The first reported cases of the successful use of extracorporeal life support systems (ECLS) occurred in the early 1970s. The circuit included a DeBakey-type roller pump and a spiral wound silicone membrane oxygenator. Until recently there have only been some small changes to the design and components of the circuit.
During the Sixth International Conference on Pediatric Mechanical Support Systems and Pediatric Cardiopulmonary Perfusion (May 6–8, Boston, MA, USA), we conducted a survey on the components of the circuits as well as the monitoring strategies of ECLS procedures. As a result, we received over 40 replies from the attendants of the conference from 32 institutions and 10 countries all over the world. Based on these replies, we have the opportunity to better understand the current changes in the ECLS circuits around the globe.
The objectives of this study are to share the recent survey results with the rest of the ECLS community and share the significant changes in our new Penn State Hershey ECLS circuitry.
The Oxygenator
The silicone membrane oxygenator consists of a flat, reinforced silicone membrane envelope that is wound in a spiral coil around a polycarbonate spool. In this oxygenator gas transfer occurs by molecular diffusion as it does in the human lung. The silicone membrane acts as a highly gas-permeable barrier separating blood and gas compartments. There is no direct blood/gas interface. The advantages of this oxygenator are that it is non-microporous, physiologically inert, biocompatible and has effective gas transfer characteristics. Some of the disadvantages include its large surface area, difficulty of priming and high transmembrane pressure.
The next type of membrane oxygenator to gain popularity is the microporous hollow-fiber oxygenator. Gas transfer is carried out by direct contact with the blood through the polypropylene hollow fibers. The hollow-fiber membrane oxygenator (HFMO) has the advantages of having a low priming volume, smaller surface area, ease of priming, low transmembrane pressure and adequate gas exchange. The primary disadvantage for long-term use is plasma leakage decreasing its gas exchange capabilities.
According to the results of the ECMO survey, for adult and pediatric patients, over 90% of the institutions are using HFMO, while referring to neonatal patients, there are still 32% (9 out of 28) institutions that are using a silicone membrane oxygenator (Table 1).
Table 1.
Clinical application of different types of oxygenator and blood pumps in ECLS
| Oxygenator (*) | Pump (†) | |||
|---|---|---|---|---|
| HFMO** | Silicone | Roller | Centrifugal | |
| Adult | 21 (6) | 2 (1) | 7 (3) | 18 (2) |
| Pediatric | 26 (7) | 2 (1) | 8 (4) | 23 (6) |
| Neonatal | 19 (0) | 9 (8) | 8 (7) | 21 (5) |
the number of the institutions that are using roller pumps;
the number of the institutions that are using servo-regulation devices prior to the pump in the circuit;
HFMO**, hollow fiber membrane oxygenator.
Even though there have been reported cases of using a HFMO successfully for 14 days, the average use is 2.5 – 3 days. Changing out an oxygenator is not a benign procedure and can result in prolonging the patient’s recovery.
With the more recent release of the polymethylpentene diffusion membrane (PMP), the issue of plasma leakage has nearly disappeared. The hollow polymethylpentene fibers are protected by a thin outer layer. Gas transfer is obtained by diffusion through this thin layer. The PMP membrane oxygenator utilizes hollow-fiber technology with a true non-microporous membrane. This low-resistance device has been introduced into clinical application because it has all the inherent advantages of hollow fiber technology without plasma leakage necessitating changeout with a maximun duration of 59 days (1–3).
The Pump
The initial pump for all ECMO circuits was the roller pump. Due to the wear on the tubing, the tubing within the raceway of the pump is advanced every several days. It is also very important that the occlusion be properly set. A bladder or compliance chamber is also inserted into the circuit prior to the inlet of the pump.
As the centrifugal pump became available, it was introduced into the adult ECLS circuit. With its reported advantages in regard to reduced damage to blood elements and reduced activation of the coagulation system, it was a logical choice for long-term support (2). There have been some questions raised concerning their heat generation and the accuracy of their flow measurements at low RPMs, but their overall ease of use and safety advantage outweigh those issues.
The newest improvement to the centrifugal pump design has been the magnetically levitated devices. They have been shown to have less shear and thus less heat generation.
