Abstract
A series of fluorine containing N-(2-methoxyphenyl)piperazine and N-(2-fluoroethoxy)piperazine analogues were synthesized and their affinities for human dopamine D2, D3 and D4 receptors were determined. Radioligand binding studies identified five compounds, 18a, 20a, 20c, 20e and 21e, which bind with high affinity at D3 (Ki = 0.17 to 5 nM) and moderate to high selectivity for D3 vs. D2 receptors (ranging from ∼25 to 163-fold). These compounds were also evaluated for intrinsic activity at D2 and D3 receptors using a forskolin-dependent adenylyl cyclase assay. This panel of compounds exhibits varying receptor subtype binding selectivity and intrinsic activity at D2 vs. D3 receptors. These compounds may be useful for behavioral pharmacology studies on the role of D2-like dopamine receptors in neuropsychiatric and neurological disorders. Furthermore, compound 20e, which has the highest binding affinity and selectivity for the D3 receptor (Ki = 0.17 nM for D3, 163-fold selectivity for D3 vs. D2 receptors) represents a candidate fluorine-18 radiotracer for in vivo PET imaging studies on the regulation of D3 receptor expression.
Introduction
Dopamine receptors are G protein-coupled receptors and are classified into two major types, the D1-like and D2-like receptors. The D1-like receptor subtypes include the D1 (rat D1a) and D5 (rat D1b) receptors, whereas the D2-like receptor subtypes include the D2, D3 and D4 receptors. Agonist stimulation of D1-like receptors results in an activation of adenylyl cyclase. Stimulation of D2-like receptors results in an inhibition of adenylyl cyclase activity, an increase in the release of arachidonic acid, activation of G protein-coupled inwardly-rectifying potassium channels (GIRKs), activation of phospholipase D (PLD) and also an increase in phosphatidylinositol hydrolysis.1 D2 and D3 receptors have ∼ 46% overall amino acid sequence homology and 78% sequence homology within the transmembrane spanning segments.2
There is a large body of evidence indicating that D3 dopamine receptors may play an important role in a number of neurological and neuropsychiatric disorders.3 First, the high density of D3 receptors in limbic regions4-7 suggests that this receptor subtype may play a role in the etiology of schizophrenia and that D3-selective antagonists may exhibit an antipsychotic profile devoid of extrapyramidal side effects.2, 5, 7 Second, prolonged treatment of 6-hydroxydopamine unilaterally lesioned rats with L-DOPA is a rodent model of L-DOPA-induced dyskinesia (LID); previous studies have suggested that there is an upregulation of D3 receptors in dyskinetic animals.8–10 D3 receptor selective ligands have been shown to be effective in attenuating L-DOPA-induced dyskinesia in rats, suggesting that the D3 receptor may be an important therapeutic target for the treatment of LID.11–15 Finally, the positive reinforcing effects of psychostimulants, such as cocaine and methamphetamine, may be mediated, in part, by the stimulation of D3 receptors. Therefore, D3 receptor selective partial agonists and/or antagonists may be useful pharmacotherapeutic agents for the treatment of substance abuse.16–21
Positron Emission Tomography (PET) is a non-invasive imaging technique that has been used to study the expression of dopamine receptors in the brain. However, the identification of D3 receptor specific PET radioligands has been challenging because of the high degree of amino acid sequence homology between D2 and D3 receptor binding sites in the ligand binding domain.1, 20,3, 22–25 A number of D3-selective ligands have served as lead compounds for PET radiotracer development (Figure 1).26–30 Unfortunately, none of the D3 receptor selective radiotracers reported to date have shown promise in in vivo imaging or brain uptake studies in rodents or nonhuman primates. One of the main limitations of many D3-selective ligands is their relatively high lipophilicity, which could compromise their ability to cross the blood-brain barrier and label D3 receptors in vivo.
Figure 1.
Representative dopamine D3 receptor ligands.
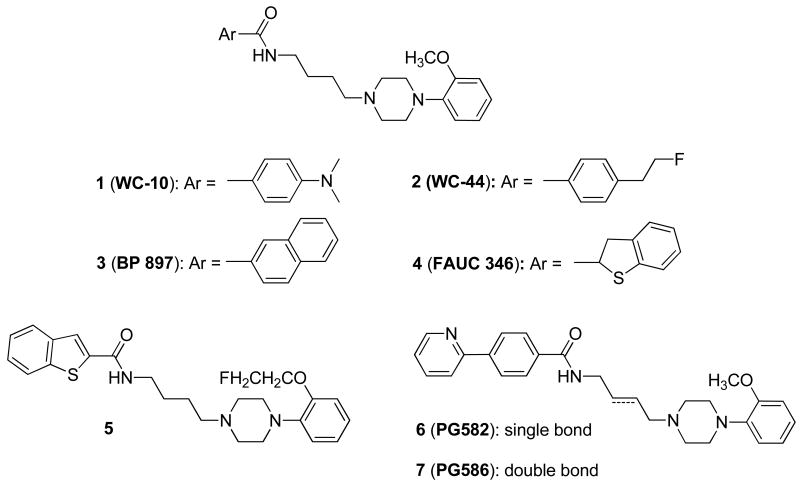
Over the past decade, our group has focused on identifying candidate ligands having the right balance between D3 receptor affinity (1-5 nM), selectivity (>50-fold selective for D3 versus D2 receptors), and lipophilicity (log P = 2.0 – 4.0) to give a suitable signal: noise ratio in PET imaging studies. We previously reported benzamide analogues, 1 (WC-10) (Ki = 0.8 nM for D3 receptor, D2/D3 ratio = 43) and 2 (WC-44) (Ki = 2.4 nM for D3, D2/D3 ratio = 23) as lead compounds for radiotracer development.24 Quantitative autoradiography studies using [3H]-1 demonstrated it has high affinity and moderate selectivity for D3 vs. D2 receptor,24, 31 which is consistent with in vitro screening data using competition binding assays.25 However, microPET studies of [11C]-1 in rhesus brain have exhibited high levels of variability for D3 imaging between subjects, and similar studies using [18F]-2 have not shown good target to non-target ratios.25
Our laboratory has continued to investigate the structure-activity relationships of conformationally flexible benzamide analogues by optimizing the structures of 1 and 2 to identify promising candidates for imaging the D3 receptor with PET. The longer half-life of 18F (t1/2 = 109.8 min) compared to 11C (t1/2 = 20.4 min) places fewer time constraints on radiotracer synthesis and permits longer scan sessions for 18F-labeled radiotracers versus 11C-labeled radiotracers. In this article, we report the synthesis and in vitro evaluation of a series of fluorine containing conformationally flexible benzamide analogues, in which the structure was altered by: 1) replacing the 2-methoxyphenyl group in the piperazinyl ring with a 2-(2-fluoroethoxyphenyl) group; 2) introducing a 2-fluoroethoxy or 2-fluoroethyl group in the 2- and 4-position of the benzamide moiety; and, 3) comparing the effect of having a double bond (trans-butenyl) within the four carbon chain that links the arylamide with the 4-phenylpiperazine moiety.
Results and Discussion
Chemistry
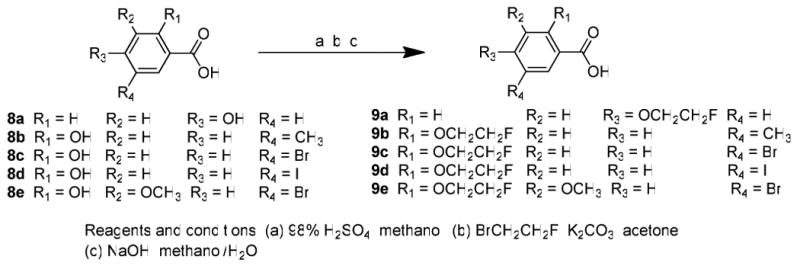
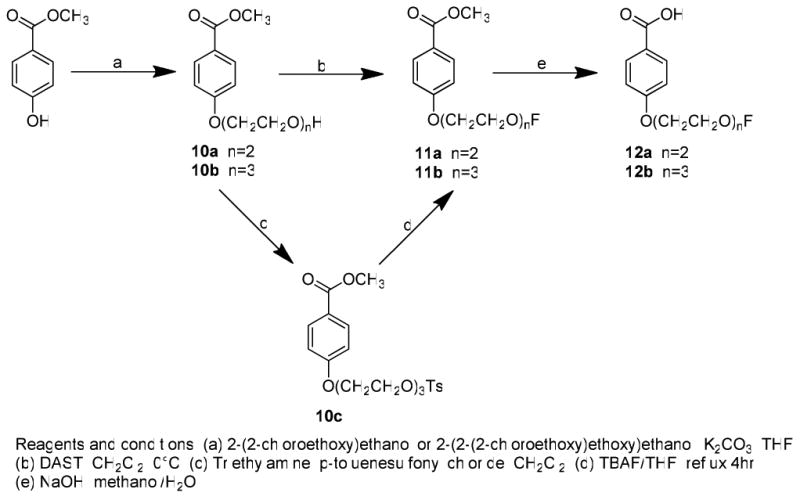
The target compounds were synthesized as depicted in Schemes 1–3. The synthesis of the substituted benzoic acids (9a-e) was accomplished as outlined in Scheme 1. The acids were first converted into the corresponding methyl esters by Fischer esterification. O-alkylation of 2-hydroxyl or 4-hydroxyl group was achieved by treatment with 1-bromide-2-fluoroethane in acetone using potassium carbonate as the base. Hydrolysis of the methyl ester with sodium hydroxide in aqueous methanol afforded the corresponding 2-fluoroethoxy benzoic acids 9a-e. The synthesis of the 4-fluoropegylated benzoic acids 12a and12b is shown in Scheme 2. O-alkylation of the phenol group of 4-hydroxy-benzoic acid methyl ester with either 2-(2-chloroethoxy)ethanol or 2-(2-(2-chloroethoxy)ethoxy)ethanol in the presence of potassium carbonate in tetrahydrofuran afforded 10a and 12b (Scheme 2). Conversion of alcohols 10a and 10b to the corresponding fluoro derivatives 11a and 11b was accomplished using two different methods. Direct conversion of the hydroxyl group of 10a with diethylaminosulfur trifluoride (DAST) gave 11a in modest yield (43%). Alternatively, conversion of the hydroxyl group of 10b to the corresponding tosylate group, 10c, followed by displacement with tetrabutylammonium fluoride (TBAF) gave 11b in an overall yield of 56%. Hydrolysis of 11a and 11b with sodium hydroxide in aqueous methanol afforded benzoic acids 12a and 12b.
