This study is the first report of successful transduction of lacrimal gland cells with an adeno-associated virus vector and demonstration of a durable reduction of inflammatory lacrimal gland and ocular surface pathology.
Abstract
Purpose.
To evaluate the effect of adeno-associated virus (AAV) vector–mediated viral (v)IL-10 gene expression on lacrimal gland (LG) immunopathology and ocular surface disease in a rabbit model of induced autoimmune dacryoadenitis (ID).
Methods.
Autologous peripheral blood lymphocytes, activated in a mixed-cell reaction when cocultured with purified rabbit lacrimal epithelial cells, induce a Sjögren's-like autoimmune dacryoadenitis when injected directly back into the donor animal's inferior LG. Four weeks after disease induction, AAV vector expressing the vIL-10 gene under control of a tetracycline-inducible promoter was injected into the inferior LG of the treatment group (ID/Rx), and doxycycline was fed orally to induce transgene expression. The ID group serving as control also received doxycycline. All LGs were removed 16 weeks after disease induction.
Results.
Clinical symptoms showed overall improvement in the ID/Rx group compared with the ID group. Histopathologic examination of the ID group's LG revealed scattered large lymphocytic foci and areas of altered or distorted acini, whereas the ID/Rx group had scattered small lymphocytic foci. The number of CD18+ cells was almost fivefold lower in the ID/Rx group than in the ID group. Although the total number of RTLA+ cells did not differ between the groups, the CD4/CD8 ratio was 16-fold smaller in the ID/Rx group.
Conclusions.
Animals with experimentally induced autoimmune dacryoadenitis appeared to benefit from AAV-mediated vIL-10 gene transfer therapy. Quantitative immunohistochemical analysis suggested that the therapy might not have been simply immunosuppressive but rather supported the induction of CD8+ regulatory cells.
Normal tear film is crucial to the integrity and function of the human cornea and conjunctiva. Disturbance of the homogeneity of the tear film gives rise to a chronic condition known as keratoconjunctivitis sicca, or dry eye. One of the most severe forms of dry eye is found in patients with Sjögren's syndrome, an inflammatory autoimmune disorder characterized by lymphocytic infiltration of the lacrimal gland (LG) and affecting approximately 2 to 4 million of the population in the United States. The lymphocytic infiltrates produce immune mediators that act as toxic factors, resulting in reduced secretory function caused by secretory tissue atrophy and dysfunction of the surviving tissue. Although the pathogenesis of the disease is yet to be defined, increasing evidence shows that immunologic, genetic, hormonal, and environmental factors play crucial roles.
Sjögren infiltrates consist primarily of CD4+ and CD8+ T cells at a ratio of 4:1, plus IgG+ B cells and plasmacytes. The proinflammatory cytokines infiltrating lymphocytes produce include interleukins (IL)-1β, -6, -12, and -18, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α. In addition to these proinflammatory cytokines, anti-inflammatory cytokines, including IL-10, transforming growth factor-β, and IL-4 also have been detected. To some extent these are produced by glandular epithelial cells rather than by lymphocytes.1 Numerous studies have shown that administering or increasing expression of specific inhibitors of proinflammatory cytokines (sTNFR, IL-ra, anti–TNF-α) or anti-inflammatory cytokines (IL-10 and IL-4) can moderate inflammatory signals and suppress harmful effector functions in autoimmune disorders.2 Among these approaches, vector-mediated transduction of IL-10 has received increased attention for its promising therapeutic potential.
IL-10 is produced by many activated immune cell types, including type 2 helper T cells, T regulatory cells, NKT cells, B cells, monocytes, and macrophages. IL-10 is pleiotropic, but it is considered an immunoregulatory cytokine because of its inhibitory effects on the expression of a large spectrum of proinflammatory cytokines and other inflammatory mediators, such as chemokines, major histocompatibility complex (MHC-II) molecules, and costimulatory molecules. IL-10 has been shown to attenuate inflammation in various experimental models, including arthritis, diabetes, uveitis, systemic lupus erythematosus, chronic renal disease, orchitis, and uveoretinitis.3–8 Injected IL-10 is short-lived and requires repeated administration, whereas viral vector–mediated IL-10 gene transfer therapy provides an extended source of therapeutic IL-10.
Previous publications have demonstrated that rabbit peripheral blood lymphocytes (PBLs) proliferate when cocultured with autologous LG epithelial cells. These activated lymphocytes induce dacryoadenitis within 2 weeks after having been injected into the donor rabbit's remaining, contralateral LG, thus providing an induced in vivo model of autoimmune dacryoadenitis.9,10 The histopathology of the induced disease is characterized by lymphocytic foci containing large proportions of CD4+ T cells. The inflammatory process is accompanied by LG dysfunction, characterized by reduced tear production, and by signs of ocular surface inflammation, characterized by reduced tear film stability and increased Rose Bengal staining. These histopathologic features and clinical manifestations mimic those associated with Sjögren syndrome. The severity of the induced disease shows no signs of abatement but, rather, increases with time over a period of 6 months after induction.11
We have reported that the transfer of viral (v)IL-10 with an adenoviral vector (AdIL-10) produces prophylaxis against LG immunopathology and ocular surface disease induced by subsequent autoadoptive transfer of activated lymphocytes.7 However, as in other tissues, adenovirus-mediated gene transfer results only in transient transgene expression, with vIL-10 detectable in tears for <2 weeks after transduction. Adeno-associated virus (AAV) vectors have been found to mediate long-term transgene expression with minimal pathogenicity in many organs.12,13 The current work was undertaken to evaluate the long-term effect of AAV vector-mediated transduction of a vIL-10 gene construct incorporating the tetracycline-inducible tetON system (AAV-tetON–vIL-10). To our knowledge, this is the first report of successful transduction of LG cells with an AAV vector and the first to demonstrate durable reductions of inflammatory LG and ocular surface disorders.
