Exposure of young human lenses to mild thermal stress results in large-scale binding of α-crystallin to cell membranes, which suggests that temperature may be a factor responsible for age-related changes to the human lens.
Abstract
Purpose.
With age, large amounts of crystallins become associated with fiber cell membranes in the human lens nucleus, and it has been proposed that this binding of protein may lead to the obstruction of membrane pores and the onset of a barrier to diffusion. This study focused on membrane binding within the barrier region and the outermost lens cortex.
Methods.
Human lenses across the age range were used, and the interaction of crystallins with membranes was examined using sucrose density gradient centrifugation, two-dimensional gel electrophoresis, and amine-reactive isobaric tagging technology. Lipids were quantified using shotgun lipidemics.
Results.
Binding of proteins to cell membranes in the barrier region was found to be different from that in the lens nucleus because in the barrier and outer cortical regions, only one high-density band formed. Most of the membrane-associated protein in this high-density band was α-crystallin. Mild thermal stress of intact young lenses led to pronounced membrane binding of proteins and yielded a sucrose density pattern in all lens regions that appeared to be identical with that from older lenses.
Conclusions.
α-Crystallin is the major protein that binds to cell membranes in the barrier region of lenses after middle age. Exposure of young human lenses to mild thermal stress results in large-scale binding of α-crystallin to cell membranes. The density gradient profiles of such heated lenses appear to be indistinguishable from those of older normal lenses. The data support the hypothesis that temperature may be a factor responsible for age-related changes to the human lens.
The human lens grows continuously throughout life, and adult lenses can be classified into two broad regions based on the age of the tissue: the nucleus is the part synthesized in utero,1,2 whereas the cortex is formed postnatally by the addition of newly differentiated cells onto the preexisting nucleus. Once fiber cells mature, there is little or no protein turnover3; thus, polypeptides endure for the lifetime of the person.
It is becoming evident that alterations to lens proteins4–8 and other lens macromolecules9,10 over time can lead to the impairment of normal lens function. For example, protein denaturation has been implicated in the onset of presbyopia.11 Similarly, large-scale association of proteins with cell membranes in the nucleus after middle age12 suggested that the formation of the lens barrier could be attributed to the occlusion of membrane pores. The barrier develops in the cortical region of the lens that immediately surrounds the nucleus,13,14 and, once it forms, age-related nuclear cataract may follow inevitably as the flow of antioxidants into the lens center declines. The lifetime of unstable molecules in the nucleus also increases, allowing more time for breakdown and consequently for posttranslational modification by reactive decomposition products.
It was the purpose of this study to investigate protein changes in the cortex of adult human lenses, with particular reference to alterations that take place with age in the barrier region. In this study, the lens cortex was dissected into two regions: the barrier zone, where the diffusion of small molecules becomes restricted after middle age,13 and the very outermost region of the lens, which contains newly synthesized tissue. By comparing the outermost and barrier regions with those parts of the lens formed in utero, the molecular basis of these changes may be revealed.
Methods
Lens Dissection
Lenses were donated to the Lions Eye Bank at the Sydney Eye Hospital. Enucleation occurred within 12 hours of death, and lenses were stored at −80°C until use. Tissue was handled in accordance with the tenets of the Declaration of Helsinki.
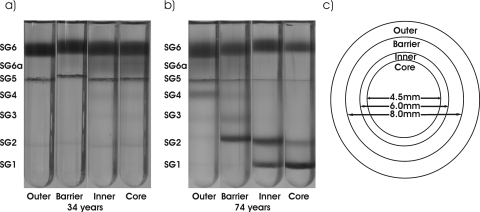
Lenses were the same as those used in a previous publication12 and were dissected as follows: trephines with diameters of 8, 6, and 4.5 mm were used to dissect lenses into the outer (>8 mm), barrier (8–6 mm), inner (6–4.5mm), and core (4.5 mm) regions, respectively (Fig. 1). As previously described,1,2 these dimensions correspond to four developmental regions of the lens: embryonic nucleus (core), infantile nucleus (inner), tissue formed in childhood (barrier), and newly synthesized cortical tissue (outer).
Figure 1.
Regional and age differences in the human lens as determined by sucrose density centrifugation. (a) 34-year-old lens and (b) 74-year-old lens dissected into outer, barrier, inner, and core regions. (c) Diagram illustrating the four regions of the lens obtained by dissection.
The trephines were precooled to −20°C. Decapsulated lenses were placed into a precooled polytetrafluoroethylene (Teflon; DuPont, Wilmington, DE) holder with a 9-mm diameter. The outer tissue was removed with an 8-mm trephine. The resultant billet was transferred to an 8-mm polytetrafluoroethylene holder, and barrier tissue was removed with a 6-mm trephine. A cold scalpel was used to remove approximately 1 mm from each end from the cylinder (inner + core), which was inserted into a 6-mm holder and refrozen. After refreezing, a 4.5-mm trephine was used to separate the core region from the inner region.