Scheme 1.
Scheme 2.
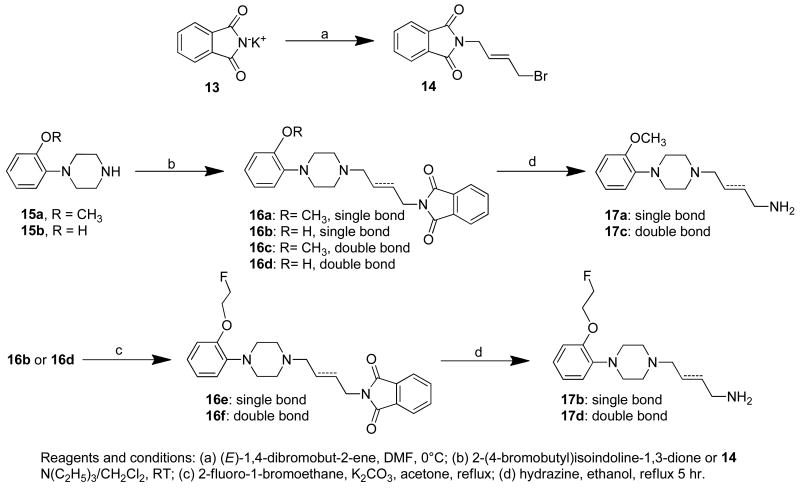
The substituted 4-(4-phenylpiperazin-1-yl)butan-1-amines (17a, b) and the substituted 4-(4-phenylpiperazin-1-yl)-trans-but-2-en-1-amines (17b, d) were synthesized according to Scheme 3. Treatment of potassium 1,3-dihydro-1,3-dioxoisoindole (13) with trans-1,4-dibromo-2-butene in N,N-dimethylformamide (DMF) gave 2-(trans-4-bromobut-2-enyl)-1,3-dihydro-1,3-dioxoisoindole, 14, in modest yield (69%). The N-alkylation of 1-(2-methoxylphenyl)piperazine (15a) or 1-(2-hydroxylphenyl)piperazine (15b) with either 2-(4-bromobutyl)-1,3-dihydro-1,3-dioxoisoindole or 14 produced 16a–d. O-alkylation of the phenol group in 16b and 16d with 1-bromo-2-fluoroethane in acetone using potassium carbonate as the base afforded compounds 16e,f. Treatment of 16a, c and 16e,f with hydrazine in refluxing ethanol (Scheme 3) afforded the corresponding amines 17a–d in variable yields.
Scheme 3.
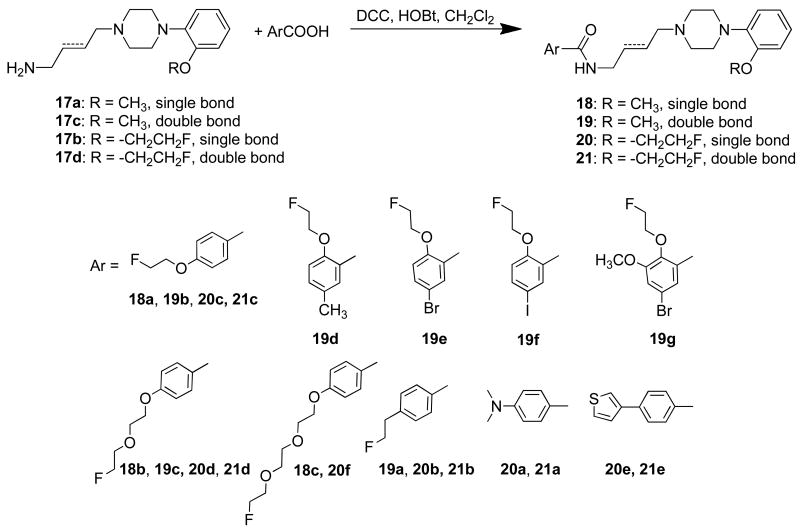
The target benzamides 18a–c, 19a–g, 20b–d,f and 21b–d, were synthesized by coupling amines 17a–d with substituted benzoic acids 9a–e, 12a–b and 4-(2-fluoroethyl)benzoic acid,25 with N,N′-dicyclohexyl-carbodiimide (DCC) in dichloromethane (Scheme 4). Benzamides 20a e and 21a,e, were prepared by coupling amines 17b,d with the corresponding commercially available benzoic acid. All final compounds were converted into the corresponding oxalic acid salt for in vitro binding studies.
Scheme 4.
In Vitro Binding Studies
Compounds were first evaluated for affinity at human D2 and D3 dopamine receptors expressed in stably transfected HEK cells. Analogues which exhibited high binding affinity at D3 receptors were further evaluated for affinity at a) D4 dopamine receptors and b) σ1 and σ2 sigma receptors. The σ receptor binding studies were undertaken because of the ubiquitous expression of sigma receptors in the CNS. Therefore, high σ1 or σ2 receptor binding affinity would preclude the usefulness of a D3- selective radiotracer for PET imaging studies. The σ receptor binding studies were included to ensure that our compounds bind with low affinity for σ receptors.
The [125I]-IABN inhibition constants (Ki) at D2 and D3 receptors are reported in Table 1. The ligand binding selectivity, in terms of a selectivity index, is calculated as Ki (D2)/Ki (D3). For the ensuing discussion, binding affinities are characterized as very high (Ki < 1.0 nM), high (Ki = 1-10 nM), moderate (Ki = 11-50 nM) or low (Ki >50 nM).
Table 1.
D2, D3 and D4 Affinities (Ki ± SD, nM) of the benzamide analogs.
| D2 | D3 | D4 | D2/D3 Ratio | Log Pa | |
|---|---|---|---|---|---|
| 1 | 34.4 ± 4.7 | 0.8 ± 0.1 | 896 ± 272 | 43 | 3.09 |
| 2 | 54.5 ± 4.4 | 2.4 ± 0.4 | 804 ± 46 | 23 | 2.94 |
| 18a | 27.1 ± 3.5 | 1.1 ± 0.1 | 1,400 ± 320 | 25 | 3.48 |
| 18b | 20.9 ± 3.7 | 6.2 ± 0.9 | ND | 3.4 | 3.08 |
| 18c | 52.0 ± 6.6 | 4.2 ± 0.2 | 2,100 ± 380 | 12.2 | 2.72 |
| 19a | 131 ± 13 | 24.9 ± 3.3 | ND | 5.2 | 3.43 |
| 19b | 55.3 ± 6.0 | 6.2 ± 0.3 | ND | 8.9 | 3.55 |
| 19c | 59.2 ± 5.8 | 18.2 ± 2.3 | ND | 3.3 | 3.16 |
| 19d | 17.8 ± 0.8 | 18.5 ± 2.4 | ND | 1 | 3.66 |
| 19e | 13.4 ± 2.3 | 13.6 ± 2.0 | ND | 1 | 4.49 |
| 19f | 13.2 ± 0.8 | 10.9 ± 1.5 | ND | 1.2 | 4.73 |
| 19g | 57.6 ± 3.7 | 13.8 ± 1.2 | ND | 4.2 | 4.15 |
| 20a | 15.1 ± 1.7 | 0.65 ± 0.2 | 890 ± 100 | 23 | 3.75 |
| 20b | 21.4 ± 2.9 | 6.9 ± 1.0 | ND | 3.1 | 3.68 |
| 20c | 15.1 ± 2.7 | 0.52 ± 0.03 | 990 ± 200 | 29 | 3.73 |
| 20d | 14.2 ± 1.9 | 2.5 ± 0.3 | ND | 5.7 | 3.34 |
| 20e | 27.7 ± 5.4 | 0.17 ± 0.01 | 246 ± 13 | 163 | 4.67 |
| 20f | 31.7 ± 2.1 | 5.2 ± 0.3 | ND | 6.1 | 2.98 |
| 21a | 35.2 ± 2.5 | 3.6 ± 0.6 | ND | 9.8 | 3.83 |
| 21b | 17.7 ± 2.7 | 1.1 ± 0.2 | 890 ± 380 | 16.1 | 3.61 |
| 21c | 37.9 ± 5.0 | 8.4 ± 1.3 | ND | 4.5 | 3.81 |
| 21d | 64.8 ± 8.4 | 19.6 ± 3.2 | ND | 3.3 | 3.41 |
| 21e | 70.5 ± 9.6 | 1.1 ± 0.2 | 182 ± 5 | 64 | 4.74 |
| 21f | 25.9 ± 2.4 | 11.2 ± 2.2 | ND | 2.3 | 3.26 |
| 21g | 10.9 ± 0.4 | 6.7 ± 1.1 | ND | 1.6 | 3.72 |
| 21h | 12.0 ± 0.6 | 3.1 ± 0.3 | ND | 3.9 | 4.36 |
Calculated using ACD log D software, Advanced Chemistry Development, Toronto, Canada.