Materials and Methods
Cell Culture Media and Reagents
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum were purchased from Omega Scientific (Tarzana, CA). Ham's F12 medium, glutamine, and antibiotic-antimycotic mixture were purchased from Invitrogen-Gibco Products (Rockville, MD). Bovine serum albumin, soybean trypsin inhibitor, and linoleic acid were purchased from Sigma-Aldrich (St. Louis, MO). Tritiated thymidine was purchased from DuPont NEN Research Products (Wilmington, DE). Antibody for neutralizing IL-10 was obtained from BD PharMingen (San Jose, CA; cat. no. 554495). Antibodies specific for rabbit CD4 (MRB4020), CD8 (MRB8020), CD18 (MRB1820), and MHCII (MRB9952) were purchased from Antigenix America (Huntington Station, NY), and antibodies specific for RTLA (rabbit T lymphocyte antigen) (CL8800) were obtained from Cedarlane Laboratories (Hornby, Ontario, Canada). Biotinylated species-specific secondary antibodies were obtained from Chemicon International (Temecula, CA). ABC reagent and aminoethylcarbazole were obtained from Vector Laboratories, Inc. (Burlingame, CA). Schirmer strip paper was purchased from Rose Stone Enterprises (Alta Loma, CA). Fluorescein strips (FUL-GLO) and Rose Bengal strips were purchased from Akorn Inc. Laboratories (Buffalo Grove, IL).
Animals, Surgical Procedures, and Clinical Assessments
Adult female New Zealand White rabbits (3.5–4 kg) were obtained from Irish Farms (Norco, CA). All animals were used in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Animals were maintained in a facility fully accredited by the American Association of Laboratory Animal Science.
Tear production was assessed by Schirmer's test with anesthesia. Tear break-up time (BUT) was evaluated by adding 5 μL of 2% fluorescein at the middle of the lower eyelid and examining the tear film with a slit-lamp biomicroscope equipped with a blue filter. Rose Bengal staining was assessed after the addition of 5 μL of 1% Rose Bengal solution to the middle of the lower eyelid; results were recorded on a cornea diagram and scored using a standardized grading system. All eyes were examined initially to establish baseline data; no corneal defects were detected, and no animals were excluded. Eyes with diseased LGs (OD) were assessed every 2 weeks initially and then every 4 weeks.10
After anesthesia, the OS LG was surgically removed from each rabbit for preparation of purified LG epithelial cells (pLGECs), as described previously.10,14 Peripheral blood was obtained for isolation of lymphocytes for the mixed-cell reactions. Bacitracin-neomycin-polymyxin veterinary ophthalmic ointment (Pharmaderm; Altana, Inc., Melville, NY) was applied after surgery. Intramuscular injections of buprenorphine HCl (Reckitt & Colman, Hull, UK), 20 μg/kg, were administered twice daily for the first 2 postoperative days.
Vectors
The generation of AAV serotype 2 vectors encoding Epstein-Barr virus-derived IL-10 under the control of the tetracycline-inducible tetON system (AAV2-tetON–vIL-10, AAV-vIL10, and AAV-GFP were from Genethon, Nantes-Evry, France) has been described elsewhere.15 Titer of tetON-inducible AAV vector was 1 × 1011 plaque-forming units (pfu)/mL. Doxycycline regulation of transgene expression by the AAV2-tetON–vIL-10 vectors used in this study has been described previously.15 In all experiments, vIL-10 expression was activated by the addition of 200 mg/kg/d doxycycline (purchased from the university pharmacy) added to the drinking water from day 1 after vector injection.
Transduction Efficiency
Descriptions of LG excision and purification, culture of LG epithelial cells (LGECs), and mixed-cell reactions between pLGEC and autologous PBL have been previously published.9
For in vitro determination of transduction efficiency with an AAV vector, a confluent monolayer of pLGECs was inoculated with AAV-GFP at a multiplicity of infection (MOI) of 500. Cells were stained with DAPI 24 hours later to label all cells; they were then examined by fluorescence microscopy to determine the percentage of the total cells expressing GFP (data not shown).
Autologous Mixed-Cell Reaction
Autologous mixed-cell reactions were performed in 96-well plates, as previously described.9 The effect of AAV-IL 10 gene expression in an autologous mixed-cell reaction was determined by [3H]thymidine incorporation assay. Briefly, pLGECs were transduced with AAV-IL-10 at an MOI of 500. Two days later an equal amount of PBL was added to wells containing nontransduced or AAV-IL-10–transduced and γ-irradiated (25 Gy) pLGECs. Controls included irradiated pLGECs alone and PBL alone. AAV-vIL-10–transduced pLGECs were also cocultured with PBL in the presence and absence of doxycycline. Neutralization studies of IL-10 were performed with three different concentrations of antibodies against human IL-10 (10, 50, and 1000 ng/mL). After 5 days of coculture, the samples were pulsed with 1 μCi/well [3H]thymidine (New England Nuclear, Boston, MA); 24 hours later, the cells were collected using a sample harvester (model 290 PHD; Brandel, Gaithersburg, MD). A beta scintillation counter (model LS 6000IC; Beckman Instruments, Inc., Fullerton, CA) was used to measure [3H]thymidine incorporation. Each condition was replicated in six wells.
To obtain activated lymphocytes for in vivo experiments, the mixed-cell reactions were carried out in 12-well plates, as described, but without the transduction of pLGECs. Parallel mixed-cell reactions were performed in 96-well plates to monitor [3H]thymidine incorporation. Only animals yielding mixed-cell reactions with stimulation indices greater than 2 were used for study.
