Abstract
Chiral PCBs have been used as molecular probes of biological metabolic processes due to their special physical, chemical and biological properties. Many animal studies showed the enantioselective biotransformation of chiral PCBs, but it is unclear whether plants can enantioselectively biotransform chiral PCBs. In order to explore the enantioselectivity of chiral PCBs in whole plants, poplars (Populus deltoides × nigra, DN34), a model plant with complete genomic sequence, were hydroponically exposed to 2,2′,3,5′,6-pentachlorobiphenyl (PCB95) and 2,2′,3,3′,6,6′-hexachlorobiphenyl (PCB136) for 20 days. PCB95 and PCB136 were shown to be absorbed, taken-up and translocated in whole poplars, and they were detected in various tissues of whole poplars. However, the enantioselectivity of poplar for PCB95 and PCB136 proved to be quite different. The first eluting enantiomer of PCB95 was enantioselectively removed in whole poplar, especially in the middle and bottom xylem. It was likely enantioselectively metabolized inside poplar tissues, in contrast to racemic mixtures of PCB95 remaining in hydroponic solutions in contact with plant roots of whole and dead poplars. Unlike PCB95, PCB136 remained nearly racemic in most parts of whole poplars after 20 days exposure. These results suggest that PCB136 is more difficult to be enantioslectively biotransformed than PCB95 in whole poplars. This is the first evidence of enantioselectivity of chiral PCBs in whole plants, and suggests that poplars can enantioselectively biotransform at least one chiral PCB.
Introduction
Polychlorinated biphenyls (PCBs) are a group of persistent organic pollutants used extensively in past decades and released worldwide into the environment (1). More importantly, these PCBs can be bioaccumulated and biomagnified in the food chain (2). Nineteen congeners with 4–8 chlorines are chiral out of a total of 209 PCBs in the environment (3). Chiral PCBs have recently received increasing attention in the environmental and toxicological fields because they were produced and released as racemic mixtures but detected as nonracemic mixtures frequently in biota (4). Two enantiomers (of an individual congener of chiral PCBs) exhibit identical physical and chemical properties in the environment. Abiotic processes can not distinguish the difference between the enantiomers. As a result, the nonracemic signature of some chiral PCBs found in environmental media suggests that the enantiomers of these chiral PCBs behave differently in biochemical processes. Namely, chiral macromolecules, such as enzymes, might prefer metabolizing or binding one of the enantiomers, which might lead to different toxicity to organisms (5–7). Therefore, the change in enantiomeric fraction (EF) of chiral PCBs is a good indicator of biological selectivity and can be used to probe the metabolic processes and toxicological differences of chiral PCBs and to further predict their environmental fate.
The enantiomeric enrichment of chiral PCBs, including 2,2′,3,5′,6-pentachlorobiphenyl (PCB95) and 2,2′,3,3′,6,6′-hexachlorobiphenyl (PCB136), was found in many environmental media, such as soil (8,9), sediment (10), aquatic and riparian biota (fish, bivalves, crayfish, water snakes, barn swallows) (11), birds (12), shark and grouper (13), dolphins (14), whale (15), human faeces and liver (16,17), suggesting that the enantioselective metabolism or accumulation of the different enantiomers occurred during their biotransformation processes following their initial racemic release into the environment. Furthermore, chiral PCBs have also been found to be transferred through the food chain (18).
During the past decades, various biological species were found to transform chiral PCBs enantioselectively in the environment. First, microorganisms were shown to cause EF changes of chiral PCBs under both aerobic conditions (19) and anaerobic conditions (20). In addition, rainbow trout and invertebrates Mysis relicta were shown to enantioselectively enrich and eliminate chiral PCBs in water (21, 22). Furthermore, Kania-Korwel et al. confirmed that rodents clearly exhibited enantioselective accumulation of chiral PCBs in different tissues, such as blood, adipose tissue and liver (23, 24). All these studies suggest that biological processes can cause the existence of nonracemic mixtures of chiral PCBs in environmental biota.