Sucrose Gradient
Lens tissues were fractionated by sucrose gradient density centrifugation, as previously described.12 In brief, sucrose concentrations of 8, 25, 45, 50, 60, 70, and 80% (wt/vol) were prepared in 10 mM Tris buffer, pH 8.0, containing 2 mM EDTA and 2 mM β-mercaptoethanol. Each sample was aspirated 30 times through a 21-gauge syringe needle in 8% sucrose (100 μL). To visualize proteins, Coomassie stain (800 μL 8% sucrose buffer plus 100 μL Coomassie stain (#23236; Pierce, Rockford, IL) was added to the sample, which was loaded onto the density gradient (sucrose: 1 mL, 80%; 2 mL, 70%; 1.5 mL, 60%; 1.5 mL, 50%; 1.5 mL, 45%; 1.5 mL, 25%). Tubes were centrifuged at 100,000g for 2 hours at 4°C using a Ti41 rotor in an L-80 centrifuge (Beckman). Sucrose gradients were photographed and quantified using densitometry with the image analysis program ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html), as described.12
Lipid Analysis
Interfaces designated SG6 to SG1 were collected,12 and each sample was diluted to an approximate concentration of 8% sucrose. A methanolic solution containing lauroyl dihydrosphingomyelin (m/z 649.6 [d18:0/12:0]) was added to each sample before lipid extraction using the method of Folch,15 with minor modifications. In brief, chloroform/methanol (2:1 vol/vol) containing 0.01% butylated hydroxytoluene was added to the diluted interfaces, vortexed, and centrifuged for 10 minutes at 2000g. The resultant organic phase was removed, and the extraction step was repeated three times. The organic phases were combined and dried under a stream of nitrogen at 37°C and reconstituted in 1 mL of 1:2 (vol/vol) chloroform/methanol.
Mass Spectrometry
Lipid extracts were analyzed using a mass spectrometer (QuattroMicro; Waters, Manchester, UK) with a z-spray electrospray ion source. Mass spectrometer conditions and details of quantification were as described.16
Heating Experiments
One intact human lens from a pair was suspended on a bed of parafilm above a reservoir of water (to maintain humidity) and incubated at 50°C for 15 hours in a sealed Eppendorf tube.11 The contralateral lens was kept frozen. Each lens was then dissected into the four regions, and tissues were separated by sucrose density centrifugation. Interfaces were removed from the gradient for protein and lipid analysis.
Two-Dimensional Gel Electrophoresis
Protein (60 μg) from SG2 was solubilized in 40 mM Tris buffer, pH 8, containing 8 M urea, 100 mM dithiothreitol, and 4% CHAPs and was centrifuged at 16,000g for 20 minutes. The first dimension was performed on a linear 17-cm IPG strip with an immobilized pH (3–10) gradient (ReadyStrip, #163–2007; Bio-Rad, Hercules, CA). The second dimension used an 18 × 25-cm SDS gel stained with colloidal Coomassie blue (G250). The identities of individual spots were confirmed by in-gel digestion with trypsin, followed by mass spectrometry (Axima TOF2 MALDI mass spectrometer; Shimadzu, Kyoto, Japan). Both PMF and MS/MS data were analyzed with a commercially available search engine (MASCOT, version 2.2.1; Matrix Science Inc., London, UK) using the Swiss-Prot database (version 51.6; made available by Swiss Institution of Bioinformatics [www.matrixscience.com]). The PMF peptide analysis tolerance was set at 0.8 Da, and MS/MS analysis peptide tolerance was set at 1.2 Da. All searches allowed for one missed cleavage with the variable modifications of acetylation (N-terminal), oxidation (M), and deamidation (NQ). PMF and MS/MS database searches were set to trypsin and semi-trypsin, respectively.
iTraq Reagent Labeling
An iTraq reagent kit (Applied Biosystems Inc. [ABI], Foster City, CA) was used to characterize changes in proteins at SG2 and SG1 from the barrier and core regions. Four lenses aged 49, 50, 71, and 72 years were dissected as described, and the barrier and core regions were loaded onto sucrose gradients, except that each gradient was run without Coomassie staining to avoid interference with the isotope labeling. SG1 and SG2 were collected and solubilized in 1% (wt/vol) SDS, and a total of 13 μg protein was labeled according to the manufacturer's instructions. A mass spectrometer (Q-Star; ABI) was used for collision-induced dissociation of labeled peptides. All mass spectral analysis was performed accordingly to the method Evans et al.17
Statistical Analysis
All data from the iTraq reagent experiments (ABI) were searched for a range of PTMs against the Swiss-Prot 54.3 database (17,400 human sequences) using MASCOT (version 2.2.03; Matrix Science, London, UK), with the enzyme specificity set to “trypsin.” Peptide tolerance was set to 8 ppm, and fragment tolerance was set to 0.6 Da, with one missed cleavage allowed. The MASCOT results were processed using the Trans-Proteomic Pipeline (version 3.2.0), with a global false discovery rate of P < 0.05 chosen using PeptideProphet statistics once the MASCOT search was fully processed by this algorithm. A minimum of two unique matching peptides were required for protein identification. To determine relative changes in protein content between SG2 and SG1 isolated from young and old lenses, a 49- and a 50-year-old lens were compared independently with a 71- and a 72-year-old lens. Each lens sample was analyzed twice, the data for both young lenses were combined and averaged, and the means were compared with the mean value of proteins from the two older lenses.
Results
Sucrose Density Gradient Centrifugation
With age, proteins in the human lens become increasingly associated with cell membranes.12,18,19 The purpose of this study was to examine this phenomenon in specific regions of the lens as a function of age, with particular attention to age-related changes in the barrier and outer cortex. A modification of the sucrose density gradient protocol described by Chandrasekar and Cendalla18 was used to separate fractions based on their density.