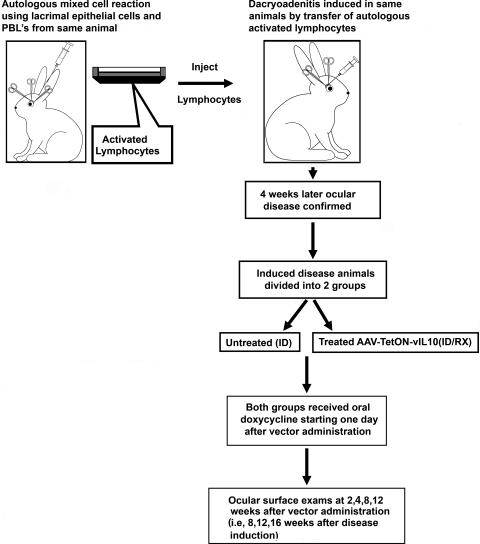
Activated lymphocytes were collected from the 12-well plates and injected back into the central regions of the inferior OD LG of each respective donor rabbit, as described previously.14 Autoimmune dacryoadenitis was induced in 16 adult New Zealand White rabbits. Eight of the 16 rabbits served as untreated disease controls (designated as the ID group). Eight of the 16 (designated as the ID/Rx group) received AAV2-tetON–vIL-10 (1 × 108 pfu in 200 μL injected into the inferior LG). Animals were killed 16 weeks after disease induction (i.e., 12 weeks after vector injection and initiation of doxycycline administration). An overview of the protocol time sequence is provided in Figure 1.
Figure 1.
Time sequence of experiments. Left: inferior lacrimal gland was removed from the rabbit, and autologous mixed-cell reaction was performed. Right: stimulated lymphocytes were injected into each donor rabbit's remaining lacrimal gland to induce dacryoadenitis. After 4 weeks, the first clinical assessments were performed. Once the disease was confirmed, the rabbits were randomly divided into two study groups. One group received AAV TetON-vIL-10 (ID/Rx), and the other group remained untreated (ID). Both groups received doxycycline in the drinking water (200 mg/kg body weight) from the next day of vector administration. The second clinical assessments were performed 2, 4, 8, and 12 weeks after vector administration (i.e., 6, 8, 12, and 16 weeks after disease induction).
ELISA for IL-10
Samples of supernatant culture media from AAV-tetON–vIL-10–transduced pLGECs were collected at different time points and diluted for the ELISA. Supernatants from transduced cells without doxycycline served as controls. Tear samples were also collected from in vivo–transduced rabbits at different time points, as described elsewhere.16 IL-10 concentrations were quantified using the IL-10 Hu+V kit (BD Bioscience, San Jose, CA) according to the manufacturer's instructions.
Tissue Collection and Histopathology
The OD inferior LGs were removed at necropsy and dissected longitudinally. One portion was fixed in 10% formalin and embedded in paraffin. The paraffin-embedded sections were stained with hematoxylin and eosin and examined in a double-blind fashion. The other portion was embedded in optimal cutting temperature compound and cryosectioned at 7 μm for immunostaining. Cryosections were fixed in chilled acetone, air dried, rehydrated in phosphate-buffered saline, and blocked in 5% bovine serum albumin for 15 minutes.11 The sections were incubated at room temperature for 1 hour with the primary antibody at the following dilutions: mouse anti-rabbit CD4 (1:200), mouse anti-rabbit CD8 (1:200), mouse-anti rabbit CD18 (1:1000), goat anti-rabbit RTLA (1:300), and mouse anti-rabbit MHCII (1:100). Sections were rinsed and incubated for 60 minutes with appropriate biotinylated secondary antibodies. After rinsing, the sections were quenched in 0.3% H2O2 in 40% methanol for 15 minutes and incubated in ABC reagent for 30 minutes, rinsed three times, and developed with AEC. The sections were again rinsed, counterstained with hematoxylin, and mounted for photography. The positive cells showed an intense brown color in the blue hematoxylin background. Entire sections were scanned (16–24 sections/antibody) and analyzed with an automated cellular imaging systems (Analysis 3.0; Olympus Soft Imaging System, Lakewood, CO). This combination of a proprietary, color-based imaging technology with an automated microscope provides quantitative data, including intensity scoring, and percentage of positively stained cells.
Statistical Analysis
Data from clinical assessments and quantitative imaging were analyzed with statistical software (SAS version 9.1; SAS Institute, Cary, NC). The paired t-test was used for within-group comparisons, and the t-test with Dunnett's correction and multiple pairwise comparisons was used for comparisons between the experimental groups and the normal control group. For thymidine incorporation assays we used ANOVA, and for pairwise comparison we used Bonferroni-adjusted P values.
Results
Efficiency of Transduction and Expression of IL-10
The efficiency of AAV vector–mediated transduction for rabbit LG cells was first determined in vitro using AAV-GFP. Approximately 50% of the cells fluoresced, indicating GFP expression, within 24 hours of inoculation (data not shown). The concentrations of IL-10 in samples of supernatant media from AAV-tetON–vIL-10–transduced pLGECs 10 and 28 days after induction with doxycycline were, respectively, twofold and fivefold higher than those from untreated, transduced pLGECs (Table 1). Thus, IL-10 expression reached a high level (2 μg/mL) rapidly and increased further over 2 weeks.
Table 1.
ELISA of vIL-10 Transgene Expression in Lacrimal Gland Cells after AAV-Mediated Transduction
| Day after Transduction | With Doxycycline Concentration (ng/mL) | Without Doxycycline Concentration (ng/mL) |
|---|---|---|
| 3 | 45.2 | 18 |
| 10 | 2025.5 | 930 |
| 28 | 2054.5 | 424 |
Cells were transduced with AAV tetON-vIL10. ELISA was performed on 10-μL samples from six different wells per time point collected on three different days.
