First- and second-order dynamics of Edinger-Westphal–stimulated accommodation in rhesus monkeys depend on the amplitude but not on the starting point of the response. The disaccommodative peak velocity for a given amplitude is lower in older than in younger monkeys.
Abstract
Purpose.
In humans, accommodative and disaccommodative dynamics depend on response amplitude and starting point. The purpose of this study was to determine the influence of amplitude and starting point on open-loop accommodative dynamics in Edinger-Westphal (EW)-stimulated, anesthetized rhesus monkeys of different ages.
Methods.
One eye each of two younger and two older iridectomized rhesus monkeys, (aged 6.8, 8.9, 15.0, and 16.3 years) were studied. The experiment was repeated in one eye of one younger monkey. Lens thickness changes were recorded by dynamic ultrasound biometry at 100 Hz. Stimuli used produced accommodative responses: (1) starting from baseline with increasing amplitudes; (2) from increasing starting points to maximum accommodation; and (3) from increasing starting points with a constant amplitude of 1 D. The lens thickness measurements were converted into accommodation and velocities and accelerations of the responses were determined by using a two-point difference algorithm.
Results.
Maximum accommodative amplitudes ranged from 4.68 to 6.37 D in the older monkeys and 9.33 to 11.59 D in the younger monkeys. The peak velocity of accommodation and disaccommodation increased linearly with response amplitude. Peak velocity and peak acceleration of accommodation and disaccommodation were independent of the response starting point. Subtle variations in disaccommodative response peak velocities were found to vary with age.
Conclusions.
The results suggest that, in anesthetized rhesus monkeys, disaccommodative rather than accommodative dynamics may be more sensitive to age-related changes and that, unlike in conscious human subjects, the starting configuration of the accommodative plant has little influence on accommodative dynamics.
The influence of starting point on focusing from far-to-near (accommodative) and near-to-far (disaccommodative) dynamics has been studied in humans.1–4 The dynamics of accommodation and disaccommodation may depend on the mechanics of the accommodative plant and the starting conditions of the response.5,6 The dynamics of disaccommodation in humans are strongly related to the response starting point, whereas the dynamics of accommodation are not.3 Bharadwaj and Schor4 suggested that disaccommodative responses in humans from all starting positions are directed to a common destination that correlates with the cycloplegic refractive state. In the present study, the accommodative and disaccommodative dynamics as a function of different starting positions were studied using Edinger-Westphal (EW)-stimulated accommodation in anesthetized adolescent and older rhesus monkeys.
Normally, in conscious human subjects, an accommodative response to a visual stimulus is closed-loop in which retinal-blur–dependent feedback plays a role. Stimulation of the EW nucleus of the brain in anesthetized monkeys allows for open-loop accommodation. In this way, perceptual influences, such as visual feedback, are eliminated, and the accommodative amplitude and response dynamics are determined only by the stimulus delivered to the brain, the response of the neural pathway from the EW nucleus to the ciliary muscle and the biomechanics of the accommodative plant. Studies of open-loop accommodation in anesthetized monkeys may help to identify the extent to which biomechanical factors associated with the accommodative plant and/or neural effects influence the accommodative dynamics, independent of visual feedback. Further, if such studies identify age-dependent changes in accommodative dynamics, it may also shed light on how aging and the progression of presbyopia affect the biomechanics of the accommodative plant.
There is substantial evidence of biomechanical age changes in the accommodative structures that may influence accommodative or disaccommodative dynamics. Several studies have demonstrated an exponential increase in stiffness of the isolated human lens with age and a decrease in focal length change with stretching.7–10 Microscopic studies of ciliary muscle specimens show a loss of compliance of the posterior attachment of the ciliary muscle in rhesus monkeys,11 as well as morphologic changes in the human ciliary muscle suggesting a loss of elasticity.12 Although no studies have directly measured loss of compliance of the aging rhesus monkey lens, there are reduced accommodative movements of the lens in aging monkeys.13–15 Together, this evidence suggests that it is likely that changes in accommodative dynamics can occur with increasing age.
Prior studies in humans have found subtle changes with age in the dynamics of accommodation only,16–18 disaccommodation only,19 both of them,20,21 or none of them.22–24 It has been suggested that the neural control of voluntary accommodation in humans compensates for any age changes in the biomechanical structures.25 A prior study in rhesus monkeys aged 14.6 to 18.6 years also showed no evidence of age-related changes in accommodative and disaccommodative dynamics.26 However, the changes in dynamics that may occur with age may be subtle, and therefore the relatively low sampling frequency of 30-Hz video photorefraction recordings used in that study together with the requirement for function-fitting to extract the response dynamics may not have permitted age differences to be detected. In the present study, we used 100-Hz acquisition frequency, high-resolution, A-scan ultrasound to extract response dynamics without the need for function-fitting.
Material and Methods
Monkey Preparation
All experiments conformed to the ARVO Statement for the Use of Animals in Vision Research and were performed in accordance with institutionally approved animal protocols. Experiments were performed on one eye each of four rhesus monkeys (Macaca mulatta), two of which were adolescents aged 6.8 (111, OS) and 8.9 (54, OS) years, and two of which were older adults aged 15.0 (34, OS) and 16.3 (96, OS) years. The experiment was repeated in the left eye of monkey 54 [referred to as 54(2)]. The monkeys had undergone total iridectomy,27 assessment of maximum pharmacologically stimulated accommodative amplitude,6,28 and stereotaxic surgical implantation of a stimulating electrode in the EW nucleus.6,29 The monkeys had been used in multiple protocols, and the justification for the iridectomies and the absence of an effect on EW-stimulated accommodation are described elsewhere.30–32 The duration of the experiment was between 2 and 3 hours, depending on the number of different stimulus conditions required.
EW-Stimulated Accommodation
The monkeys were anesthetized with intramuscular ketamine (Ketaset; Fort Dodge Animal Health, Fort Dodge, IA) 10 mg/kg and acepromazine (Vedco, St. Joseph, MO) 0.5 mg/kg. Surgical-depth anesthesia was induced with a bolus injection of 1.5 mg/kg intravenous propofol (PropoFlo; Abbott Laboratories, Abbott Park, IL) and was maintained with a continuous intravenous infusion of propofol 0.5 mg/kg/min.
The monkey's head was placed in a headholder upright and facing forward. The eye was held open by a lid speculum. To reduce convergence eye movements during accommodation, 4-0 nylon sutures were placed through the medial and lateral rectus muscles and held under light tension. A rigid, gas-permeable contact lens was placed on the cornea. Baseline resting refraction was measured with a Hartinger coincidence refractometer.
Static Measurement of Accommodation
Accommodation was stimulated with a digital stimulator (model DS-8000; World Precision Instruments, Sarasota, FL) connected to a linear stimulus isolator (model A-395; World Precision Instruments). Four-second-duration stimulus trains were used (frequency, 72 Hz; pulse width, 600 μs), ranging in amplitude from 0 μA up to the current amplitude needed to produce the maximum accommodative response available to each eye. This level was determined by increasing the stimulus amplitude until there was no further increase in response amplitude for three consecutive increasing stimulus current amplitudes. Pulse frequency of 72 Hz is routinely used for EW-stimulated accommodation.6,33–35 At this frequency, a maximum stimulus amplitude is achieved for a given stimulus current while minimizing convergence eye movements.34 Step-stimulation of accommodation with increasing amplitude stimulus pulses has been used in multiple studies and shown to produce reliable accommodative responses, although it may not be totally representative of the EW spike frequencies in awake, behaving monkeys.36,37 For each stimulus amplitude, five consecutive 4-second stimulus trains with 4-second interstimulus intervals were delivered. Refraction was measured during the last second of each 4-second stimulus train with the Hartinger coincidence refractometer. The static accommodative response amplitude was calculated by subtracting the accommodated refraction from the baseline refraction. The means and SDs of the response amplitudes achieved in the last three of the five stimulus trains were calculated and used to obtain a static stimulus–response curve. For each experiment, a unique calibration curve was obtained relating the stimulus current necessary to produce a given accommodative response amplitude.
