Abstract
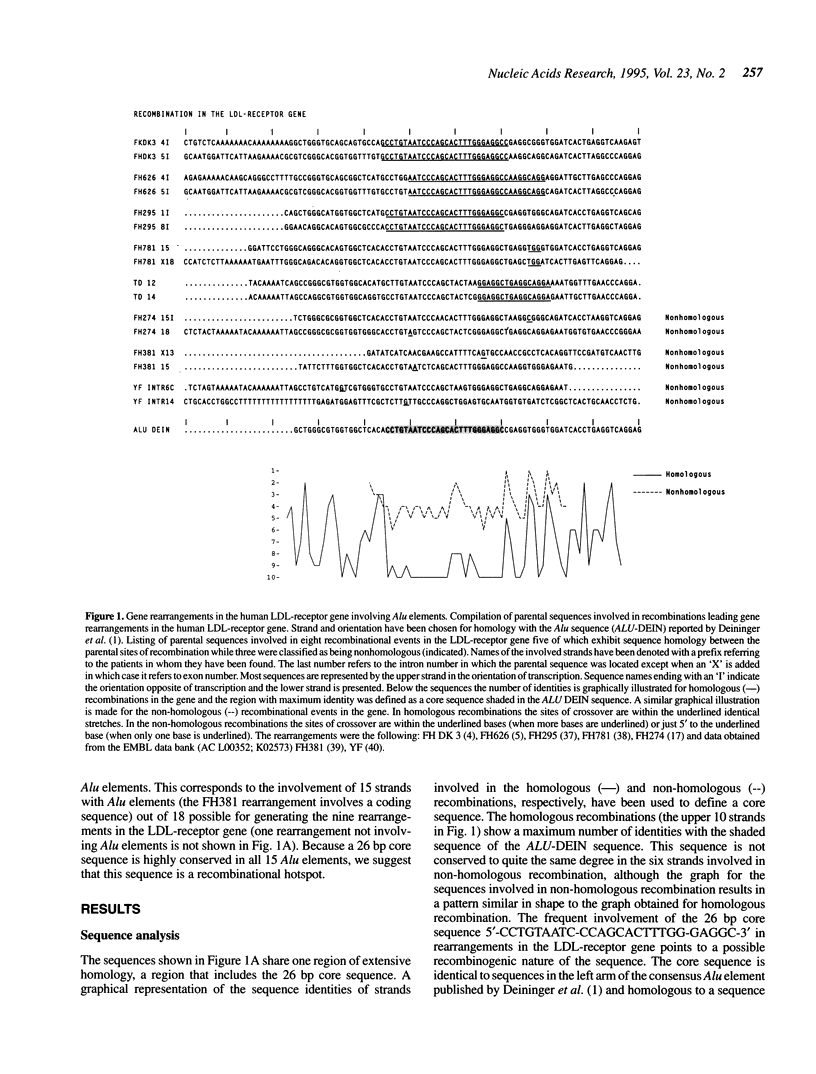
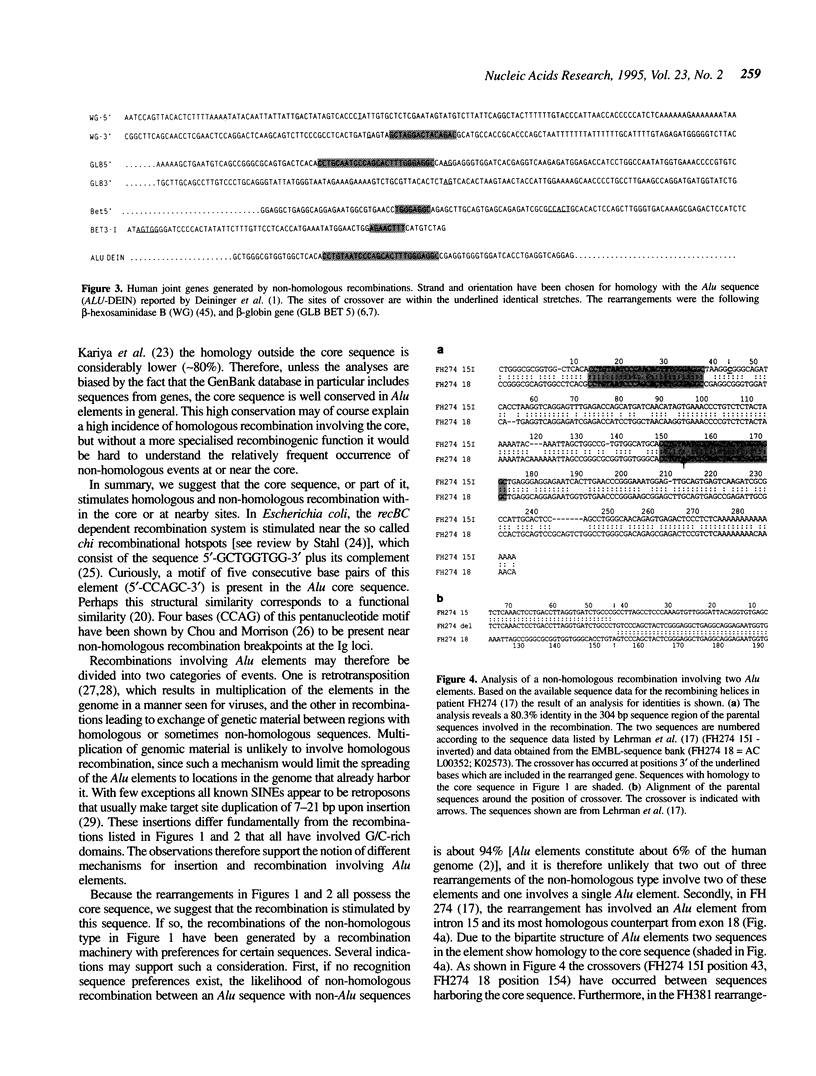
Alu elements have repeatedly been found involved in gene rearrangements in humans. Although these elements have been suggested to stimulate gene rearrangements, sparse information is available for the possible mechanism(s) of these events. Here we present a compilation of Alu elements that have been involved in recombinational events leading to gene rearrangements, indicating the presence of a common 26 bp core sequence at or close to the sites of recombination. Besides the obvious possibility of retrotransposition, gene rearrangements may be induced by sequences that stimulate genetic recombination. We suggest that the core sequence stimulates recombination and may thereby cause the frequent involvement of these elements in gene rearrangements. Curiously, the core sequence contains the pentanucleotide motif CCAGC, which is also part of chi, an 8 bp sequence known to stimulate recBC mediated recombination in Escherichia coli.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariga T., Carter P. E., Davis A. E., 3rd Recombinations between Alu repeat sequences that result in partial deletions within the C1 inhibitor gene. Genomics. 1990 Dec;8(4):607–613. doi: 10.1016/0888-7543(90)90246-q. [DOI] [PubMed] [Google Scholar]
- Bae Y. S., Kawasaki I., Ikeda H., Liu L. F. Illegitimate recombination mediated by calf thymus DNA topoisomerase II in vitro. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2076–2080. doi: 10.1073/pnas.85.7.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsh G. S., Seeburg P. H., Gelinas R. E. The human growth hormone gene family: structure and evolution of the chromosomal locus. Nucleic Acids Res. 1983 Jun 25;11(12):3939–3958. doi: 10.1093/nar/11.12.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock P., Champoux J. J., Botchan M. Association of crossover points with topoisomerase I cleavage sites: a model for nonhomologous recombination. Science. 1985 Nov 22;230(4728):954–958. doi: 10.1126/science.2997924. [DOI] [PubMed] [Google Scholar]
- Bullock P., Miller J., Botchan M. Effects of poly[d(pGpT).d(pApC)] and poly[d(pCpG).d(pCpG)] repeats on homologous recombination in somatic cells. Mol Cell Biol. 1986 Nov;6(11):3948–3953. doi: 10.1128/mcb.6.11.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabretta B., Robberson D. L., Barrera-Saldaña H. A., Lambrou T. P., Saunders G. F. Genome instability in a region of human DNA enriched in Alu repeat sequences. Nature. 1982 Mar 18;296(5854):219–225. doi: 10.1038/296219a0. [DOI] [PubMed] [Google Scholar]
- Chou C. L., Morrison S. L. A common sequence motif near nonhomologous recombination breakpoints involving Ig sequences. J Immunol. 1993 Jun 15;150(12):5350–5360. [PubMed] [Google Scholar]
- Deininger P. L., Jolly D. J., Rubin C. M., Friedmann T., Schmid C. W. Base sequence studies of 300 nucleotide renatured repeated human DNA clones. J Mol Biol. 1981 Sep 5;151(1):17–33. doi: 10.1016/0022-2836(81)90219-9. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Fuscoe J. C., Zimmerman L. J., Lippert M. J., Nicklas J. A., O'Neill J. P., Albertini R. J. V(D)J recombinase-like activity mediates hprt gene deletion in human fetal T-lymphocytes. Cancer Res. 1991 Nov 1;51(21):6001–6005. [PubMed] [Google Scholar]
