The results of this study demonstrate that diabetic eNOS−/− mice develop an accelerated diabetic retinopathy, including increased retinal vessel leakage, gliosis, an increased number of acellular retinal capillaries, and basement membrane thickening. Moreover, these pathologic changes occur at an accelerated rate compared with those in wild-type STZ-treated diabetic mice, supporting a role for the deficiency in eNOS-derived NO production in the pathogenesis of diabetic retinopathy.
Abstract
Purpose.
Dysfunction of endothelial nitric oxide synthase (eNOS) has been implicated in the pathogenesis of diabetic vascular complications. This study was undertaken to determine the role of eNOS in the development of diabetic retinopathy (DR), by investigating the functional consequences of its deficiency in the diabetic state.
Methods.
Diabetes was induced in eNOS-knockout (eNOS−/−) and C57B/6 mice by streptozotocin (STZ) injection. Retinal vasculature was evaluated by albumin extravasation, to quantitatively measure vascular permeability, and by trypsin-digested retinal vascular preparations, to quantify acellular capillaries. Gliosis was evaluated by immunofluorescent techniques. Retinal capillary basement membrane thickness was assessed by transmission electron microscopy. Total retinal nitric oxide level was assessed by measuring nitrate/nitrite using a fluorometric-based assay, iNOS expression was examined by real-time PCR.
Results.
Diabetic eNOS−/− mice exhibit more severe retinal vascular permeability than age-matched diabetic C57BL/6 mice, detectable as early as 3 weeks after diabetes induction. Diabetic eNOS−/− mice also show earlier onset and an increased number of acellular capillaries, sustained gliosis, and increased capillary basement membrane thickness. Total nitric oxide (NO) level was also increased, concomitant with elevated iNOS expression in diabetic eNOS−/− retina.
Conclusions.
Diabetic eNOS−/− mice exhibit A significantly wider range of advanced retinal vascular complications than the age-matched diabetic C57BL/6 mice, supporting the notion that eNOS-derived NO plays an essential role in retinal vascular function. This mouse model also faithfully replicates many of the hallmarks of vascular changes associated with human retinopathy, thus providing a unique model to aid in understanding the pathologic mechanisms of and to develop effective therapeutic strategies for diabetic retinopathy.
Diabetic retinopathy (DR) is the most common form of diabetic vascular complication and is the leading cause of severe vision loss in the working-age group.1 Vision loss in DR develops by slow and progressive alterations in the retinal vasculature, including increased capillary basement membrane thickening, increased vascular permeability, pericyte loss, and acellular capillary development, all of which contribute to breakdown of the blood–retinal barrier and subsequent pathologic angiogenesis.
Despite extensive research, the mechanisms underlying the pathogenesis of DR are still not fully understood. Endothelial cell derived nitric oxide (NO), synthesized continuously in the endothelium from l-arginine by endothelial nitric oxide synthase (eNOS), plays an important role in modulating retinal vascular tone. Constitutive production and release of NO are critical in maintaining vascular homeostasis. Diabetes in both humans and animal models has been associated with reduced bioavailability of NO and impaired endothelium-dependent relaxation.2–4 Furthermore, decreased expression of eNOS concomitant with increased expression of iNOS and nitrotyrosine during diabetes progression in rats has been reported.5 Studies of mice genetically deficient in one of the constitutive NOS isoforms suggest that NO from both neuronal NOS (n)NOS and eNOS provide mutually compensating pathways under normal conditions. In the eye, the retinal vasculature develops normally in eNOS−/− mice, and this development is associated with increases in vascular-associated nNOS activity compensating for the eNOS deficiency in the developing and adult mutant retina.6 However, these knockout mice develop more severe diseases under stress conditions.7,8
To further investigate the pathogenic role of eNOS dysfunction in the development of DR, we induced diabetes in eNOS−/− and C57B/6 control mice and evaluated their retinal vasculature. The diabetic eNOS−/− mice developed more severe DR symptoms than did the diabetic control mice and exhibited most of the pathologic vascular changes seen in human patients. This finding supports a role for the deficiency in eNOS-derived NO production in the pathogenesis of both diabetic nephropathy and retinopathy. Further understanding the underlying mechanisms of pathogenesis for these diabetic complications has important implications in designing effective therapies, and diabetic eNOS−/− mice also provide a good animal model for achieving that end.
Materials and Methods
Animals and General Procedures
Wild-type C57B6/J mice (6–8 weeks old) and breeding pairs of eNOS−/− mice were purchased from Jackson Laboratories (Bar Harbor, ME) and maintained at the Animal Care Service at the University of Florida. All procedures adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the protocol was approved by the Animal Care and Use Committee of the University of Florida. The animals were fed standard laboratory chow and allowed free access to water in an air-conditioned room with a 12–12-hour light–dark cycle.
Diabetes was induced by two consecutive intraperitoneal injections of streptozotocin (STZ, 100 mg/kg body weight, freshly made in 0.05 M citrate buffer, pH 4.5), confirmed 1 week after induction by measuring the blood glucose level (defined as >200 mg/dL) using a glucomonitor (FreeStyle; Abbott Diabetes Care, Alameda, CA) and test strip according to the manufacturer's instructions. Blood pressure was measured in conscious mice at room temperature with a tail-cuff monitor (BP2000 Analysis System; Visitech Systems, Apex, NC).
Fluorescein Angiography
The mouse's eyes were dilated with 1% atropine and phenylephrine (AK-Dilate; Akorn, Inc., Buffalo Grove, IL). Fluorescein angiography was performed after intraperitoneal injection of 10% fluorescein sterile solution (1 mL/kg body weight, AK-Fluor; Akorn, Inc.), and fundus photographs were captured (Genesis fundus camera; Kowa Optimed, Inc., Torrance, CA).