Dynamic Measurements of Accommodation
Dynamic A-Scan Ultrasound Biometry.
A-scan ultrasound measured accommodative changes in lens thickness have been shown in a previous study to be linearly correlated (r2 = 0.937–0.996) with the accommodative refractive changes over the whole range of accommodation and therefore can be used to study accommodative dynamics.33 A continuous A-scan ultrasound biometry system (CUB) developed by Rob van der Heijde2 was used to measure dynamic accommodative changes in lens thickness.
The CUB has a 10-MHz transducer, is able to detect a movement of ±2 μm and records ocular biometry data to a computer via the RS-232 port with an acquisition frequency of up to 100 Hz.33 A 1-cm-long rubber tubing stand-off sleeve was placed over the transducer tip and filled with ultrasound transmission gel (Liquasonic Ultrasound Gel; Chester Laboratories Inc., Cincinnati, OH). The transducer was clamped in a micromanipulator (D-10 Positioner; Research Instruments, London, UK) in front of the eye. After the contact lens was removed from the eye, the tip of the rubber tube was positioned in contact with a bead of ultrasound transmission gel on the cornea to give sharp A-scan peaks for all ocular surfaces. The instrument records the time between the peaks associated with the different intraocular interfaces. Times were subsequently converted to millimeters by multiplication with accepted sound velocities (anterior chamber depth [ACD] = 1532 m s−1; lens thickness [LT] = 1641 m s−1).28,33,38–41 Anterior chamber depth, lens thickness, and vitreous chamber depth were measured and automatically stored to the computer for subsequent analysis.
Relationship between Accommodative Changes in Lens Thickness and Refraction.
From the Hartinger-measured static stimulus–response curve, five stimulus amplitudes producing accommodative responses up to the maximum accommodative amplitude available were selected for calibration, to convert the lens thickness measurements in millimeters into accommodative response in diopters. For each of these stimulus amplitudes for which the accommodative response was measured with the Hartinger, responses to five 4-second stimulus trains were recorded with the CUB. The mean lens thickness measurements of the unaccommodated eye and mean lens thickness of the accommodated eye from the last three stimulus trains was subsequently plotted against the corresponding mean Hartinger-measured refraction values from the static stimulus–response measurements. The linear function fitted to these data was used as the calibration function, to convert lens thickness into refraction.
Stimulations with Predefined Starting Points and Amplitudes
Calculation of Stimulus Amplitudes.
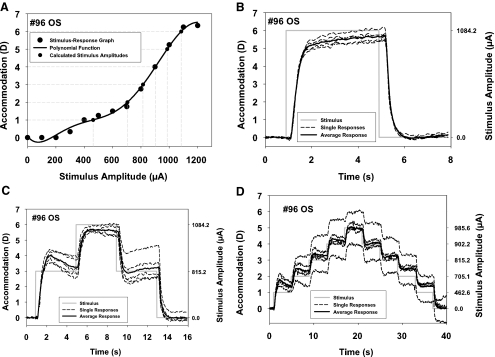
The intention of the experiment was to produce accommodative and disaccommodative responses of predictable starting points and amplitudes. Because of the different ages of the monkeys, the maximum amplitude in each of them differed, and different stimulus amplitudes were needed to achieve maximum response amplitude. Therefore, for comparison between monkeys, it was necessary to predetermine the stimulus amplitudes that would produce response amplitudes that could be directly compared across the monkeys. The maximum stimulus amplitude used in the experiment for each monkey was selected to produce an accommodative response amplitude that was a multiple of 1 D. The necessary stimulus amplitudes were calculated by fitting polynomials of third order or higher to the static Hartinger stimulus–response curves (Fig. 1A). From these functions, the stimulus amplitudes necessary to produce the desired accommodative amplitudes were selected. In this way, the stimulus amplitudes producing accommodative responses from 1 D in 1-D steps up to the maximum response amplitude available were determined for each monkey. The maximum stimulus amplitude in each experiment was selected in such a way that the response amplitude was below the absolute maximum available to the monkey, to avoid supramaximum stimulation and ensure that all stimulations were in the linear range of the peak velocity versus amplitude relation.
Figure 1.
(A) Example of a stimulus–response curve (monkey 96) with the polynomial function (equation: y = 5.0560 × 10−17x6 − 2.3096 × 10−13x5 + 3.8076 × 10−10x4 − 2.7989 × 10−7x3 + 9.4723 × 10−5x2 − 1.0101 × 10−2x + 7.0795 × 10−2; r2 = 0.996) used to calculate stimulus amplitudes for accommodative responses in 1-D steps (gray dashed lines originating on the y-axis). (B) Example of a 6-D, 4-second step stimulus and the responses as a function of time. (C) Example of a 3-D, 6-D, 3-D, three-step stimulus and the responses as a function of time. (D) Example of a staircase stimulus with five successive incrementing and five successive decrementing 1-D steps and the responses as a function of time. In each graph, the stimulus is shown as a solid gray line. On the right y-axis, the corresponding stimulus amplitudes calculated from the polynomial function in (A) are shown. Dashed lines: five individual, consecutive responses. Solid line: the average of these five responses. The individual responses show various amplitudes and the average falls close to the intended amplitude.
Design of the Stimulus Shapes.
Stimuli were constructed to systematically alter the starting point, the ending point, and the amplitudes of the accommodative and disaccommodative responses. These stimuli were designed to produce accommodative responses in 1-D steps from the unaccommodated resting state (baseline) to maximum accommodation and disaccommodative responses from maximum accommodation back to baseline in 1-D steps. Three different stimulus shapes were used: (1) simple step stimuli, (2) three-step stimuli, and (3) staircase stimuli. Each of the constructed stimuli was delivered five times in succession, each with a 4-second interstimulus interval, to obtain five similar responses that could be averaged (Figs. 1B–D). The details and purposes of the three different stimulus shapes are as follows.
Simple Step Stimuli.
Accommodation was first stimulated with 4-second stimulus trains from baseline to a stimulus amplitude known to produce a response in 1-D multiples up to the closest 1-D multiple of the maximum response amplitude and then back to baseline. For example, if the monkey had a maximum of 4.6 D of accommodation, these stimuli were designed to produce responses (in diopters) of: 0-1-0; 0-2-0; 0-3-0; and 0-4-0. Figure 1B shows an example of the simple step stimulus 0-6-0, the five individual responses, and the averaged response.
Three-Step Stimuli.
Next a stimulus train consisting of three steps, each with a duration of 4 seconds was delivered for 12 seconds. In step 1, accommodation was stimulated from baseline to a preselected amplitude between 1 D and 1 D less than the maximum amplitude in 1-D multiples. Step 2 was a stimulus amplitude to produce the closest 1-D multiple of the maximum response amplitude. In step 3, the stimulus amplitude returned to the same level as in step 1. After this, the stimulus was terminated and accommodation returned to baseline. These three-step, 12-second-duration stimulus trains were repeated five times with 4-second interstimulus intervals between each train. In each subsequent stimulus train, the amplitude of the lower step was increased by 1 D compared with the previous train until the lower step reached an amplitude of 1 D less than the maximum amplitude. For example, if the monkey had a maximum of 4.6 D of accommodation, these stimuli were designed to produce responses (in diopters) of 0-1-4-1-0; 0-2-4-2-0; and 0-3-4-3-0. Figure 1C shows an example of the 3-step stimulus 0-3-6-3-0, the five individual responses, and the averaged response.
Staircase Stimuli.
The final stimulus train in each experiment consisted of staircase stimulations with each subsequent 4-second stimulus step increasing from 0 up to the closest 1-D multiple of the maximum response amplitude in 1-D steps and then similarly decreasing to 0. The number of steps and the overall duration of this stimulus train depended on the maximum accommodative amplitude of each monkey. For example, if the monkey had a maximum of 4.6 D of accommodation, these stimuli were designed to produce responses (in diopters) of 0-1-2-3-4-3-2-1-0. This stimulus would last 28 seconds (seven steps × 4 seconds/step). Figure 1D shows an example of the staircase stimulus from 0 to 5 D in 1-D steps, the five individual responses and the averaged response.