The radioligand binding assays identified a number of potentially useful structure-activity trends as well as several promising fluorinated analogues which could serve as potential PET radiotracers. First, in a comparison of 1 and 18a–c, it was observed that replacing the 4-dimethylamino group in the benzamide moiety with a 4-(2-fluoroethoxy) group (compound 18a) resulted in a D3 binding affinity (Ki = 1.1 nM) comparable to 1 (Ki = 0.8 nM). However, there was a decrease in D3 receptor affinity when the 4-(2-fluoroethoxy) group was homologated to the corresponding fluoropegylated groups, 18b and 18c. Thus, the D3 vs. D2 selectivity for 18b and 18c, was <15-fold, whereas the D3 vs. D2 selectivity for 18a was ∼25 fold.
To further explore the structure-activity relationships of this series, the structures of the amides were modified by introducing the 2-fluoroethoxyl group in the 2-position compared to 4-position of the benzamide moiety. These analogs also had a methyl or halogen atom (Br or I) in the 5-position, and the trans double bond 4-carbon spacer group. This approach generally resulted in compounds with only moderate affinity for both D3 and D2 receptors (compounds 19d-f), except for compound 19g which displayed a 4-fold selectivity for D3 versus D2 receptors. Based on these results, a higher D3 receptor affinity and selectivity was observed when the 2-fluoroethoxy is in the 4-position of the benzamide region (compound 19b) rather than the 2-position.
The next substitution involved replacing the 2-methoxy group with a 2-fluoroethoxy group in the N-phenylpiperazinyl moiety. The replacement of a methoxy group with a 2-fluoroethoxy group is a standard method for preparing a potential 18F-labeled radiotracer. This modification generally resulted in compounds having a slightly increased D3 binding affinity when compared with the corresponding N-(2-methoxyphenyl)piperazinyl analogs (e.g., 20a vs. 1, 20c vs. 18a and 20d vs. 18c). However, one exception to this trend was noticed, with 20b having a lower D3 affinity than its corresponding 2-methoxy analog, 2. An interesting observation was in the substitution of the 4-position of the benzamide group with a 3-thiophene ring to give compound 20e; this analog displayed both the highest D3 binding affinity (0.17 nM) and greatest D3 vs. D2 receptor selectivity (163-fold) among the compounds reported in this article.
When the saturated 4-carbon spacer linking the arylamide with the N-(4-methoxyphenyl)piperazine moiety was replaced with a trans double bond, the binding affinity at both D2 and D3 receptors generally decreased. However, replacing the single bond with a trans double bond caused a larger reduction in D3 affinity relative to the reduction in D2 affinity. This trend was also observed with the N-(2-fluoroethoxyphenyl)piperazine containing analogues shown in Table 1. These results are consistent with the N-(2-methoxyphenyl)piperazinyl analogs described by Taylor et al.32 but opposite to what has been reported with the corresponding N-(2,3-dichlorophenyl)piperazinyl benzamide analogs.20, 33 Therefore, the effect of the single vs. double bond replacement on binding selectivity appears to be governed by the type of substitution on the N-phenylpiperazine group. The only analog showing an increase in D3 affinity when the trans double bond was introduced was 21b, which had a 6-fold higher affinity at D3 receptors when compared with its saturated analog 20b.
Affinity at dopamine D4 receptors was determined on compounds having a high affinity (Ki < 5 nM) for D3 receptors and high selectivity for D3 vs. D2 receptors (>10 fold). All compounds that were tested exhibited low binding affinity at D4 receptors (Table 1).
Since many dopamine ligands have been shown to bind to σ1 and σ2 receptors, we determined the σ receptor binding affinities for compounds having a high D3 receptor affinity and good selectivity for D3 vs. D2 receptors. All of the compounds tested exhibited low binding affinities at σ1 and σ2 receptors. The affinity ratios of D3 to σ receptors were >260-fold (Table 2). Compound 20e, which has the highest D3 affinity and D3 vs. D2 selectivity ratio, binds with low affinity at both σ1 and σ2 receptors. This observation eliminates any concern that σ receptor binding might interfere with the imaging signal when a 18F-radiolabeled derivative of 20e is made for PET imaging studies of the D3 receptor.
Table 2. Sigma Receptor Affinities (Ki ± SD [nM]) of Selected Analogs.
| D3 | σ1 | σ2 | D3/ σ1 Ratio | D3/ σ2 ratio | |
|---|---|---|---|---|---|
| 1 | 0.8 ± 0.1 | 1,260 ± 290 | 1,570 ± 310 | 1,573 | 1,970 |
| 2 | 2.4 ± 0.4 | 3,540 ± 2500 | 2,210 ± 260 | 1,476 | 919 |
| 18a | 1.1 ± 0.1 | 4,780 ± 730 | 660 ± 36 | 4344 | 601 |
| 18c | 4.2 ± 0.2 | 4,870 ± 470 | 1,120 ± 30 | 1,159 | 266 |
| 20a | 0.65 ± 0.2 | 1,960 ± 50 | 650 ± 38 | 3,017 | 1,002 |
| 20c | 0.52 ± 0.03 | 4,360 ± 570 | 794 ± 14 | 8,377 | 1,527 |
| 20e | 0.17 ± 0.01 | 20,900 ± 5250 | 5,960 ± 360 | 122,706 | 35,047 |
| 21b | 1.1 ± 0.2 | 7,200 ± 880 | 2,020 ± 260 | 6,541 | 1,839 |
| 21e | 1.1 ± 0.2 | 7,780 ± 540 | 1,320 ± 33 | 7,076 | 1,200 |
| Haloperidol | – | 1.5 ± 0.3 | 24.2 ± 3.0 | – | – |
Intrinsic Activity at Dopamine Receptors
The intrinsic activity of compounds 18a, 18c, 20a, 20c, 20e, 21b, and 21e at D3 and D2 receptors was also evaluated. This assay measures the ability of the compounds to inhibit forskolin-dependent stimulation of adenylyl cyclase activity in stably transfected HEK-293 cells expressing human D2 or D3 dopamine receptors. For each compound, the inhibition was compared to the intrinsic efficacy of the full agonist quinpirole and the antagonist haloperidol. The compounds that were evaluated were all partial agonists at D3 dopamine receptors, displaying intrinsic efficacy from 34.5 ± 1.7 % (20e) to 68.8 ± 5.6% (18a) (Table 3). As previously reported, the constituent at the para position of the benzamide group plays a pivotal role in determining the intrinsic activity of our compounds. For example, 1, 2, 18a and 18c each contain an 4-(2-methoxyphenyl)piperazine moiety with a saturated 4-carbon spacer, yet their efficacy compared to quinpirole varies from 34% to 64% at D2 receptors and 18% to 96% at D3 receptors. In addition, the structure of the 4-carbon spacer influences efficacy. For example, substitution of a trans double bond (21e) for the saturated spacer (20e) had minimal effect on efficacy at D2 receptors (29% vs. 21% maximal efficacy), while efficacy at D3 receptors increased almost 60% (35% to 55% maximal efficacy) (Table 3). The diverse range of D3 and D2 receptor affinities and intrinsic activities at these receptors indicates that these compounds are useful probes for studying the behavioral pharmacology of D3 and D2 receptors in animal models of substance abuse, schizophrenia, and L-DOPA induced dyskinesia. In addition, since most dopamine receptor imaging agents have been either antagonists (e.g., [11C]raclopride and [18F]fallypride) or full agonists (e.g., [11C]-(+)-4-propyl-9-hydroxynaphthoxazine ([11C](+)-PHNO) and [11C]-N-propylapomorphine ([11C]NPA)) at both D2 and D3 receptors, it will be of interest to see if the partial agonists described here are capable of serving as radiotracers for imaging the D3 receptor in vivo with PET.