To date, the studies of enantioselective biotransformation of chiral PCBs focused on animal species (21–24) and microorganisms (19, 20). Little is known about the enantioselective biotransformation of chiral PCBs in plants, although the total biomass of plants is far greater than that of animals on earth, and plants are well known for the “green-liver” model of metabolism in the transformation and degradation of xenobiotic contaminants (25, 26). Although a few papers have mentioned chiral PCBs in phytoplankton in the aquatic food web, conflicting conclusions were reported whether phytoplankton can transform PCB enantiomers selectively. For example, Wong et al. (27) found PCB enatiomers were racemic in phytoplankton, but Asher et al. (28) found PCB95 was significantly nonracemic in phytoplankton due to uptake from the surrounding aquatic environment. Therefore, In order to clearly understand the biotransformation mechanisms in the plants for the application of phytoremediation, it is desirable to investigate whether woody whole plants like poplar can transform chiral PCBs enantioselectively.
Poplars as a model plant in the field of phytoremediation have been shown to take-up and translocate some lower chlorinated PCBs (29) and to metabolize PCB3 and PCB77 to their hydroxlated PCBs (30, 31) in our previous work. However, whether whole poplars can take-up and/or biotransform chiral PCBs enantioselectively was still unknown. In order to test the hypothesis that, like in animal species, chiral PCBs can be enantioselectively taken-up and/or biotransformed in whole poplars in vivo, highly neurotoxic congeners PCB95 and PCB136 (32) found in many environmental media were selected as model chiral PCBs for this work. At the same time, the time-dependent changes of uptake and enantioselectivity of PCB95 and PCB136 were investigated in detail.
Experimental Section
Chemicals
The standards (99% purity or better) of PCB95 and PCB136 were obtained from AccuStandard (New Haven, CT). Stock solutions of PCB95 and PCB136 were prepared in hexane at 1.0 mg mL−1. Working solutions of PCB95 and PCB136 were prepared by gradual dilution of the stock solution with hexane. All standards and solutions of PCB95 and PCB136 were stored in amber glass vials at 4 °C.
Silica gel (70–230 mesh, Fisher Scientific) was activated at 450 °C for 12 h and acidified silica gel was prepared by adding 50 g of concentrated sulfuric acid (95–98%, Sigma-Aldrich) into 100 g of activated silica gel. Methyl-tert butyl ether (MTBE) (HPLC grade), acetone (pesticide grade) and hexane (pesticide grade) were from Fisher Scientific.
Exposure of Chiral PCBs
Cuttings of the adult Imperial Carolina hybrid poplar tree (Populus deltoides × nigra, DN34) were grown hydroponically for about 25 days before they were used as the model plant in this work. The healthy, actively growing, whole poplar plants were used in chiral PCBs exposure experiments. The exposure setup was the same as described in previous papers (29–31). Hoagland solution (400 mL) and a suitable amount of PCB95 or PCB136 were added to the autoclaved reactors. The exposure of PCB95 and PCB136 to poplars was performed separately in each experiment. Except for the blank poplar control without PCBs, the starting concentration of PCB95 in each reactor was 0.003 mg L−1 and the starting concentration of PCB136 in each reactor was 0.002 mg L−1. A variety of reference “controls” were tested at each time point with the following rationale: blank plant control- triplicate whole poplar plants without PCBs (contamination control); dead plant control- triplicate wilted, dead whole poplar plants exposed for 4 days with PCB95 or PCB136 (inactive plant control); and whole poplar plant- triplicate treatments of whole, growing, intact poplar plants with PCB95 or PCB136.