Albumin Extravasation Assay
Retinal vascular permeability was evaluated by albumin extravasation. Anesthetized mice received intravenous (IV) injection of FITC-labeled albumin (100 mg/kg body weight; Sigma-Aldrich, St. Louis, MO), and blood samples were collected before and immediately after the injection. After 30 minutes, the animals were killed, their eyes enucleated, fixed in 4% paraformaldehyde (freshly made in PBS, pH 7.4), and embedded in optimal cutting temperature (OCT) compound. Frozen sections (12 μm) were cut and mounted on slides in such a way that adjacent sections were mounted on eight different slides, five sections per slide, so that adjacent sections on the same slide were ∼100 μm apart, and each eye was cut in three sets of 5 × 8 slide sections. Extravasation of FITC-albumin from the retinal vessels was evaluated in serial cross sections (10 sections, representing total 500-μm thickness) by fluorescence imaging and quantified from representative sections by measuring the fluorescent intensity, then normalized to the plasma level of FITC determined by fluorometer (Molecular Device, Sunnyvale, CA). For direct visualization of leakage, alkaline phosphatase (AP)-conjugated anti-FITC antibody (Sigma-Aldrich) was used and detected with an AP-substrate kit from Vector Laboratories (Vector Laboratories, Burlingame, CA), according to the manufacturer's instruction. The endogenous AP activity was blocked (Levamisole Solution; Vector Laboratories), according to the manufacturer's instruction.
Trypsin Digest Preparation of Retinal Vasculature
Retinal vasculature was prepared by using the method described by Kuwabara and Cogan9 with minor modifications. Briefly, the eyes were fixed in 4% paraformaldehyde freshly made in PBS overnight after enucleation. The retinas were dissected from the eye cups, washed in water overnight, and digested in 3% trypsin (Invitrogen-Gibco, Grand Island, NY) for 2 to 3 hours at 37°C. The tissue was then transferred into water and the network of vessels was freed from adherent retinal tissue by gentle shaking and manipulation under a dissection microscope. The vessels were then mounted on a clean slide, allowed to dry, and stained with PAS-H&E (periodic acid Schiff–hematoxylin & eosin; Gill No.3; Sigma-Aldrich) according to the instruction manual. After the tissue was stained and washed in water, it was then dehydrated and mounted (Permount mounting medium; Fisher Scientific, Pittsburgh, PA). The prepared retinal vessels were photographed by microscope equipped with a high-resolution digital camera (AxioCam MRC5, Axiovert 200; Carl Zeiss Meditec, Inc., Dublin, CA), using both 20× and 40× objective lenses. Six to eight representative, nonoverlapping fields from each quadrant of the retina were imaged. Acellular capillaries are counted from images for each retina and expressed as the number of acellular vessels per square millimeter.
Retinal Capillary Basement Membrane Evaluation
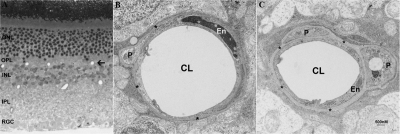
The basement membrane thickness of retinal capillaries was evaluated by transmission electron microscopy (TEM). Anesthetized mice (two nondiabetic and two diabetic mice at 2 months after induced diabetes) were perfused with fixative containing 2% paraformaldehyde and 2% glutaraldehyde and the eyes were enucleated and immersed in the same fixative overnight, after the cornea and lens were removed. The eyes were then postfixed in 2% OsO4, dehydrated in ethanol series, embedded in epoxy resin. Thin sections (0.5 μm) were stained with toluidine blue for orientation and identification of capillaries. Ultrathin sections (60 nm) were counterstained with uranyl acetate and lead citrate and examined by TEM. Retinal capillary basement membrane thickness (CBMT) was measured from TEM images captured from deep capillaries residing between the outer plexiform (OPL) and inner nuclear (INL) layers (see Fig. 5A, arrow). Minimally, 10 capillaries from the central, mid, and peripheral retina were measured for each eye, at least 30 measurements were taken per capillary.
Figure 5.
Micrograph of retinal plastic section (A) and transmission electron micrographs of retinal capillaries (B, C) from a nondiabetic eNOS−/− mouse (B) and an eNOS−/− mouse at 2 months after STZ induction of diabetes mellitus (C). Both micrographs of capillaries were imaged from the deep vascular bed within the layer between the OPL and INL (A, arrow). ONL, outer nuclear layer; OPL, outer plexiform layer; INL, Inner nuclear layer; IPL, Inner plexiform layer; RGC, retinal ganglion cell; CL, capillary lumen; En, endothelial cell; P, pericyte. *Capillary basement membrane.
Measurement of Retinal NO Levels
Nitric oxide (NO) production in the retina was examined by measuring the total level of NO. Since NO is a gaseous free radical with a short half-life in vivo of a few seconds or less, levels of the more stable NO metabolites nitrite (NO2−) and nitrate (NO3−) have been used in the indirect measurement of NO in biological fluids.10 A nitrate/nitrite fluorometric assay kit (Cayman Chemical, Ann Arbor, MI) was used for total nitrite/nitrate measurement in the retina, according to the manufacturer's instructions.