The dynamics of accommodative and disaccommodative responses were analyzed for three conditions, as in a prior study on human subjects.3 Each condition considered both accommodative and disaccommodative responses. These three conditions were a fixed far condition, a fixed near condition, and a fixed-amplitude condition.
Fixed Far Condition
For accommodation, the fixed far condition considered all the accommodative responses that started from the far point (unaccommodated rest state). For disaccommodation, the fixed far condition considered and all the disaccommodative responses that ended at the far point (unaccommodated rest state). Far (the unaccommodated resting state) was always fixed in this condition.
Fixed Near Condition
For accommodation, the fixed near condition considered all accommodative responses to step stimulations starting from various far positions that ended at maximum accommodation. For disaccommodation, the fixed near condition considered all disaccommodative responses that started from maximum accommodation and ended at various far positions. Near (maximum accommodation) was always fixed in this condition.
Fixed Amplitude Condition
The fixed amplitude condition considered all accommodative and disaccommodative responses intended to be of 1 D. Amplitude was always fixed (at the intended 1 D) in this condition. These responses were all of a similar (1 D) amplitude, but to and from different starting and ending positions.
Any and all accommodative and disaccommodative responses that met these criteria were considered in each condition from all the different stimulus shapes delivered.
Data Analysis
The data were analyzed by using custom macros written in two commercial programs (Excel; Microsoft, Redmond, WA, and MatLab; The MathWorks, Natick, MA). The dynamic lens thickness measurements were converted into refraction values with the polynomial calibration functions described earlier. The 100-Hz data were smoothed using a running average over 10 data points (a 100-ms interval). The accommodative responses were plotted against time. The baseline refraction before each stimulus was determined by calculating the average refraction of 20 data points before stimulus onset. The refraction measurements were converted into accommodation by subtracting the refraction at each time point from this baseline refraction. Because of limitations of the stimulator, it was not possible to record to the output data file the exact time point of onset or termination of the stimulus steps starting from or ending at points other than 0. These time points would normally provide a reference to allow the preceding data to be averaged to calculate the response amplitude. Therefore, accommodative response amplitudes from starting points higher than baseline were calculated from the 20 data points immediately preceding each accommodative step response. For disaccommodation, the amplitude of each step was similarly calculated from the 20 data points immediately preceding each disaccommodative step response. Velocity of accommodation was calculated as the rate of change of the accommodative/disaccommodative response using a two-point difference algorithm on the smoothed data. The beginning of a response was identified as the first of 15 consecutive sample values of an accommodative/disaccommodative velocity of 0.5 D/s−1 or more. This point was confirmed by visual inspection of the data from each response. For each of the three conditions, peak velocity was plotted as a function of response amplitude and starting point.
Acceleration profiles were also calculated by averaging five consecutive responses to the same stimulus. The velocity profile from the average response was again differentiated using the two-point difference algorithm to obtain an acceleration profile.
For both velocity and acceleration profiles, a dynamic signal-to-noise ratio was computed by dividing the velocity and acceleration by the root mean square of 100 data points starting 300 ms after the end of the response when no stimulus was present. Peak velocity and peak acceleration values with a signal-to-noise ratio lower than 5 were excluded from subsequent analyses.
Statistical analysis was performed for each condition by using multiple linear regression with peak velocity and acceleration as dependent variables and accommodative amplitude and as the numerical independent variable. The binary variable younger/older monkey was dummy coded with 0 for younger monkeys and 1 for older monkeys. An interaction term was included in each model to estimate the effect of age on the slope of the relationship between accommodative amplitude and peak velocity or peak acceleration, respectively.
Results
Static Measurements
The maximum EW-stimulated accommodative amplitudes and standard deviations as measured with the Hartinger refractometer and calculated for the CUB data for each experiment are shown in Table 1. Standard deviations of response amplitudes achieved by trains of five consecutive equal stimuli ranged from 0.04 to 1.00 D in the older monkeys and from 0.04 to 1.77 D in the younger monkeys. The two younger monkeys had significantly higher accommodative amplitudes than did the two older monkeys. The r2 values for the linear calibration functions used to convert lens thickness into refraction ranged from 0.964 to 0.982.
Table 1.
Maximum Accommodative Amplitudes Measured with the Hartinger Coincidence Refractometer and with Continuous Ultrasound Biometry (CUB) Converted into Diopters
| Experiment | 34 OS | 96 OS | 54(1) OS | 54(2) OS | 111 OS |
|---|---|---|---|---|---|
| Age of monkey, y | 15.0 | 16.3 | 8.0 | 8.9 | 6.8 |
| Maximum accommodative amplitude, (D) Hartinger | 5.25 ± 0.00 | 6.33 ± 0.14 | 10.67 ± 0.00 | 9.83 ± 0.14 | 8.67 ± 0.14 |
| Maximum accommodative amplitude, (D) CUB | 4.68 | 6.37 | 9.46 | 9.33 | 11.59 |
The static Hartinger measurements are the mean values of the last three of five consecutive stimulus trains with the same stimulus amplitude. The CUB measurements are the maximum values from the running-averaged data converted into diopters using the calibration procedure described.
Dynamic Measurements: Amplitudes and Starting Points
Intended versus Achieved Amplitudes.
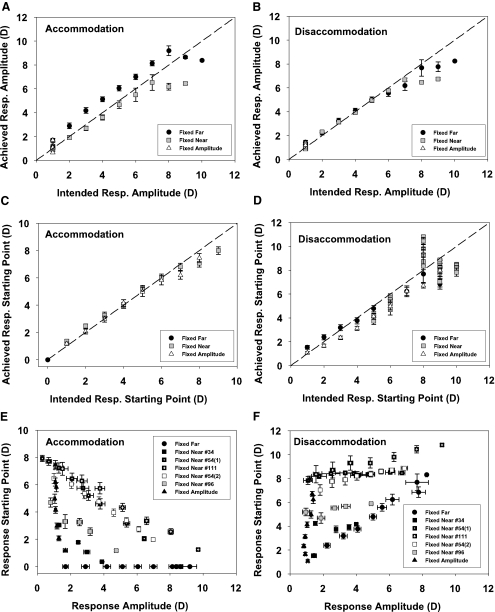
The experiments, as performed, successfully achieved the response amplitudes and response starting points intended. As there were no differences between the older and younger monkeys in this regard, the data from both groups were pooled. The averages of the accommodative (Fig. 2A) and disaccommodative (Fig. 2B) response amplitudes achieved from all experiments for each condition are plotted against the intended response amplitudes together with the 1:1 line. Except for some of the highest response amplitudes of the younger monkeys, the accommodative and disaccommodative responses for the fixed far and fixed near conditions fall close to the 1:1 lines. In the fixed-amplitude condition with an intended amplitude of 1 D, the average accommodative amplitude achieved was 1.16 ± 0.40 D with a range from 0.30 to 2.68 D and the average disaccommodative amplitude was 1.05 ± 0.30 D ranging from 0.35 to 1.87 D.
Figure 2.
Relationships between intended and achieved amplitudes of accommodation (A) and disaccommodation (B) and intended and achieved starting points of accommodation (C) and disaccommodation (D) for the three conditions are shown. The error bars, ±SEM (E) and (F) show the accommodative and disaccommodative amplitudes plotted against the starting point. For the fixed near condition, the experiments are plotted separately, because a different near point was used in each experiment. The average response amplitude of accommodation and disaccommodation in the fixed-amplitude condition was about the same for proximal and distal starting points.
Intended versus Achieved Starting Points.
