Abstract
Objectives
To examine whether high insulin resistance versus high inflammation identifies subtypes of preeclampsia.
Methods
A cytokine panel, glucose and insulin were measured in 37 preeclampsia plasma samples. Wilcoxon rank sum assessed median concentration of HOMAIR by pro-inflammatory: anti-inflammatory ratio. Regression stratifying by BMI and preterm birth was conducted.
Results
There was no difference in median HOMAIR by the pro-inflammatory: anti-inflammatory ratio (p = 0.16). No subsets scatterplot clusters emerged. A positive correlation between HOMAlog and the ratio was significant (p = 0.04).
Conclusions
No dichotomous subsets of preeclampsia by inflammation versus insulin resistance were detected. Contrary to our hypothesis, insulin resistance was higher as inflammation increased in preeclampsia.
Keywords: Multi-plex cytokines, Subtypes, Systems biology, Pregnancy complication
INTRODUCTION
Hypertensive disorders persist as a leading cause of maternal mortality worldwide (1). Preeclampsia affects approximately 4–8% of all pregnant women (2, 3) and the frequency appears to be increasing in the U.S. with the obesity epidemic (4). Defined by the new onset of hypertension and proteinuria after 20 weeks gestation, preeclampsia is a heterogeneous disorder manifesting different phenotypes. Identification of subsets of preeclampsia would increase understanding of the disorder (5).
We were intrigued by the unexpected finding that the pro-inflammatory cytokine tumor necrosis factor alpha (TNFα) was not elevated in obese compared to lean women with preeclampsia (6). We had anticipated that adipocyte secretion would augment the levels of inflammatory biomarkers in preeclampsia (7–11) but this was not the case. Others in our group demonstrated that approximately one third of the total effect of body mass index (BMI) on preeclampsia risk is mediated through inflammation and triglyceride levels (12). This led us to conjecture that perhaps insulin resistance, another component of the metabolic syndrome mediated by BMI, would account for additional variance in preeclampsia.
Robillard had suggested that preeclampsia might be separated into either an immunological or metabolic pathogenesis in different populations. In developed countries, prepregnancy maternal metabolic factors in older (≥30 years), mildly obese pregnant women with near term onset may play a greater role in preeclampsia pathogenesis (13). However, preeclampsia remains even more common in settings of under nutrition (1, 14). Preeclampsia in young women (15–25 years) from developing countries and certain lower socioeconomic groups in developed countries may be more likely to originate with immune maladaptation (13). An immune etiology is supported by evidence indicating that maternal exposure to paternal antigens reduces the incidence of preeclampsia (15). For example, an extended period of sexual cohabitation with a new male partner before conception may protect against pregnancy-induced hypertension, including preeclampsia (16). The semiallogeneic fetus in normal pregnancy stimulates an increased maternal inflammatory status compared to non-pregnancy and inflammation is further increased in preeclampsia compared to normal pregnancy (17). A shift to pro-inflammatory Th1 cytokine predominance over anti-inflammatory Th2 may account for this excessive inflammation in preeclampsia (18, 19).
Metabolic origins are implicated by differences in preeclampsia compared to normal pregnancy. Maternal hyperinsulinemia and insulin resistance which support the growing conceptus in normal pregnancy (20) are accentuated in preeclampsia. Preeclamptic pregnancies demonstrated 37% lower insulin sensitivity and 70% higher free fatty acid concentration at 29 to 39 weeks gestation than was present in control women (21). Moreover, women entering pregnancy with metabolic syndrome are more likely to develop preeclampsia (22). Obesity triples the risk of preeclampsia, yet 90% of obese pregnant women do not develop the disease (15). Insulin resistance also occurs in non-obese metabolic syndrome (23). Although inflammation and insulin resistance commonly are associated, lack of correlation between these processes has been found in studies of hypertensive disorders of pregnancy (24–26).