Each lens was dissected reproducibly into four regions by the use of trephines (see Fig. 1). The nucleus of the lens was divided into two parts, with a 4.5-mm equatorial diameter (core region) and a 6-mm equatorial diameter (inner region) that correspond to the fetal lens and the infantile lens, respectively, and were examined in a previous study.12 The cortex was dissected in two using a trephine with a diameter of 8 mm. Lens tissue between 6 and 8 mm was defined as the barrier region, which is the area where an age-related impediment to diffusion occurs.13,14 The cortical tissue outside 8 mm was referred to as the outer region and is composed of newly synthesized lens tissue.1 All four regions were examined by sucrose density gradient centrifugation.
Considerable changes were apparent when density gradients of the same regions from a young lens (34 years) and an older lens (74 years) were compared (Fig. 1). In the young lens, all four regions were similar. In the older lens, two distinct regional density patterns were observed. The core and inner regions displayed similar patterns, with large amounts of protein detected at two dense (SG1 and SG2) interfaces. By comparison, the barrier and outer cortical regions were different, displaying fewer changes in overall protein density and, notably, no SG1 band in either region.
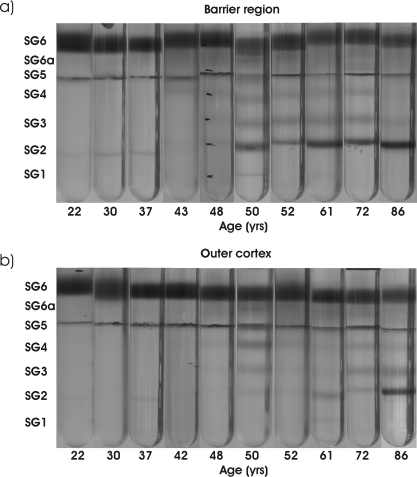
Previously, protein density changes in two nuclear tissues (core and inner regions) of the lens were found to be caused by the membrane binding of proteins.12 In the present study, the two cortical regions were examined to determine whether similar phenomena occurred. Figure 2 shows the patterns of protein density in the barrier (Fig. 2a) and the outer cortex (Fig. 2b) of the lens with age, as observed by sucrose density centrifugation. Before age 30, most protein in each lens region was located in two interfaces, SG6 and SG5 (Figs. 3a, 3b), which correspond to water-soluble protein (WSP) and “native” membranes, respectively.12 Between ages 30 and 45, the SG6 interface became more dispersed, and a new band between SG5 and SG6 (SG6a) appeared (Fig. 3c). Examination of SG6a by size-exclusion HPLC revealed it was composed primarily of high molecular weight protein complexes.12 Coinciding with the appearance of SG6a (Fig. 3c), a slight increase in staining was observed at the SG5 interface (Fig. 3b), suggesting an increased protein content of the membranes.
Figure 2.
Sucrose density gradient patterns as a function of age in (a) the barrier and (b) the outer regions of the lens. Lens ages are shown at the bottom of each tube.
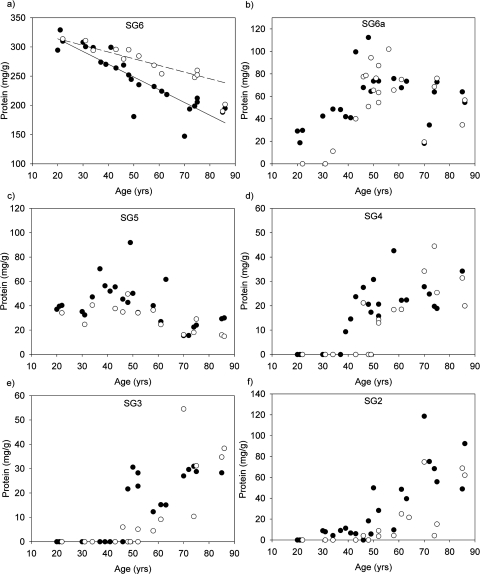
Figure 3.
Densitometric analysis of protein at each interface of the sucrose gradients as a function of age. (a) SG6, (b) SG6a, (c) SG5, (d) SG4, (e) SG3, and (f) SG2. Barrier (●) and outer (○) regions.
After age 40, other changes in protein density were observed in the barrier region. Protein at the SG4 and SG3 interfaces was detected (Figs. 3d, 3e), and, as the amount of protein in these interfaces increased with age, there was a corresponding loss of protein from SG6 (Fig. 3a).
After ages 45 to 50, protein was detected at the SG2 interface, increasing linearly with age (Fig. 3f). In marked contrast to the core and inner regions, there was little, or no, SG1 in any of the lenses examined (Fig. 2). Trends in the outer cortex mirrored those in the barrier region, though with an approximately 10-year lag period.
Lipid Analysis
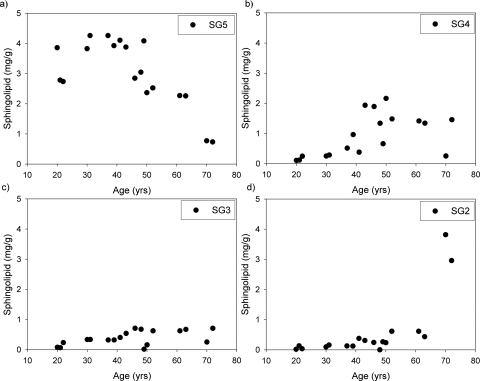
In the nucleus, substantial binding of proteins to lens membranes occurs at middle age.12 Shotgun lipidemics was used to examine lipid content in each interface of the sucrose gradients from the barrier region. Because sphingomyelins are a major (∼50%) component of phospholipids in the human lens,16,20 their presence was used as a marker for membranes. Before age 45, most sphingomyelins were found in the native membrane interface (SG5) (Fig. 4a). It was only after age 45 that a decrease in sphingomyelins in SG5 was observed; concomitantly, sphingomyelins increased in SG3 and SG4 (Figs. 4b, 4c) as well as in SG2. The largest increase in the content of sphingomyelins of SG2 (Fig. 4d) was observed after the age of 70.