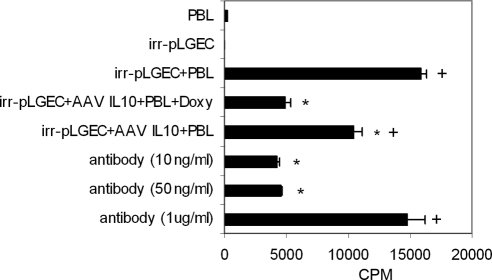
Incorporation of [3H]thymidine by PBL in mixed-cell reactions was reduced in reactions performed with AAV-tetON–vIL-10–transduced pLGEC compared with reactions using nontransduced pLGECs (P ≥ 0.05; Fig. 2). When mixed-cell reactions were performed with AAV-tetON–vIL-10 in the absence of doxycycline, there was significantly greater lymphocyte proliferation than with doxycycline (P ≤ 0.05). In addition, a high concentration of anti–IL-10 antibody significantly neutralized the suppression of lymphocyte proliferation in the presence of IL-10 (P ≤ 0.05). These findings indicated that expression of the AAV-transduced vIL-10 transgene product suppressed the ability of pLGECs to stimulate lymphocyte proliferation in the presence of doxycycline.
Figure 2.
The ability of irradiated pLGECs to stimulate lymphocyte proliferation was suppressed by the expression of IL-10 after transduction. Incorporation of [3H]thymidine by peripheral blood lymphocytes (1 × 105/well) cocultured with AAV-vIL-10-tetON transduced or nontransduced and irradiated pLGECs (1 × 105/well) in an autologous mixed-cell reaction (n = 6). Transduced pLGECs without doxycycline treatment showed increased lymphocytic proliferation compared with the doxycycline-treated group (n = 6). Suppression of lymphocytic proliferation in mixed-cell reaction with transduced pLGECs with doxycycline treatment was neutralized by IL-10 antibody (n = 6). *Statistically significant differences between irradiated nontransduced pLGEsC + PBL and all other groups. For multiple pairwise comparison, Bonferroni-adjusted P-value was used (P ≤ 0.05). +Significant differences between values for the AAV IL-10–transduced pLGECs + PBL + doxycycline and all other groups (P ≤ 0.05). CPM, cycles per minute.
ELISA revealed that tears from the ID/Rx group contained more IL-10 than did tears from the uninjected ID group (Table 2). The amount of IL-10 in tears remained constant for the ID group (55–56 pg/mL), whereas it increased over time in the ID/RX group; the significantly highest titer, 164 pg /mL, was detected 12 weeks after vector injection.
Table 2.
ELISA of IL-10 Transgene Expression in Tears from ID and ID/Rx Rabbits
| Weeks | ID (IL-10 pg/mL) | ID/Rx (IL-10 pg/mL) |
|---|---|---|
| 4 | 54.9 ± 17.9 | 73.0 ± 3.7 |
| 8 | 55.8 ± 1 | 74.4 ± 5.4 |
| 12 | ND | 164.1 ± 12.9* |
Tears were collected 6 weeks after disease induction (i.e., 4 weeks after AAV-IL-10 transduction and doxycycline feeding in ID/RX rabbits. ID rabbits were not transduced but were fed with doxycycline. Paired t-test was performed for within-group differences. Independent sample t-test was performed for comparison between AAV and ID groups. ND, not done.
Significant increase in 12 weeks.
Tear Production
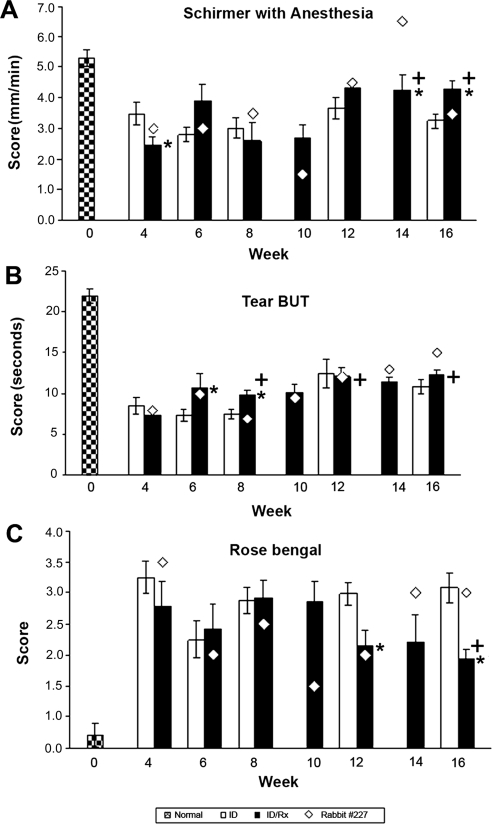
As in previous studies, aqueous tear production was significantly decreased in rabbits with induced dacryoadenitis (Fig. 3A). The animals with induced disease were randomly separated into two groups 4 weeks after disease induction. Tear production was reduced by 51% in the group that had been selected to receive treatment and by 37% in the group that was to remain untreated. Tear production in the untreated ID group remained essentially unchanged throughout the period of observation. As described in detail, one rabbit in the ID/Rx group (rabbit 227) responded differently from the remaining seven rabbits, and values for this rabbit are presented separately. Schirmer scores for the ID/Rx animals underwent a sequence of changes. Typically, improvement was obvious by week 12 and remained stable through week 16, by which time they had improved by 64%. Scores for rabbit 227 fluctuated but appeared to have deteriorated further by week 10, increased above the normal value by week 14, and deteriorated nearly to the pretreatment value by week 16 (week 10 and 14 data not shown for the ID group).
Figure 3.