In order to elucidate the dynamic processes of uptake, translocation, distribution and transformation of these two chiral PCBs in different tissues of whole poplar plants, three time points of exposure were set: day 5, day 10 and day 20 when each reactor specimen was divided into hydroponic solution, root, bottom bark, bottom xylem, middle bark, middle xylem, top bark, top xylem and leaf as shown in Figure S1 A. For whole poplar samples at day 20, the bark was divided into cork, phelloderm and phloem for further study (Figure S1 B). Roots were extracted twice: whole roots were extracted initially, which mainly consisted of extracting PCBs adsorbed outside the root (root first); a second extraction of root (root second) was the ground-up root from root-first samples, which mainly extracted PCBs inside the root (operationally). Roots and leaves were ground in liquid nitrogen. Other parts of the poplar plants were cut into very small pieces (~0.2 cm or below) for efficient extraction of PCBs.
Extraction and Cleanup
The extraction and cleanup procedure for PCBs was modified from the previous literature for poplar plants (29). In brief, hydroponic solution samples were added to 100 mL of hexane/MTBE (1:1 v/v) and shaken overnight to extract PCBs. The organic phase was transferred and the hydroponic solution samples were extracted again with 50 mL of hexane/MTBE (1:1 v/v) and then shaken for 30 min. The combined extracts were evaporated to dryness in a rotary evaporator at 40 °C. Then the extracts were re-dissolved in 3 mL of hexane. Then the extracts were added into 1 mL of concentrated sulfuric acid to remove the macromolecular impurity and trace water. The organic phase and concentrated sulfuric acid were partitioned at 4000 rpm of centrifugation for 5 min. The organic phase was transferred and sulfuric acid phase was extracted again with 3 mL of hexane. The combined organic phase was concentrated under the gentle nitrogen flow and finally made up to 1 mL for GC analysis.
Plant tissue samples were extracted with 10 mL of (1:1, v/v) hexane/acetone g−1 of sample (wet weight) and vigorously shaken overnight. The organic phase was transferred after the centrifugation at 4000 rpm for 5 min. Then the samples were extracted again with 10 mL of (1:1, v/v) hexane/acetone g−1 of sample (wet weight) and vigorously shaken 30 min. The combined organic phase was evaporated to dryness in a rotary evaporator. Then the extracts were re-dissolved in 3 mL of hexane and added into 2 mL of concentrated sulfuric acid for the primary cleanup. The organic phase was transferred after the centrifugation at 4000 rpm for 5min. The sulfuric acid phase was extracted again with 3 mL of hexane and then the organic phase was combined after the centrifugation. The combined organic phase was concentrated about 2 mL and transferred to silica gel column (1 g of acidified silica gel on the top and 0.1 g of activated silica gel on the bottom) for further cleanup. PCBs were eluted from the column with 10 mL of hexane. The eluent was concentrated under the gentle nitrogen flow and finally made up to 1 mL for GC analysis.
Analysis
Qualitative and quantitative analysis of PCB95 and PCB136 was performed on GC-μECD (Agilent 6890) with an autosampler. The capillary column to separate the enantiomers was Chirasil-DEX CB (25m×0.25 mm i.d.×0.25 μm film thickness) from Varian, USA. The injection volume was 1 μL. The inlet mode was pulsed splitless at 250 °C and the carrier gas was helium at a flow rate of 1.0 mL min−1. The temperature of μECD was set at 250 °C with makeup gas (argon:methane=95:5) flow rate of 30 mL min−1. The oven program was the following: starting temperature 80 to 130 °C at 15 °C min−1; 130 to 165 °C at 0.3 °C min−1; post run at 200 °C held for 10 min. At above conditions, the enantiomers of PCB95 and PCB136 gained almost baseline separation and the enantiomeric retention times were 62.7 and 63.7 min for PCB95 and 82.2 and 83.1 min for PCB136. The detection limits (S/N = 3) of enantiomers of PCB95 and PCB136 were 0.25 ng mL−1 and 1.00 ng mL−1, respectively.