We attempted to address the hypothesis of immune versus metabolic origins of preeclampsia by comparing pro-inflammatory and anti-inflammatory markers and insulin resistance in preeclamptic women to see if there was any evidence of clustering into separate subtypes. The inflammatory status was determined by a ratio of pro- versus anti-inflammatory cytokines. Metabolic status was assessed by the homeostasis model assessment of insulin resistance (HOMAIR) (27), which has been validated for use with lean and obese pregnant women (28, 29). A negative correlation of the two variables was predicted if high insulin resistance and high inflammation identified different subtypes of preeclampsia.
MATERIALS AND METHODS
Design
A cross-sectional study was conducted of preeclamptic women who consented to protocols approved by the University of Pittsburgh Institutional Review Board. Preeclamptic cases were hypertensive, hyperuricemic and proteinuric. Hypertension was defined as blood pressure greater than or equal to 140 or 90 and an increase of greater than 30 systolic or increase of 15 diastolic after 20 weeks gestation in previously normotensive women, returning to usual BP by 12 weeks postpartum. Proteinuria was defined as ≥300 mg per 24-hour urine collection, ≥1+ protein on a catheterized or 2+ on a voided urine sample or a protein/creatinine ratio ≥0.3 (30). Hyperuricemia was included to increase specificity of the diagnosis of preeclampsia and was defined as uric acid >1 standard deviation above the mean for gestational age. Hyperuricemia, which occurs in 75% of preeclamptic pregnancies, is associated with an increased risk of iatrogenic prematurity and SGA infants (31). Women with medical or other obstetric problems were excluded. Prepregnancy BMI (kg/m2) was based on measured height and maternal self-report of prepregnancy weight at the initial visit.
Samples
Samples were obtained as part of a prospective study of preeclampsia (PEPP study). Plasma samples were available from 40 women with preeclampsia who were fasting at least 6 hours when sampled prior to the onset of labor. These restrictions were applied to allow the determination of insulin resistance and to avoid the confounding inflammatory effects of labor. Blood had been drawn in EDTA and stored at −80°C. Participants were primiparous. With the 40 available samples, we estimated we could detect an r of 0.5 with 80% power and p=0.05.
Assays
Samples were analyzed by a Cytokine Ultrasensitive Human 10-Plex Panel (Catalog #LHC6004, Biosource; Invitrogen, Carlsbad, CA) using Luminex 100 instrumentation (Luminex Corporation; Austin, TX). Analytes in the panel included granulocyte-macrophage colony stimulating factor (GM-CSF), interferon gamma (IFNγ), TNFα, interleukin 1-beta (IL1β), IL2, IL4, IL5, IL6, IL8, and IL10. The intra-assay (n = 16) coefficient of variation (CV %) ranged 6.6–9.3 and the inter-assay (n = 32) CV ranged 7.0–9.6 for each singleplex assay, excluding IFNγ and IL1β (not available).
Glucose was measured by glucose oxidase colorimetric assay (Pointe Scientific, Inc.). Intra-assay CV averaged 0.6 and inter-assay CV averaged 1.9. Insulin was measured by ELISA (Linco, St. Charles, Missouri) with intra-assay CV 6.0, inter-assay CV 10.3, and sensitivity was 2 μU/ml. Insulin resistance was calculated using the homeostasis model assessment (HOMAIR = Insulin μU/ml x [Glucose mM/L/22.5]) (27).
Analysis
Means and percentages of demographic and clinical variable values were determined for descriptive purposes. The distribution of each cytokine was assessed. A pro-inflammatory group was created to assess the effects of the two inflammation stimulating chemokines, GM-CSF and IL8, along with Th1 cytokines IL2, IL6, IFNγ, and TNFα. IL4, IL5, IL10 were grouped as Th2 or anti-inflammatory cytokines. The pro-inflammatory: anti-inflammatory ratio was calculated using the mean of the multiple of the median (MOM) for each relevant cytokine to normalize the data for comparison. HOMAIR was not normally distributed (Shapiro-Wilk p < 0.01). Therefore, the correlation between the median concentration of HOMAIR and each chemokine as well as the pro-inflammatory:anti-inflammatory ratio was tested with Wilcoxon rank sum test. In addition HOMAIR was logarithmically transformed (log10) in linear regression models evaluating the relationship with the pro-inflammatory: anti-inflammatory ratio. Results were also stratified by BMI (<25 vs. ≥25 kg/m2) and preterm birth (<37 vs. ≥37 weeks).