Table 3. Intrinsic Efficacy of Selected Analogues at Dopamine D2 and D3 Receptorsa.
| Compound | hD2 HEK | hD3 HEK |
|---|---|---|
| Haloperidol | -0.6 ± 1.6 | 4.0 ± 5.5 |
| 1 | 33.5 ± 3.1 | 18.7 ± 2.2 |
| 2 | 35.3 ± 1.0 | 96.2 ± 4.2 |
| 18a | 58.6 ± 1.1 | 68.8 ± 5.6 |
| 18c | 63.8 ± 4.2 | 59.9 ± 7.4 |
| 20a | 66.3 ± 1.0 | 64.5 ± 8.3 |
| 20c | 73.2 ± 0.7 | 50.3 ± 5.2 |
| 20e | 29.3 ± 7.3 | 34.5 ± 1.7 |
| 21b | 65.8 ± 0.2 | 65.5 ± 7.2 |
| 21e | 21.2 ± 5.5 | 55.4 ± 4.2 |
| Quinpirole | 100 | 100 |
The intrinsic efficacy of the test compounds was evaluated by determining the percent inhibition of a forskolin-dependent whole cell adenylyl cyclase assay. The results were normalized to the percent inhibition obtained using the full agonist quinpirole at human D2 (1 μM) and D3 (100 nM) receptors expressed in stably transfected HEK 293 cells. For D2 receptors the maximum inhibition was >90% and for D3 receptors the maximum inhibition ranged from 38 to 53%. The test drug was used at a concentration equal to approximately 10 × the Ki value that was determined from the radioligand binding analysis. The mean ± the S.E.M. values are reported for n ≥ 3.
In summary, we observed that the 2-methoxy group in the 4-(2-methoxyphenyl)piperazinyl moiety can be replaced with a 2-fluoroethoxy group, a commonly-used strategy for preparing 18F-labeled PET radiotracers, without causing a significant change in D3 receptor affinity or D3 vs. D2 selectivity ratio. An exception to this trend were the structural congeners which contained the 4-(2-fluoroethyl)benzamide moiety: 21b had a much higher D3 affinity than its 4-(2-methoxyphenyl)piperazine analogue, 19a (Ki = 1.1 ± 0.2 nM vs. 24.9 ± 3.3 nM, respectively), and 20b, which had a lower D3 affinity and poorer D3 vs.D2 selectivity ratio than its corresponding 4-(2-methoxyphenyl)piperazine analogue, 2. Replacing the saturated 4-carbon spacer that links the benzamide and the 4-phenylpiperazinyl moieties with a trans double bond reduced the binding affinity at D3 and D2 receptors. However, the presence of the trans double bond can modulate the intrinsic efficacy of the analogue. Finally, although increasing the length of the 2-fluoroethoxy side chain by pegylation did not dramatically alter the D3 binding affinity or the D3 vs. D2 receptor binding selectivity, it did decrease the log P value, which may facilitate the penetration of blood-brain-barrier.
Conclusion
In the present study, we have reported the synthesis and pharmacological evaluation of a series of benzamides which have high binding affinity for D3 receptors and good selectivity for D3 vs. D2 receptors. Within the series, 5 compounds exhibited high D3 binding affinity (≤ 5.0 nM) and/or moderate to high selectivity for D3 vs. D2 receptors, including 18a, 20a, 20c, 20e and 21e. Moreover, all of these analogues contain a fluorine atom, thus providing candidates ligands for PET imaging studies via the corresponding 18F-labeled analogs.
Since recent studies indicate that the density of D3 receptors in the striatal regions of brain is ∼40% that of the D2 receptor32, ligands having a high D3 versus D2 selectivity (>50-fold) will likely be needed in order to image D3 versus D2 receptors in the CNS. Among the 5 compounds described above, 20e displayed the highest D3 affinity (0.17 nM) and selectivity for D3 vs. D2 receptors (163-fold). However, the high lipophilicity of this analog (log P = 4.67) may limit its utility as a PET radiotracer because of its predicted low brain uptake and relatively high level of nonspecific binding. In vivo evaluation of a number of the fluorinated ligands described above are currently ongoing to assess their suitability for use as PET tracers for studying the in vivo expression and regulation of D3 dopamine receptors in the CNS.
Experimental Section
General
4-Dimethylaminobenzoic acid was purchased from Sigma-Aldrich (Milwaukee, WI) and 3-thienylbenzoic acid Matrix Scientific (Columbia, SC). All other synthetic intermediates were purchased from Sigma-Aldrich and used as received unless otherwise stated. Tetrahydrofuran (THF) was distilled from sodium hydride immediately prior to use.
All air-sensitive reactions were carried out in oven-dried glassware under an inert nitrogen atmosphere unless otherwise stated. Standard handling techniques for air sensitive materials were employed throughout this study. Yields were not optimized. Melting points were determined on a Haake-Buchler or Mel-Temp melting point apparatus and are uncorrected. 1H NMR spectra were recorded at 300 MHz on a Varian Mercury-VX spectrometer with CDCl3 as the solvent and tetramethylsilane (TMS) as the internal standard. The following abbreviations were used to describe peak patterns wherever appropriate: b = broad, d = doublet, t = triplet, q = quartet, m = multiplet. Analytical thin layer chromatography (TLC) was performed on Analtech GHLF silica gel glass plates, and visualization was aided by UV. Elemental analyses (C, H, N) were determined by Atlantic Microlab, Inc. (Norcross, GA) and the results are within 0.4% of the calculated values unless otherwise noted. The purity of the target compounds was determined by elemental analysis and by HPLC methods. All the compounds reported in this article have a purity ≥ 95%. The synthesis of benzoic acid intermediates 9a–e, 12a and 12b, and the amine intermediates 17a–d can be found in the Supporting Data section.
General Method for Preparing the Substituted Benzamide Analogs
4-(2-Fluoroethoxy)-N-(4-(4-(2-methoxyphenyl)-piperazin-1-yl)-butyl)benzamide (18a)
A mixture of compound 17a (379 mg, 1.44 mmol) and 9a (221 mg, 1.20 mmol) in dichloromethane (20 mL) was stirred at 0 °C (ice-water bath). Dicyclohexylcarbodiimide (DCC) (356 mg, 1.73 mmol) and hydroxybenzotriazole (HOBT) (234 mg, 1.73 mmol) were added to the above solution. Then the ice bath was removed and the reaction mixture was stirred at ambient temperature for 15 h. Dichloromethane (60 mL) was added into the reaction mixture and the solution was washed with saturated aqueous NaHCO3 solution (3 × 10 mL). The organic layer was dried over Na2SO4, concentrated under reduced pressure and the crude product was purified by silica gel column chromatography using dichloromethane/methanol (20/1, v/v) as the mobile phase to give 18a (443 mg, 86%). Mp (oxalate salt): 151.5–152.6 °C. 1H NMR (300 MHz, free base, CDCl3): δ 1.61–1.69 (m, 4H), 2.48 (t, J = 5.2 Hz, 2H), 2.66 (s, 4H), 3.08 (s, 4H), 3.48 (q, J = 5.7 Hz, 2H), 3.85 (s, 3H), 4.19 (t, J = 4.2 Hz, 1H), 4.28 (t, J = 4.2 Hz, 1H), 4.68 (t, J = 4.2 Hz, 1H), 4.84 (t, J = 4.2 Hz, 1H), 6.61 (br s, 1H), 6.82– 7.04 (m, 6H), 7.74 (d, J = 8.7 Hz, 2H). Anal. (C24H32FN3O3·1.5H2C2O4) C, H, N.
4-(2-(2-Fluoroethoxy)ethoxy)-N-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)benzamide (18b)
18b was made from 12a and 17a. Yield: 84%. Mp (oxalate salt): 127.9–129.0 °C. 1H NMR (free base, CDCl3): δ 1.66–1.68 (m, 4H), 2.47 (t, J = 3.6 Hz, 2H), 2.65 (s, 4H), 3.08 (s, 4H), 3.47 (q, J = 5.4 Hz, 2H), 3.76 (t, J = 4.8 Hz, 1H), 3.85 (s, 3H), 3.91 (t, J = 3.6 Hz,, 2H), 4.17 (t, J = 4.8 Hz, 2H), 4.52 (t, J = 4.2 Hz, 1H), 4.67 (t, J = 4.2 Hz, 1H), 6.58 (t, J = 10.0 Hz, 1H), 6.83–7.04 (m, 6H), 7.72 (d, J = 9.0 Hz, 2H). Anal. (C26H36FN3O4·H2C2O4) C, H, N.
4-(2-(2-(2-Fluoroethoxy)ethoxy)ethoxy)-N-(4-(4-(2-methoxyphenyl)piperazin-1-l)butyl)benzamide (18c)
18c was prepared from 12b and 17a. Yield: 98%. Mp (oxalate salt): 103.0–103.9 °C. 1H NMR (free base, CDCl3): δ 1.60 (s, 4H), 1.67 (t, J = 3.3 Hz, 4H), 2.47 (t, J = 4.8 Hz, 2H), 2.66 (s, 4H), 3.08 (s, 4H), 3.47 (q, J = 5.7 Hz, 2H), 3.69–3.76 (m, 4H), 3.80 (t, J = 4.2 Hz, 1H), 3.86 (s, 3H), 3.88 (t, J = 4.8 Hz, 2H), 4.16 (t, J = 4.8 Hz, 2H), 4.48 (t, J = 4.2 Hz, 1H), 4.64 (t, J = 4.2 Hz, 1H), 6.54 (t, J = 9.0 Hz, 1H), 6.83–7.04 (m, 6H), 7.72 (d, J = 9.0 Hz, 2H). Anal. (C28H40FN3O5·H2C2O4·0.4H2O) C, H, N.