Enantiomer fraction or enantiomeric fraction (EF) (33) was used to calculate the enantiomer composition in this work:
where A and B are the concentrations of the (+)- and (−)-enantiomers for PCB136, respectively, or are the concentrations of the first-eluting enantiomer (E1) and the second-eluting enantiomer (E2) on the enantioselective chromatographic column for PCB95 because elution order is unknown. The standards of PCB95 and PCB136 were racemic with EF values of 0.499±0.001 (n=12) and 0.506±0.003 (n=12) for PCB95 and PCB136, respectively.
The data of statistical analysis are presented in Tables 1–2 and S1–2 as mean ± standard deviation. Differences in EFs of PCB95 and PCB136 with their standards at different time points for dead poplars and whole poplars were analyzed for significant differences by one way ANOVA with Tukey test at a = 0.05.
Table 1.
Masses (ng), concentrations (ng g−1 wet weight) and EFs of PCB95 in hydroponic solutions and different parts of dead poplar plants (n=3)
| sample | day 5 |
day 10 |
day 20 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| ng | ng g−1 | EF b | ng | ng g−1 | EF | ng | ng g−1 | EF | |
| middle xylem | 0.74±0.36 | 0.29±0.15 | 0.494±0.001* | 0.80±0.69 | 0.33±0.25 | 0.483±0.012* | 0.60±0.05 | 0.19±0.02 | 0.487±0.006* |
| middle bark | 115±26.9 | 34.6±2.88 | 0.499±0.001 | 227±108 | 109±44.8 | 0.500±0.001 | 193±2.04 | 92.8±7.79 | 0.498±0.001 |
| bottom xylem | 20.9±0.87 | 6.00±0.19 | 0.498±0.0002 | 8.18±3.33 | 2.56±1.52 | 0.496±0.002 | 20.6±0.18 | 4.88±0.44 | 0.497±0.001 |
| bottom bark | 656±35.7 | 187±2.31 | 0.499±0.0004 | 653±93.8 | 240±59.9 | 0.499±0.001 | 754±15.4 | 319±38.9 | 0.501±0.003 |
| solution | 94.6±10.2 | 0.24±0.03 | 0.501±0.002 | 67.3±49.0 | 0.17±0.12 | 0.499±0.0003 | 23.2±0.60 | 0.058±0.001 | 0.504±0.002 |
| total recovery mass | 887±52.1 | 957±69.5 | 991±16.6 | ||||||
| Recovery (%) a | 74.0±4.3 | 79.7±5.8 | 82.6±1.4 | ||||||
Total added mass of PCB95 was 1200 ng;
EF of PCB95 standard is 0.499±0.001 (n=12);
Significant difference of EFs from standard by one way ANOVA at α = 0.05.
Table 2.
Masses (ng), concentrations (ng g−1 wet weight) and EFs of PCB95 in hydroponic solutions and different parts of whole poplar plants (n=3)
| sample | day 5 |
day 10 |
day 20 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ng | ng g−1 | EF e | ng | ng g−1 | EF | ng | ng g−1 | EF | Mass of E2-E1 (ng) | |
| leaf | ND d | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| top xylem | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| top bark | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| middle xylem | 0.79±0.44 | 0.31±0.17 | 0.488±0.011* | 1.56±2.04 | 0.61±0.76 | 0.485±0.016* | 0.64±0.09 | 0.29±0.07 | 0.307±0.051* | 0.494±0.127 |
| cork b | 106±46.3 | 48.4±25.1 | 0.498±0.001 | 161±97.7 | 56.3±37.3 | 0.499±0.001 | 305±99.4 | 554±195 | 0.496±0.001* | 4.97±1.23 |
| middle bark a | ||||||||||
| phelloderm | 19.8±9.95 | 49.5±14.9 | 0.482±0.006* | 1.39±0.900 | ||||||
| phloem | 7.33±3.66 | 6.56±3.68 | 0.457±0.028* | 0.990±0.323 | ||||||
| bottom xylem | 4.27±1.63 | 1.24±0.60 | 0.493±0.007* | 6.85±4.52 | 2.18±1.47 | 0.493±0.004* | 2.65±1.04 | 0.96±0.19 | 0.449±0.012* | 0.514±0.112 |