Forty samples were analyzed. Three participants were deleted because most cytokine (pro-inflammatory and anti-inflammatory) values fell below the range of sensitivity as determined by all MOM values <0.5. IL1β was omitted from analysis because the concentration in a majority of samples was out of range of sensitivity.
RESULTS
The average participant was a 25-year-old White nonsmoker, who was sampled and delivered preterm at 35 weeks gestation and whose infant was not growth restricted (>10th percentile for gestational age; Table 1). The mean, median, range and MOM for each cytokine were determined (Table 2). Each cytokine concentration was tested for correlation with HOMAIR (Table 3). Pro-inflammatory Th1 cytokines IFNγ and TNFα and anti-inflammatory Th2 cytokine IL4 were significantly and positively correlated to HOMAIR.
Table 1.
Demographic and clinical characteristics.
| Factor | Mean (range) or count (%) | Factor | Mean (range) or count (%) |
|---|---|---|---|
| Gestational age at sampling | 34.8 weeks (25.3–41.3) | Smoke | Yes 5 (13.5 %) No 32 (86.5 %) |
| Gestational age at delivery | 34.98 weeks (25.4–41.4) | BP in labor | 158/94 (130–189/76–124) |
| Maternal age | 24.96 years (15.9–37.2) | Uric acid | 6.63 mg/dL (4–11) |
| BMI | 27.82 (kg/m2) (16.7–36.7) | Birthweight | 2262.14 grams (704–4224) |
| Race | 5 Black (14%) 32 White (86%) |
Birthweight centiles of growth restricted (N = 5 <10th) | 2.6 (1–5) |
Table 2.
Mean and median concentrations of cytokines and HOMAIR.
| Cytokine | Mean (pg/ml) | Median (pg/ml) | Range (pg/ml) | MOMa | MOM range |
|---|---|---|---|---|---|
| GM-CSF | 32.4 | 22.8 | 0.51–194 | 1.4 | 0.02–8.5 |
| IL8 | 32.8 | 18.3 | 2.98–566.3 | 1.9 | 0.32–30.9 |
| IFNγ | 13.0 | 11.0 | 0.83–62.9 | 1.2 | 0.08–5.7 |
| TNFα | 7.8 | 6.3 | 0.17–45.9 | 1.3 | 0.03–7.3 |
| IL2 | 12.0 | 10.2 | 2.0–41.9 | 1.2 | 0.20–4.10 |
| IL6 | 14.0 | 7.6 | 0.75–118.7 | 1.9 | 0.10–15.6 |
| IL4 | 15.2 | 12.9 | 0.64–60.3 | 1.2 | 0.05–4.7 |
| IL5 | 9.1 | 8.9 | 0.23–27.3 | 1.1 | 0.15–3.1 |
| IL10 | 17.1 | 8.9 | 1.57–109.5 | 2.0 | 0.31–12.3 |
| HOMAIR | 2.6 | 1.9 | 0.22–11.2 |
Multiple of the median (MOM).
Table 3.
Correlation of HOMAIR and cytokines.
| Cytokine | Type | Correlationa with HOMAIR | P-value |
|---|---|---|---|
| GM-CSF | Pro-inflammatory | 0.14 | 0.42 |
| IL8 | Pro-inflammatory | −0.07 | 0.66 |
| IFNγ | Pro-inflammatory | 0.36 | 0.03* |
| TNFα | Pro-inflammatory | 0.35 | 0.04* |
| IL2 | Pro-inflammatory | 0.27 | 0.11 |
| IL6 | Pro-inflammatory | −0.16 | 0.34 |
| IL4 | Anti-inflammatory | 0.48 | <0.01* |
| IL5 | Anti-inflammatory | 0.21 | 0.25 |
| IL10 | Anti-inflammatory | −0.19 | 0.25 |
| Pro-inflammatory: Anti-inflammatory | 0.37 | 0.02 |
Spearman.