Achieved response starting points are plotted together with the intended starting points for accommodation (Fig. 2C) and disaccommodation (Fig. 2D). In the fixed far condition, all accommodative responses were calculated to start from the local unaccommodated baseline and all disaccommodative responses ended at the unaccommodated baseline, so for older and younger monkeys, the starting point of the accommodative responses and the ending points of the disaccommodative responses in this condition was 0 by definition. In the fixed near and fixed-amplitude conditions, the averaged accommodative response starting points fell close to the 1:1 line. The achieved average disaccommodative starting points of the fixed far and fixed-amplitude conditions were close to that intended. In the fixed near condition, the intended starting point of the disaccommodative response was the nearest 1-D multiple below the maximum accommodative amplitude. These intended amplitudes (monkey) were 5 D (34), 6 D (96), 10 D (54[1]), 9 D (54 [2]), and 8 D (111). The achieved mean starting points ± SD (monkey) were 4.00 ± 0.23 D (34), 5.39 ± 0.47 D (69), 8.32 ± 0.21 D (54 [1]), 8.17 ± 0.60 D (54 [2]), and 9.52 ± 0.89 D (111). The range of differences between achieved and intended starting points was −3.00 and +3.25 D with a mean (±SD) difference of −0.54 ± 1.41 D.
Although the average achieved amplitudes matched the intended amplitudes fairly well, both accommodative and disaccommodative responses showed overshoots of variable size (Figs. 1B, 1C).
Amplitude versus Starting Point.
For accommodation, the fixed near condition would require that as response amplitude increased, starting point decreased. Younger monkeys with higher accommodative amplitudes would have higher starting points than the older monkeys. For the fixed-amplitude condition, there should be a range of starting points for fixed-amplitude responses of 1 D, with the younger monkeys having a larger range of starting points. For the fixed far condition, the starting point would always be 0, so a range of amplitudes with 0 D starting points should be seen. In the starting point versus amplitude graphs, the response data for each monkey should appear as a similar, right angled, isosceles triangle with the right angle anchored at the bottom left coordinate (1,0), with smaller triangles for older monkeys, the general pattern in Figure 2E.
For disaccommodation, the fixed near condition should have a range of response amplitudes from fixed near starting points. The younger monkeys with higher amplitudes would have higher starting points than the older monkeys. For the fixed-amplitude condition, there should be a range of starting points for fixed-amplitude responses of 1 D, with the younger monkeys having a larger range of starting points. For the fixed far condition, as response amplitude increases, starting point should increase with the younger monkeys having a wider range of response amplitudes and starting points than the older monkeys. In the starting point versus amplitude graphs, the response data for each monkey should appear as a similar, right angled, isosceles triangle with the right angle at top left but the triangles anchored at bottom left coordinate (1,1), with smaller triangles for older monkeys, the general pattern in Figure 2F.
Peak Velocity
The results of the multiple regression of peak velocity against amplitude and age for the cumulated data from each condition are shown in Table 2.
Table 2.
Results of the Multiple Regression Analysis of Peak Velocity versus Amplitude and Age
| R2 (Adjusted) | Predictor | b | β | P |
|---|---|---|---|---|
| Fixed far | ||||
| Accommodation | ||||
| 0.85 | A | 2.289 | 0.890 | <0.001 |
| Z | −0.665 | 0.042 | 0.414 | |
| A*Z | −0.142 | 0.035 | 0.456 | |
| Disaccommodation | ||||
| 0.88 | A | 6.795 | 0.964 | <0.001 |
| Z | 6.145 | 0.145 | <0.001 | |
| A*Z | −2.182 | −0.186 | <0.001 | |
| Fixed Near | ||||
| Accommodation | ||||
| 0.76 | A | 2.296 | 0.813 | <0.001 |
| Z | −1.378 | −0.104 | 0.322 | |
| A*Z | −0.201 | −0.046 | 0.639 | |
| Disaccommodation | ||||
| 0.89 | A | 6.120 | 0.930 | <0.001 |
| Z | 3.456 | 0.118 | 0.086 | |
| A*Z | −2.209 | −0.241 | <0.001 | |
| Fixed Amplitude | ||||
| Accommodation | ||||
| 0.39 | A | 2.359 | 0.582 | <0.001 |
| Z | 0.350 | 0.109 | 0.567 | |
| A*Z | −1.186 | −0.435 | 0.023 | |
| Disaccommodation | ||||
| 0.57 | A | 5.763 | 0.766 | <0.001 |
| Z | 1.720 | 0.367 | 0.063 | |
| A*Z | −3.1329 | −0.721 | <0.001 |
R2, coefficient of determination, adjusted for number of predictors; b, unstandardized partial regression coefficient; β, standardized partial regression coefficient; A, amplitude of accommodation/disaccommodation; Z, dummy variable for age group. A*Z, interaction term.
Fixed Far Condition
Peak Velocity in Relation to Amplitude.
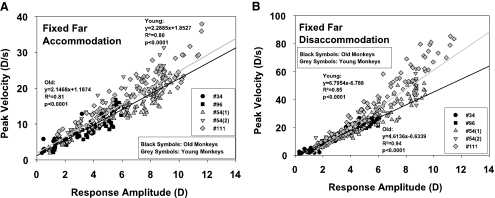
For the fixed far condition, the peak velocity of accommodation in each experiment showed an increasing linear relationship (Fig. 3A) to the accommodative response amplitude calculated from the local baseline of each response.
Figure 3.
Peak velocity plotted against the response amplitude of accommodation (A) and disaccommodation (B) in the fixed far condition. The regression lines have been extrapolated to the edges of the graphs for better visibility.
The peak velocity of disaccommodation showed a linearly increasing relationship to the disaccommodative response amplitude (Fig. 3B).
The multiple regressions showed no significant influence of age group on the peak velocity of accommodation, but a significant decreasing influence of the age group on the regression slope for disaccommodation (Table 2, Z term).
Peak Velocity in Relation to Starting Point.
In the fixed far condition, since accommodation always started from 0 and returned to 0, there was no relationship between peak velocity and starting point.
Fixed Near Condition
Peak Velocity in Relation to Amplitude.
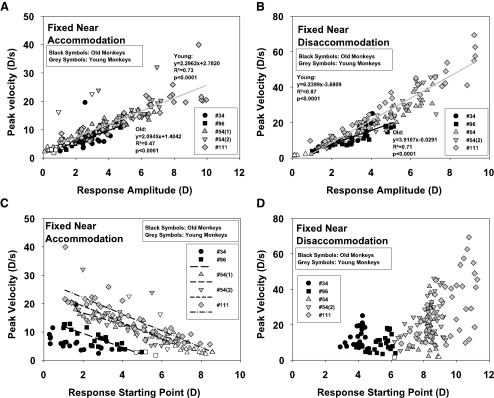
For the fixed near condition, the peak velocity of accommodation showed an increasing linear relationship (Fig. 4A) to the accommodative response amplitude from the non-zero starting points. The peak velocity of disaccommodation (Fig. 4B) likewise showed an increasing linear relationship with response amplitude in older and younger monkeys.
Figure 4.
Peak velocity of the accommodative and disaccommodative response versus amplitude (A, B) and starting point (C, D) of the response in the fixed near condition. For accommodation, the peak velocity showed a decrease with increasing starting point (C). The intercepts of the linear regressions for the different experiments varied according to the maximum response amplitude available to each monkey (regression equations: monkey 34: no significant fit; 96: y = −2.4735x + 14.3841, r2 = 0.75, P < 0.0001; 54(1): y = −2.0138x + 20.6698, r2 = 0.85, P < 0.0001; 54(2): y = −2.8977x + 24.9081, r 2 =0.57, P < 0.0001; 111: y = −2.9271x + 27.9468, r 2 = 0.73, P < 0.0001). For disaccommodation (D), peak velocity is not related to starting point of the response. The peak velocities are separated along the various starting points of the experiments.
The multiple regressions showed no significant influence of age group on the peak velocity for accommodation and disaccommodation (Table 2).
Peak Velocity in Relation to Starting Point.
For the fixed near condition, when plotted against the starting point of the accommodative response, the peak velocity showed a decreasing linear relationship for each monkey (Fig. 4C) that depends on the maximum relative accommodative amplitude available to each monkey. The data for the older monkeys is effectively shifted down and to the left because the peak velocities and starting points for the older monkeys were less than those for the younger monkeys, owing to the lower response amplitudes of the older monkeys.