4-(2-Fluoroethyl)-N-(4-(4-(2-methoxyphenyl)piperazin-1-yl)-trans-butyl-2-enyl)benzamide (19a)
19a was prepared from 4-(2-fluoroethyl)benzoic acid and 17c. Yield: (397 mg, 98%). Mp (oxalate salt): 116.7–121.3 °C. 1H NMR (free base, CDCl3): δ 2.63 (s, 4H), 3.03 (t, J = 4.8 Hz, 2 H), 3.08– 3.12 (m, 6H), 3.85 (s, 3H), 4.06–4.08 (t, J = 6.2 Hz, 2H), 4.57 (t, J = 4.8 Hz, 1H), 4.72 (t, J = 4.8 Hz, 1H), 5.78 (t, J = 5.4 Hz, 2H), 6.80 (t, J = 6.3 Hz, 1H), 6.82–7.04 (m, 4H), 7.31 (d, J = 8.4 Hz, 2H), 7.73 (d, J = 8.4 Hz, 2H). Anal. (C24H30FN3O2 0.5H2C2O4·H2O) C, H, N.
4-(2-Fluoroethoxy)-N-(4-(4-(2-methoxyphenyl)piperazin-1-yl)-trans-butyl-2-enyl)benzamide (19b)
19b was prepared from 9a and 17c. Yield: 77%. Mp (oxalate salt): 133.6–134.9 °C. 1H NMR (free base, CDCl3): δ 2.67 (s, 4H), 3.08–3.09 (m, 6H), 3.86 (s, 3H), 4.09 (t, J = 4.3 Hz, 2H), 4.22 (t, J = 4.2 Hz, 1H), 4.30 (t, J = 4.2 Hz, 1H), 4.69 (t, J = 4.2 Hz, 1H), 4.85 (t, J = 4.2 Hz, 1H), 5.78 (t, J = 5.3 Hz, 2H), 6.13 (t, J = 6.3 Hz, 1H), 6.83–7.04 (m, 6H), 7.75 (d, J = 9.3 Hz, 2H). Anal. (C24H30FN3O3) C, H, N.
4-(2-(2-Fluoroethoxy)ethoxy)-N-(4-(4-(2-methoxyphenyl)piperazin-1-yl)-trans-butyl-2-enyl) benzamide (19c)
19c was prepared from 12a and 17c. Yield: 99%. Mp (oxalate salt): 108.3–109.8 °C. 1H NMR (free base, CDCl3): δ 2.67 (s, 4H), 3.07–3.10 (m, 6H), 3.77 (t, J = 4.2 Hz, 1H), 3.85 (s, 3H), 3.86–3.94 (m, 3H), 4.06–4.10 (m, 2H), 4.19 (m, 2H), 4.52 (t, J = 4.1 Hz, 1H), 4.68 (t, J = 4.1 Hz, 1H), 5.78 (t, J = 3.3 Hz, 2H), 6.10 (t, J = 5.3 Hz, 1H), 6.82–7.04 (m, 6H), 7.73 (d, J = 8.7 Hz, 2H). Anal. (C26H34FN3O4·H2C2O4) C, H, N.
2-(2-Fluoroethoxy)-5-methyl-N-(4-(4-(2-methoxyphenyl)piperazin-1-yl)-trans-butyl-2-enyl) benzamide (19d)
19d was prepared from 9b and 17c. Yield: 79%. Mp (oxalate salt): 151.1–152.3 °C. 1H NMR (free base, CDCl3): δ 2.33 (s, 3H), 2.67 (s, 4H), 3.07–3.09 (m, 6H), 3.85 (s, 3H), 4.06–4.14 (m, 2H), 4.26 (t, J = 4.1 Hz, 1H), 4.35 (t, J = 4.1 Hz, 1H), 4.70 (t, J = 4.1 Hz, 1H), 4.86 (t, J = 4.1 Hz, 1H), 5.79 (t, J = 2.4 Hz, 2H), 6.80–7.04 (m, 5H), 7.214 (dd, J = 2.4, 8.7 Hz, 1H), 7.98 (br s, 1H), 8.00 (dd, J = 2.4, 8.7 Hz, 1H), Anal. (C25H32FN3O3·H2C2O4) C, H, N.
5-Bromo-2-(2-fluoroethoxy)-N-(4-(4-(2-methoxyphenyl)piperazin-1-yl)-trans-butyl-2-enyl)-benzamide (19e)
19e was prepared from 9c and 17c. Yield: 35%. Mp (oxalate salt): 157.6–158.7 °C. 1H NMR (free base, CDCl3): δ 2.65(s, 4H), 3.06–3.10 (m, 6H), 3.85 (s, 3H), 4.09 (t, J = 5.1 Hz, 2H), 4.27 (t, J = 4.1 Hz, 1H), 4.37 (t, J = 4.1 Hz, 1H), 4.71 (t, J = 4.1 Hz, 1H), 4.87 (t, J = 4.1 Hz, 1H), 5.76– 5.80 (m, 2H), 6.80–7.04 (m, 5H), 7.52(dd, J = 2.4, 8.4 Hz, 1H), 7.61 (br s, 1H), 8.32(d, J = 5.4 Hz, 1H). Anal. (C24H29BrFN3O3) C, H, N.
2-(2-Fluoroethoxy)-5-iodo-N-(4-(4-(2-methoxyphenyl)piperazin-1-yl)-trans-butyl-2-enyl)-benzamide (19f)
19f was prepared from 9d and 17c. Yield: 60%. Mp (oxalate salt): 162.2–163.6 °C. 1H NMR: (free base, CDCl3): δ 2.65 (s, 4H), 3.07–3.10 (m, 6H), 3.86 (s, 3H), 4.09 (t, J = 5.1 Hz, 2H), 4.27 (t, J = 4.1 Hz,1H), 4.36 (t, J = 4.1 Hz, 1H), 4.72 (t, J = 4.1 Hz, 1H), 4.88 (t, J = 4.1 Hz, 1H), 5.74– 5.82 (m, 2H), 6.71 (d, J = 8.7 Hz, 1H), 6.84–7.04 (m, 4H), 7.71 (dd, J = 2.4, 8.4 Hz, 1H), 7.83 (br s, 1H), 8.49 (d, J = 2.1 Hz, 1H). Anal. (C24H29FIN3O3·H2C2O4·0.5H2O) C, H, N.
5-Bromo-2-(2-fluoroethoxy)-3-methoxy-N-(4-(4-(2-methoxyphenyl)piperazin-1-yl)-trans-butyl-2-enyl)benzamide (19g)
19g was prepared from 9e and 17c. Yield: 84%. Mp (oxalate salt): 193.5–195.5 °C. 1H NMR (free base, CDCl3): δ 2.66 (s, 4H), 3.06–3.10 (m, 6H), 3.86 (s, 3H), 3.87 (s, 3H), 4.06 (t, J = 4.2 Hz, 2H), 4.27 (t, J = 4.1 Hz, 1H), 4.36 (t, J = 4.1 Hz, 1H), 4.62 (t, J = 3.9 Hz, 1H), 4.78 (t, J = 3.9 Hz, 1H), 5.76 (t, J = 3.3 Hz, 2H), 6.84–7.04 (m, 4H), 7.14 (d, J = 2.4 Hz, 1H), 7.88 (d, J = 2.1 Hz, 1H), 8.04 (br s, 1H). Anal. (C25H31BrFN3O4·H2C2O4) C, H, N.
4-(Dimethylamino)-N-(4-(4-(2-(2-fluoroethoxy)phenyl)piperazin-1-yl)butyl)benzamide (20a)
20a was prepared from 4-dimethylaminobenzoic acid and 17b. Yield: 80%. Mp (oxalate salt): 103.4–106.3 °C. 1H NMR (free base, CDCl3): δ 1.60–1.80 (m, 4H), 2.45 (t, J = 2.1 Hz, 2H), 2.65 (s, 4H), 3.00 (s, 6H), 3.12 (s, 4H), 3.46 (q, J = 5.4 Hz, 2H), 4.19 (t, J = 4.1 Hz, 1H), 4.32 (t, J = 4.1 Hz, 1H), 4.69 (t, J = 4.1 Hz, 1H), 4.85 (t, J = 4.1 Hz, 1H), 6.36 (br s, 1H), 6.66 (d, J = 9.2 Hz, 2H); 6.83–7.00 (m, 4H), 7.676 (d, J = 9.2 Hz, 2H). Anal. (C25H35FN4O2·H2C2O4) C, H, N.
4-(2-Fluoroethyl)-N-(4-(4-(2-(2-fluoroethoxy)phenyl)piperazin-1-yl)-butyl)benzamide (20b)
20b was prepared from 4-(2-fluoroethyl)benzoic acid and 17b. Yield: 95%. Mp (oxalate salt): 140.5–142.1 °C. 1H NMR (free base, CDCl3): δ 1.60–1.78 (m, 4H), 2.46 (t, J = 6.8 Hz, 2H), 2.63 (s, 4H), 3.00 (t, J = 6.4 Hz, 2H) 306–3.10 (m, 4H), 3.48 (q, J = 5.4 Hz, 2H), 4.20 (t, J = 4.2 Hz, 1H), 4.29 (t, J = 4.2 Hz, 1H), 4.54 (t, J = 6.4 Hz, 1H), 4.67–4.71 (m, 2H), 4.85 (t, J = 4.1 Hz, 1H), 6.72 (br s, 1H), 6.83–6.90 (m, 2H), 6.95–6.98 (m, 2H), 7.28 (d, J = 8.1 Hz, 2H), 7.71(dd, J = 2.1, 6.3 Hz, 2H). Anal. (C25H33F2N3O2·0.5H2C2O4) C, H, N.