Significant; p < 0.05.
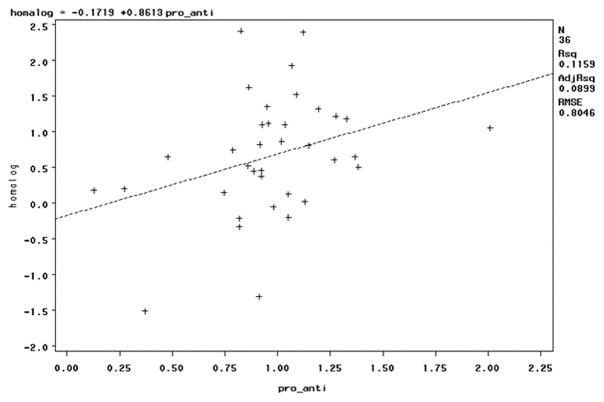
The correlation between HOMAIR and the pro-inflammatory:anti-inflammatory ratio was significant with one outlier observation excluded (IL8 = 566 pg/ml; Table 2 and Figure 1; r = 0.34, p = 0.04). Contrary to our hypothesis, the correlation was positive. The pro-inflammatory ratio was highly correlated with the Th1:Th2 ratio (r = 0.66; p < 0.0001), and the Th1:Th2 ratio was even more strongly positively correlated with HOMAIR (Spearman r = 0.45, p < 0.01). There were no clusters by scatterplot. There was no difference in median HOMAIR by pro-inflammatory:anti-inflammatory ratio above and below 1 (p = 0.33; Table 4). Similarly, these relationships were unchanged when limited to White women (n = 32) or nonsmokers (n = 28). Stratifying by overweight status versus normal weight or the occurrence of preterm preeclampsia did not identify a subset with a negative relationship between HOMAIR and the pro-inflammatory:anti-inflammatory ratio. For none of these subsets was the relationship to HOMAIR significant (p > 0.05 for all subsets).
Figure 1.
The positive correlation between insulin resistance measured by HOMAIR and the pro-inflammatory:anti-inflammatory ratio was significant with p=0.04. Scatterplot displays no clustering of data points.
Table 4.
Median concentrations of HOMAIR according to pro-inflammatory: anti-inflammatory ratio.
| Median (IQR) HOMAIR | P-valuea | |
|---|---|---|
| Pro-inflammatory: Anti-inflammatory ≤1 (n = 20) | 1.6 (1.1, 2.6) | 0.16 |
| Pro-inflammatory: Anti-inflammatory >1 (n = 17) | 2.4 (1.7, 3.4) |
Wilcoxon rank sum.
IQR = Interquartile range.
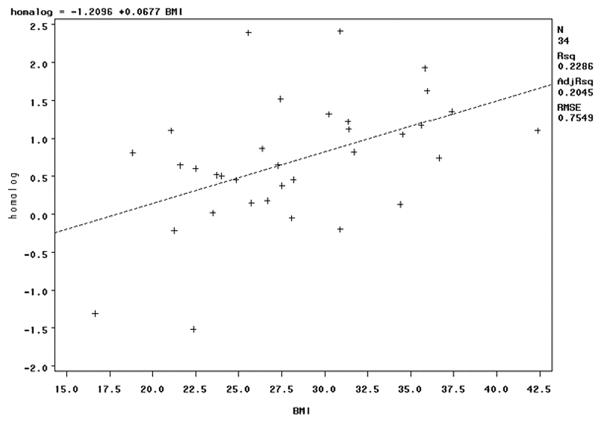
HOMAlog was correlated with prepregnancy BMI (Figure 2); the pro-inflammatory:anti-inflammatory ratio was not (r = −0.06, p = 0.74). The pro-inflammatory:anti-inflammatory ratio positively correlated with HOMA-log among both normal weight and overweight women, but did not achieve the chosen level of significance in either group (p = 0.11 in both groups). Despite the strong positive correlation between IL4 and HOMAIR (Table 3), IL4 did not relate to BMI (r = 0.13, p = 0.47). HOMAlog and the pro-inflammatory:anti-inflammatory ratio were positively correlated with gestational age at delivery (r = 0.46, p < 0.01; r = 0.36, p = 0.03).