For the fixed near condition, the peak velocity of disaccommodation showed no clear relationship to the starting point (Fig. 4D). The response starting points depend on the amplitude available to each monkey. For each disaccommodative starting point, there is a range of response amplitudes available and hence a range of possible peak velocities. The highest peak velocities were reached in the monkeys with the highest response amplitudes.
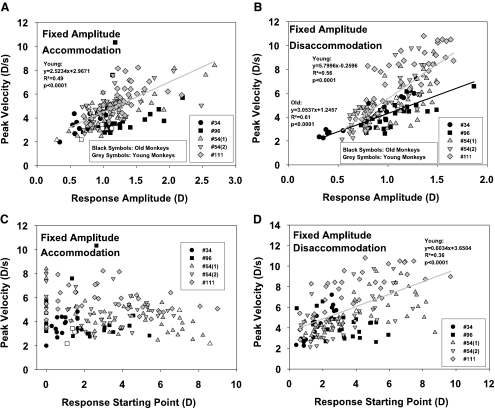
Fixed Amplitude Condition
Peak Velocity in Relation to Amplitude.
In the fixed-amplitude condition, the accommodative response amplitudes were clustered around the intended 1-D response amplitude (Fig. 5A). The peak velocity measurements were likewise clustered around a velocity value of approximately 4 D/s. In the old monkeys in this condition, peak velocity did not show a significant relationship to response amplitude. The mean peak velocity in the older monkeys was 3.90 D/s. In the younger monkeys in this condition, peak velocity showed an increasing linear relationship to response amplitude (Table 2). The peak velocity from the regression line for an accommodative response of 1 D was 5.49 D/s. The relation between amplitude and starting point in this fixed-amplitude condition was similar to that found for the other two conditions. The multiple regression showed no significant influence of age group on the relationship between accommodative amplitude and peak velocity (Table 2).
Figure 5.
Peak velocity of accommodation and disaccommodation plotted against amplitude (A, B) and starting point (C, D) of the response in the fixed-amplitude condition.
In the fixed-amplitude condition, disaccommodative peak velocity was linearly related to the accommodative response amplitude for the older and for the younger monkeys (Fig. 5B). There was greater variation in the disaccommodative than in the accommodative amplitudes and peak velocities. The multiple regressions showed a significant influence of age group on the slope and intercept of the relationship between disaccommodative amplitude and peak velocity of disaccommodation in the fixed far and fixed near condition. In the fixed-amplitude condition, there was a significant influence on the slope but not the intercept (Table 2).
Peak Velocity in Relation to Starting Point.
In the fixed-amplitude condition, there was no relationship between peak velocity and starting point for accommodation in older and younger monkeys (Fig. 5C). The disaccommodation data (Fig. 5D) showed a significant linear relationship of peak velocity to the starting point of the response for the younger monkeys. However, there was a linear increase in the disaccommodative response amplitude of the younger monkeys with starting point in this condition (data not shown; regression equation: 0.6957x + 0.0983, r2 = 0.57, P < 0.0001), and this explains why peak velocity increased with starting point. When comparing only responses with roughly the same amplitude (e.g., all responses between 1 and 2 D), there was no dependence of peak velocity on the starting point. The ratio of peak velocity/amplitude was 3.73 ± 1.47 seconds−1 in the older monkeys and 4.34 ± 1.05 seconds−1 in the younger monkeys for accommodative responses. For disaccommodative responses, it was 4.22 ± 1.27 seconds−1 in the older monkeys and 5.45 ± 1.51 seconds−1 in the younger monkeys. For both accommodation and disaccommodation there was no significant correlation of this ratio to the response starting point.
Excluded Responses.
In the analysis of amplitude versus peak velocity, 17 fixed near responses and 2 fixed-amplitude responses of the younger monkeys and 3 fixed near responses and 3 fixed-amplitude responses of the older monkeys were excluded because of a low signal-to-noise ratio. These data points are not shown in the graphs.
Peak Acceleration.
Generally, peak acceleration showed patterns similar to those of peak velocity. There was a linear increase of peak acceleration with the response amplitude of accommodation and disaccommodation in all three conditions. Linear regression showed significant differences between older and younger monkeys in accommodation or disaccommodation (data not shown).
The peak acceleration data showed a pattern very similar to that of the peak velocity data. In the fixed near condition, the plots of starting point versus accommodative response amplitude showed a linear decrease of peak acceleration with the amplitude (slope: −20.4878 seconds−2, r 2 =0.66, P = 0.0139 for the older monkeys; −26.5919 seconds−2, r 2 = 0.84, P < 0.0001 for the younger monkeys; graphs not shown). The regression line of the older monkeys was shifted down and to the left because of the lower maximum accommodation amplitudes. The slopes of the regression lines for accommodation for older and younger monkeys were not significantly different (P = 0.4473). As in the peak velocity plot, the accommodative and disaccommodative peak acceleration linearly increased with response amplitude. There was no significant difference between older and younger monkeys.
The regression analyses for both peak velocity and peak acceleration were repeated with the results from all conditions pooled together. This procedure confirmed the result of the separate analysis (Table 3).
Table 3.
Results of the Multiple Regression Analysis of Peak Velocity versus Amplitude and Age for All Conditions Pooled
| R2 (Adjusted) | Predictor | b | β | P |
|---|---|---|---|---|
| Accommodation | ||||
| 0.88 | A | 2.249 | 0.914 | <0.001 |
| Z | −0.899 | −0.058 | 0.020 | |
| A*Z | −0.168 | 0.034 | 0.155 | |
| Disaccommodation | ||||
| 0.91 | A | 6.142 | 0.960 | <0.001 |
| Z | 2.132 | 0.057 | 0.006 | |
| A*Z | −1.728 | −0.252 | <0.001 |
Abbreviations are as defined in Table 2.
Discussion
Amplitudes and Starting Points of the Responses
At rest, under propofol anesthesia, the eyes are essentially in an unaccommodated state. A previous study in which the same anesthesia was used showed that atropine cycloplegia resulted in a nonsignificant hyperopic shift in refraction in the eyes of iridectomized monkeys.42 The unaccommodated state under propofol anesthesia is similar to that found with other anesthesia regimens in monkeys.43,44
Generally, in all three conditions, the average intended and achieved response amplitudes and starting points corresponded well. However, there was variability in the responses of a single monkey to the same stimulus amplitude, even within the same experiment. There was also a difference between the older and the younger monkeys, with the younger monkeys tending to achieve higher amplitudes than intended and the older monkeys tending to achieve lower amplitudes than intended, probably due to variability in the responses from relatively long-duration experiments, as the variability of the responses with time and stimulus number occurred in the raw lens thickness measurement data.
In both accommodative and disaccommodative responses, initial overshoots were visible (Figs. 1B–D). These cannot be attributed to a blur-dependent feedback mechanism, as all visual feedback was excluded by general anesthesia. At present, the reason for this phenomenon is not entirely clear. Recently, anatomic evidence for proprioceptive nerve endings in the ciliary muscle has been identified.45 It cannot be ruled out that biomechanical neural feedback regulation mechanisms operating at the ciliary muscular level or even convergence-related feedback from the horizontal rectus muscles are responsible for this behavior, although the inconsistent nature of these overshoot characteristics makes this possibility seem unlikely.
Peak Velocity of Accommodation and Disaccommodation
As described in prior studies in anesthetized monkeys,6,33 an increasing linear relationship exists between accommodative and disaccommodative response amplitude and peak velocity. In the results of our study, for accommodation, this relationship was not significantly different in the older and younger monkeys in all three conditions. For disaccommodation, in the fixed far and fixed-amplitude conditions, the peak velocity showed a decrease with age when responses over the whole accommodative range of each monkey were analyzed. The youngest monkey in the study (111) showed the steepest slope in the main sequence relationship on all conditions. To confirm the presence of age-related differences in the disaccommodative main sequences under all conditions, experiments with larger sample sizes may be needed. However, this may be difficult to achieve because of the complex nature of the experiment and the limited number of available animals.