Figure 4.
Amount of sphingomyelin detected at (a) SG5, (b) SG4, (c) SG3, and (d) SG2 as a function of age in the barrier region.
Characterization of Proteins in the High-Density Bands
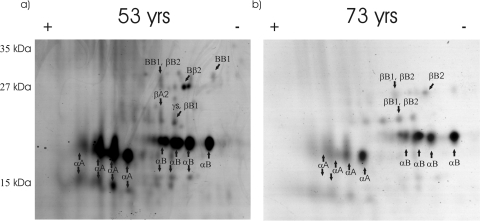
Two-dimensional electrophoresis showed clearly that the major protein component of SG2 is alpha crystallin. Both αA and αB crystallins were present as major spots in 2D gels of SG2 from a 53-year-old lens (Fig. 5a). More negatively charged forms of both αA and αB crystallin were also observed as prominent spots, and their identities were confirmed using in-gel digestion. Two possibilities to explain the characteristic series of spots are phosphorylation8,21,22 and deamidation of the α-crystallin subunits.6,23 The amount of protein in SG2 increased with age (Fig. 3f), but the appearance of the 2D gels did not alter significantly (Fig. 5b). This suggests that the composition of the proteins binding to the membranes is, to a large extent, independent of age. Truncated forms of both αA and αB crystallins were also observed in the gels of SG2.
Figure 5.
Two dimensional SDS-PAGE gels of SG2 isolated from the barrier regions of a (a) 53-year-old and a (b) 73-year-old lens. The identities of spots were confirmed using in-gel tryptic digestion followed by mass spectrometry.
To look in greater detail at changes in the other more minor protein components associated with SG2, amine-reactive isobaric tagging technology (iTRAQ)24 was used. In the absence of labeled internal standards, iTraq technology can provide information on the relative abundance of individual polypeptides within samples. The four-plex approach was used, and the proteomic data from SG2 isolated from two younger lenses (ages 49 and 50) was compared with SG2 isolated from two older lenses (ages 71 and 72). In this analysis, the results from the two younger lenses were averaged and compared with the corresponding data from the two older lenses. These particular ages were chosen to enable a comparison of the protein composition of SG2 at its earliest stage of formation (at around age 50) with that of SG2 from older lenses.
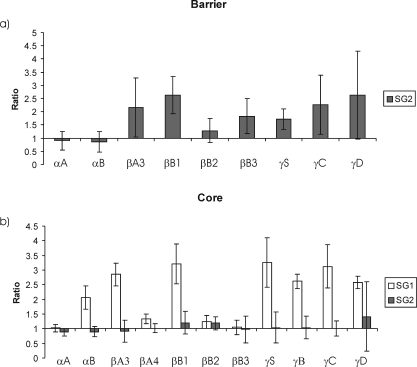
The results demonstrated that the other major β- and γ-crystallins could be detected in SG2 and that their amounts increased with age (Fig. 6). The exceptions were the αA and αB crystallins, each of which showed small apparent declines.
Figure 6.
Changes in the relative abundance of lens crystallins in (a) SG2 from the barrier region and (b) SG1 and SG2 from the core. The four-plex iTraq technology technique24 was used to compare the relative amount of each crystallin present in SG2 (or SG1 in b) from a 71- and a 72-year-old lens with the amount present in the same fraction isolated from a 49- and a 50-year-old lens. Error bars represent the combined SD between age groups from duplicate experiments. In this method of analysis, a value of 1 on the y-axis means that there has been no change in relative abundance of that protein between young and old groups. A value >1 indicates a larger relative amount of that polypeptide.
When the data sets from Figures 5 and 6 are compared, it is apparent that αA and αB crystallins are the dominant polypeptides in SG2 at both ages but that there is a small increase in the relative amounts of the other crystallins in the older lenses. This reflects the fact that reagent technology provides a relative comparison of the amounts of each individual crystallin polypeptide. The iTraq technology analyses revealed a small relative decrease in a large absolute amount of αA and αB crystallins. Therefore, increases with age in the amounts of the β- and γ-crystallins, though small in absolute terms, resulted in apparent relative decreases in the content of the α-crystallins. βB2-crystallin increased to a lesser extent than the other crystallins.
To determine whether the age-dependent binding of crystallins in the high-density (SG2) fraction from the barrier region was a general phenomenon throughout the lens, SG2 from the core of the same lenses was examined. A similar pattern for αA and αB crystallins was observed (Fig. 6b); however, in this case, the changes in γ- and β-crystallins were less pronounced. βA3 responded in the same way as did the α-crystallins, displaying a lower relative abundance in SG2 from the older lenses. The core region of older lenses is more complex because it contains both SG1 and SG2, whereas the barrier contains only SG2. Therefore, a more valid comparison was to compare the changes with age in SG2 from the barrier, with the age-related changes in both SG1 and SG2 from the core. When this was done, a closer match in terms of the proteins that increase in relative abundance with age in the higher density bands was obtained (Fig. 6b). The core data also showed that some crystallin subunits appear to be preferentially localized at SG1. All γ- and most β-crystallins increased in relative abundance in (SG1 + SG2) from the core region of older lenses compared with SG1+ SG2 from the younger lenses; βB3 was the exception, and βB2 increased to a lesser extent.