| cork b | 432±53.9 | 144±26.9 | 0.501±0.0004 | 388±79.1 | 129±17.2 | 0.498±0.002 | 346±105 | 414±52.1 | 0.495±0.002* | 6.90±5.09 |
| bottom bark a | ||||||||||
| phelloderm | 13.8±14.3 | 37.1±36.9 | 0.468±0.008* | 1.75±1.81 | ||||||
| phloem | 19.6±18.1 | 12.1±10.0 | 0.475±0.010* | 1.50±0.812 | ||||||
| root first | 103±28.5 | 87.6±29.2 | 0.499±0.001 | 94.7±42.7 | 51.3±36.6 | 0.500±0.001 | 86.2±31.2 | 23.9±4.9 | 0.496±0.001* | 1.25±0.25 |
| root second | 150±37.4 | 123±9.28 | 0.499±0.001 | 260±117 | 135±87.8 | 0.501±0.010 | 177±62.9 | 49.3±10.4 | 0.511±0.001* | −(7.72±2.09) |
| solution | 166±106 | 0.42±0.27 | 0.500±0.002 | 56.1±27.2 | 0.14±0.07 | 0.497±0.004 | 44.5±8.47 | 0.11±0.02 | 0.499±0.014 | 0.32±2.35 12.36±1.69 (Sum of E2-E1 ng) |
| total recovery mass | 962±88.1 | 969±101 | 1022±142 | |||||||
| Recovery (%) c | 80.2±7.34 | 80.8±8.44 | 85.2±11.8 | |||||||
Middle bark and bottom bark at day 20 were divided three parts: cork, phelloderm and phloem (Figure S1);
The values in cork row at day 5 and 10 are the relative bark values;
total added mass of PCB95 was 1200 ng;
ND=not detectable;
EF of PCB95 standard is 0.499±0.001 (n=12);
Significant difference of EFs from standard by one way ANOVA at α = 0.05.
Results and Discussion
EFs and Distribution of PCB95 in Poplar Plants
The change in EFs has proven to be a powerful metric to indicate selective biotransformation of PCBs in biota; thus it was used in this work to show the enantioselectivity of PCB95 in poplar plants. PCB95 was not detected in any of the parts of the “blank poplar controls” at all the time points, indicating that the reactors and poplars had not been contaminated during the course of the experiment. At the same time, possible background interference was checked in the blank poplar samples because signals of GC-ECD were more sensitive and easily disturbed by other compounds. Results showed no interference at the retention times of PCB95 enantiomers (Figure S2 A and B) with clean baseline.
Data from a first experiment appear in Tables 1 and 2 on mass uptake, translocation, and enantiomeric fraction with PCB95 exposed to hybrid poplar plants in hydroponic solution. Table 1 includes the results from a negative control (dead poplar plants) where living plant tissues were not present, but physical absorption (uptake) and microbial enantioselective transformation by microorganisms were possible. Transformation products (metabolites) were not measured directly in this research; rather we were interested in the selection and concentration of one enantiomer over another in plant tissues, which would indicate an enzymatic selectivity for one enantiomer over another.
Table 2 gives the results for the main experimental treatment, i.e., whole, intact poplar plants exposed to PCB95. Approximately 74.0±4.3 to 82.6±1.4 % of the PCB95 mass added on day zero was recovered from the negative control (Table 1), while 80.2±7.34 to 85.2±11.8 % of the mass added was recovered from the treatment at day 5, 10 and 20. It is likely that the remainder (the unrecovered mass) was due to volatilization through the reactor seal, unextractable or irreversible binding of PCB95 and its metabolites to plant tissues, and/or experimental error during the course of the 20-day experiment.