- Gordenin D. A., Malkova A. L., Peterzen A., Kulikov V. N., Pavlov Y. I., Perkins E., Resnick M. A. Transposon Tn5 excision in yeast: influence of DNA polymerases alpha, delta, and epsilon and repair genes. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3785–3789. doi: 10.1073/pnas.89.9.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes S. R., Toomey T. P., Leinwand L., Jelinek W. R. The Chinese hamster Alu-equivalent sequence: a conserved highly repetitious, interspersed deoxyribonucleic acid sequence in mammals has a structure suggestive of a transposable element. Mol Cell Biol. 1981 Jul;1(7):573–583. doi: 10.1128/mcb.1.7.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henthorn P. S., Mager D. L., Huisman T. H., Smithies O. A gene deletion ending within a complex array of repeated sequences 3' to the human beta-globin gene cluster. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5194–5198. doi: 10.1073/pnas.83.14.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs H. H., Brown M. S., Goldstein J. L., Russell D. W. Deletion of exon encoding cysteine-rich repeat of low density lipoprotein receptor alters its binding specificity in a subject with familial hypercholesterolemia. J Biol Chem. 1986 Oct 5;261(28):13114–13120. [PubMed] [Google Scholar]
- Hobbs H. H., Lehrman M. A., Yamamoto T., Russell D. W. Polymorphism and evolution of Alu sequences in the human low density lipoprotein receptor gene. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7651–7655. doi: 10.1073/pnas.82.22.7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsthemke B., Beisiegel U., Dunning A., Havinga J. R., Williamson R., Humphries S. Unequal crossing-over between two alu-repetitive DNA sequences in the low-density-lipoprotein-receptor gene. A possible mechanism for the defect in a patient with familial hypercholesterolaemia. Eur J Biochem. 1987 Apr 1;164(1):77–81. doi: 10.1111/j.1432-1033.1987.tb10995.x. [DOI] [PubMed] [Google Scholar]
- Huang L. S., Ripps M. E., Korman S. H., Deckelbaum R. J., Breslow J. L. Hypobetalipoproteinemia due to an apolipoprotein B gene exon 21 deletion derived by Alu-Alu recombination. J Biol Chem. 1989 Jul 5;264(19):11394–11400. [PubMed] [Google Scholar]
- Kariya Y., Kato K., Hayashizaki Y., Himeno S., Tarui S., Matsubara K. Revision of consensus sequence of human Alu repeats--a review. Gene. 1987;53(1):1–10. doi: 10.1016/0378-1119(87)90087-4. [DOI] [PubMed] [Google Scholar]
- Konopka A. K. Compilation of DNA strand exchange sites for non-homologous recombination in somatic cells. Nucleic Acids Res. 1988 Mar 11;16(5):1739–1758. doi: 10.1093/nar/16.5.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornreich R., Bishop D. F., Desnick R. J. Alpha-galactosidase A gene rearrangements causing Fabry disease. Identification of short direct repeats at breakpoints in an Alu-rich gene. J Biol Chem. 1990 Jun 5;265(16):9319–9326. [PubMed] [Google Scholar]