Changes in Protein Density Reproduced by Heating
A likely cause of the increased binding of proteins to cell membranes from older lenses observed in this study is protein denaturation. Interaction of the hydrophobic portions of unfolded proteins with membrane lipids would be expected to be a favorable process. To examine this hypothesis, intact young human lenses were incubated at 50°C to promote thermal denaturation of proteins.
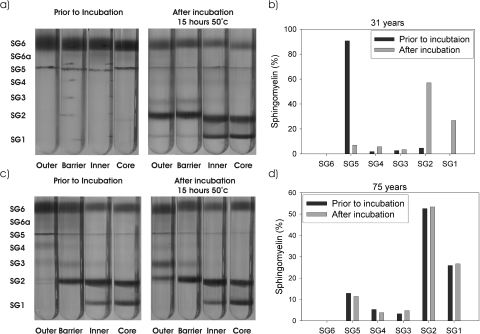
Sucrose density gradients from a 31-year-old and a 75-year-old lens pair before and after incubation at 50°C are depicted in Figure 7. As is clear, on heating there were major changes in protein density in the 31-year-old lens when compared with the nonincubated lens (Fig. 7a). Consistent with previous data, in the nontreated young lens, all protein in the outer, barrier, inner, and core regions was found in two interfaces, SG6 and SG5, which correspond to water-soluble protein and native membranes. After thermal stress, two distinct high-density bands were observed that appeared identical to those observed in older lenses (Fig. 1). This was found to be reproducible in a number of young lenses after incubation (data not shown).
Figure 7.
The effect of mild thermal stress on the appearance of sucrose density gradient profiles. Sucrose gradients of the four regions of a (a) 31-year-old lens pair and a (c) 75-year-old lens pair before (left) and after (right) incubation at 50°C for 15 hours. (b, d) Sphingomyelin content at each interface of the sucrose gradients from the core regions before and after incubation.
In the core and inner regions, approximately 50% of the water-soluble protein was lost after heating, with a similar loss of native membrane. Correspondingly, there was an increase of protein at the SG1 and SG2 interfaces, with smaller amounts of protein observed at SG3 or SG4.
The changes observed in the barrier and outer regions after incubation were also similar to those observed in the older normal lenses, with large amounts of protein sedimenting at SG2. Examination of the proteins in SG2 by 1D electrophoresis showed the presence of α-crystallins as the major components, with βB1 and βA3/A1 crystallins in lower levels (data not shown). This was further confirmed by 2D gel electrophoresis of SG2 from a heated young lens. The gel appeared almost identical with SG2 from the aged lenses used in this study (data not shown). Interestingly, as was also observed in the aged lenses, no protein was detected at SG1 in the barrier region of the heated lens.
To confirm that the increase in protein density in the heated lens was caused by membrane binding, each interface in the lens core was also examined for sphingomyelin content. As depicted in Figure 7b, the increase in protein density in the incubated lens of both SG1 and SG2 was matched by an increase in the amount of sphingomyelin. This coincided with a loss of sphingomyelin from the native membrane (SG5). Again, these changes mimic those observed in the aging human lens.
If heat were indeed responsible for this transformation in older normal human lenses, it would be predicted that thermally stressing an older lens may result in few changes given that these should have already taken place in the eye as a result of exposure to body temperature over a period of decades.
To test this hypothesis, a 75-year-old lens was subjected to the same thermal stress protocol. In this case, no changes in either protein density or sphingomyelin content were observed, suggesting that no further protein denaturation or membrane association can be induced in the barrier, inner, or core regions of an older lens (Figs. 7c, 7d). By contrast, since the outer part of an aged lens consists of recently synthesized proteins and membranes, it would be expected that the thermal stress might induce changes in the appearance of the sucrose gradient profile of this region. Indeed this was the only part of the older lens to display a noticeable change, which mirrored that observed in the oldest normal lens (Fig. 1).
Discussion
Major binding of crystallins to membranes occurs after middle age; however, the results differ in the lens cortex and in the nucleus. In the nucleus of the human lens this process takes place between the ages of 40 and 50.12 In the cortex, the major high-density band (SG2) became more obvious in the barrier region slightly later, at around age 50, and a delay was more evident in the more recently synthesized outer region, which showed few density changes except in the oldest lenses.
In the two nuclear regions, approximately 50% of crystallins become bound to fiber cell membranes from older lenses, resulting in the formation of two high-density bands, SG1 and SG2.12 In the innermost cortical zone (the barrier), only one high-density band, SG2, was formed (Fig. 2).