Clinical ocular assessments. (A) Basal tear production. Schirmer test was performed on OD eyes of 16 rabbits (day 0). Stimulated lymphocytes from the autologous mixed-cell reactions were then injected into the remaining OD inferior LGs to induce disease. The LGs of the normal control group were not manipulated. To measure total tear production, a Schirmer strip was inserted in the lower fornix of OD eye for 1 minute, and the length of the wetted area of the strip was measured. Tear production was measured at indicated time points until 16 weeks after disease induction (further clarification for time points is given in Fig. 1). *P ≤ 0.05; statistically significant difference between the ID and ID/Rx animals at the indicated time points. P-values were calculated using independent sample t-tests. +P ≤ 0.05; significant differences between values for the AAV-treated animals at different times. P-values were calculated by paired t-test. (B) Tear break-up time (BUT) demonstrates tear instability. Group designations and statistical analyses were the same as for Figure 3A. BUT was evaluated by adding 5 μL of 2% fluorescein to the lower conjunctiva OD; the animal was allowed to blink several times to distribute the fluorescein on the cornea and was examined with a slit-lamp biomicroscope equipped with a blue filter. The time from opening of the eyes to the appearance of the first dry spot in the central cornea was measured three times, and the mean was recorded. (C) Rose Bengal. Designations for groups and statistical analyses are the same as in Figure 3A. Detection of deficiency in preocular tear film protection with Rose Bengal stain. Rose Bengal staining was assessed after the addition of 5 μL of 1% Rose Bengal solution to the OD lower conjunctiva. Results were recorded on a cornea diagram and scored using a standardized grading system.
Tear Break-up Time
As summarized in Figure 3B, by week 4 after disease induction, tear BUT was decreased to roughly 37% of the control value, both in the animals randomly assigned to the ID group and in the animals randomly assigned to the ID/Rx group. Tear BUT appeared to improve in the ID group, reaching 49% of the control value by week 16. It appeared to improve more in the ID/Rx group, reaching 58% of the control value by week 16. Thus, AAV treatment appeared to be responsible for reversing roughly 17% of lost tear BUT.
Rose Bengal Staining
Rose Bengal staining scores increased 11-fold by week 4 after disease induction, with no significant difference between the animals randomly assigned to the ID and the ID/Rx groups (Fig. 3C). Scores for the ID group fluctuated but by week 16 did not differ from the scores at week 4. Scores for the typical ID/Rx group appeared to undergo a series of changes mirroring the changes in Schirmer test scores. Animals' conditions appeared to improve by week 6, recrudesce between weeks 8 and 10, improve by week 12, and remain stable through week 16. Although scores in the typical ID/Rx group remained seven-fold greater than the control value by week 16, they were 40% lower than in the ID group (P < 0.05). Like the Shirmer test scores, Rose Bengal staining scores for rabbit 227 fluctuated but did not appear to differ from the pretreatment value by weeks 14 and 16 (weeks 10 and 14 data not shown for the ID group).
Histopathology
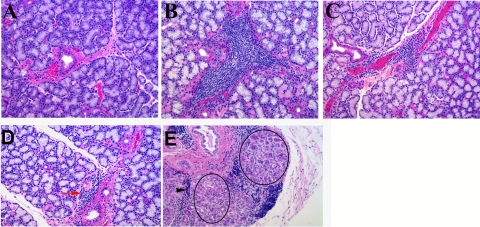
Representative hematoxylin and eosin–stained paraffin sections from normal, ID, and ID/Rx LGs are presented in Figures 4A, 4B, and 4C, respectively. The LGs of normal animals contained occasional small immune cell aggregates. Scattered focal periductal lymphocytic infiltrates appeared frequently in the glands of ID animals. Foci sizes were variable, and some lobules appeared atrophic, with altered or distorted acinar cells. Some abnormal ductlike structures were present, and these contained cells that morphologically resembled eosinophils within their lumens. Streaming lymphocytes were also observed in this group (Fig. 4B). Histopathology was consistently diminished in 7 of 8 glands in group ID/Rx (Fig. 4C). Although the ID/Rx glands still contained periductal/perivenular lymphocytic foci, these were considerably smaller and appeared less frequently than ID glands. The gland from rabbit 227 was largely normal (Fig. 4D), but it contained one abnormal area, with a cluster of ductlike structures and streaming lymphocytes such as are generally indicative of proliferation (Fig. 4E).
Figure 4.
Histopathology features of treated and untreated animals. (A) Normal LG showed occasional small lymphocytic aggregates. (B) After 16 weeks, dacryoadenitis induction resulted in substantial lymphocytic infiltration in the LG, primarily periductal and perivenular. (C) Sections from ID/Rx 12 weeks after the treatment period revealed small to medium foci around ducts and venules that were considerably smaller and less frequent than in the ID group. (D) LG of rabbit 227 from the ID/Rx group was largely normal in appearance and contained small lymphocytic foci (red arrow). (E) One area of the rabbit 227 gland was dramatically altered, presenting with clusters of duct-like structures (circles) and evidence of streaming lymphocytes (black arrowhead) suggesting proliferation.