Figure 2.
The positive correlation between insulin resistance measured by HOMAlog and prepregnancy body mass index was significant, p < 0.01.
COMMENT
In a disorder as broadly defined and presenting with such heterogeneous findings it is likely that subsets of the disorder exist. Evaluating subsets of preeclampsia by differences in pathophysiology should enhance understanding of the disorder and provide information on women who would be likely to benefit from specific novel therapies as they become available (15). We pursued this concept of subsets by expanding on the suggestion that preeclampsia could be caused by immunological versus metabolic origins (13). It was anticipated that one group of preeclamptic women would demonstrate a high pro-inflammatory:anti-inflammatory balance with a low insulin resistance measured by HOMAIR, whereas another group would demonstrate lower inflammation and higher insulin resistance.
Our results do not support the hypothesis that these subsets are detectable in our sample of preeclamptic women. There was no clustering of data points nor was the correlation of the immunologic and metabolic factors negative as would have been predicted by the existence of two subsets. In fact, there was a significant positive association between inflammation and insulin resistance (p = 0.04; Figure 1). This is consistent with a positive relationship between TNFα and insulin resistance demonstrated in normal pregnancy (32). However, others showed no relationship between markers of insulin resistance and markers of inflammation in preeclampsia in less extensive evaluations (24, 25). We examined the possibility that the metabolic and immunological subtypes might be evident in obvious subsets of preeclampsia, overweight versus normal weight women, and early versus late onset preeclampsia. These stratifications did not indicate the hypothesized negative relationship of HOMAIR and the pro-inflammatory:anti-inflammatory ratio.
We found that insulin resistance increased with higher prepregnancy BMI, but higher pro-inflammatory and Th1 status in preeclampsia were not related to BMI. This finding was consistent with our previous work. Although TNFα was higher in preeclampsia compared with control subjects, concentrations were not associated with obesity in either group (6). Either prepregnancy BMI is not an adequate proxy for obesity when assessing metabolic factors in pregnancy or the inflammatory effect of pregnancy and preeclampsia blunt the inflammatory effect of obesity.
In this study we attempted to compare several relevant pro-inflammatory and anti-inflammatory cytokines. Previous studies demonstrated conflicting results in individual cytokine concentrations in preeclampsia (19, 33, 34). The ratio of pro-inflammatory:anti-inflammatory cytokines predicted inflammatory status in preeclampsia better than either type of cytokine alone (18, 35). Multiplexed immunoassays provided the opportunity to examine a panel of cytokines in preeclampsia and their concentrations were normalized by a summary MOM ratio to strengthen a more systemic view of the innate and Th1 and Th2 network with less influence of any one particular cytokine on the pro-inflammatory:anti-inflammatory balance. Importantly, the cytokine milieu was examined in the plasma of fasted women who met strict criteria for preeclampsia without confounding effects of infection or onset of labor (36). Neither race nor smoking affected the pro-inflammatory:anti-inflammatory ratio in this sample, We demonstrated the pro-inflammatory shift and Th1 predominance characteristic of preeclampsia (18, 19), unlike two other studies that used multiplexed cytokine immunoassays in preeclampsia (33, 37). Although these studies measured concentrations of many inflammatory markers, they included fewer cytokines.