Tissue Process, Immune Fluorescence, and Histochemistry
For immune-fluorescent staining, the eyes were fixed in 4% paraformaldehyde freshly made in phosphate-buffered saline (PBS) at 4°C overnight. The eye cups were cryoprotected in 30% sucrose/PBS for several hours or overnight before quick freezing in OCT compound. Then 12-μm-thick sections were cut at −20 to –22°C. A rabbit polyclonal antibody against GFAP (1:1000; Sigma-Aldrich) was used. Secondary antibodies conjugated with Alexa594 and Alexa488 were from Invitrogen-Molecular Probes (Carlsbad, CA) and were used according to the manufacturer's instruction. The sections were coverslipped with antifade medium (Vectashield; Vector Laboratories), and examined with a microscope (AxioVision; Carl Zeiss MicroImaging, Inc., Thornwood, NY) equipped with epifluorescence illumination and a high-resolution digital camera.
For RT-PCR, total RNA was isolated from freshly dissected retinas (Trizol Reagent; Invitrogen), according to the manufacturer's instructions. RT-PCR was performed (Enhanced Avian HS RT-PCR kit; Sigma-Aldrich). Primer pairs for iNOS sequence were TCGCTTTGCCACGGACGAGA (forward) and TGGCCAGCTGCTTTTGCAGG (reverse), and primer pairs for β-actin were CTACAATGAGCTGCGTGTGG (forward) and CGGTGAGGATCTTCATGAGG (reverse).
Statistics
All values are presented as the mean ± SD. A paired Student's t-test was used to assess the significance of the difference between two groups. One-way ANOVA followed by the post hoc Tukey (Fisher's protected least-significant difference) test was used to assess statistical significance between multiple groups. Differences were significant at P < 0.05.
Results
Development of Diabetes in Wild-Type and eNOS−/− Mice: General Parameters
As originally reported,11 the eNOS−/− mice are viable and fertile, but have blood pressure that is approximately 20 mm Hg higher than that in their normal wild-type siblings (Table 1). In the eye, the retinal vasculature develops normally in eNOS−/− mice and is associated with increases in vascular-associated nNOS activity that compensate for the eNOS deficiency in the developing and adult mutant retina.6 Moreover, these mice demonstrate increased iNOS immunoreactivity in the retina.12 STZ treatment resulted in hyperglycemia in both C57B/6 and eNOS−/− mice (Table 1) and progressive loss of body weight. The weight loss in the diabetic eNOS−/− mice was more severe than that in the age-matched diabetic C57B/6 mice, as reported previously.7
Table 1.
General Parameters Compared in Diabetic and Nondiabetic C57B/6 and eNOS−/− Mice
| C57/B6 |
eNOS−/− |
|||
|---|---|---|---|---|
| NDM | DM | NDM | DM | |
| 2 mo after induced DM | ||||
| Body weight, g | 28.7 ± 3.5 | 21.5 ± 2.4* | 23.2 ± 2.1 | 17.6 ± 1.7* |
| Glucose, mg/dL | 117 ± 8 | 331 ± 58* | 112 ± 26 | 389 ± 55* |
| SBP, mm Hg | 119 ± 8 | 136 ± 13* | 139 ± 8 | 153 ± 14 |
| 4 mo after induced DM | ||||
| Body weight, g | 30.2 ± 3.1 | 22.8 ± 2.9* | 27.8 ± 1.9 | 19.6 ± 1.7* |
| Glucose, mg/dL | 115 ± 9 | 347 ± 51* | 115 ± 13 | 396 ± 41* |
| SBP, mm Hg | 120 ± 8 | 133 ± 14* | 141 ± 9 | 139 ± 9 |
n = 5, nondiabetic group; n = 10, diabetic group.
P < 0.01 versus NDM.
Progressive Vascular Changes and Increased Retinal Vascular Permeability in Diabetic eNOS−/− Mice
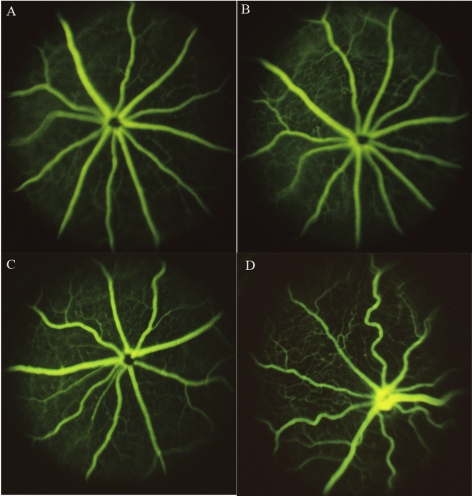
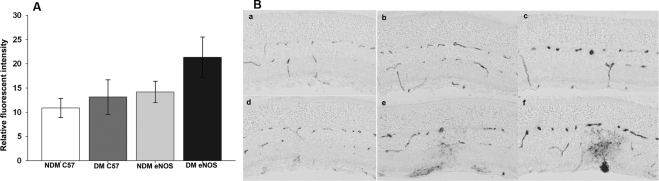
Abnormal vascular changes were clearly detectable on fundus fluorescein angiography in diabetic eNOS animals, including increased vessel tortuosity, whereas similar changes were not observed in C57/B6 mice, even after longer periods of diabetes (Fig. 1). Retinal vascular permeability was evaluated by albumin extravasations and quantified by measuring the fluorescence intensity in serial sections from both nondiabetic and diabetic control C57B/6 and eNOS−/− mice. Diabetic eNOS−/− mouse retina showed increased vascular leakage of FITC-albumin compared with nondiabetic retina (Fig. 2A). Increased retinal vascular permeability was detectable at 2 weeks after STZ treatment in the eNOS−/− mice and numerous focal vascular lesions were present, as evidenced by intense albumin leakage, whereas similar permeability change occurred much later and less severely in diabetic C57/B6 mouse retinas (Fig. 2B).
Figure 1.
Fundus fluorescein angiography of nondiabetic C57/B6 (A), eNOS−/− (C), diabetic C57/B6 (6-month diabetes, B), and eNOS−/− (2-month diabetes, D, which demonstrates increased tortuosity compared with A, B, C) mouse eyes.