According to the results of our survey, magnetically levitated centrifugal pumps have been the most popular pump in ECLS circuits. 72%, 74% and 72% of all the institutions involved in our survey reported there clinical application of centrifugal pump for adult, pediatric and neonatal patients, respectively. Almost all institutions that are still using silicone membrane oxygenators are the ones using conventional roller pumps, especially for neonatal patients, with only one exception (Table 1). Venous servo-regulation devices (bladders/compliance chambers) prior to the arterial pump are used in most ECLS centers utilizing roller pumps and some centers utilizing centrifugal pumps. These devices are more commonly used in neonatal/pediatric roller pump ECLS. (Table 1)
Pressure Monitoring
Pressure monitoring across the ECLS circuit is one of the main safety controlling methods due to the occlusion of the roller pump. Though centrifugal pumps are considered safer by virtue of their afterload and preload sensitivity, inlet and outlet line pressures are still being monitored in some institutions to avoid hemolytic or cavitation situations. In clinical, three sites (pre-membrane, post-membrane and arterial line) are usually selected to monitor the circuit pressure. Additionally, when using a centrifugal pump, the pressure at the inlet of the pump or the CVP should also be monitored to prevent the possible oversuction in the right atrium and the occurrence of cavitation. The results of our ECLS survey on the pressure monitoring are shown in table 2. For adult patients, 64% of institutions monitor the pre-membrane pressure during the ECLS, while at the post-membrane site and arterial line (system pressure), the percentage drops to lower than 50%. Lower percentages were also found in pediatric and neonatal patients for system pressure, but all the institutions perform both pre-membrane and post-membrane pressure monitoring simultaneously in pediatric and neonatal patients. In terms of negative pressure monitoring at the inlet of the centrifugal pump, the pediatric group has the highest percentage of 70%, compared to the percentage of 61% and 57% in the adult and neonatal group, respectively.
Table 2.
Report of Circuit pressure monitoring at different sites. (the number of the institutions doing the pressure monitoring / the number of total institutions reported)
| Pre-membrane Pressure |
Post- membrane Pressure |
Total System (arterial line) Pressure |
Pump Inlet Negative pressure |
|
|---|---|---|---|---|
| Adult | 16 / 25 | 11 / 24 | 9 / 21 | 11 / 18 |
| Pediatric | 20 / 29 | 20 / 29 | 11 / 24 | 16 / 23 |
| Neonatal | 20 / 28 | 20 / 28 | 11 / 23 | 12 / 21 |
Anticoagulation Monitoring
One of the most challenging and frequent complications to manage during ECLS is bleeding and circuit thrombosis. Over the past few years, the ECLS circuits with complete blood handling surface modification have been brought to the marketplace and introduced into clinical application. These artificial coatings can be divided into heparin based and non-heparin biopassive surfaces such as amphiphilic polymers. The results of our survey has shown that 84% of (21 out of 25 ) institutions are using ECLS circuits with surface coatings, among which 16 institutions are using heparin based coatings, 2 institutions are using non-heparin based coatings and 3 institutions are using both of them, depending on the different products.
Even though the biocompatibility of the surface has been improved, heparin is still needed to keep the patients undergoing ECLS at a higher anticoagulative level than normal. The activated clotting time (ACT) and partial thromboplastin time (PTT) tests are being widely used in clinical conditions to monitor the effects of the anticoagulation therapy. The ACT is a rapid test that can be performed at the patients’ bedside and allows relatively rapid changes in heparin infusion, helping to achieve and maintain a constant level of anticoagulation. But the sensitivity of the ACT test to heparin depends on the method being used. The PTT is a performance indicator measuring the efficacy of both the intrinsic and the common coagulation pathways, and it is also used to monitor the treatment effects with heparin. Besides the effects of heparin, there are several factors that could also cause prolonged PTT, such as the antiphospholipid antibody and coagulation factor deficiency in the serum. Another shortcoming of the PTT is that it cannot be used as a clinical monitoring tool beyond a certain level of heparinization. Currently there is not a widespread agreement of the target range of these two values in clinical application.
According to the results of our ECLS survey (Table 3), most institutions use ACT as the anticoagulation monitoring tool, for adult patients, 5 institutions are using PTT as the monitoring tool, but in regard to pediatric and neonatal patients, only one institution is using PTT as the monitoring tool. In addition, there are 8 institutions that are using both ACT and PTT to monitor the effects of anticoagulation therapy clinically.
Table 3.
Clinical Application of ACT and PTT as a monitoring tool of anticoagulation therapy (the number of institutions)
ACT*, Activated clotting time;
PTT**, partial thromboplastin time.