A few potential reasons for the lack of support for inflammation and metabolic etiological subsets in this study can be identified. The sample of preeclamptic women was from a single region in the U.S. populations in different regions or countries might better demonstrate these different etiologies in development of preeclampsia (13). There are likely to be immunological explanations for our results. Evidence is beginning to accumulate that insulin changes T cell polarization to an anti-inflammatory Th2-type response through extracellular signal-regulated kinase (ERK) phosphorylation (38). Concomitantly, the Th1:Th2 paradigm probably oversimplifies the complex immunology of pregnancy and preeclampsia (39).
We attempted to address this issue by evaluating the pro-inflammatory: anti-inflammatory ratio. IL6 is mainly produced by monocytes and macrophages and also guides naïve T-helper cells towards the Th17 lineage (40). Grouping the pro-inflammatory stimulating molecules GM-CSF and IL8 with pleiotropic IL6 integrated innate with adaptive immunity in the pro-:anti-inflammatory ratio. IL-6 exerts both beneficial and detrimental effects on insulin resistance, in part through the NF-kB and Jun kinases pathway (40–42), which may account for the stronger relationship we found between HOMAIR and the Th1:Th2 ratio compared to HOMAIR and the pro-:anti-inflammatory ratio.
Limitations
The cross-sectional design of women with preeclampsia and the small sample size may limit our findings. Subsets of inflammation versus insulin resistance may become apparent in larger longitudinal studies. Prospective study of insulin sensitivity/resistance by IV- or oral glucose tolerance testing may provide more reliable results. Comparison of each cytokine and the pro-inflammatory:anti-inflammatory ratios versus insulin resistance between preeclampsia and control groups would add information on each factor.
CONCLUSION
This study indicated no evidence of a dichotomy in preeclampsia by inflammation and insulin resistance as the data do not identify subtypes in this sample. Insulin resistance was higher as inflammation increased in preeclampsia. The pro-inflammatory:anti-inflammatory ratio is a novel approach for a more systemic view of the cytokine milieu. Although the findings did not support the hypothesis of subsets, the investigation points toward pathways-oriented thinking about the interactions of immunologic and metabolic systems in preeclampsia.
Acknowledgments
This work was supported by the University of Pittsburgh School of Nursing and its Center for Research and Evaluation funding to Dr. Founds, NIH PO1 HD30367 to Dr. Roberts, and the BIRCWH-K12HD043441-06 to Dr. Catov.
Footnotes
Presentation information: This study was presented in poster format at the International Society for the Study of Hypertension in Pregnancy’s XVIth World Congress in Washington DC on September 22, 2008.
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Khan K, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367(9516):10661074. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 2.ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33. Obstet Gynecol. 2002;99(1):159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 3.Martin J, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S. Births: final data for 2004. Natl Vital Stat Rep. 2006;55(1):1–101. [PubMed] [Google Scholar]
- 4.Wallis A, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Amer J Hyperten. 2008;21(5):521–526. doi: 10.1038/ajh.2008.20. [DOI] [PubMed] [Google Scholar]
- 5.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Preg. 2003;22(2):143–148. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 6.Founds S, Powers RW, Patrick TE, Ren D, Harger GF, Markovic N, Roberts JM. A comparison of circulating TNF-alpha in obese and lean women with and without preeclampsia. Hypertens Preg. 2008;27(1):39–48. doi: 10.1080/10641950701825838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schipper E, Bolte AC, Schalkwijk CG, Van Geijn HP, Dekker GA. TNF-receptor levels in preeclampsia—results of a longitudinal study in high-risk women. J Matern Fetal Neonatal Med. 2005;18(5):283–287. doi: 10.1080/14767050500246466. [DOI] [PubMed] [Google Scholar]