Previous studies in anesthetized monkeys indicate that the peak velocities of accommodation and disaccommodation are related to the absolute response amplitude rather than to the fraction of the maximum response amplitude. It was also shown that the peak velocity of accommodation in EW-stimulated monkeys did not depend on the amplitude of the stimulus input but on the absolute accommodative response amplitude. Thus, different stimulus amplitudes may be necessary in different monkeys to reach the same response amplitude. The responses with the same amplitude will nevertheless have the same peak velocity.6,33,46 An analysis of the stimulus amplitudes used in young and old monkeys in this study reveals that, on average, a higher stimulus amplitude was necessary in the older monkeys than in the younger monkeys to reach the same amplitude/peak velocity of the accommodative response (data not shown). Therefore, it is possible that the stimulus paradigms used in this study concealed additional differences in accommodative dynamics between the young and old monkeys. However, the relationship between accommodative response amplitude and peak velocity was the same in younger and older monkeys, indicating that both are influenced to the same extent by the stimulus amplitude. Furthermore, the peak velocity was the same for step responses with the same amplitude and different starting points, which indicates that it is in fact determined by the response amplitude and not by the stimulus amplitude. The peak velocity and acceleration of the disaccommodative responses were unaffected by the stimulus amplitude as the disaccommodative response occurred passively with cessation of the stimulus in the anesthetized monkeys.
It has long been recognized that the human lens becomes stiffer with increasing age7–10,21,47–50 and that this stiffening is a cause of presbyopia.7,8,10,51 Although similar in vitro stiffness studies have not been performed on monkey lenses, reduced accommodative lens movements in aging monkeys have been demonstrated in vivo.13,14 Further, histologic studies show a loss of compliance of the posterior attachment of the ciliary muscle in older monkeys.11,52 The results shown in this study are in accordance with these observations. In anesthetized monkeys, on cessation of the stimulus to the EW nucleus, the lens is passively pulled into an unaccommodated state via the elasticity of the posterior attachments of the ciliary muscle, the posterior zonular fibers and increased tension on the zonular fibers around the lens equator, and the molding forces of the capsule.32 It is possible that disaccommodation is slower in older monkeys than in younger monkeys, and this would suggest an age-related loss of compliance and/or elasticity of the tissues involved in this process. Dynamics of accommodation were not found to be affected by age in the present study. Peak velocity of accommodation continues to increase in young and older monkeys with supramaximum EW stimuli (i.e., a stimulus greater than that required to achieve maximum accommodation) above the peak velocity achieved for the maximum response amplitude but without any further increase in acc ommodative response amplitude.26,46 Thus, EW-stimulated accommodation may be subject to lower-degree rate limitations than disaccommodation, and passive disaccommodation in anesthetized monkeys could be a more sensitive indicator of age-dependent changes in the accommodative plant.
An earlier study described an age-dependent decrease in the velocity of ciliary body and lens movement during accommodation and disaccommodation in rhesus monkeys.53 In that study, however, instead of measuring peak velocity, the velocity during the approximately linear phase of accommodation and disaccommodation was determined by fitting of a linear function. The same supramaximum stimulus amplitude was used with each monkey, thereby avoiding potential confounds associated with different accommodative response amplitudes in the younger and older monkeys.
As in previous studies of EW-stimulated accommodative dynamics in anesthetized rhesus monkeys,6,33,35,42,46 in this present study a strong linear dependence of the accommodative and disaccommodative peak velocity on the response amplitude was found. There was no such relationship to the response starting point. In a recent study in which accommodation was stimulated in rhesus monkeys after shifting the response starting point and the available amplitude by treatment with either atropine or pilocarpine,42 the peak velocity of disaccommodation was independent of the starting point, and the main sequence of accommodation and disaccommodation was unchanged after instillation of atropine or pilocarpine. In contrast to the results presented herein, however, accommodative responses of the same amplitude showed a decrease in peak velocity with the response starting point after treatment with pilocarpine (i.e., the responses became slower in the more proximal range). In the present study, the response starting point of accommodation was altered only by EW stimulation and not by pharmacologic action on the receptor and did not influence the accommodative response dynamics. Apparently, with pharmacologic shifting of the starting point, the same stimulus current produces the same response amplitude, regardless of the more distant or proximal range, and this response amplitude is added to the baseline refractive shift caused by the pharmacologic treatment. If the starting point is altered by EW stimulation in a first step, the amplitude of the second step–response may be the same but with a higher stimulus pulse. So far, the cause of this difference between pharmacologically and EW-stimulated accommodation is not known.
Investigations on awake, behaving monkeys have shown an increase in firing frequency of the accommodation-related neurons in the EW nucleus during accommodation.36,37 In this study, accommodation was stimulated using different stimulus amplitudes with a constant pulse frequency rather than different frequencies. Varying the accommodative amplitude using different stimulus frequencies with a constant amplitude results in accommodative dynamics that are no different from the amplitude-dependent responses presented here (Wendt M, et al. IOVS 2006;47:ARVO E-Abstract 5850).
Because of the number of different stimulation conditions used in this study, the duration of the experiments was up to 3 hours. It is possible that during this period, changes in the accommodative response occurred. Prior experiments with repeated accommodation in conscious humans and with EW-stimulated accommodation in anesthetized monkeys show no systematic effects of fatigue on accommodative amplitude and accommodative dynamics.34 In the present study, the effective time of consecutive stimulation was shorter, and the resting intervals between stimulations were longer. Therefore, it is unlikely that accommodative fatigue would have affected the results of this study. The accommodative response may be affected over time by subtle physiological changes, such as reduced blood pressure or depressed heart rate due to prolonged anesthesia.44,54 However, vital signs monitoring performed during the experiments provides no indication of this effect.
The sequence of stimulus types (simple step stimuli, three-step stimuli, and staircase stimuli) was not randomized between the different monkeys. However, the lack of randomization and the long duration of the experiments are unlikely to have any systematic influence on the results presented. The analysis of each condition (fixed far, fixed near, and fixed amplitude) includes responses recorded from different stimulus types and from different times during each experiment. For example, fixed-amplitude responses were analyzed from all three stimulus types.
In human studies of the influence of amplitude and starting point on accommodative dynamics,3 fixed-amplitude accommodative responses showed a lag at higher stimulus levels and higher amplitude responses to more proximal than to more distal stimuli. In this study in anesthetized monkeys, there was no consistent relationship between starting point and accommodative amplitude in the fixed-amplitude condition. Although for the young monkeys the accommodative amplitude increased slightly with a higher starting point, on average, EW stimulation with the same stimulus amplitude reliably produced the same response amplitude, regardless of the starting point. This result suggests that biomechanical factors that could be linked to the starting configuration of the accommodative structures are not the cause of this variability in conscious human subjects. The lags and differences in accommodative response amplitude between distal and proximal responses in human subjects may be caused by neural factors rather than by biomechanical factors.
The results in our study of EW-stimulated accommodation in anesthetized monkeys are strikingly different from those obtained in studies in conscious human subjects.3,4,18,55 In humans, peak velocity of accommodation increase with the accommodative amplitude but saturate at amplitudes higher than approximately 3D,18,55 and this saturation occurs at lower response amplitudes with increasing age.18 For disaccommodation, peak velocity correlates linearly with amplitude.18,55 Further, in humans the accommodative and disaccommodative dynamics correlate strongly with response starting point and peak velocity of accommodation, showing very different rates of increase with amplitude in the fixed far and fixed-amplitude conditions and a decrease with amplitude in the fixed near condition.3 In humans, peak velocity of disaccommodation increases at different rates for the three conditions, and in the fixed near and fixed-amplitude conditions, accommodative peak velocity increases with starting point (Fig. 4D in Kasthurirangan and Glasser3). For the fixed near condition, response amplitude decreases with increasing starting point, but peak velocity increases. For the fixed-amplitude condition, more proximal 1-D responses are faster than more distal 1-D responses. The data shown here from EW-stimulated accommodation in anesthetized monkeys is strikingly different. Peak velocity for both accommodation and disaccommodation increased linearly with amplitude over the full response range available. In the fixed-amplitude condition, the monkeys showed no change in peak velocity with increasing proximity for the 1-D response amplitudes. Further, in the fixed near condition, the monkeys showed a decrease in peak velocity with increasing starting point. These results suggest that the accommodative plant in anesthetized monkeys is not influenced by the starting point's proximity and that peak velocity is entirely dictated by the response amplitude. It has been suggested that the mechanical starting configuration of the accommodative plant could influence the accommodative dynamics.3 The results from the present study in anesthetized monkeys show that this was not so. However, in conscious humans, there may be saturation of the neural control of accommodation at higher amplitudes25 and evidently the neural control or some other component of conscious accommodation influences the peak velocity.4,56 With EW-stimulated accommodation in anesthetized rhesus monkeys, visual feedback is eliminated and the number of neurons firing for an accommodative response is determined only by the stimulus to the midbrain. Disaccommodation in anesthetized monkeys is a consequence of cessation of the stimulus to the brain and consequently cessation of any neural activity and the purely passive process of the inherent elasticity of the posterior attachment of the ciliary muscle52 and the posterior zonular fibers,32 pulling the lens into an unaccommodated state. The differences in results for disaccommodation between anesthetized monkeys and conscious humans could indicate that the dependency of the disaccommodative peak velocity on the starting point of the response in conscious human subjects is due to conscious neural feedback rather than to the mechanics of the accommodative plant.4,56 This possibility may have clinical implications for further understanding of the etiology and development of presbyopia, although it is difficult to draw direct clinical conclusions from these results.