Blood Flow
Accurate blood flow readings are more critical in smaller patients. By design, centrifugal pumps utilize a flow probe to indicate flow rates. Roller pumps rely on tubing size and RPMs to derive a calculated flow. Accurate flow rates require proper occlusion settings, tubing size and calibrated RPMs. Incorporating any devices utilizing an arterial to venous shunt, such as a hemoconcentrator or inline blood gas device, requires the use of an additional flow measurement device to assure accurate blood flow delivery.
Newest Circuits
Within the past few years adult ECLS has taken on a new look in particular in the United States. It has been simplified to a small circuit comprised mainly of a PMP oxygenator, magnetically-levitated centrifugal pump, coated tubing and cannulae. The circuit no longer requires fulltime surveillance and can be managed by the patient’s ICU nurse. The recent H1N1 outbreak resulted in a surge of patients requiring ECLS even in small community hospitals stressing the importance for a simpler, affordable, easier to manage circuit.
Some institutions have employed the PMP oxygenators with a centrifugal pump for their neonatal and pediatric cases, but most centers are reluctant to change from the silicone membrane oxygenator and roller pump especially for neonates. Although the newer circuits would have a smaller surface area, less prime, and probably not require changeout over the length of the support period, the need for a shunt around the oxygenator and additional access ports in the infant have delayed its adoption by most centers.
The recent release in the United States of the pediatric Quadrox-iD from Maquet Cardiopulmonary may help to change that current trend. It has a recommended minimum blood flow of only 200 ml/min and a maximum recommended blood flow rate of 2.8 L/min. (Figure 1).
Figure 1.
Quadrox-iD Pediatric oxygenator
A research group from Japan has developed a heparin-free ECLS system using a Rotaflow centrifugal pump and a new oxygenator consisting of plasma leakage-tight polymer fibers and a new biocompatible coating. By using this ECLS system, the researchers were able to conduct animal ECLS experiments for over 30 days without any systemic anticoagulation, and 151 days with only trivial heparin infusion, without plasma leakage or any component replacement in the circuit (4, 5). During the experiments, the ACT, aPTT, platelet count and fibrinogen level were within normal limits. Only a small amount of thrombus was found after the experiments, at the top of the oxygenator where the blood flow was supposed to be slowest or stagnant. There were no clots observed in the other components of the circuit. According to researchers’ recent report (6), this new heparin-free ECLS system has progressed into clinical application in both adult and pediatric patients and gained encouraging outcomes.
Recent Laboratory Studies at the Penn State Hershey
In Vitro studies
Over the past few years, with the goal to improve the outcomes for children undergoing ECLS procedures and minimize the adverse effects of the ECLS, several translational research projects aimed at the evaluation and the selection of every single component in the ECLS circuit have been performed in our Penn State Pediatric Cardiovascular Research Center (http://www.pennstatehershey.org/web/pedscardiacresearch).
For the oxygenator, we compared two neonatal ECLS circuits with the new PMP HFMO or the conventional silicone oxygenator (7), we also evaluated three different HFMOs in terms of perfusion quality. (8) For the pump, we not only compared the magnetically levitated centrifugal pump to the conventional roller pump in regard to the hemodynamic energy delivery as well as the air bubble handling (9), but also evaluated the mechanical performance between two widely-used centrifugal pumps (10). Furthermore, to determine the impact of arterial cannulae and tubing length, we evaluated the performance of a minimized neonatal ECMO circuit with different length of tubing (11), as well as different combination of arterial and venous cannulae (manuscript in preparation).