- 8.Hauner H. The new concept of adipose tissue function. Physiol Behav. 2004;83(4):653–658. doi: 10.1016/j.physbeh.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi MUY, Yamaguchi T, Sohma R, Shibazaki M, Ohkura T, Inaba N. Tumor necrosis factor-alpha in the placenta is not elevated in pre-eclamptic patients despite its elevation in peripheral blood. Am J Reprod Immunol. 2005;53(3):113–119. doi: 10.1111/j.1600-0897.2005.00253.x. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi K. Adipokines: therapeutic targets for metabolic syndrome. Curr Drug Targets. 2005;6(4):525–529. doi: 10.2174/1389450054021972. [DOI] [PubMed] [Google Scholar]
- 11.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92(3):347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 12.Bodnar L, Ness RB, Harger GF, Roberts JM. Inflammation and triglycerides partially mediate the effect of prepregnancy body mass index on the risk of preeclampsia. Am J Epidemiol. 2005;162(12):1198–1206. doi: 10.1093/aje/kwi334. [DOI] [PubMed] [Google Scholar]
- 13.Dekker G, Robillard PY. Pre-eclampsia: Is the immune maladaptation hypothesis still standing? An epidemiological update. J Reprod Immunol. 2007;76(1–2):8–16. doi: 10.1016/j.jri.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Leeners B, Rath W, Kuse S, Irawan C, Neumaier-Wagner P. The significance of under- or overweight during childhood as a risk factor for hypertensive diseases in pregnancy. Early Hum Dev. 2006;82(10):663–668. doi: 10.1016/j.earlhumdev.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Ilekis J, Reddy UM, Roberts JM. Preeclampsia--a pressing problem: an executive summary of a National Institute of Child Health and Human Development workshop. Reprod Sci. 2007;14(6):508–523. doi: 10.1177/1933719107306232. [DOI] [PubMed] [Google Scholar]
- 16.Robillard P, Hulsey TC, Périanin J, Janky E, Miri EH, Papiernik E. Association of pregnancy-induced hypertension with duration of sexual cohabitation before conception. Lancet. 1994;344(8928):973–975. doi: 10.1016/s0140-6736(94)91638-1. [DOI] [PubMed] [Google Scholar]
- 17.Redman C, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 18.Dong M, Wang Z, He J. Serum T helper 1- and 2-type cytokines in preeclampsia. Int J Gynaecol Obstet. 2005;89(3):288–290. doi: 10.1016/j.ijgo.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 19.Saito S, Sakai M. Th1/Th2 balance in preeclampsia. J Reprod Immunol. 2003;59(2):161–173. doi: 10.1016/s0165-0378(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 20.Seely E, Solomon CG. Insulin resistance and its potential role in pregnancy-induced hypertension. J Clin Endocrinol Metab. 2003;88(6):2393–2398. doi: 10.1210/jc.2003-030241. [DOI] [PubMed] [Google Scholar]
- 21.Kaaja R, Laivuori H, Laakso M, Tikkanen MJ, Ylikorkala O. Evidence of a state of increased insulin resistance in preeclampsia. Metabolism. 1999;48(7):892–896. doi: 10.1016/s0026-0495(99)90225-1. [DOI] [PubMed] [Google Scholar]
- 22.Ness R, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am J Obstet Gynecol. 2006;195(1):40–49. doi: 10.1016/j.ajog.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 23.Oda E. The metabolic syndrome as a concept of adipose tissue disease. Hypertens Res. 2008;31(7):1283–1291. doi: 10.1291/hypres.31.1283. [DOI] [PubMed] [Google Scholar]
- 24.Kaaja R, Laivuori H, Pulkki P, Tikkanen MJ, Hiilesmaa V, Ylikorkala O. Is there any link between insulin resistance and inflammation in established preeclampsia? Metabolism. 2004;53(11):1433–1435. doi: 10.1016/j.metabol.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Anim-Nyame N, Sooranna SR, Jones J, Alaghband-Zadeh J, Steer PJ, Johnson MR. Insulin resistance and pre-eclampsia: a role for tumor necrosis factor-alpha? Gynecol Endocrinol. 2004;18(3):117–123. doi: 10.1080/09513590410001667832. [DOI] [PubMed] [Google Scholar]