A recent study of age-related changes of the dynamics of accommodation in humans showed a decrease in the peak velocity of accommodation with age but no change in the peak velocity of disaccommodation.18 This difference in the findings between conscious humans and anesthetized monkeys is most likely explained by the fact that the step stimulus to the midbrain is an artificial and somewhat nonphysiological stimulus. Theories about the neurophysiology of accommodation indicate that accommodation and disaccommodation in conscious humans is driven by a pulse-step stimulus.57 The step stimulus train of pulses delivered via the EW electrode could overwhelm subtle age-related differences that would be apparent with a more physiological stimulus. To clarify this issue and to detect possible interspecies differences, it would be interesting to conduct the same kind of experiment in awake, behaving monkeys. Conversely, the existing models of dual-mode or pulse-step neural control of human accommodation could be applied in anesthetized monkeys to find out whether the responses correspond to the predictions of the model. A preliminary study investigating pulse-step stimuli in anesthetized monkeys has been conducted by the authors. The results suggest that it may be possible to modify the dynamics of the accommodative response, independent of the response amplitude, by using a pulse-step stimulation paradigm (Baumeister M, et al. IOVS 2008;49:ARVO E-Abstract 4561).
Peak Acceleration of Accommodation and Disaccommodation
Previous studies of second-order dynamics of accommodation and disaccommodation in humans found that acceleration properties are different between accommodation and disaccommodation.4,56 For both accommodation and disaccommodation, acceleration was found to be invariant of the stimulus amplitude. However, for accommodation, the time to peak velocity increased with response amplitude and for disaccommodation, acceleration increased, independent of the response amplitude, with increasing starting point of the response. Schor and Bharadwaj57 suggested a model based on an independent control of first- and second-order dynamics of accommodation in which peak velocity of accommodation is increased by holding an amplitude-invariant peak acceleration for a longer duration. In the present study in anesthetized monkeys, both peak velocity and peak acceleration increased linearly with response amplitude and were independent of the response starting point. This finding was true of accommodation and disaccommodation in all conditions. The time to peak velocity likewise showed a slight increase with response amplitude and no dependence on the response starting point. These findings suggest that an independent control of first- and second-order dynamics, which may be present in conscious accommodation to visual stimuli, is not present in EW-stimulated accommodation with a step stimulus and that in this study, first- and second-order dynamics were controlled in the same way by the step stimulus of the EW nucleus.
Acknowledgments
The authors thank Rob van der Heijde for providing the CUB.
Footnotes
Supported in part by National Eye Institute Grant 1 R01 EY014651 (AG) and by DFG Grant BA 3443/1-1 (MB).
Disclosure: M. Baumeister, None; M. Wendt, None; A. Glasser, None
References
- 1. Shirachi D, Liu J, Lee M, Jang J, Wong J, Stark L. Accommodation dynamics I. Range nonlinearity. Am J Optom Physiol Opt. 1978;55:631–641 [DOI] [PubMed] [Google Scholar]
- 2. Beers AP, Van Der Heijde GL. In vivo determination of the biomechanical properties of the component elements of the accommodation mechanism. Vision Res. 1994;34:2897–2905 [DOI] [PubMed] [Google Scholar]
- 3. Kasthurirangan S, Glasser A. Influence of amplitude and starting point on accommodative dynamics in humans. Invest Ophthalmol Vis Sci. 2005;46:3463–3472 [DOI] [PubMed] [Google Scholar]
- 4. Bharadwaj SR, Schor CM. Initial destination of the disaccommodation step response. Vision Res. 2006;46:1959–1972 [DOI] [PubMed] [Google Scholar]
- 5. Fisher RF. The significance of the shape of the lens and capsular energy changes in accommodation. J Physiol. 1969;201:21–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vilupuru AS, Glasser A. Dynamic accommodation in rhesus monkeys. Vision Res. 2002;42:125–141 [DOI] [PubMed] [Google Scholar]
- 7. Glasser A, Campbell MC. Biometric, optical and physical changes in the isolated human crystalline lens with age in relation to presbyopia. Vision Res. 1999;39:1991–2015 [DOI] [PubMed] [Google Scholar]
- 8. Heys KR, Cram SL, Truscott RJ. Massive increase in the stiffness of the human lens nucleus with age: the basis for presbyopia? Mol Vis. 2004;10:956–963 [PubMed] [Google Scholar]
- 9. Weeber HA, Eckert G, Soergel F, Meyer CH, Pechhold W, van der Heijde RG. Dynamic mechanical properties of human lenses. Exp Eye Res. 2005;80:425–434 [DOI] [PubMed] [Google Scholar]
- 10. Weeber HA, Eckert G, Pechhold W, van der Heijde RG. Stiffness gradient in the crystalline lens. Graefes Arch Clin Exp Ophthalmol. 2007;245:1357–1366 [DOI] [PubMed] [Google Scholar]
- 11. Tamm E, Croft MA, Jungkunz W, Lütjen-Drecoll E, Kaufman PL. Age-related loss of ciliary muscle mobility in the rhesus monkey: role of the choroid. Arch Ophthalmol. 1992;110:871–876 [DOI] [PubMed] [Google Scholar]
- 12. Tamm S, Tamm E, Rohen JW. Age-related changes of the human ciliary muscle: a quantitative morphometric study. Mech Ageing Dev. 1992;62:209–221 [DOI] [PubMed] [Google Scholar]
- 13. Croft MA, Glasser A, Heatley G, et al. The zonula, lens, and circumlental space in the normal iridectomized rhesus monkey eye. Invest Ophthalmol Vis Sci. 2006;47:1087–1095 [DOI] [PubMed] [Google Scholar]
- 14. Croft MA, Glasser A, Heatley G, et al. Accommodative ciliary body and lens function in rhesus monkeys, I: normal lens, zonule and ciliary process configuration in the iridectomized eye. Invest Ophthalmol Vis Sci. 2006;47:1076–1086 [DOI] [PubMed] [Google Scholar]
- 15. Croft MA, McDonald JP, Nadkarni NV, Lin TL, Kaufman PL. Age-related changes in centripetal ciliary body movement relative to centripetal lens movement in monkeys. Exp Eye Res. 2009;89:824–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun FC, Stark L, Nguyen A, Wong J, Lakshminarayanan V, Mueller E. Changes in accommodation with age: static and dynamic. Am J Optom Physiol Optics. 1988;65:492–498 [DOI] [PubMed] [Google Scholar]
- 17. Temme LA, Morris A. Speed of accommodation and age. Optom Vis Sci. 1989;66:106–112 [DOI] [PubMed] [Google Scholar]
- 18. Kasthurirangan S, Glasser A. Age related changes in accommodative dynamics in humans. Vision Res. 2006;46:1507–1519 [DOI] [PubMed] [Google Scholar]
- 19. Heron G, Winn B. Binocular accommodation reaction and response times for normal observers. Ophthal Physiol Opt. 1989;9:176–183 [DOI] [PubMed] [Google Scholar]