Results from the negative control (dead poplar) in Table 1 indicate that most of the PCB95 was removed from solution (from 1200 ng at day zero to 23.2±0.60 ng on day 20), and it was uptaken by the plant along the bottom and middle bark directly exposed to PCB95 in the hydroponic solution or headspace. This mass movement is consistent with physical absorption to bark tissues. EFs were not significantly different in hydroponic solution and various tissues, most values being 0.494–0.499 with the range from 0.487±0.006 to 0.504±0.002 in dead poplars during the 20 day exposure. Compared with an EF of PCB95 standard (0.499±0.001), the EFs of PCB95 in middle xylem of the whole poplar at different time points were significantly different (α=0.05) as shown in Table 2. However, among the time point at day 5, day 10 and day 20, EFs of PCB95 in middle xylem of the dead poplar showed no significant differences (α=0.05), which meant that it was the translocation of PCB95, not microbial degradation and/or binding which led to the EF differences between middle xylems and standard. Moreover, EFs of PCB95 in hydroponic solution and other tissues were quite consistent with the racemic mixture originally added and didn’t deviate significantly (α=0.05) compared with the standard and among the different time points for the hydroponic solution and same tissue of dead poplar. Therefore, microorganisms had no enantioselective influence on PCB95 in dead poplar plants.
In Table 2 for whole poplar plants, PCB95 was not detected in plant tissues outside the aqueous exposure of the reactors, including leaf, top xylem and top bark, in all the samples, suggesting that PCB95 was not easily translocated compared to less chlorinated PCBs, such as PCB3 (29), in whole poplars. PCB95 was detected in the remaining parts of whole poplar plants and the results are shown in Table 2. The results indicated a greater absorption of PCB95 to healthy root tissues (root first and root second extraction), uptake and translocation in the xylem, and lower concentrations in the bottom bark (cork). Once again, a large fraction of PCB95 was removed from solution (from 1200 ng at day zero to 44.5±8.47 ng at day 20), but much more resided on the middle cork (305±99.4 ng at day 20), bottom cork (346±105 ng at day 20), outside the roots (“root first” at 86.2±31.2 ng at day 20), and inside the roots (“root second” at 177±62.9 ng at day 20).
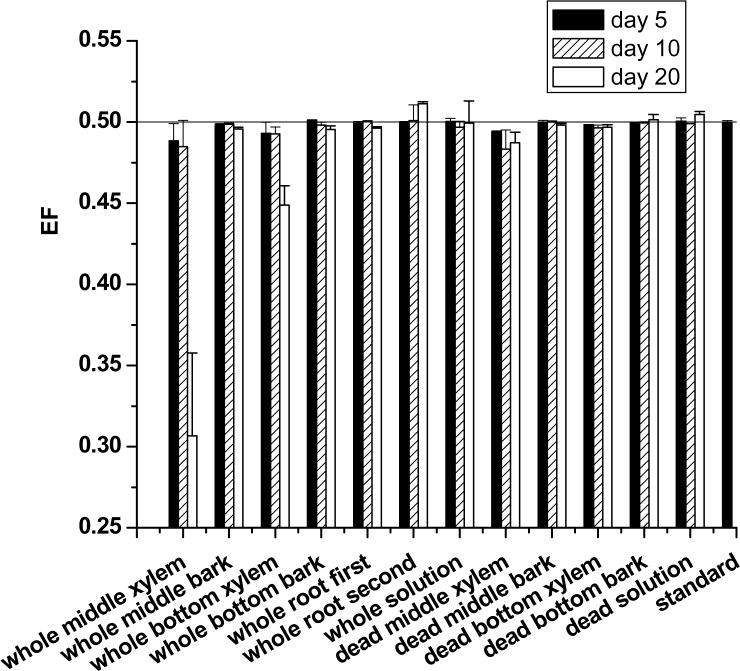
Significantly, some EF factors of PCB95 changed during the course of the experiment as shown in Table 2 and Figure 1. In the solution of the reactor, the EFs of PCB95 remained unchanged with the values of 0.500±0.002, 0.497±0.004 and 0.499±0.014 at day 5, day 10 and day 20, respectively. Therefore, there are no significant EF differences in the hydroponic solutions compared to the initial EF of PCB95 (α=0.05) and among EFs at the different time points (α=0.05), suggesting that whole poplar plants equally take-up and absorb the two enantiomers of PCB95 from hydroponic solutions. In addition, whole poplars were observed to enantioselectively remove the first-eluting enantiomer in the samples of middle xylem and bottom xylem (Table 2, Figures 1 and S3). The masses of PCB95 in middle xylem increased from 0.79±0.44 ng at day 5 to 1.56±2.01 ng at day 10 and then decreased to 0.64±0.09 ng at day 20; The masses of PCB95 in bottom xylem increased from 4.27±1.63 ng at day 5 to 6.85±4.52 ng at day 10 and then decreased to 2.65±1.04 ng at day 20. Furthermore, the concentrations of PCB95 in middle xylem and bottom xylem displayed the same tendency as the masses of PCB95, which implied that PCB95 was biologically transformed in whole poplars. The masses and concentrations of PCB95 in the bottom xylem were about 4 times greater than those in the middle xylem because the bottom xylem was connected to the roots and its bottom bark in contact with the hydroponic solution.