Figure 2.
Retinal vascular permeability in nondiabetic and diabetic C57B/6 and eNOS−/− mice. (A) Retinal vascular permeability was evaluated by FITC-labeled albumin extravasations and quantified by measuring the fluorescent intensity in serial sections and normalized to plasma level of FITC from nondiabetic and 2-month diabetic eNOS−/− and C57B/6 mice. Data are presented as the mean ± SD of results in six eyes in each group. *P < 0.01. (B) Representative images of retinal vascular leakage visualized by AP-labeled anti-FITC antibody in nondiabetic C57B/6 (Ba), eNOS−/− (Bd), and diabetic C57B/6 (Bb, Bc) and eNOS−/− (Be, Bf) at 3 weeks (Bb, Be) and 2 months (Bc, Bf) after induced diabetes.
Glial Cell Changes
Müller glial cells play a vital role in maintaining normal retinal vascular and neuronal function. Glial activation (gliosis) is a common feature of many retinal diseases including diabetic retinopathy. A key feature of retinal gliosis is the upregulation of the intermediate filament, glial fibrillary acidic protein (GFAP). Increased GFAP expression has been shown in diabetic rats,13–16 as well as in humans with diabetic retinopathy.17 However, no change or transient change of GFAP activation has been detected in diabetic C57/B6 mouse retina.18,19
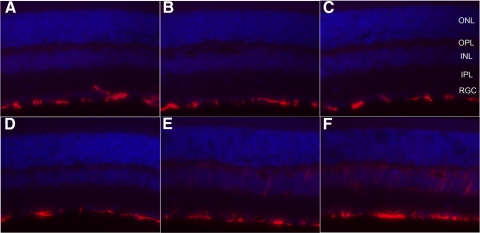
Glial reactivity in diabetic eNOS−/− mouse retina was detected by immune fluorescence labeling with antibody against GFAP. In nondiabetic eNOS−/− retina, GFAP expression was detectable only in retinal astrocytes (Fig. 3D) as in wild-type C57B/6 retinas (Figs. 3A, 3B), but was highly elevated in diabetic eNOS−/− retina (Figs. 3E, 3F). The elevated GFAP expression can be detected as early as 3 weeks after diabetes induction (data not shown).
Figure 3.
GFAP expression in diabetic and nondiabetic eNOS−/− and control mouse retina. GFAP expression is present only in retinal astrocytes in nondiabetic (A) and diabetic C57B6/J (B, 1 month; C, 3-month diabetes) and non-eNOS−/− retina (D), but elevated in Müller glial cells in diabetic eNOS−/− mouse retinas (E, 1 month diabetes; F, 3-month diabetes).
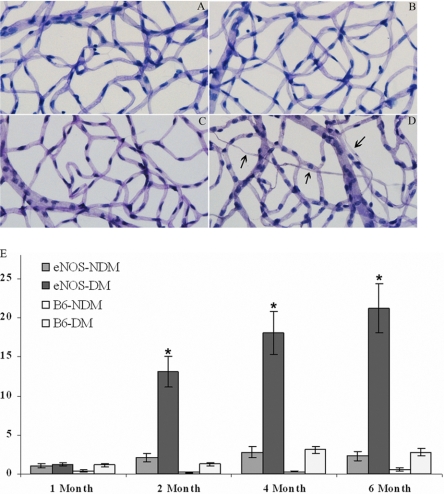
Increased Acellular Capillaries in Diabetic eNOS−/− Mouse Retina
Nondiabetic eNOS−/− mouse retinas showed normal vasculature indistinguishable from that of age-matched C57B/6 mice (Figs. 4A, 4B). Diabetic C57B/6 mouse retinas exhibited only a slight (nonsignificant) increase in the number of acellular capillaries per unit area over 6 months of diabetes. In contrast, diabetic eNOS−/− mouse retinas exhibited much earlier onset and increased the number of acellular capillaries (Fig. 4B), even more than that of diabetic C57B/6 mouse after 13 month of diabetes (data not shown). Because of the increased mortality of diabetic eNOS−/− mice, retinas from mice with diabetes duration longer than 6 months were not examined.
Figure 4.
Diabetic eNOS−/− mice exhibit increased retinal capillary loss. Representative images of retinal vessels from nondiabetic C57B/6 (A), diabetic C57B/6 (B, 6 months after induced diabetes), nondiabetic eNOS−/− (C), and diabetic eNOS−/− mouse retinas (D, 4 month after induced diabetes). (E) Quantitative measurements of acellular capillaries (n = 6 for each data point). The values on the y-axis represent the number of acellular capillaries per square millimeter of retina. B6: wild-type C57/B6.
Increased Basement Membrane Thickness in Retinal Vascular Capillaries
Thickening of microvascular basement membrane represents a histopathologic hallmark of diabetic complications and has been observed in human diabetes and in several different animal models of experimental diabetes.20–22 The basement membrane of retinal capillaries from the diabetic eNOS−/− animals at 2 months after STZ induction of diabetes was significantly thicker than those from age-matched, nondiabetic animals (95.72 ± 20 nm in DM versus 71.29 ± 19 nm in NDM eNOS retina, P < 0.01). Representative TEM micrographs of retinal capillaries from diabetic and nondiabetic eNOS−/− mouse retinas are shown in Figure 5.