- Kudo S., Fukuda M. Structural organization of glycophorin A and B genes: glycophorin B gene evolved by homologous recombination at Alu repeat sequences. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4619–4623. doi: 10.1073/pnas.86.12.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman M. A., Goldstein J. L., Russell D. W., Brown M. S. Duplication of seven exons in LDL receptor gene caused by Alu-Alu recombination in a subject with familial hypercholesterolemia. Cell. 1987 Mar 13;48(5):827–835. doi: 10.1016/0092-8674(87)90079-1. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Russell D. W., Goldstein J. L., Brown M. S. Alu-Alu recombination deletes splice acceptor sites and produces secreted low density lipoprotein receptor in a subject with familial hypercholesterolemia. J Biol Chem. 1987 Mar 5;262(7):3354–3361. [PubMed] [Google Scholar]
- Lehrman M. A., Russell D. W., Goldstein J. L., Brown M. S. Exon-Alu recombination deletes 5 kilobases from the low density lipoprotein receptor gene, producing a null phenotype in familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3679–3683. doi: 10.1073/pnas.83.11.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman M. A., Schneider W. J., Südhof T. C., Brown M. S., Goldstein J. L., Russell D. W. Mutation in LDL receptor: Alu-Alu recombination deletes exons encoding transmembrane and cytoplasmic domains. Science. 1985 Jan 11;227(4683):140–146. doi: 10.1126/science.3155573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerowitz R., Hogikyan N. D. A deletion involving Alu sequences in the beta-hexosaminidase alpha-chain gene of French Canadians with Tay-Sachs disease. J Biol Chem. 1987 Nov 15;262(32):15396–15399. [PubMed] [Google Scholar]
- Neote K., McInnes B., Mahuran D. J., Gravel R. A. Structure and distribution of an Alu-type deletion mutation in Sandhoff disease. J Clin Invest. 1990 Nov;86(5):1524–1531. doi: 10.1172/JCI114871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls R. D., Fischel-Ghodsian N., Higgs D. R. Recombination at the human alpha-globin gene cluster: sequence features and topological constraints. Cell. 1987 May 8;49(3):369–378. doi: 10.1016/0092-8674(87)90289-3. [DOI] [PubMed] [Google Scholar]
- Orita M., Sekiya T., Hayashi K. DNA sequence polymorphisms in Alu repeats. Genomics. 1990 Oct;8(2):271–278. doi: 10.1016/0888-7543(90)90282-y. [DOI] [PubMed] [Google Scholar]
- Rouyer F., Simmler M. C., Page D. C., Weissenbach J. A sex chromosome rearrangement in a human XX male caused by Alu-Alu recombination. Cell. 1987 Nov 6;51(3):417–425. doi: 10.1016/0092-8674(87)90637-4. [DOI] [PubMed] [Google Scholar]
- Rüdiger N. S., Hansen P. S., Jørgensen M., Faergeman O., Bolund L., Gregersen N. Repetitive sequences involved in the recombination leading to deletion of exon 5 of the low-density-lipoprotein receptor gene in a patient with familial hypercholesterolemia. Eur J Biochem. 1991 May 23;198(1):107–111. doi: 10.1111/j.1432-1033.1991.tb15992.x. [DOI] [PubMed] [Google Scholar]