Immunohistochemistry
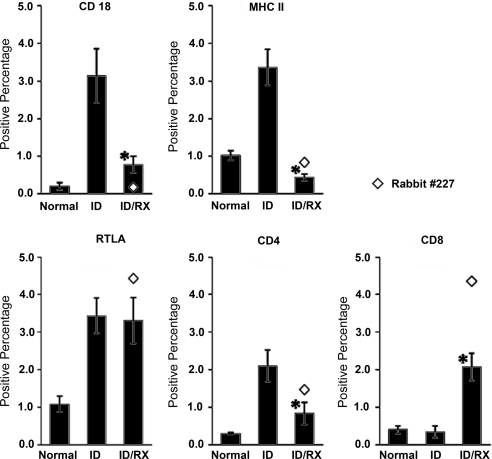
Percentages of cells expressing MHCII, CD18, RTLA, CD4, and CD8 are presented in Figure 5. MHCII+ CD18+, RTLA+, and CD4+ cells were markedly more abundant in the ID group than in the normal group. In contrast, the numbers of CD8+ cells were similar in the ID and normal groups. Typical glands from the ID/Rx group had 87% fewer MHCII+ cells than glands from the ID group, 75% fewer CD18+ cells, and 60% fewer CD4+ cells. The respective improvements in the rabbit 227 gland were 75%, 94%, and 30%. The positively stained cells in both ID and ID/Rx glands were found primarily in foci around ducts and venules, but, as suggested by the images in Figure 4, the foci were smaller and less frequent in the ID/Rx glands. Typical ID/Rx glands contained as many RTLA+ cells as glands from the ID group, and they contained sevenfold more CD8+ cells; the rabbit 227 gland contained 13-fold more CD8+ cells. Thus, though the CD4+/CD8+ ratio was approximately 1:1 in normal animals, it was 8:1 in the ID group, 0.4:1 in the typical ID/Rx group, and 0.2:1 in the ID/Rx rabbit 227 gland.
Figure 5.
LG sections from three study groups (normal, ID, ID/Rx) were stained for CD4, CD8, RTLA, CD18, and MHC II expression. For each marker studied, 16 to 24 sections per group were stained by the ABC method and analyzed by a cellular imaging system. Data are shown as mean ± SE positive percentage (i.e., area stained for specific antigen divided by total area of tissue scanned × 100 = positive percentage). *P ≤ 0.05; statistically significant difference between ID and ID/Rx animals.
Discussion
The present findings clearly demonstrate that expression of vIL-10 fundamentally altered the immunopathological process in a rabbit model of induced autoimmune dacryoadenitis. In 7 of 8 treated animals, this intervention partially restored the lacrimal fluid production that had been lost because of the inflammatory process, and ocular surface inflammation appeared to decrease as indicated by a reduction in Rose Bengal staining. It also improved several measures of LG immunopathology in the same animals, decreasing the size and frequency of focal infiltrates, decreasing the number of infiltrating bone marrow-derived cells marked by CD18, and reversing acinar atrophy. One additional treated LG appeared to respond anomalously.
Adenovirus vector-mediated gene transfer therapy has previously been evaluated in this rabbit model of induced autoimmune dacryoadenitis.7,16 Those studies showed that the expression of vIL-10 was prophylactic in that it prevented the induction of LG inflammation and that expression of the TNF inhibitor reduced established immunopathology and promoted improvement of tear production and tear stability.17 However, use of adenovirus vectors raises at least two important issues. The first is whether a troublesome immune response against the vector will occur in the recipient. The second is whether the duration of transgene expression will be long enough to support a significant therapeutic response. Regarding the immunogenicity issue, our earlier study showed that transduction of normal rabbit LGs with an adenoviral vector for GFP did not cause significant histopathologic changes or clinical signs of ocular surface inflammation.16 These findings contrast markedly with the severe neutrophilic infiltration that occurs when adenovirus vectors are introduced into rat salivary glands.18 They also suggest that immunophysiological differences between rodent LGs and LGs in other species1 may influence the outcomes of experimental gene transfer therapies. Regarding the duration of transgene expression and the durability of the therapeutic response, our previous study17 found that the therapeutic response in rabbit LGs persisted for weeks, whereas transgene expression subsided by 2 weeks, suggesting that even transient transgene expression permitted a durable response.
AAV vectors have emerged as preferred vehicles for gene transfer therapy. One reason is that a transgene delivered by an AAV vector is likely to be incorporated into the host cell genome, allowing for the possibility of long-term expression. A second reason is that AAV vectors are less immunogenic than adenovirus vectors.19,20 Other desirable features of AAV include their low toxicity, ability to infect nondividing cells, broad host range, and ability to carry larger constructs. For these reasons we selected an AAV vector to transfer a tetracycline-inducible vIL-10 gene. Our in vitro studies reveal a degree of leakiness in vIL-10 expression in that vIL-10 was expressed at low levels in the absence of doxycycline. We have not yet examined the influence of varying tetracycline doses on either the level of vIL-10 expression or the magnitudes of the therapeutic responses in rabbits with induced disease. Nevertheless, the availability of the inducible promoter system remains attractive because it potentially provides a means of modulating the level of transgene expression within some definable range. Moreover, doxycycline, a tetracycline derivative, has anti-inflammatory properties that are known to be beneficial.21
The in vitro experiments in the present study demonstrated that AAV vectors transduce LGECs efficiently, with approximately 50% of primary LG cells expressing transduced GFP. Moreover, like cells that had been transduced with Ad-vIL-10,7 cells that had been transduced with AAV-Tet-On vIL-10 had a markedly diminished capacity to support the proliferation of autologous lymphocytes in mixed-cell reactions (Fig. 2).
Other investigators have previously reported that AAV vector-mediated transduction of vIL-10 modulates inflammatory processes in a mouse model for Sjögren's-like salivary gland disease22 and in experimental models for other autoimmune diseases such as diabetes,12 atherosclerosis,23 arthritis,15 uveoretinitis,6,24 and orchitis.25 Our study used an AAV2 serotype, which other studies indicate may have to reach a certain dose threshold before useful proteins are produced at therapeutic levels.26,27 Our findings suggest that this dose threshold must have been attained.