Retinal NO Levels
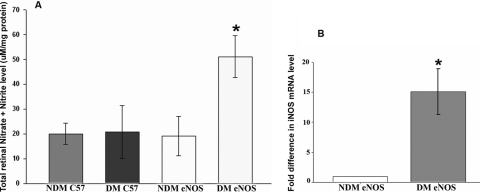
NO is constitutively produced by endothelial cells and maintained at physiological levels under normal conditions. Diabetes has been associated with reduced bioavailability of NO and impaired endothelium-dependent relaxation, both in patients and in animal models.2–4 We measured the NO level with an indirect fluorometric-based assay to determine total nitrite/nitrate levels in retinas isolated from NDM and DM mice 2 months after diabetes induction. The total retinal NO levels in nondiabetic eNOS−/− mouse retina were similar to those in age-matched C57B/6 mice (diabetic or nondiabetic), but were significantly increased in the diabetic retina (Fig. 6A), and this NO increase was associated with a concomitant increase in iNOS expression that was undetectable in nondiabetic conditions (Fig. 6B).
Figure 6.
Total nitrite/nitrate levels and iNOS expression in eNOS−/− retina. (A) Total NO level was measured from retinal protein extracts isolated from nondiabetic (NDM, n = 5) and diabetic (2 months after induced diabetes, n = 6) mice by using indirect fluorometric-based assays to measure total nitrite/nitrate levels in the retina. (B). iNOS mRNA detection in eNOS−/− retinas by RT-PCR (n = 3).
Discussions
Animal models of diabetes have been valuable tools for shaping our understanding of the pathophysiological mechanisms of diabetic retinopathy and discovering and assessing new potential therapeutic agents. Although numerous diabetic animal models have been described (see a recent review and references therein23), none of these models replicates the key features of the disease in humans, which is characterized by progressive alternations to the retinal vasculature, including capillary dilation and leakage, capillary occlusion, pericyte/capillary loss, and subsequent new vessel formation.24,25 Most of these models exhibit early features of retinal complications of diabetes, but none of the advanced changes.26–32 In this study, we examined the role of eNOS in the development of diabetic retinopathy by inducing diabetes in the eNOS−/− mice and characterized the ensuing retinal vascular changes. In the present study diabetic eNOS−/− mice developed a significantly wider range of severe retinal vascular complications than did age-matched STZ-induced diabetic C57B6 mice. These complications resemble most of the major vascular hallmarks of DR, including increased retinal vessel leakage, gliosis, an increased number of acellular retinal capillaries, and increased basement membrane thickening of retinal capillaries. Moreover, the increase in retinal vessel leakage, gliosis, and number of acellular retinal capillaries occurred at an accelerated rate compared with wild-type STZ-treated diabetic mice. These observations strongly support the essential role of NO in retinal vascular function.
Several pathologic pathways could contribute to accelerated retinopathy in this model: First, a deficiency in endothelial-derived NO, coupled with concomitant activation of iNOS, which can produce a larger amount of NO which, when encountering reactive oxygen species (ROS), can generate highly reactive nitrogen species, leading to oxidative stress, and accelerated retinopathy. Endothelial cell–derived NO plays an essential role in maintaining vascular homeostasis. Diabetes both in human and animal models has been associated with reduced NO bioavailability and impaired endothelium-dependent relaxation.2–4 Recent studies have shown that eNOS gene polymorphisms are associated with increased risk of developing DR.33–37 Furthermore, decreased expression of eNOS concomitant with increased expression of iNOS and nitrotyrosine during diabetes progression in rats have been reported.5 The nondiabetic eNOS−/− mice are viable, fertile, and exhibit no gross anatomic abnormalities, despite the absence of detectable eNOS mRNA, protein, or enzymatic activity. Further analysis showed that eNOS−/− mice exhibit abnormalities in vascular relaxation, blood pressure regulation, and cardiac contractility, and increased propensity to form neointima in response to vessel injury.38,39 Studies of genetically deficient mice in one of the constitutive NOS isoforms suggest that the NO from both nNOS and eNOS provide mutually compensating pathways in normal conditions40 that probably account for the lack of gross anatomic abnormalities in these knockout mice. In the eye, the retinal vasculature develops normally in eNOS−/− mice, and that normal development is associated with increases in vascular-associated nNOS activity compensating for the eNOS deficiency in the developing and adult mutant retina.6 Leukocyte–endothelial cell interactions, which are normally modulated by the eNOS isoform, are also replaced by nNOS in eNOS−/− mice.41 However, nNOS cannot completely compensate for eNOS in oxidative stress conditions, where there is insufficient NO produced by nNOS to overcome leukocyte recruitment elicited by such stress.41 In our results, the total NO level in the nondiabetic eNOS−/− retina was similar to that in the wild-type C57/B6 retina, consistent with that reported in the literature.6 However, NO levels were highly increased during hyperglycemia, accompanied by increased iNOS expression, whereas no further increase in nNOS expression was evident in high-glucose conditions (data not shown).This finding would explain the increased levels of NO products reported in diabetic retinas.42,43 Increased expression of iNOS has been observed in retinas of both human and experimental animals,42–45 and such high NO concentrations produced by iNOS can be toxic. NO toxicity has been attributed to various mechanisms including peroxynitrate-mediated oxidative damage to macromolecules and cells and energy failure.46
A second pathologic process, increased gliosis, as evidenced by increased GFAP expression in Müller cells in diabetic eNOS−/− retina, may also contribute to the observed retinopathy in this model. Reactive changes in Müller cells, such as upregulation of GFAP occur early in the course of the disease and precede the onset of overt vascular changes in both human and STZ-treated rat retinas during early diabetes.13,15,17,47 However, increased GFAP expression is not dramatically altered in STZ-diabetic rats48 and is also not observed in the Ins2Akita mouse model.31 This finding is consistent with the fact that diabetic C57B/6 mice induced by STZ lack many biochemical changes that are clearly manifested in the retina of STZ-induced diabetic rats.32 In our study, diabetic eNOS−/− retinas also exhibited sustained increased GFAP expression in Müller cells. The onset of abnormal Müller cell GFAP expression in diabetic eNOS−/− retinas was earlier than that in the STZ-treated rat retina, which is detectable only after 6 months of induced diabetes.49 Müller glial cells play a crucial role in maintaining normal retinal function and regulating retinal vasculature (reviewed in Refs. 50, 51). Early and transient Müller glial activation is thought to be neuroprotective response—for example, by leading to the release neurotrophic factors and antioxidants. However, activated Müller glial also release increased levels of VEGF, which may contribute to increased vascular permeability and progressive neovascularization in the diabetic retina,50 as well as the expression of iNOS in early diabetic retinopathy.43,52 Thus, the diabetic eNOS−/− mouse model also provides a valuable tool for further understanding the cellular and molecular mechanisms of gliotic responses in Müller cells; it should be valuable in developing therapeutic strategies for DR.