- 20. Schaeffel F, Wilhelm H, Zrenner E. Inter-individual variability in the dynamics of natural accommodation in humans: relation to age and refractive errors. J Physiol. 1993;461:301–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beers AP, van der Heijde GL. Age-related changes in the accommodation mechanism. Optom Vis Sci. 1996;73:235–242 [DOI] [PubMed] [Google Scholar]
- 22. Heron G, Charman WN, Gray LS. Accommodation dynamics as a function of age. Ophthal Physiol Opt. 2002;22:389–396 [DOI] [PubMed] [Google Scholar]
- 23. Heron G, Charman WN. Accommodation as a function of age and the linearity of the response dynamics. Vision Res. 2004;44:3119–3130 [DOI] [PubMed] [Google Scholar]
- 24. Mordi JA, Ciuffreda KJ. Dynamic aspects of accommodation: age and presbyopia. Vision Res. 2004;44:591–601 [DOI] [PubMed] [Google Scholar]
- 25. Schor CM, Bharadwaj SR. A pulse-step model of accommodation dynamics in the aging eye. Vision Res. 2005;45:1237–1254 [DOI] [PubMed] [Google Scholar]
- 26. Baumeister M, Wendt M, Glasser A. Edinger-Westphal stimulated accommodative dynamics in anesthetized, middle-aged rhesus monkeys. Exp Eye Res. 2008;86:25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaufman PL, Lütjen-Drecoll E. Total iridectomy in the primate in vivo: surgical technique and postoperative anatomy. Invest Ophthalmol. 1975;14:766–771 [PubMed] [Google Scholar]
- 28. Koretz JF, Bertasso AM, Neider MW, True-Gabelt BA, Kaufman PL. Slit-lamp studies of the rhesus monkey eye: II. Changes in crystalline lens shape, thickness and position during accommodation and aging. Exp Eye Res. 1987;45:317–326 [DOI] [PubMed] [Google Scholar]
- 29. Crawford K, Terasawa E, Kaufman PL. Reproducible stimulation of ciliary muscle contraction in the cynomolgus monkey via a permanent indwelling midbrain electrode. Brain Res. 1989;503:265–272 [DOI] [PubMed] [Google Scholar]
- 30. Bito LZ, Kaufman PL, DeRousseau CJ, Koretz J. Presbyopia: an animal model and experimental approaches for the study of the mechanism of accommodation and ocular ageing. Eye. 1987;1:222–230 [DOI] [PubMed] [Google Scholar]
- 31. Crawford KS, Kaufman PL, Bito LZ. The role of the iris in accommodation of rhesus monkeys. Invest Ophthalmol Vis Sci. 1990;31:2185–2190 [PubMed] [Google Scholar]
- 32. Glasser A, Kaufman PL. The mechanism of accommodation in primates. Ophthalmology. 1999;106:863–872 [DOI] [PubMed] [Google Scholar]
- 33. Vilupuru AS, Glasser A. The relationship between refractive and biometric changes during Edinger-Westphal stimulated accommodation in rhesus monkeys. Exp Eye Res. 2005;80:349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vilupuru AS, Kasthurirangan S, Glasser A. Dynamics of accommodative fatigue in rhesus monkeys and humans. Vision Res. 2005;45:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ostrin LA, Glasser A. Edinger-Westphal and pharmacologically stimulated accommodative refractive changes and lens and ciliary process movements in rhesus monkeys. Exp Eye Res. 2007;84:302–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gamlin PD, Zhang Y, Clendaniel RA, Mays LE. Behavior of identified Edinger-Westphal neurons during ocular accommodation. J Neurophysiol. 1994;72:2368–2382 [DOI] [PubMed] [Google Scholar]
- 37. Judge SJ, Cumming BG. Neurons in the monkey midbrain with activity related to vergence eye movement and accommodation. J Neurophysiol. 1986;55:915–930 [DOI] [PubMed] [Google Scholar]
- 38. Wallman J, Adams JI. Developmental aspects of experimental myopia in chicks: susceptibility, recovery and relation to emmetropization. Vision Res. 1987;27:1139–1163 [DOI] [PubMed] [Google Scholar]
- 39. Vilupuru AS, Glasser A. Dynamic accommodative changes in Rhesus monkey eyes assessed with A-scan ultrasound biometry. Optom Vis Sci. 2003;80:383–394 [DOI] [PubMed] [Google Scholar]
- 40. Troilo D, Judge SJ. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus). Vision Res. 1993;33:1311–1324 [DOI] [PubMed] [Google Scholar]
- 41. van der Heijde GL, Weber J. Accommodation used to determine ultrasound velocity in the human lens. Optom Vis Sci. 1989;66:830–833 [DOI] [PubMed] [Google Scholar]
- 42. Ostrin LA, Glasser A. Effects of pharmacologically manipulated amplitude and starting point on edinger-westphal-stimulated accommodative dynamics in rhesus monkeys. Invest Ophthalmol Vis Sci. 2007;48:313–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Westheimer G, Blair SM. Accommodation of the eye during sleep and anesthesia. Vision Res. 1973;13:1035–1040 [DOI] [PubMed] [Google Scholar]
- 44. Crawford K, Gabelt BT, Kaufman PL, Bito LZ. Effects of various anesthetic and autonomic drugs on refraction in monkeys. Curr Eye Res. 1990;9:525–532 [DOI] [PubMed] [Google Scholar]
- 45. Flugel-Koch C, Neuhuber WL, Kaufman PL, Lutjen-Drecoll E. Morphologic indication for proprioception in the human ciliary muscle. Invest Ophthalmol Vis Sci. 2009;50:5529–5536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ostrin LA, Glasser A. Comparisons between pharmacologically and Edinger-Westphal–stimulated accommodation in rhesus monkeys. Invest Ophthalmol Vis Sci. 2005;46:609–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fincham EF. The mechanism of accommodation. Br J Ophthalmol. 1937;Monograph VIII:7–76 [Google Scholar]
- 48. Fisher RF. The elastic constants of the human lens. J Physiol. 1971;212:147–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fisher RF. Presbyopia and the changes with age in the human crystalline lens. J Physiol. 1973;228:765–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pau H, Kranz J. The increasing sclerosis of the human lens with age and its relevance to accommodation and presbyopia. Graefes Arch Clin Exp Ophthalmol. 1991;229:294–296 [DOI] [PubMed] [Google Scholar]
- 51. Glasser A, Campbell MC. Presbyopia and the optical changes in the human crystalline lens with age. Vision Res. 1998;38:209–229 [DOI] [PubMed] [Google Scholar]
- 52. Tamm E, Lütjen-Drecoll E, Jungkunz W, Rohen JW. Posterior attachment of ciliary muscle in young, accommodating old, presbyopic monkeys. Invest Ophthalmol Vis Sci. 1991;32:1678–1692 [PubMed] [Google Scholar]
- 53. Croft MA, Kaufman PL, Crawford KS, Neider MW, Glasser A, Bito LZ. Accommodation dynamics in aging rhesus monkeys. Am J Physiol. 1998;275:R1885–R1897 [DOI] [PubMed] [Google Scholar]
- 54. Tornqvist G. Accommodation in monkeys: some pharmacological and physiological aspects. Acta Ophthalmol. 1967;45:429–460 [DOI] [PubMed] [Google Scholar]
- 55. Kasthurirangan S, Vilupuru AS, Glasser A. Amplitude dependent accommodative dynamics in humans. Vision Res. 2003;43:2945–2956 [DOI] [PubMed] [Google Scholar]
- 56. Bharadwaj SR, Schor CM. Dynamic control of ocular disaccommodation: First and second-order dynamics. Vision Res. 2006;46:1019–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schor CM, Bharadwaj SR. Pulse-step models of control strategies for dynamic ocular accommodation and disaccommodation. Vision Res. 2006;46:242–258 [DOI] [PubMed] [Google Scholar]