Right after the approval of the Quadrox-iD PMP HFMO by the FDA in 2010, we evaluated the hemodynamic performance of this new oxygenator in both the conventional circuit with roller pump and the minimized circuit with centrifugal pump. The results have shown an extremely low pressure drop as well as an excellent performance in hemodynamic energy preservation compared to all other oxygenators we have tested in our laboratory (manuscript in preparation)
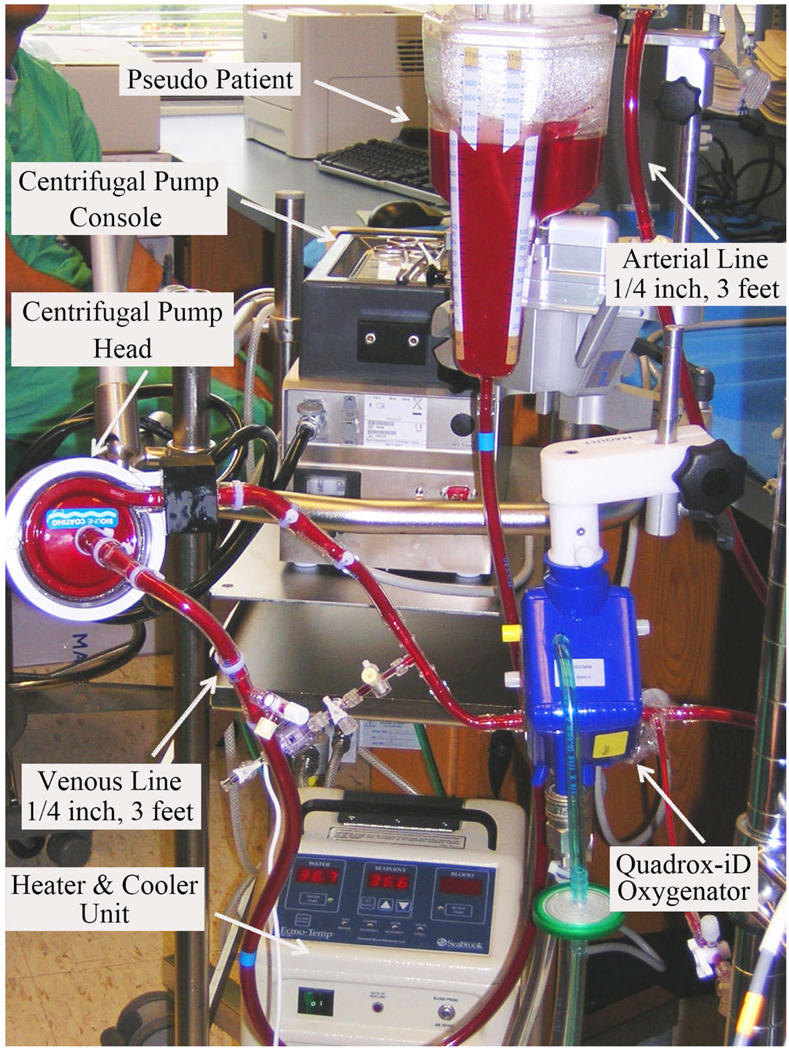
Based on the results of our several translational research projects and input from the entire clinical team including perfusionists, nurses, surgeons and engineers, we have created a Penn State pediatric ECMO circuit, which is composed of a Rotaflow centrifugal pump, a Quadrox-iD membrane oxygenator, two Bio-Medicus ECMO cannulae (arterial and venous), and three feet of ¼ inch tubing for both the arterial and venous line (Figure 2). All the blood handling surface of the circuit is completely coated. The advantages of this new circuit include minimized blood contact surface area and priming volume (less than 200ml), extremely low resistance to blood flow (means lower RPMs under the same pressure and flow condition), better safety and portability.
Figure 2.
New set-up of pediatric ECLS circuit (Rotaflow centrifugal pump and Quadrox-iD Pediatric oxygenator) at the Penn State Hershey Children s Hospital.
In Vivo
After the evaluation in the laboratory, all the selected products were tested in animal experiments before the clinical application in Penn State Hershey Medical Center.
With the significant change in ECLS equipment and the inherent safety of the new circuit, we have provided both didactic and “hands on” education for our clinical ECLS team including the clinicians as well as the ICU nurses. (12) The educational project was performed both in the wet laboratory and the animal experiments.
Conclusions
The ECLS circuit will continue to evolve. For the use of ECLS to continue to be safer and effective with a more wide-spread utilization, the circuit needs to be simple, have less foreign surface area, have a lower priming volume with its resultant hemodilution and transfusion requirements and endure for the duration of the support period.
A conventional ECLS circuit is no longer a roller pump and silicone membrane oxygenator, despite their historic success saving thousands of children’s lives. All new products (pumps, oxygenators, cannulae) should be compared to these new generation products.
Our efforts will continue on this underserved research area and we are eager to conduct more translational research when new products become available. In addition, we will host several one-day ECMO workshops for other members of the ECLS community in 2010 as well as in 2011 at the Milton S. Hershey Medical Center.