4-(2-Fluoroethoxy)-N-(4-(4-(2-(2-fluoroethoxy)phenyl)piperazin-1-yl)butyl)benzamide (20c)
20c was prepared from 9a and 17b. Yield: 80%. Mp (oxalate salt): 110.3–112.8 °C. 1H NMR (free base, CDCl3): δ 1.60–1.72 (m, 4H), 2.45 (t, J = 6.8 Hz, 2H), 2.63 (s, 4H), 3.10 (s, 4H), 3.47 (q, J = 5.7 Hz, 2H), 4.20 (t, J = 4.5 Hz, 2H), 4.28 (t, J = 4.5 Hz, 2H), 4.69 (t, J = 4.5 Hz, 2H), 4.83 (t, J = 4.5 Hz, 2H), 6.59 (br s, 1H), 6.83–7.00 (m, 6H), 7.73 (d, J = 9.0 Hz, 2H). Anal. (C25H33F2N3O3·H2C2O4) C, H, N.
4-(2-(2-Fluoroethoxy)ethoxy)-N-(4-(4-(2-(2-fluoroethoxy)phenyl)piperazin-1-yl)butyl)benzamide (20d)
20d was prepared from 12a and 17b. Yield: 77%. Mp (oxalate salt): 110.5–112.6 °C. 1H NMR (free base, CDCl3): δ 1.67–1.71 (m, 4H), 2.50 (t, J = 6.3 Hz, 2H), 2.68 (s, 4H), 3.13 (s, 4H), 3.47 (q, J = 5.4 Hz, 2H), 3.77 (t, J = 4.1 Hz, 1H), 3.85–3.91 (m, 3H), 4.16–4.213.47 (m,3H), 4.29 (t, J = 4.4 Hz, 1H), 4.51 (t, J = 4.1 Hz, 1H), 4.66–4.7169 (m,2H), 4.85 (t, J = 4.1 Hz, 1H), 6.6159 (br s, 1H), 6.83–7.04 (m, 6H), 7.73 (d, J = 9.0 Hz, 2H). Anal. (C27H37F2N3O4·H2C2O4) C, H, N.
4-(Thiophen-3-yl)-N-(4-(4-(2-(2-fluoroethoxy)phenyl)piperazin-1-yl)butyl)benzamide (20e)
20e was prepared from 4-(thiophen-3-yl)benzoic acid and 17b. Yield: 62%. Mp (oxalate salt): 193.3–194.1 °C. 1H NMR (free base, CDCl3): δ 1.68–1.72 (m, H), 2.48 (t, J = 6.0 Hz, 2H), 2.65 (s, 4H), 2.10 (s, 4H), 3.50 (q, J = 5.7 Hz, 2H), 4.19 (t, J = 4.2 Hz, 1H), 4.28 (t, J = 4.2 Hz, 1H), 4.69 (t, J = 4.2 Hz, 1H), 4.84 (t, J = 4.2 Hz, 1H), 6.79 (br s, 1H), 6.82–7.00 (m, 4H), 7.41 (d, J = 2.7 Hz, 2H), 7.52 (t, J = 2.7 Hz, 1H), 7.64 (d, J = 8.7 Hz, 2H), 7.80 (d, J = 8.7 Hz, 2H). Anal. (C27H32FN3O2S·H2C2O4) C, H, N.
4-(2-(2-(2-Fluoroethoxy)ethoxy)ethoxy)-N-(4-(4-(2-(2-fluoroethoxy)phenyl)piperazin-1-yl)butyl) benzamide (20f)
20f was prepared from 12b and 17b. Yield: 82%. Mp (oxalate salt): 110.7–111.6 °C. 1H NMR (free base, CDCl3): δ 1.67–1.69 (m, 4H), 2.48 (t, J = 5.9 Hz, 3H), 2.67 (s, 4H), 3.12 (s, 4H), 3.47 (q, J = 5.4 Hz, 2H), 3.68–3.76 (m, 4H), 3.80 (t, J = 4.2 Hz, 1H), 3.87 (t, J = 4.8 Hz, 2H), 4.12 (t, J = 4.8 Hz, 2H), 4.20 (t, J = 4.2 Hz, 1H), 4.31 (t, J = 4.2 Hz, 1H), 4.48 (t, J = 4.2 Hz, 1H), 4.64 (t, J = 4.2 Hz, 1H), 4.69 (t, J = 4.2 Hz, 1H), 4.85 (t, J = 4.2 Hz, 1H), 6.55 (br s, 1H), 6.82–7.02 (m, 6H), 7.73 (d, J = 9.0 Hz, 2H). Anal. (C29H41F2N3O5·H2C2O4) C, H, N.
4-(Dimethylamino)-N-(4-(4-(2-(2-fluoroethoxy)phenyl)piperazin-1-yl)-trans-butyl-2-enyl)-benzamide (21a)
21a was prepared from 4-dimethylaminobenzoic acid and 17d. Yield: 50%. Mp (oxalate salt): 84.9–85.9 °C. 1H NMR (free base, CDCl3) δ 2.66 (s, 4H), 3.01 (s, 6H), 3.07 (d, J = 4.5 Hz, 2H), 3.14 (s, 4H), 4.09 (t, J = 5.4 Hz, 2H), 4.21 (t, J = 4.2 Hz, 1H), 4.30 (t, J = 4.2 Hz, 1H), 4.70 (t, J = 4.2 Hz, 1H), 4.86 (t, J = 4.2 Hz, 1H), 5.76–5.80 (m, 2H), 6.05 (s, 1H), 6.66 (d, J = 9.0 Hz, 2H), 6.82–6.87 (m, 1H), 6.94–6.97 (m, 3H), 7.68 (d, J = 9.0 Hz, 2H). Anal. (C25H33FN4O2· H2C2O4·H2O) C, H, N.
4-(2-Fluoroethyl)-N-(4-(4-(2-(2-Fluoroethoxy)phenyl)piperazin-1-yl)-trans-butyl-2-enyl)benzamide (21b)
21b was prepared from 4-(2-fluoroethyl)benzoic acid and 17d. Yield: 72%. Mp (oxalate salt): 155.0–156.1 °C. 1H NMR (free base, CDCl3): δ 2.67 (s, 4H), 3.01 (t, J = 4.1 Hz, 2H), 3.07– 3.13 (m, 6H), 4.10 (t, J = 4.1 Hz, 2H), 4.20–4.20 (t, J = 4.1 Hz, 1H), 4.30 (t, J = 4.1 Hz, 1H), 4.57 (t, J = 6.3 Hz, 1H), 4.68–4.76 (m, 2H), 4.84 (t, J = 4.1 Hz, 1H), 5.76–5.80 (m, 2H), 6.18 (br s, 1H), 6.82–6.88 (m, 1H), 6.95–6.97 (m, 3H), 7.29 (d, J = 8.1 Hz, 2H), 7.704 (d, J = 8.1 Hz, 2H). Anal. (C25H31F2N3O2·0.5H2C2O4) C, H, N.
4-(2-Fluoroethoxy)-N-(4-(4-(2-(2-fluoro-ethoxy)-phenyl)-piperazin-1-yl)-trans-butyl-2-enyl)-benzamide (21c)
21c was prepared from 9a and 17d. Yield: 48%. Mp (oxalate salt): 112.8–125.1 °C. 1H NMR (free base, CDCl3): δ 2.68 (s, 4H), 3.09 (s, 3H), 3.15 (s, 3H), 4.10 (t, J = 4.2 Hz, 2H), 4.20 (t, J = 4.2 Hz, 2H), 4.30 (t, J = 4.2 Hz, 2H), 4.70 (t, J = 4.2 Hz, 2H), 4.86 (t, J = 4.2 Hz, 2H), 5.70–5.90 (m, 2H), 6.170 (br s, 1H), 6.81–6.85 (m, 1H), 6.93–7.00 (m, 5H), 7.75 (d, J = 10.5 Hz, 2H). Anal. (C25H31F2N3O3·2H2C2O4) C, H, N.
4-(2-(2-fluoroethoxy)ethoxy)-N-(4-(4-(2-(2-fluoroethoxy)phenyl)piperazin-1-yl)-trans-but-2-enyl)-benzamide (21d)
21d was prepared from 12a and 17d. Yield: 76%. Mp (oxalate salt): 113.4–115.0 °C. 1H NMR (free base, CDCl3): δ 2.67 (s, 4H), 3.08 (d, J = 2.7Hz, 2H), 3.84–3.14 (s, 4H), 3.77 (t, J = 4.2 Hz, 1 H), 3.86–3.96 (m, 3H), 4.08 (t, J = 4.2 Hz, 2H), 4.17–4.22 (m, 3H), 4.30 (t, J = 4.2 Hz, 1H), 4.52 (t, J = 4.2 Hz, 1H), 4.66–4.70 (m, 2H), 4.85 (t, J = 4.2 Hz, 1H), 5.77–5.80 (m, 2H), 6.11 (t, J = 8.7 Hz, 1H), 6.84–6.88 (m, 1H), 6.93–6.99(m, 5H), 7.74 (td, J = 2.4, 9.0 Hz, 2H). Anal. (C27H35F2N3O4·1.5H2C2O4) C, H, N.