A third process involves the observation that eNOS−/− mice also develop hypertension, one of the major risk factors for type 2 diabetes53 and also an important independent risk factor for both the initial development and its subsequent progression of diabetic retinopathy.54,55 Control of hypertension in patients with type 2 diabetes has been shown to help prevent retinopathy and other microvascular complications.56 The possible mechanisms by which hypertension contributes to diabetic retinopathy are thought to be both direct hemodynamic processes including impaired autoregulation and hyperperfusion resulting in endothelial damage in the retinal vasculature57 and independently through increased expression of VEGF.58 It has been shown that hypertension independent of hyperglycemia increases VEGF expression in retinal endothelial cells and ocular fluids.59 In animal models, combined diabetes and hypertension by STZ treatment of spontaneous hypertensive rats exacerbates some of the earlier pathologic changes such as inflammation and increased oxidative stress60,61; however, detailed characterization of retinal vascular changes in this model has not been reported.
Finally, we note that in addition to retinopathy, eNOS−/− mice develop more severe diseases in other organs under stress conditions, including severe and early-onset diabetic nephropathy,7,8 consistent with clinical studies that overt nephropathy is strongly associated with proliferative diabetic retinopathy in both type 1 and 2 diabetic patients.62–64 Thus, diabetic eNOS−/− model provides a valuable tool to further investigate the pathophysiological mechanisms at multiple levels in both retina and kidney.
Footnotes
Supported in part by National Institutes of Health Grants EY13729, EY11123, EY08571, and EY016073 and by grants from the Macular Vision Research Foundation, Foundation Fighting Blindness, Juvenile Diabetes Research Foundation, and Research to Prevent Blindness, Inc.
Disclosure: Q. Li, None; A. Verma, None; P. Han, None; T. Nakagawa, None; R.J. Johnson None; M.B. Grant, None; M. Campbell-Thompson, None; Y.P.R. Jarajapu, None; B. Lei, None; W.W. Hauswirth, None
References
- 1. Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102:527–532 [DOI] [PubMed] [Google Scholar]
- 2. Durante W, Sen AK, Sunahara FA. Impairment of endothelium-dependent relaxation in aortae from spontaneously diabetic rats. Br J Pharmacol. 1988;94:463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnstone MT, Creager SJ, Scales KM, Cusco JA, Lee BK, Creager MA. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation. 1993;88:2510–2516 [DOI] [PubMed] [Google Scholar]
- 4. Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27:567–574 [DOI] [PubMed] [Google Scholar]
- 5. Nagareddy PR, Xia Z, McNeill JH, MacLeod KM. Increased expression of iNOS is associated with endothelial dysfunction and impaired pressor responsiveness in streptozotocin-induced diabetes. Am J Physiol Heart Circ Physiol. 2005;289:H2144–H2152 [DOI] [PubMed] [Google Scholar]
- 6. Al-Shabrawey M, El-Remessy A, Gu X, et al. Normal vascular development in mice deficient in endothelial NO synthase: possible role of neuronal NO synthase. Mol Vis. 2003;9:549–558 [PubMed] [Google Scholar]
- 7. Nakagawa T, Sato W, Glushakova O, et al. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J Am Soc Nephrol. 2007;18:539–550 [DOI] [PubMed] [Google Scholar]
- 8. Zhao HJ, Wang S, Cheng H, et al. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol. 2006;17:2664–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuwabara T, Cogan DG. Studies of retinal vascular patterns. I. Normal architecture. Arch Ophthalmol. 1960;64:904–911 [DOI] [PubMed] [Google Scholar]
- 10. Tsikas D. Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radic Res. 2005;39:797–815 [DOI] [PubMed] [Google Scholar]
- 11. Shesely EG, Maeda N, Kim HS, et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1996;93:13176–13181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guthrie SM, Curtis LM, Mames RN, Simon GG, Grant MB, Scott EW. The nitric oxide pathway modulates hemangioblast activity of adult hematopoietic stem cells. Blood. 2005;105:1916–1922 [DOI] [PubMed] [Google Scholar]
- 13. Rungger-Brandle E, Dosso AA, Leuenberger PM. Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2000;41:1971–1980 [PubMed] [Google Scholar]
- 14. Agardh E, Bruun A, Agardh CD. Retinal glial cell immunoreactivity and neuronal cell changes in rats with STZ-induced diabetes. Curr Eye Res. 2001;23:276–284 [DOI] [PubMed] [Google Scholar]
- 15. Lieth E, Barber AJ, Xu B, et al. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes. 1998;47:815–820 [DOI] [PubMed] [Google Scholar]