Reference
- 1.Horton S, Thuys C, Bennett M, Augustin S, Rosenberg M, Brizard C. Experience with the Jostra Rotaflow and QuadroxD oxygenator for ECMO. Perfusion. 2004;19(1):17–23. doi: 10.1191/0267659104pf702oa. [DOI] [PubMed] [Google Scholar]
- 2.Thiara AP, Høyland V, Norum H, Aasmundstad TA, Karlsen HM, Fiane AE, Geiran O. Extracorporeal membrane oxygenation support for 59 days without changing the ECMO circuit: a case of Legionella pneumonia. Perfusion. 2009;24(1):45–47. doi: 10.1177/0267659109106297. [DOI] [PubMed] [Google Scholar]
- 3.Thiara AP, Hoel TN, Kristiansen F, Karlsen HM, Fiane AE, Svennevig JL. Evaluation of oxygenators and centrifugal pumps for long-term pediatric extracorporeal membrane oxygenation. Perfusion. 2007;22(5):323–326. doi: 10.1177/0267659107086270. [DOI] [PubMed] [Google Scholar]
- 4.Nishinaka T, Tatsumi E, Taenaka Y, Katagiri N, Ohnishi H, Shioya K, Fukuda T, Oshikawa M, Sato K, Tsukiya T, Homma A, Takewa Y, Takano H, Sato M, Kashiwabara S, Tanaka H, Sakai K, Matsuda T. At least thirty-four days of animal continuous perfusion by a newly developed extracorporeal membrane oxygenation system without systemic anticoagulants. Artif Organs. 2002;26(6):548–551. doi: 10.1046/j.1525-1594.2002.06886_3.x. [DOI] [PubMed] [Google Scholar]
- 5.Nishinaka T, Tatsumi E, Katagiri N, Ohnishi H, Mizuno T, Shioya K, Tsukiya T, Homma A, Kashiwabara S, Tanaka H, Sato M, Taenaka Y. Up to 151 days of continuous animal perfusion with trivial heparin infusion by the application of a long-term durable antithrombogenic coating to a combination of a seal-less centrifugal pump and a diffusion membrane oxygenator. J Artif Organs. 2007;10(4):240–244. doi: 10.1007/s10047-007-0390-3. [DOI] [PubMed] [Google Scholar]
- 6.Tatsumi E, Katagiri N, Takewa Y, Mizuno T, Tsukiya T, Homma A, Taenaka Y, Hayashi T, Yagihara T. Development of an Ultra-Durable Heparin-Free ECMO System and Its Clinical Application to Pediatric and Adult Patients in Japan. Artif Organs. 2010;34(4):A7. [Google Scholar]
- 7.Haines N, Wang SG, Myers JL, Ündar A. Comparison of two types of neonatal extracorporeal life support systems with pulsatile and nonpulsatile flow. Artif Organs. 2009;33:958–966. doi: 10.1111/j.1525-1594.2009.00934.x. [DOI] [PubMed] [Google Scholar]
- 8.Talor J, Yee S, Rider A, Kunselman AR, Guan YL, Ündar A. Comparison of perfusion quality in hollow-fiber membrane oxygenators for neonatal extracorporeal life support. Artif Organs. 2010;34(4):E110–E116. doi: 10.1111/j.1525-1594.2009.00971.x. [DOI] [PubMed] [Google Scholar]
- 9.Yee S, Qiu F, Su XW, Rider A, Kunselman AR, Guan YL, Ündar A. Evaluation of HL-20 roller pump and rotaflow centrifugal pump on perfusion quality and gaseous microemboli delivery. Artif Organs. 2010 doi: 10.1111/j.1525-1594.2010.01079.x. (in press). [DOI] [PubMed] [Google Scholar]
- 10.Guan YL, Su XW, McCoach R, Kunselman AR, El-Banayosy A, Ündar A. Mechanical performance comparison between RotaFlow and CentriMag centrifugal blood pumps in an adult ECLS model. Perfusion. 2010;25(2):71–76. doi: 10.1177/0267659110365366. [DOI] [PubMed] [Google Scholar]
- 11.Qiu F, Uluer MC, Kunselman A, Clark JB, Myers JL, Ündar A. Impact of the length of the tubing on hemodynamics in a simulated neonatal ECLS circuit. Artif Organs. 2010 doi: 10.1111/j.1525-1594.2010.01132.x. (in press). [DOI] [PubMed] [Google Scholar]
- 12.McCoach R, Weaver B, Carney E, Clark JB, Pauliks L, Guan YL, Qiu F, Chang D, Reed-Thurston D, Myers JL, Ündar A. Pediatric extracorporeal life support (ECLS) systems: education and training at Penn State Hershey Children’s Hospital. Artif Organs. 2010 doi: 10.1111/j.1525-1594.2010.01113.x. (in press). [DOI] [PubMed] [Google Scholar]