- 26.Wolf M, Sandler L, Jimenez-Kimble R, Shah A, Ecker JL, Thadhani R. Insulin resistance but not inflammation is associated with gestational hypertension. Hypertens Preg. 2002;40(6):886–891. doi: 10.1161/01.hyp.0000042085.65467.9f. [DOI] [PubMed] [Google Scholar]
- 27.Matthews D, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 28.Cohen O, Epstein GS, Weisz B, Homko CJ, Sivan E. Longitudinal assessment of insulin sensitivity in pregnancy. Validation of the homeostasis model assessment. Clin Endocrinol. 2006;64(6):640–644. doi: 10.1111/j.1365-2265.2006.02519.x. [DOI] [PubMed] [Google Scholar]
- 29.Kirwan J, Huston-Presley L, Kalhan SC, Catalano PM. Clinically useful estimates of insulin sensitivity during pregnancy: validation studies in women with normal glucose tolerance and gestational diabetes mellitus. Diabetes Care. 2001;24:1602–1607. doi: 10.2337/diacare.24.9.1602. [DOI] [PubMed] [Google Scholar]
- 30.NHBPEP. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183(1):S1–S22. [PubMed] [Google Scholar]
- 31.Roberts J, Bodnar LM, Lain KY, Hubel CA, Markovic N, Ness RB, Powers RW. Uric acid is as important as proteinuria in identifying fetal risk in women with gestational hypertension. Hypertens Preg. 2005;46:1263–1269. doi: 10.1161/01.HYP.0000188703.27002.14. [DOI] [PubMed] [Google Scholar]
- 32.Kirwan J, Hauguel-De Mouzon S, Lepercq J, Challier JC, Huston-Presley L, Friedman JE, Kalhan SC, Catalano PM. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes Care. 2002;51(7):2207–2213. doi: 10.2337/diabetes.51.7.2207. [DOI] [PubMed] [Google Scholar]
- 33.Brewster J, Orsi NM, Gopichandran N, Ekbote UV, Cadogan E, Walker JJ. Host inflammatory response profiling in preeclampsia using an in vitro whole blood stimulation model. Hypertens Preg. 2008;27(1):1–16. doi: 10.1080/10641950701826067. [DOI] [PubMed] [Google Scholar]
- 34.Freeman D, McManus F, Brown EA, Cherry L, Norrie J, Ramsay JE, Clark P, Walker ID, Sattar N, Greer IA. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertens Preg. 2004;44(5):708–714. doi: 10.1161/01.HYP.0000143849.67254.ca. [DOI] [PubMed] [Google Scholar]
- 35.Sakai M, Shiozaki A, Sasaki Y, Yoneda S, Saito S. The ratio of interleukin (IL)-18 to IL-12 secreted by peripheral blood mononuclear cells is increased in normal pregnant subjects and decreased in pre-eclamptic patients. J Reprod Immunol. 2004;61(2):133–143. doi: 10.1016/j.jri.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Omu A, Al-Qattan F, Diejomaoh ME, Al-Yatama M. Differential levels of T helper cytokines in preeclampsia: pregnancy, labor and puerperium. Acta Obstet Gynecol Scand. 1999;78(8):675–680. [PubMed] [Google Scholar]
- 37.Jonsson Y, Rubèr M, Matthiesen L, Berg G, Nieminen K, Sharma S, Ernerudh J, Ekerfelt C. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J Reprod Immunol. 2006;70(1–2):83–91. doi: 10.1016/j.jri.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Viardot A, Grey ST, Mackay F, Chisholm D. Potential antiinflammatory role of insulin via the preferential polarization of effector T cells toward a T helper 2 phenotype. Endocrinology. 2007;148(1):346–353. doi: 10.1210/en.2006-0686. [DOI] [PubMed] [Google Scholar]
- 39.Visser N, van Rijn BB, Rijkers GT, Franx A, Bruinse HW. Inflammatory changes in preeclampsia: current understanding of the maternal innate and adaptive immune response. Obstet Gynecol Surv. 2007;62(3):191–201. doi: 10.1097/01.ogx.0000256779.06275.c4. [DOI] [PubMed] [Google Scholar]
- 40.Naugler W, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14(3):109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Shoelson S, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14(3–4):222–231. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]