- 16. Li Q, Zemel E, Miller B, Perlman I. Early retinal damage in experimental diabetes: electroretinographical and morphological observations. Exp Eye Res. 2002;74:615–625 [DOI] [PubMed] [Google Scholar]
- 17. Mizutani M, Gerhardinger C, Lorenzi M. Müller cell changes in human diabetic retinopathy. Diabetes. 1998;47:445–449 [DOI] [PubMed] [Google Scholar]
- 18. Feit-Leichman RA, Kinouchi R, Takeda M, et al. Vascular damage in a mouse model of diabetic retinopathy: relation to neuronal and glial changes. Invest Ophthalmol Vis Sci. 2005;46:4281–4287 [DOI] [PubMed] [Google Scholar]
- 19. Gaucher D, Chiappore JA, Paques M, et al. Microglial changes occur without neural cell death in diabetic retinopathy. Vision Res. 2007;47:612–623 [DOI] [PubMed] [Google Scholar]
- 20. Frank RN. On the pathogenesis of diabetic retinopathy. Ophthalmology. 1984;91:626–634 [DOI] [PubMed] [Google Scholar]
- 21. Chakrabarti S, Sima AA. Pathogenetic heterogeneity in retinal capillary basement membrane thickening in the diabetic BB-rat. Diabetologia. 1987;30:966–968 [DOI] [PubMed] [Google Scholar]
- 22. Stitt AW, Anderson HR, Gardiner TA, Archer DB. Diabetic retinopathy: quantitative variation in capillary basement membrane thickening in arterial or venous environments. Br J Ophthalmol. 1994;78:133–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thompson CS. Animal models of diabetes mellitus: relevance to vascular complications. Curr Pharm Des. 2008;14:309–324 [DOI] [PubMed] [Google Scholar]
- 24. Aiello LP, Gardner TW, King GL, et al. Diabetic retinopathy. Diabetes Care. 1998;21:143–156 [DOI] [PubMed] [Google Scholar]
- 25. Sjolie AK. Ocular complications in insulin treated diabetes mellitus: an epidemiological study. Acta Ophthalmol Suppl. 1985;172:1–77 [PubMed] [Google Scholar]
- 26. Engerman RL, Kern TS. Retinopathy in animal models of diabetes. Diabetes Metab Rev. 1995;11:109–120 [DOI] [PubMed] [Google Scholar]
- 27. Kern TS, Engerman RL. Comparison of retinal lesions in alloxan-diabetic rats and galactose-fed rats. Curr Eye Res. 1994;13:863–867 [DOI] [PubMed] [Google Scholar]
- 28. Kern TS, Engerman RL. Galactose-induced retinal microangiopathy in rats. Invest Ophthalmol Vis Sci. 1995;36:490–496 [PubMed] [Google Scholar]
- 29. Kern TS, Engerman RL. A mouse model of diabetic retinopathy. Arch Ophthalmol. 1996;114:986–990 [DOI] [PubMed] [Google Scholar]
- 30. Kakehashi A, Saito Y, Mori K, et al. Characteristics of diabetic retinopathy in SDT rats. Diabetes Metab Res Rev. 2006;22:455–461 [DOI] [PubMed] [Google Scholar]
- 31. Barber AJ, Antonetti DA, Kern TS, et al. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci. 2005;46:2210–2218 [DOI] [PubMed] [Google Scholar]
- 32. Obrosova IG, Drel VR, Kumagai AK, Szabo C, Pacher P, Stevens MJ. Early diabetes-induced biochemical changes in the retina: comparison of rat and mouse models. Diabetologia. 2006;49:2525–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Awata T, Neda T, Iizuka H, et al. Endothelial nitric oxide synthase gene is associated with diabetic macular edema in type 2 diabetes. Diabetes Care. 2004;27:2184–2190 [DOI] [PubMed] [Google Scholar]
- 34. Taverna MJ, Elgrably F, Selmi H, Selam JL, Slama G. The T−786C and C774T endothelial nitric oxide synthase gene polymorphisms independently affect the onset pattern of severe diabetic retinopathy. Nitric Oxide. 2005;13:88–92 [DOI] [PubMed] [Google Scholar]
- 35. Ezzidi I, Mtiraoui N, Mohamed MB, Mahjoub T, Kacem M, Almawi WY. Endothelial nitric oxide synthase Glu298Asp, 4b/a, and T–786C polymorphisms in type 2 diabetic retinopathy. Clin Endocrinol (Oxf). 2008;68:542–546 [DOI] [PubMed] [Google Scholar]
- 36. Chen Y, Huang H, Zhou J, et al. Polymorphism of the endothelial nitric oxide synthase gene is associated with diabetic retinopathy in a cohort of West Africans. Mol Vis. 2007;13:2142–2147 [PubMed] [Google Scholar]
- 37. Frost D, Chitu J, Meyer M, Beischer W, Pfohl M. Endothelial nitric oxide synthase (ecNOS) 4 a/b gene polymorphism and carotid artery intima-media thickness in type-1 diabetic patients. Exp Clin Endocrinol Diabetes. 2003;111:12–15 [DOI] [PubMed] [Google Scholar]
- 38. Moroi M, Zhang L, Yasuda T, et al. Interaction of genetic deficiency of endothelial nitric oxide, gender, and pregnancy in vascular response to injury in mice. J Clin Invest. 1998;101:1225–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang L, Fishman MC, Huang PL. Estrogen mediates the protective effects of pregnancy and chorionic gonadotropin in a mouse model of vascular injury. Arterioscler Thromb Vasc Biol. 1999;19:2059–2065 [DOI] [PubMed] [Google Scholar]