The amount of protein detected in SG2 from the barrier region increased in an age-dependent manner. Most protein was shown by 2D gel electrophoresis to be α-crystallin. This finding is consistent with the literature in that this crystallin is well known to have binding affinity for lipids25–27 and to associate with lens membranes.19,28–31 Interaction of α-crystallin may well begin in young lenses30 and may undergo age-related modifications thereafter. Multiple more negatively charged forms of both αA and αB crystallin were also evident as major spots in the 2D gels (Fig. 5). Deamidation or phosphorylation could be responsible for these isoforms.5,22,23 Deamidation may contribute to the denaturation of crystallins in the aged lens because insoluble protein contains higher amounts6 of deamidated crystallins. In agreement with Chandrasekher and Cenedella18 in our 2D gels of SG2, the relative abundances of the negatively charged forms of both α-crystallins appear to be greater than seen in published reports of 2D gels of lens proteins.1,5 This could indicate preferential membrane association of the phosphorylated/deamidated isoforms. Truncated forms of α-crystallins, analogous to those described in older human lenses,32 were also evident in 2D gels of SG2, and this may be related to the recent finding that peptides derived from α-crystallin can promote crystallin aggregation.33
Compared with middle-aged (49/50 years) lenses, the relative quantities of other crystallins in SG2 increased with age. SG2 purified from older (72/73 years) lenses was found, using iTraq technology, to contain relatively higher amounts of the most abundant β- and γ-crystallins. There appeared to be a degree of selectivity in terms of the particular crystallins involved, with relatively lower amounts of βB2 and βB3 crystallins present in the higher density band (Fig. 6). Despite these increases in β- and γ-crystallins in older lenses, α-crystallin remained the major protein component of the high-density band. One interpretation of these data is that high molecular weight protein, which consists largely of α-crystallin complexed with other denatured crystallins,34,35 could be binding initially to the cell membranes36 and that, in older lenses, most other β- and γ-crystallins become progressively more abundant because they associate with the membrane-bound aggregates.
One distinctive finding was that aging of the barrier region resulted in the formation of just a single high-density band, SG2. In the nuclear regions from the same lenses, SG2 was always accompanied by SG1. The reason for this difference is not yet clear. One possibility is that the composition of the membrane phospholipids in the two regions is different. Major changes occur in the lipid composition of the human lens with age,10,37–39 and these are being more fully characterized in lens regions using newly developed lipidomic procedures (manuscript in preparation). Differences in protein composition in the two regions40–42 and rates of posttranslational modification of integral membrane proteins such as aquaporin 043 may also play a role.
The consequences of such large-scale binding of proteins to cell membranes in older lenses are being investigated. It has been proposed that crystallin binding occludes membrane pores, for example those of gap junctions, which normally facilitate cell-to-cell movement of small molecules. In this manner, a barrier to diffusion could be formed. This barrier not only diminishes the flux of glutathione from the lens cortex,13 it also reduces the movement of reactive molecules in the reverse direction. Thus, decomposition of spontaneously unstable species in the nucleus is increased, and this inevitably promotes posttranslational modification44 and ultimately age-related nuclear cataract.
It is clear from our data that if occlusion of membrane pores results from membrane binding of proteins, this process is not confined to the barrier region. Rather, it occurs throughout the interior of the older lens because age-related membrane association was observed in the inner and core regions. Substantial blocking of cell membrane pores could, therefore, ultimately result in nuclear cataract by inducing oxidative conditions in the lens center. It is also possible that large-scale protein-membrane association in the lens center could lead to physical changes, such as stiffening, which has been linked with the onset of presbyopia.11
A remarkable feature of the present study was that major changes in the appearance of the sucrose density gradients could be induced by mild thermal stress (50°C, 15 hours). By exposing an intact young lens to this temperature, crystallin binding to membranes was initiated, and, in this way, the protein pattern from a heated young lens was converted to one that appeared identical with that of an older lens (Fig. 7). In effect, a young lens was converted overnight into an “old lens” by mild thermal stress. We observed similar changes, over a longer period, by exposing lenses to 45°C (data not shown).
Exposure of human lenses to 50°C can be considered mild stress because the lenticular temperature recorded in the eyes of monkeys exposed to the midday sun in New Delhi reached 42°C within minutes.45 A surprising feature of the lens is that its temperature seems to be remarkably sensitive to changes in ambient temperature and to irradiation. If one can extrapolate from animal data,45,46 the range in temperatures of the human lens under conditions present on earth may span at least 13°C!
One conclusion is that exposure to heat over a period of decades may be responsible for human age-related lens changes. Although in this experiment temperature was the only agent used, it could be argued that any factor that promotes protein denaturation within the eye could also be implicated in lens changes. For example, deamidation,6,23 racemization,47,48 and other abundant posttranslational modifications44,49–51 may contribute to the protein denaturation that occurs gradually in the human lens. Although these factors undoubtedly contribute, other changes to membrane lipids that we have recently characterized support the contention that temperature may indeed be a primary agent causing deterioration (manuscript in preparation).
Therefore, a tentative conclusion is that exposure of the lifelong proteins and membranes in the human lens to ocular temperature over a period of decades may be responsible for the age-related changes to this tissue. Further experiments are under way to test the proposition that interactions between denatured proteins and older cell membranes may ultimately underpin the formation of age-related nuclear cataract and possibly other age-related visual disorders, such as presbyopia.
Acknowledgments
The authors thank Jane Deeley for help with lipid analysis, and Raj Devasahayam and Meidong Zhu of the Sydney Lions Eye Bank for providing the human lenses.
Footnotes
Supported by National Institutes of Health Grant EY013570 and National Health and Medical Research Council Grant 512334. RJWT is an NHMRC Senior Research Fellow.