- Rüdiger N. S., Heinsvig E. M., Hansen F. A., Faergeman O., Bolund L., Gregersen N. DNA deletions in the low density lipoprotein (LDL) receptor gene in Danish families with familial hypercholesterolemia. Clin Genet. 1991 Jun;39(6):451–462. doi: 10.1111/j.1399-0004.1991.tb03057.x. [DOI] [PubMed] [Google Scholar]
- Schiestl R. H., Dominska M., Petes T. D. Transformation of Saccharomyces cerevisiae with nonhomologous DNA: illegitimate integration of transforming DNA into yeast chromosomes and in vivo ligation of transforming DNA to mitochondrial DNA sequences. Mol Cell Biol. 1993 May;13(5):2697–2705. doi: 10.1128/mcb.13.5.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smidt M., Kirsch I., Ratner L. Deletion of Alu sequences in the fifth c-sis intron in individuals with meningiomas. J Clin Invest. 1990 Oct;86(4):1151–1157. doi: 10.1172/JCI114820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. R., Kunes S. M., Schultz D. W., Taylor A., Triman K. L. Structure of chi hotspots of generalized recombination. Cell. 1981 May;24(2):429–436. doi: 10.1016/0092-8674(81)90333-0. [DOI] [PubMed] [Google Scholar]
- Sperry A. O., Blasquez V. C., Garrard W. T. Dysfunction of chromosomal loop attachment sites: illegitimate recombination linked to matrix association regions and topoisomerase II. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5497–5501. doi: 10.1073/pnas.86.14.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl F. W. Special sites in generalized recombination. Annu Rev Genet. 1979;13:7–24. doi: 10.1146/annurev.ge.13.120179.000255. [DOI] [PubMed] [Google Scholar]
- Stoppa-Lyonnet D., Carter P. E., Meo T., Tosi M. Clusters of intragenic Alu repeats predispose the human C1 inhibitor locus to deleterious rearrangements. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1551–1555. doi: 10.1073/pnas.87.4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T. C., Goldstein J. L., Brown M. S., Russell D. W. The LDL receptor gene: a mosaic of exons shared with different proteins. Science. 1985 May 17;228(4701):815–822. doi: 10.1126/science.2988123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treco D., Arnheim N. The evolutionarily conserved repetitive sequence d(TG.AC)n promotes reciprocal exchange and generates unusual recombinant tetrads during yeast meiosis. Mol Cell Biol. 1986 Nov;6(11):3934–3947. doi: 10.1128/mcb.6.11.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanin E. F., Henthorn P. S., Kioussis D., Grosveld F., Smithies O. Unexpected relationships between four large deletions in the human beta-globin gene cluster. Cell. 1983 Dec;35(3 Pt 2):701–709. doi: 10.1016/0092-8674(83)90103-4. [DOI] [PubMed] [Google Scholar]
- Wallace M. R., Andersen L. B., Saulino A. M., Gregory P. E., Glover T. W., Collins F. S. A de novo Alu insertion results in neurofibromatosis type 1. Nature. 1991 Oct 31;353(6347):864–866. doi: 10.1038/353864a0. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- White M. A., Wierdl M., Detloff P., Petes T. D. DNA-binding protein RAP1 stimulates meiotic recombination at the HIS4 locus in yeast. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9755–9759. doi: 10.1073/pnas.88.21.9755. [DOI] [PMC free article] [PubMed] [Google Scholar]