4-(Thiophen-3-yl)-N-(4-(4-(2-(2-fluoroethoxy)phenyl)piperazin-1-yl)-trans-but-2-enyl)benzamide (21e)
21e was prepared from 4-(thiophen-3-yl)benzoic acid and 17d. Yield: 62%. Mp (oxalate salt): 140.5–142.1 °C. 1H NMR (free base, CDCl3): δ 2.66(s, 4H), 3.08 (d, J = 4.0 Hz, 2H), 3.14 (s, 4H), 4.12 (t, J = 4.0 Hz, 2H), 4.22 (t, J = 4.0 Hz, 1H), 4.30 (t, J = 4.0 Hz, 1H), 4.70 (t, J = 4.0 Hz, 1H), 4.86 (t, J = 4.0 Hz, 1H), 5.80–5.82 (m, 2H), 6.23 (br s, 1H), 6.83–6.88 (m, 1H), 6.93–7.00 (m, 3H), 7.42 (d, J = 10.2 Hz, 2H), 7.54 (t, J = 2.1 Hz, 1H), 7.66 (td, J = 3.6, 10.5 Hz, 2H), 7.81 (td, J = 3.3, 13.2 Hz, 2H). The elemental analysis was conducted on free base of 21e. Anal. (C27H30FN3O2S) C, H, N.
In Vitro Binding Studies
Dopamine receptor binding assays
The binding properties of membrane-associated receptors were characterized by a filtration binding assay.34 For human D2long, D3, and D4 dopamine receptors expressed in HEK 293 cells, 50 μL of membrane homogenates were suspended in 50 mM Tris–HCl/150 mM NaCl/10 mM EDTA buffer, pH = 7.5 and incubated with 50 μL of [125I]IABN34 at 37°Cfor 60 min, using 20 μM (+)-butaclamol to define the non-specific binding. The radioligand concentration was equal to approximately 0.5 times the Kd value and the concentration of the competitive inhibitor ranged over 5 orders of magnitude for competition experiments. For each competition curve, two concentrations of inhibitor per decade was used and triplicates were performed. Binding was terminated by the addition of the cold wash buffer (10mM Tris–HCl/150mM NaCl, pH = 7.5) and filtration over a glass-fiber filter (Schleicher and Schuell No. 32). A Packard Cobra gamma counter was used to measure the radioactivity. The equilibrium dissociation constant and maximum number of binding sites were generated using unweighted non-linear regression analysis of data modeled according to the equation describing mass action binding. The concentration of inhibitor that inhibits 50% of the specific binding of the radioligand (IC50 value) was determined by using nonlinear regression analysis to analyze the data of competitive inhibition experiments. Competition curves were modeled for a single site and the IC50 values were converted to equilibrium dissociation constants (Ki values) using the Cheng and Prusoff35 correction. Mean Ki values ± S.E.M. are reported for at least three independent experiments.
Sigma Receptor Binding Assays
Before determining the σ1 and σ2 receptor binding assays, the compounds were dissolved in either DMF, DMSO, or ethanol and then diluted in 50 mM Tris-HCl buffer containing 150 mM NaCl and 100 mM EDTA at pH = 7.4. The procedures for isolating the membrane homogenates and performing the σ1 and σ2 receptor binding assays have been described previously.24, 33
Briefly, the σ1 receptor binding assays were conducted in 96-well plates using guinea pig brain membrane homogenates (∼300 μg protein) and ∼5 nM (+)-[3H]-pentazocine (34.9 Ci/mmol, Perkin Elmer, Boston, MA). The total incubation time was 90 min at room temperature. Nonspecific binding was determined from samples that contained 10 μM of cold haloperidol. After 90 min, the reaction was terminated by the adding 150 μL of ice-cold wash buffer (10 mM Tris-HCl, 150 mM NaCl, pH 7.4) using a 96 channel transfer pipette (Fisher Scientific, Pittsburgh, PA). The samples were harvested and filtered rapidly through a 96-well fiber glass filter plate (Millipore, Billerica, MA) that had been presoaked with 100 μL of 50 mM Tris-HCl buffer at pH = 8.0 for 1 h. Each filter was washed 3 times with 200 μL of ice-cold wash buffer, and the filter counted in a Wallac 1450 MicroBeta liquid scintillation counter (Perkin Elmer, Boston, MA).
The σ2 receptor binding assays were conducted using rat liver membrane homogenates (∼300 μg protein) and ∼5 nM [3H]-DTG (58.1 Ci/mmol, Perkin Elmer, Boston, MA) in the presence of 1 μM (+)-pentazocine to block σ1 sites. The incubation time was 2 h at room temperature. Nonspecific binding was determined from samples that contained 10 μM of cold haloperidol. All other procedures were identical to those described above for the σ1 receptor binding assay.
Data from the competitive inhibition experiments were modeled using nonlinear regression analysis to determine the concentration that inhibits 50% of the specific binding of the radioligand (IC50 value). Competitive curves were best fit to a one-site fit and gave pseudo-Hill coefficients of 0.6–1.0. Ki values were calculated using Cheng and Prusoff method35 and were presented as the mean ± S.E.M. For these calculations, we used a Kd value of 7.89 nM for (+)-[3H]-pentazocine and guinea pig brain and a Kd value of 30.73 nM for [3H]-DTG and rat liver.24
Whole cell adenylyl cyclase assay
The accumulation of 3H-cyclic AMP in HEK cells was measured by a modification of the Shimizu et al's method.36 Transfected HEK cells were treated with serum-free media containing 2,8-[3H]adenine (ICN Pharmaceutical Inc., Costa Mesa, CA) and cells were incubated at 37°C for 75 min. Cells and drugs diluted in serum-free media containing 0.1 mM 3-isobutyl-1-methylxanthine (Sigma) were mixed to give a final volume of 500 μL and cells were incubated for 20 min at 37°C. The reaction was stopped by addition of 500 μL of 10% trichloroacetic acid and 1 mM cyclic AMP. After centrifugation, the supernatants were fractionated using Dowex AG1-X8 and neutral alumina to separate the [3H]ATP and the [3H]cyclic AMP. Individual samples were corrected for column recovery by monitoring the recovery of the cyclic AMP using spectrophotometric analysis at OD 259 nm.34, 36
Supplementary Material
Acknowledgments
This work was supported by NIH grants DA16181 (RHM), DA23957 (RRL), and DA029840 (RHM).