Disclosure: M.G. Friedrich, None; R.J.W. Truscott, None
References
- 1. Garland DL, Duglas TY, Jimenez AJ, et al. The nucleus of the human lens: demonstration of a highly characteristic protein pattern by two-dimensional electrophoresis and introduction of a new method of lens dissection. Exp Eye Res. 1996;62:285–291 [DOI] [PubMed] [Google Scholar]
- 2. Kuszak JR. The development of lens sutures. Prog Retin Eye Res. 1995;14:567–591 [Google Scholar]
- 3. Lynnerup N, Kjeldsen H, Heegaard S, et al. Radiocarbon dating of the human eye lens crystallines reveal proteins without carbon turnover throughout life. PLoS ONE. 2008;3:e1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng R, Feng Q, Argirov OK, et al. Structure elucidation of a novel yellow chromophore from human lens protein. J Biol Chem. 2004;279:45441–45449 [DOI] [PubMed] [Google Scholar]
- 5. Lampi KJ, Ma Z, Hanson SRA, et al. Age-related changes in human lens crystallins identified by two-dimensional electrophoresis and mass spectrometry. Exp Eye Res. 1998;67:31–43 [DOI] [PubMed] [Google Scholar]
- 6. Wilmarth PA, Tanner S, Dasari S, et al. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: does deamidation contribute to crystallin insolubility? J Proteome Res. 2006;5:2554–2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ball LE, Little M, Nowak MW, et al. Water permeability of C-terminally truncated aquaporin 0 (AQP0 1–243) observed in the aging human lens. Invest Ophthalmol Vis Sci. 2003;44:4820–4828 [DOI] [PubMed] [Google Scholar]
- 8. Zhang Z, Smith DL, Smith JB. Human beta-crystallins modified by backbone cleavage, deamidation and oxidation are prone to associate. Exp Eye Res. 2003;77:259–272 [DOI] [PubMed] [Google Scholar]
- 9. Yappert MC, Borchman D. Sphingolipids in human lens membranes: an update on their composition and possible biological implications. Chem Phys Lipids. 2004;129:1–20 [DOI] [PubMed] [Google Scholar]
- 10. Huang L, Grami V, Marrero Y, et al. Human lens phospholipid changes with age and cataract. Invest Ophthalmol Vis Sci. 2005;46:1682–1689 [DOI] [PubMed] [Google Scholar]
- 11. Heys K, Friedrich M, Truscott RJW. Presbyopia and heat: changes associated with aging of the human lens suggest a functional role for the small heat shock protein, alpha-crystallin, in maintaining lens flexibility. Aging Cell. 2007;6:807–815 [DOI] [PubMed] [Google Scholar]
- 12. Friedrich MG, Truscott RJW. Membrane association of proteins in the aging human lens: profound changes take place in the 5th decade of life. Invest Ophthalmol Vis Sci. 2009;50:4786–4793 [DOI] [PubMed] [Google Scholar]
- 13. Sweeney MH, Truscott RJ. An impediment to glutathione diffusion in older normal human lenses: a possible precondition for nuclear cataract. Exp Eye Res. 1998;67:587–595 [DOI] [PubMed] [Google Scholar]
- 14. Moffat BA, Landman KA, Truscott RJ, et al. Age-related changes in the kinetics of water transport in normal human lenses. Exp Eye Res. 1999;69:663–669 [DOI] [PubMed] [Google Scholar]
- 15. Folch J, Lees M, Stanley G. A simple method for the isolation and purification of total lipides from animal tissue. J Biol Chem. 1957;226:497–509 [PubMed] [Google Scholar]
- 16. Deeley JM, Mitchell TW, Wei X, et al. Human lens lipids differ markedly from those of commonly used experimental animals. Mol Cell Biol Lipids. 2008;1781:288–298 [DOI] [PubMed] [Google Scholar]
- 17. Evans FF, Raftery MJ, Egan S, et al. Profiling the secretome of the marine bacterium Pseudoalteromonas tunicata using amine-specific isobaric tagging (iTRAQ). J Proteome Res. 2007;6:967–975 [DOI] [PubMed] [Google Scholar]
- 18. Chandrasekher G, Cenedella RJ. Protein associated with human lens ‘native’ membrane during aging and cataract formation. Exp Eye Res. 1995;60:707–717 [DOI] [PubMed] [Google Scholar]
- 19. Boyle DL, Takemoto L. EM immunolocalization of alpha-crystallins: association with the plasma membrane from normal and cataractous human lenses. Curr Eye Res. 1996;15:577–582 [DOI] [PubMed] [Google Scholar]
- 20. Byrdwell WC, Borchman D, Porter RA, et al. Separation and characterization of the unknown phospholipid in human lens membranes. Invest Ophthalmol Vis Sci. 1994;35:4333–4343 [PubMed] [Google Scholar]
- 21. Colvis C, Garland D. Posttranslational modification of human alpha A-crystallin: correlation with electrophoretic migration. Arch Biochem Biophys. 2002;397:319–323 [DOI] [PubMed] [Google Scholar]
- 22. Spector A, Chiesa R, Sredy J, et al. cAMP-dependent phosphorylation of bovine lens alpha-crystallin. Proc Natl Acad Sci U S A. 1985;82:4712–4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hains P, Truscott RJ. Age-dependent deamidation of life-long proteins in the human lens. Invest Ophthalmol Vis Sci. 2010;51:3107–3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zieske LR. A perspective on the use of iTRAQ reagent technology for protein complex and profiling studies. J Exp Bot. 2006;57:1501–1508 [DOI] [PubMed] [Google Scholar]
- 25. Borchman D, Tang D. Binding capacity of alpha-crystallin to bovine lens lipids. Exp Eye Res. 1996;63:407–410 [DOI] [PubMed] [Google Scholar]