- 40. Liu VW, Huang PL. Cardiovascular roles of nitric oxide: a review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc Res. 2008;77:19–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sanz MJ, Hickey MJ, Johnston B, et al. Neuronal nitric oxide synthase (NOS) regulates leukocyte-endothelial cell interactions in endothelial NOS deficient mice. Br J Pharmacol. 2001;134:305–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Du Y, Smith MA, Miller CM, Kern TS. Diabetes-induced nitrative stress in the retina, and correction by aminoguanidine. J Neurochem. 2002;80:771–779 [DOI] [PubMed] [Google Scholar]
- 43. Abu El-Asrar AM, Desmet S, Meersschaert A, Dralands L, Missotten L, Geboes K. Expression of the inducible isoform of nitric oxide synthase in the retinas of human subjects with diabetes mellitus. Am J Ophthalmol. 2001;132:551–556 [DOI] [PubMed] [Google Scholar]
- 44. do Carmo A, Lopes C, Santos M, Proenca R, Cunha-Vaz J, Carvalho AP. Nitric oxide synthase activity and L-arginine metabolism in the retinas from streptozotocin-induced diabetic rats. Gen Pharmacol. 1998;30:319–324 [DOI] [PubMed] [Google Scholar]
- 45. Kowluru RA, Engerman RL, Kern TS. Abnormalities of retinal metabolism in diabetes or experimental galactosemia VIII. Prevention by aminoguanidine. Curr Eye Res. 2000;21:814–819 [DOI] [PubMed] [Google Scholar]
- 46. Dawson VL, Dawson TM. Nitric oxide neurotoxicity. J Chem Neuroanat. 1996;10:179–190 [DOI] [PubMed] [Google Scholar]
- 47. Barber AJ, Antonetti DA, Gardner TW. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. The Penn State Retina Research Group. Invest Ophthalmol Vis Sci. 2000;41:3561–3568 [PubMed] [Google Scholar]
- 48. Asnaghi V, Gerhardinger C, Hoehn T, Adeboje A, Lorenzi M. A role for the polyol pathway in the early neuroretinal apoptosis and glial changes induced by diabetes in the rat. Diabetes. 2003;52:506–511 [DOI] [PubMed] [Google Scholar]
- 49. Fletcher EL, Phipps JA, Ward MM, Puthussery T, Wilkinson-Berka JL. Neuronal and glial cell abnormality as predictors of progression of diabetic retinopathy. Curr Pharm Des. 2007;13:2699–2712 [DOI] [PubMed] [Google Scholar]
- 50. Bringmann A, Pannicke T, Grosche J, et al. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25:397–424 [DOI] [PubMed] [Google Scholar]
- 51. Bringmann A, Iandiev I, Pannicke T, et al. Cellular signaling and factors involved in Müller cell gliosis: neuroprotective and detrimental effects. Prog Retin Eye Res. 2009;28:423–451 [DOI] [PubMed] [Google Scholar]
- 52. Goureau O, Hicks D, Courtois Y, De Kozak Y. Induction and regulation of nitric oxide synthase in retinal Müller glial cells. J Neurochem. 1994;63:310–317 [DOI] [PubMed] [Google Scholar]
- 53. Abuissa H, O'Keefe J., Jr The role of renin-angiotensin-aldosterone system-based therapy in diabetes prevention and cardiovascular and renal protection. Diabetes Obes Metab. 2008;10:1157–1166 [DOI] [PubMed] [Google Scholar]
- 54. Wong TY, Mitchell P. The eye in hypertension. Lancet. 2007;369:425–435 [DOI] [PubMed] [Google Scholar]
- 55. DellaCroce JT, Vitale AT. Hypertension and the eye. Curr Opin Ophthalmol. 2008;19:493–498 [DOI] [PubMed] [Google Scholar]
- 56. Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol. 2004;122:1631–1640 [DOI] [PubMed] [Google Scholar]
- 57. Hsueh WA, Anderson PW. Hypertension, the endothelial cell, and the vascular complications of diabetes mellitus. Hypertension. 1992;20:253–263 [DOI] [PubMed] [Google Scholar]
- 58. Suzuma I, Hata Y, Clermont A, et al. Cyclic stretch and hypertension induce retinal expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2: potential mechanisms for exacerbation of diabetic retinopathy by hypertension. Diabetes. 2001;50:444–454 [DOI] [PubMed] [Google Scholar]
- 59. Srivastava BK, Rema M. Does hypertension play a role in diabetic retinopathy? J Assoc Physicians India. 2005;53:803–808 [PubMed] [Google Scholar]
- 60. Pinto CC, Silva KC, Biswas SK, Martins N, De Faria JB, De Faria JM. Arterial hypertension exacerbates oxidative stress in early diabetic retinopathy. Free Radic Res. 2007;41:1151–1158 [DOI] [PubMed] [Google Scholar]
- 61. Silva KC, Pinto CC, Biswas SK, de Faria JB, de Faria JM. Hypertension increases retinal inflammation in experimental diabetes: a possible mechanism for aggravation of diabetic retinopathy by hypertension. Curr Eye Res. 2007;32:533–541 [DOI] [PubMed] [Google Scholar]
- 62. Klein R, Moss SE, Klein BE. Is gross proteinuria a risk factor for the incidence of proliferative diabetic retinopathy? Ophthalmology. 1993;100:1140–1146 [DOI] [PubMed] [Google Scholar]
- 63. Kofoed-Enevoldsen A, Jensen T, Borch-Johnsen K, Deckert T. Incidence of retinopathy in type I (insulin-dependent) diabetes: association with clinical nephropathy. J Diabet Complications. 1987;1:96–99 [DOI] [PubMed] [Google Scholar]
- 64. Phoksunthorn T, Thatsnarong D. Retinopathy and macro-albuminuria in type 2 diabetic patients. J Med Assoc Thai. 2007;90:684–687 [PubMed] [Google Scholar]