes in conjunctiva during ocular infection: limiting dilution analysis. Reg Immunol. 1990–91;3:171–176 [PubMed] [Google Scholar]
- 59. Whittum-Hudson JA, Taylor HR, Farazdaghi M, Prendergast RA. Immunohistochemical study of the local inflammatory response to chlamydial ocular infection. Invest Ophthalmol Vis Sci. 1986;27:64–69 [PubMed] [Google Scholar]
- 60. Taylor HR, Maclean IW, Brunham RC, Pal S, Wittum-Hudson J. Chlamydial heat shock proteins and trachoma. Infect Immun. 1990;58:3061–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Patton DL, Sweeney YT, Kuo CC. Demonstration of delayed hypersensitivity in Chlamydia trachomatis salpingitis in monkeys: a pathogenic mechanism of tubal damage. J Infect Dis. 1994;169:680–683 [DOI] [PubMed] [Google Scholar]
- 62. Frikha-Gargouri O, Gdoura R, Znazen A, Gargouri J, Hammami A. Diagnostic value of enzyme-linked immunosorbent assays using hypothetical proteins CT226 and CT795 as antigens in Chlamydia trachomatis serodiagnosis. Diagn Microbiol Infect Dis. 2009;65:224–231 [DOI] [PubMed] [Google Scholar]
- 63. Sharma J, Bosnic AM, Piper JM, Zhong G. Human antibody responses to a Chlamydia-secreted protease factor. Infect Immun. 2004;72:7164–7171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kawana K, Quayle AJ, Ficarra M, et al. CD1d degradation in Chlamydia trachomatis-infected epithelial cells is the result of both cellular and chlamydial proteasomal activity. J Biol Chem. 2007;282:7368–7375 [DOI] [PubMed] [Google Scholar]
- 65. Dong F, Su H, Huang Y, Zhong Y, Zhong G. Cleavage of host keratin 8 by a Chlamydia-secreted protease. Infect Immun. 2004;72:3863–3868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pirbhai M, Dong F, Zhong Y, Pan KZ, Zhong G. The secreted protease factor CPAF is responsible for degrading pro-apoptotic BH3-only proteins in Chlamydia trachomatis-infected cells. J Biol Chem. 2006;281:31495–31501 [DOI] [PubMed] [Google Scholar]
- 67. Li W, Murthy AK, Guentzel MN, et al. Antigen-specific CD4+ T cells produce sufficient IFN-gamma to mediate robust protective immunity against genital Chlamydia muridarum infection. J Immunol. 2008;180:3375–3382 [DOI] [PubMed] [Google Scholar]
- 68. Paschen SA, Christian JG, Vier J, et al. Cytopathicity of Chlamydia is largely reproduced by expression of a single chlamydial protease. J Cell Biol. 2008;182:117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]