- 26. Tang D, Borchman D, Yappert MC, et al. Influence of cholesterol on the interaction of alpha-crystallin with phospholipids. Exp Eye Res. 1998;66:559–567 [DOI] [PubMed] [Google Scholar]
- 27. Cobb BA, Petrash JM. Factors influencing α-crystallin association with phospholipid vesicles. Mol Vis. 2002;8:85–93 [PMC free article] [PubMed] [Google Scholar]
- 28. Cenedella RJ, Fleschner CR. Selective association of crystallins with lens ‘native’ membrane during dynamic cataractogenesis. Curr Eye Res. 1992;11:801–815 [DOI] [PubMed] [Google Scholar]
- 29. Cobb BA, Petrash JM. Structural and functional changes in the alpha-A-crystallin R116C mutant in hereditary cataracts. Biochemistry. 2000;39:15791–15798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cobb BA, Petrash JM. Characterization of alpha-crystallin-plasma membrane binding. J Biol Chem. 2000;275:6664–6672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mulders JW, Wajcik E, Bloemendal H, et al. Loss of high-affinity membrane binding of bovine nuclear alpha-crystallin. Exp Eye Res. 1989;49:149–152 [DOI] [PubMed] [Google Scholar]
- 32. Harrington V, McCall S, Huynh S, et al. Crystallins in water soluble-high molecular weight protein fractions and water insoluble protein fractions in aging and cataractous human lenses. Mol Vis. 2004;10:476–489 [PubMed] [Google Scholar]
- 33. Santhoshkumar P, Udupa P, Murugesan R, et al. Significance of interactions of low molecular weight crystallin fragments in lens aging and cataract formation. J Biol Chem. 2008;283:8477–8485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rao PV, Huang QL, Horwitz J, et al. Evidence that alpha-crystallin prevents non-specific protein aggregation in the intact eye lens. Biochim Biophys Acta. 1995;1245:439–447 [DOI] [PubMed] [Google Scholar]
- 35. Srivastava K, Chaves JM, Srivastava OP, et al. Multi-crystallin complexes exist in the water-soluble high molecular weight protein fractions of aging normal and cataractous human lenses. Exp Eye Res. 2008;87:356–366 [DOI] [PubMed] [Google Scholar]
- 36. Cobb BA, Petrash JM. Alpha-crystallin chaperone-like activity and membrane binding in age-related cataracts. Biochemistry. 2002;41:483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li LK, So L, Spector A. Membrane cholesterol and phospholipid in consecutive concentric sections of human lenses. J Lipid Res. 1985;26:600–609 [PubMed] [Google Scholar]
- 38. Borchman D, Byrdwell WC, Yappert MC. Regional and age-dependent differences in the phospholipid composition of human lens membranes. Invest Ophthalmol Vis Sci. 1994;35:3938–3942 [PubMed] [Google Scholar]
- 39. Li LK, So L, Spector A. Age-dependent changes in the distribution and concentration of human lens cholesterol and phospholipids. Biochim Biophys Acta. 1987;917:112–120 [DOI] [PubMed] [Google Scholar]
- 40. Thomson JA, Augusteyn RC. Ontogeny of human lens crystallins. Exp Eye Res. 1985;40:393–410 [DOI] [PubMed] [Google Scholar]
- 41. Li LK, Roy D, Spector A. Changes in lens protein in concentric fractions from individual normal human lenses. Curr Eye Res. 1986;5:127–135 [DOI] [PubMed] [Google Scholar]
- 42. Fagerholm PP, Philipson BT, Lindstrom B. Normal human lens—the distribution of protein. Exp Eye Res. 1981;33:615–620 [DOI] [PubMed] [Google Scholar]
- 43. Korlimbinis A, Berry Y, Thibault D, et al. Protein aging: truncation of aquaporin 0 in human lens regions is a continuous age-dependent process. Exp Eye Res. 2008;88:966–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Korlimbinis A, Truscott RJW. Identification of 3-hydroxykynurenine bound to proteins in the human lens: a possible role in age-related nuclear cataract. Biochemistry. 2006;45:1950–1960 [DOI] [PubMed] [Google Scholar]
- 45. Al-Ghadyan AA, Cotlier E. Rise in lens temperature on exposure to sunlight or high ambient temperature. Br J Ophthalmol. 1986;70:421–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schwartz B. Environmental temperature and the ocular temperature gradient. Arch Ophthalmol. 1965;74:237–243 [DOI] [PubMed] [Google Scholar]
- 47. Masters PM, Bada JL, Zigler J., Jr Aspartic acid racemisation in the human lens during ageing and in cataract formation. Nature. 1977;268:71–73 [DOI] [PubMed] [Google Scholar]
- 48. Fujii N, Ishibashi Y, Satoh K, et al. Simultaneous racemization and isomerization at specific aspartic acid residues in alpha B-crystallin from the aged human lens. Biochim Biophys Acta. 1994;1204:157–163 [DOI] [PubMed] [Google Scholar]
- 49. Korlimbinis A, Aquilina JA, Truscott RJW. Protein-bound and free UV filters in cataract lenses: the concentration of UV filters is much lower than in normal lenses. Exp Eye Res. 2007;85:219–225 [DOI] [PubMed] [Google Scholar]
- 50. Ahmed N, Thornalley PJ, Dawczynski J, et al. Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins. Invest Ophthalmol Vis Sci. 2003;44:5287–5292 [DOI] [PubMed] [Google Scholar]
- 51. Linetsky M, Shipova E, Cheng R, et al. Glycation by ascorbic acid oxidation products leads to the aggregation of lens proteins. Biochim Biophys Acta. 2008;1782:22–34 [DOI] [PMC free article] [PubMed] [Google Scholar]