Abbreviations
- CIMS
Chemical ionization mass spectrometry
- DCC
N,N′-Dicyclohexylcarbodiimide
- DMF
N,N-Dimethylformamide
- DMSO
Dimethyl sulfoxide
- DAST
diethylaminosulfur trifluoride
- DTG
1,3-Di-tolylguanidine
- GIRKs
G protein-coupled inwardly-rectifying potassium channels
- HEK cells
Human Embryonic Kidney 293 cells
- [125I]IABN
[125I]N-benzyl-5-iodo-2,3,-dimethoxy-[3.3.1]azabicyclononan-3-β-yl-benzamide
- LID
L-DOPA-induced dyskinesia
- PET
Positron Emission Tomography
- PLD
Phospholipase D
- SPECT
Single photon emission computed tomography
- TBAF
Tetra-n-butylammonium fluoride
Footnotes
Supporting Information Available. Experimental procedures and analytical data for compounds 9a–e, 10a–c, 11a, 11b, 12a, 12b, 14, 16a–f and 17a–d, HPLC conditions to confirm the purity of final compounds, and elemental analysis data on all new compounds are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Luedtke RR, Mach RH. Progress in developing D3 dopamine receptor ligands as potential therapeutic agents for neurological and neuropsychiatric disorders. Curr Pharm Des. 2003;9(8):643–71. doi: 10.2174/1381612033391199. [DOI] [PubMed] [Google Scholar]
- 2.Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347(6289):146–51. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- 3.Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY, Boyfield I, Branch CL, Cilia J, Coldwell MC, Hadley MS, Hunter AJ, Jeffrey P, Jewitt F, Johnson CN, Jones DN, Medhurst AD, Middlemiss DN, Nash DJ, Riley GJ, Routledge C, Stemp G, Thewlis KM, Trail B, Vong AK, Hagan JJ. Pharmacological actions of a novel, high-affinity, and selective human dopamine D3 receptor antagonist, SB-277011-A. J Pharmacol Exp Ther. 2000;294(3):1154–65. [PubMed] [Google Scholar]
- 4.Murray AM, Ryoo HL, Gurevich E, Joyce JN. Localization of dopamine D3 receptors to mesolimbic and D2 receptors to mesostriatal regions of human forebrain. Proc Natl Acad Sci U S A. 1994;91(23):11271–5. doi: 10.1073/pnas.91.23.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, Schott D, Morgat JL, Schwartz JC, Sokoloff P. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci U S A. 1992;89(17):8155–9. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaz J, Levesque D, Lammers CH, Griffon N, Martres MP, Schwartz JC, Sokoloff P. Phenotypical characterization of neurons expressing the dopamine D3 receptor in the rat brain. Neuroscience. 1995;65(3):731–45. doi: 10.1016/0306-4522(94)00527-c. [DOI] [PubMed] [Google Scholar]
- 7.Hall H, Halldin C, Dijkstra D, Wikstrom H, Wise LD, Pugsley TA, Sokoloff P, Pauli S, Farde L, Sedvall G. Autoradiographic localisation of D3-dopamine receptors in the human brain using the selective D3-dopamine receptor agonist (+)-[3H]PD 128907. Psychopharmacology (Berl) 1996;128(3):240–7. doi: 10.1007/s002130050131. [DOI] [PubMed] [Google Scholar]
- 8.Bordet R, Ridray S, Schwartz JC, Sokoloff P. Involvement of the direct striatonigral pathway in levodopa-induced sensitization in 6-hydroxydopamine-lesioned rats. Eur J Neurosci. 2000;12(6):2117–23. doi: 10.1046/j.1460-9568.2000.00089.x. [DOI] [PubMed] [Google Scholar]
- 9.Bordet R, Ridray S, Carboni S, Diaz J, Sokoloff P, Schwartz JC. Induction of dopamine D3 receptor expression as a mechanism of behavioral sensitization to levodopa. Proc Natl Acad Sci U S A. 1997;94(7):3363–7. doi: 10.1073/pnas.94.7.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bordet R. Central dopamine receptors: general considerations (Part 1) Rev Neurol (Paris) 2004;160:8–9. 862–70. doi: 10.1016/s0035-3787(04)71067-x. [DOI] [PubMed] [Google Scholar]
- 11.Kumar R, Riddle L, Griffin SA, Grundt P, Newman AH, Luedtke RR. Evaluation of the D3 dopamine receptor selective antagonist PG01037 on l-dopa-dependent abnormal involuntary movements in rats. Neuropharmacology. 2009;56:6–7. 944–955. doi: 10.1016/j.neuropharm.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cenci MA. Dopamine dysregulation of movement control in L-DOPA-induced dyskinesia. Trends Neurosci. 2007;30(5):236–43. doi: 10.1016/j.tins.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Mela F, Millan MJ, Brocco M, Morari M. The selective D3 receptor antagonist, S33084, improves parkinsonian-like motor dysfunction but does not affect l-DOPA-induced dyskinesia in 6-hydroxydopamine hemi-lesioned rats. Neuropharmacology. 2010;58(2):528–536. doi: 10.1016/j.neuropharm.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Visanji NP, Fox SH, Johnston T, Reyes G, Millan MJ, Brotchie JM. Dopamine D3 receptor stimulation underlies the development of L-DOPA-induced dyskinesia in animal models of Parkinson's disease. Neurobiology of Disease. 2009;35(2):184–192. doi: 10.1016/j.nbd.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Vingerhoets FJG. Dyskinesia in Parkinson disease: Back for the future. Neurology. 2009;72(14):1202–1203. doi: 10.1212/01.wnl.0000345658.54511.31. [DOI] [PubMed] [Google Scholar]
- 16.Sokoloff P, Le Foll B, Perachon S, Bordet R, Ridray S, Schwartz JC. The dopamine D3 receptor and drug addiction. Neurotox Res. 2001;3(5):433–41. doi: 10.1007/BF03033202. [DOI] [PubMed] [Google Scholar]
- 17.Van Kampen JM, Eckman CB. Dopamine D3 receptor agonist delivery to a model of Parkinson's disease restores the nigrostriatal pathway and improves locomotor behavior. J Neurosci. 2006;26(27):7272–80. doi: 10.1523/JNEUROSCI.0837-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz JC, Everitt BJ, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400(6742):371–5. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- 19.Khaled MATM, Farid Araki K, Li B, Coen KM, Marinelli PW, Varga J, Gaál J, Le Foll B. The selective dopamine D3 receptor antagonist SB 277011-A, but not the partial agonist BP 897, blocks cue-induced reinstatement of nicotine-seeking. Int J Neuropsychopharmacol. 2009:1–10. doi: 10.1017/S1461145709991064. [DOI] [PubMed] [Google Scholar]
- 20.Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Newman AH. Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. J Med Chem. 2005;48(3):839–48. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Ladona FJ, Cox BF. BP 897, a selective dopamine D3 receptor ligand with therapeutic potential for the treatment of cocaine-addiction. CNS Drug Reviews. 2003;9(2):141–158. doi: 10.1111/j.1527-3458.2003.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carr KD, Yamamoto N, Omura M, Cabeza de Vaca S, Krahne L. Effects of the D3 dopamine receptor antagonist, U99194A, on brain stimulation and d-amphetamine reward, motor activity, and c-fos expression in ad libitum fed and food-restricted rats. Psychopharmacology (Berl) 2002;163(1):76–84. doi: 10.1007/s00213-002-1132-0. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh B, Antonio T, Zhen J, Kharkar P, Reith MEA, Dutta AK. Development of (S)-N6-(2-(4-(isoquinolin-1-yl)piperazin-1-yl)ethyl)-N6-propyl-4,5,6,7-tetrahydrobenzo[d]-thiazole-2,6-diamine and its analogue as a D3 receptor preferring agonist: Potent in vivo activity in Parkinson's disease animal models. J Med Chem. 2010;53(3):1023–1037. doi: 10.1021/jm901184n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu W, Tu Z, McElveen E, Xu J, Taylor M, Luedtke RR, Mach RH. Synthesis and in vitro binding of N-phenyl piperazine analogs as potential dopamine D3 receptor ligands. Bioorg Med Chem. 2005;13(1):77–87. doi: 10.1016/j.bmc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Collins GT, Zhang J, Yang CY, Levant B, Woods J, Wang S. Design, synthesis, and evaluation of potent and selective ligands for the dopamine 3 (D3) receptor with a novel in vivo behavioral profile. J Med Chem. 2008;51(19):5905–8. doi: 10.1021/jm800471h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bettinetti L, Schlotter K, Hubner H, Gmeiner P. Interactive SAR studies: rational discovery of super-potent and highly selective dopamine D3 receptor antagonists and partial agonists. J Med Chem. 2002;45(21):4594–7. doi: 10.1021/jm025558r. [DOI] [PubMed] [Google Scholar]
- 27.Hackling A, Ghosh R, Perachon S, Mann A, Holtje HD, Wermuth CG, Schwartz JC, Sippl W, Sokoloff P, Stark H. N-(ω-(4-(2-methoxyphenyl)piperazin-1-yl)alkyl)carboxamides as dopamine D2 and D3 receptor ligands. J Med Chem. 2003;46(18):3883–99. doi: 10.1021/jm030836n. [DOI] [PubMed] [Google Scholar]
- 28.Hocke C, Prante O, Salama I, Hubner H, Lober S, Kuwert T, Gmeiner P. 18F-Labeled FAUC 346 and BP 897 derivatives as subtype-selective potential PET radioligands for the dopamine D3 receptor. ChemMedChem. 2008;3(5):788–93. doi: 10.1002/cmdc.200700327. [DOI] [PubMed] [Google Scholar]
- 29.Leopoldo M, Lacivita E, De Giorgio P, Colabufo NA, Niso M, Berardi F, Perrone R. Design, synthesis, and binding affinities of potential positron emission tomography (PET) ligands for visualization of brain dopamine D3 receptors. J Med Chem. 2006;49(1):358–65. doi: 10.1021/jm050734s. [DOI] [PubMed] [Google Scholar]
- 30.Gao M, Wang M, Hutchins GD, Zheng QH. Synthesis of new carbon-11-labeled carboxamide derivatives as potential PET dopamine D3 receptor radioligands. Appl Radiat Isot. 2008;66(12):1891–7. doi: 10.1016/j.apradiso.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Chu W, Tu Z, Jones LA, Luedtke RR, Perlmutter JS, Mintun MA, Mach RH. [3H]4-(Dimethylamino)-N-[4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl]benzamide, a selective radioligand for dopamine D3 receptors. I. In vitro characterization. Synapse. 2009;63(9):717–28. doi: 10.1002/syn.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor M, Grundt P, Griffin SA, Newman AH, Luedtke RR. Dopamine D3 receptor selective ligands with varying intrinsic efficacies at adenylyl cyclase inhibition and mitogenic signaling pathways. Synapse. 2010;64(3):251–66. doi: 10.1002/syn.20725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grundt P, Prevatt KM, Cao J, Taylor M, Floresca CZ, Choi JK, Jenkins BG, Luedtke RR, Newman AH. Heterocyclic analogues of N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl) arylcarboxamides with functionalized linking chains as novel dopamine D3 receptor ligands: Potential substance abuse therapeutic agents. J Med Chem. 2007;50(17):4135–4146. doi: 10.1021/jm0704200. [DOI] [PubMed] [Google Scholar]
- 34.Luedtke RR, Freeman RA, Boundy VA, Martin MW, Huang Y, Mach RH. Characterization of 125I-IABN, a novel azabicyclononane benzamide selective for D2-like dopamine receptors. Synapse. 2000;38(4):438–49. doi: 10.1002/1098-2396(20001215)38:4<438::AID-SYN9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 35.Cheng Y, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22(23):3099–108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu H, Daly JW, Creveling CR. A radioisotopic method for measuring the formation of adenosine 3′,5′-cyclic monophosphate in incubated slices of brain. J Neurochem. 1969;16(12):1609–19. doi: 10.1111/j.1471-4159.1969.tb10360.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.