The hypothesis that expression of vIL-10 supports development of a cell-mediated regulatory response is consistent with the findings that, though the number of CD4+ cells decreased, the number of CD8+ cells increased, and the total number of T cells, marked by RTLA, remained unchanged. Most reports indicate that the number of CD8+ cells populating normal LGs exceeds the number of CD4+ cells several-fold, and Mircheff et al.28 have proposed that the CD8+ cells might be regulatory cells that prevent inflammatory autoimmune responses to autoantigens that are constantly present in the glands' stromal spaces. In addition to decreasing the expression of proinflammatory cytokines,24,29 IL-10 plays major roles in the differentiation and function of regulatory T cells.30 The authors have found that the reduction of lacrimal histopathology induced by treatment with topical ophthalmic cyclosporine occurs after a similar lag time and that it also is associated with such an increase in the CD8+/CD4+ ratio.11 The authors also have found that the abundance of transcripts for CD8 and IL-10 increase as rabbit LGs maintain immunohomeostasis during responses to desiccating environments (Mircheff AK, et al. IOVS. 2010;51:ARVO E-Abstract 5196). The notion that therapeutic interventions permit intrinsic, cell-mediated regulatory mechanisms to become established also suggests an explanation for the finding that intervals of 4 weeks or longer were often required before the therapeutic efficacy of systemic androgen replacement became evident.31
The anomalous behavior of rabbit 227 raises questions that must be answered before the promise of AAV-mediated vIL-10 gene transfer therapy can be evaluated. By week 16, Schirmer test scores, tear BUT scores, and Rose Bengal staining scores in rabbit 227 were no worse than in untreated animals. However, the hypothesis that this animal simply failed the therapy is not sufficient. Rather, the findings that most of the gland appeared histologically normal while one area presented with pronounced pathologic changes seems to indicate that one immunopathologic process replaced another in this animal. It will be necessary to study greater numbers of animals to address the hypothesis that the apparent alternative process is causally related to the therapeutic intervention. Similarly, it will be necessary to study animals over a longer post-transduction period to determine whether the alternative immunopathologic process is progressive and, therefore, poses an unacceptable risk.
The finding that the improvements in Rose Bengal staining scores were similar to improvements in lacrimal fluid production in 7 of 8 treated animals suggests that the beneficial changes in the LGs promoted quantitative improvements in the status of the ocular surface. The finding that restoration of lacrimal fluid production rates was accompanied by a decline in Rose Bengal staining scores and an improvement of tear BUT warrants further consideration. Tear BUT depends not only on the quality of the hydrophilic substratum the corneal epithelium provides for the tear film but also on the quality of the superficial lipid layer of the tear film, which is produced by the meibomian glands. The impact the present model of induced autoimmune dacryoadenitis has on the meibomian glands is yet to be determined. Rose Bengal staining is thought to reflect deficiencies of the mucinous substratum at the surfaces of the corneal and conjunctival epithelia induced by underlying inflammatory processes. It is possible that the continuing presence of increased IL-10 in the tear film over an extended period will suppress the ocular surface sequelae of induced LG disease, but it also is possible that additional therapeutic modalities will be needed.
Acknowledgments
The authors thank Laurie LaBree Dustin for statistical analysis and Tamako Nakamura and Ernesto Barron for technical assistance.
Footnotes
Supported by National Institutes of Health Grants EY12689, EY05801, EY10550, and EY03040; an unrestricted grant from Research to Prevent Blindness, Inc.; and a grant from Allergan (MT).
Disclosure: P.B. Thomas, None; D.M. Samant, None; S. Selvam, None; R.H. Wei, None; Y. Wang, None; D. Stevenson, None; J.E. Schechter, None; F. Apparailly, None; A.K. Mircheff, None; M.D. Trousdale, None
References
- 1. de Saint Jean M, Nakamura T, Wang Y, Trousdale MD, Schechter JE, Mircheff AK. Suppression of lymphocyte proliferation and regulation of dendritic cell phenotype by soluble mediators from rat lacrimal epithelial cells. Scand J Immunol. 2009;70:53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rehman KK, Trucco M, Wang Z, Xiao X, Robbins PD. AAV8-mediated gene transfer of interleukin-4 to endogenous beta-cells prevents the onset of diabetes in NOD mice. Mol Ther. 2008;16:1409–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Apparailly F, Verwaerde C, Jacquet C, Auriault C, Sany J, Jorgensen C. Adenovirus-mediated transfer of viral IL-10 gene inhibits murine collagen-induced arthritis. J Immunol. 1998;160:5213–5220 [PubMed] [Google Scholar]
- 4. Johnston LC, Su X, Maguire-Zeiss K, et al. Human interleukin-10 gene transfer is protective in a rat model of Parkinson's disease. Mol Ther. 2008;16:1392–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perez N, Plence P, Millet V, et al. Tetracycline transcriptional silencer tightly controls transgene expression after in vivo intramuscular electrotransfer: application to interleukin 10 therapy in experimental arthritis. Hum Gene Ther. 2002;13:2161–2172 [DOI] [PubMed] [Google Scholar]
- 6. Smith JR, Verwaerde C, Rolling F, et al. Tetracycline-inducible viral interleukin-10 intraocular gene transfer, using adeno-associated virus in experimental autoimmune uveoretinitis. Hum Gene Ther. 2005;16:1037–1046 [DOI] [PubMed] [Google Scholar]
- 7. Zhu Z, Stevenson D, Schechter JE, et al. Prophylactic effect of IL-10 gene transfer on induced autoimmune dacryoadenitis. Invest Ophthalmol Vis Sci. 2004;45:1375–1381 [DOI] [PubMed] [Google Scholar]
- 8. Nonaka-Sarukawa M, Okada T, Ito T, et al. Adeno-associated virus vector-mediated systemic interleukin-10 expression ameliorates hypertensive organ damage in Dahl salt-sensitive rats. J Gene Med. 2008;10:368–374 [DOI] [PubMed] [Google Scholar]
- 9. Guo Z, Song D, Azzarolo AM, et al. Autologous lacrimal-lymphoid mixed-cell reactions induce dacryoadenitis in rabbits. Exp Eye Res. 2000;71:23–31 [DOI] [PubMed] [Google Scholar]
- 10. Zhu Z, Stevenson D, Schechter JE, Mircheff AK, Atkinson R, Trousdale MD. Lacrimal histopathology and ocular surface disease in a rabbit model of autoimmune dacryoadenitis. Cornea. 2003;22:25–32 [DOI] [PubMed] [Google Scholar]
- 11. Thomas PB, Samant DM, Zhu Z, et al. Long term topical cyclosporine treatment improves tear production and reduces keratoconjunctivitis in rabbits with induced autoimmune dacryoadenitis. JOPT. 2009;25:285–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Flotte T, Agarwal A, Wang J, et al. Efficient ex vivo transduction of pancreatic islet cells with recombinant adeno-associated virus vectors. Diabetes. 2001;50:515–520 [DOI] [PubMed] [Google Scholar]
- 13. Rehman KK, Wang Z, Bottino R, et al. Efficient gene delivery to human and rodent islets with double-stranded (ds) AAV-based vectors. Gene Ther. 2005;12:1313–1323 [DOI] [PubMed] [Google Scholar]
- 14. Guo Z, Azzarolo AM, Schechter JE, et al. Lacrimal gland epithelial cells stimulate proliferation in autologous lymphocyte preparations. Exp Eye Res. 2000;71:11–22 [DOI] [PubMed] [Google Scholar]
- 15. Apparailly F, Millet V, Noel D, Jacquet C, Sany J, Jorgensen C. Tetracycline-inducible interleukin-10 gene transfer mediated by an adeno-associated virus: application to experimental arthritis. Hum Gene Ther. 2002;13:1179–1188 [DOI] [PubMed] [Google Scholar]
- 16. Zhu Z, Stevenson D, Schechter JE, et al. Tumor necrosis factor inhibitor gene expression suppresses lacrimal gland immunopathology in a rabbit model of autoimmune dacryoadenitis. Cornea. 2003;22:343–351 [DOI] [PubMed] [Google Scholar]
- 17. Trousdale MD, Zhu Z, Stevenson D, Schechter JE, Ritter T, Mircheff AK. Expression of TNF inhibitor gene in the lacrimal gland promotes recovery of tear production and tear stability and reduced immunopathology in rabbits with induced autoimmune dacryoadenitis. J Autoimmune Dis. 2005;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adesanya MR, Redman RS, Baum BJ, O'Connell BC. Immediate inflammatory responses to adenovirus-mediated gene transfer in rat salivary glands. Hum Gene Ther. 1996;7:1085–1093 [DOI] [PubMed] [Google Scholar]
- 19. Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129 [DOI] [PubMed] [Google Scholar]
- 20. Rabinowitz JE, Samulski J. Adeno-associated virus expression systems for gene transfer. Curr Opin Biotechnol. 1998;9:470–475 [DOI] [PubMed] [Google Scholar]
- 21. Beardsley RM, De Paiva CS, Power DF, Pflugfelder SC. Desiccating stress decreases apical corneal epithelial cell size–modulation by the metalloproteinase inhibitor doxycycline. Cornea. 2008;27:935–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kok MR, Yamano S, Lodde BM, et al. Local adeno-associated virus-mediated interleukin 10 gene transfer has disease-modifying effects in a murine model of Sjögren's syndrome. Hum Gene Ther. 2003;14:1605–1618 [DOI] [PubMed] [Google Scholar]
- 23. Yoshioka T, Okada T, Maeda Y, et al. Adeno-associated virus vector-mediated interleukin-10 gene transfer inhibits atherosclerosis in apolipoprotein E-deficient mice. Gene Ther. 2004;11:1772–1779 [DOI] [PubMed] [Google Scholar]
- 24. Broderick CA, Smith AJ, Balaggan KS, et al. Local administration of an adeno-associated viral vector expressing IL-10 reduces monocyte infiltration and subsequent photoreceptor damage during experimental autoimmune uveitis. Mol Ther. 2005;12:369–373 [DOI] [PubMed] [Google Scholar]
- 25. Watanabe M, Kashiwakura Y, Kusumi N, et al. Adeno-associated virus-mediated human IL-10 gene transfer suppresses the development of experimental autoimmune orchitis. Gene Ther. 2005;12:1126–1132 [DOI] [PubMed] [Google Scholar]
- 26. Lee HC, Kim SJ, Kim KS, Shin HC, Yoon JW. Remission in models of type 1 diabetes by gene therapy using a single-chain insulin analogue. Nature. 2000;408:483–488 [DOI] [PubMed] [Google Scholar]
- 27. Song S, Morgan M, Ellis T, et al. Sustained secretion of human alpha-1-antitrypsin from murine muscle transduced with adeno-associated virus vectors. Proc Natl Acad Sci U S A. 1998;95:14384–14388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mircheff AK, Warren DW, Wood RL. Hormonal support of lacrimal function, primary lacrimal deficiency, autoimmunity, and peripheral tolerance in the lacrimal gland. Ocular Immunol Inflamm. 1996;4:145–172 [DOI] [PubMed] [Google Scholar]
- 29. Ma Y, Thornton S, Duwel LE, et al. Inhibition of collagen-induced arthritis in mice by viral IL-10 gene transfer. J Immunol. 1998;161:1516–1524 [PubMed] [Google Scholar]
- 30. Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765 [DOI] [PubMed] [Google Scholar]
- 31. Scott G, Yiu SC, Wasilewski D, Song J, Smith RE. Combined esterified estrogen and methyltestosterone treatment for dry eye syndrome in postmenopausal women. Am J Ophthalmol. 2005;139:1109–1110 [DOI] [PubMed] [Google Scholar]