Figure 1.
Comparison of enantiomeric fractions (EFs) of PCB95 in different parts of dead and whole poplar plants and at different time points
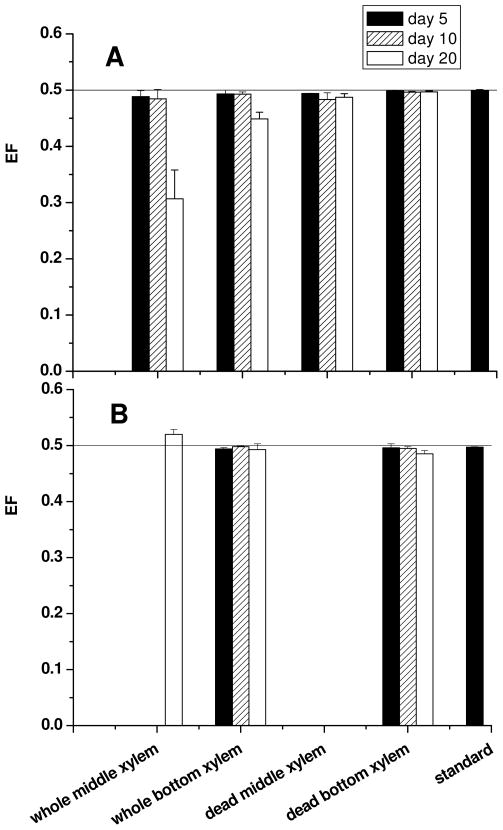
Most importantly, as shown in Table 2 and Figure 2A, EFs in middle xylem and bottom xylem exhibited a large, significant change (a=0.05), both compared with EF of PCB95 standards and among the EFs of PCB95 in the same tissue at different time points, from 0.488±0.011 and 0.493±0.007 at day 5 to 0.307±0.051 and 0.449±0.012 at day 20. Especially in the middle xylem at day 20, the concentration of E1-PCB95 was less than half of the concentration of E2-PCB95, which indicated a preferential loss (binding) of E1-PCB95 as it was translocated from the bottom to the middle tissues for PCB95. No such change was observed in the dead plant control (Table 1) suggesting that live plant tissues actively transformed more E1-PCB95 than E2-PCB95, either by enzymatic reaction in solution (e.g., formation of hydroxy-PCB95 compound/s) or by enzyme complex formation and binding to cellular tissues (e.g., complexation with reduced glutathione, GSH), or both. Also, bark samples had higher masses and concentrations of PCB95 in whole poplars; especially the bottom bark showed the highest masses and concentrations because the bottom bark in hydroponic solution directly contacted aqueous PCB95 and was in closer proximity to the root system.
Figure 2.
Comparison of enantiomeric fractions (EFs) of PCBs in middle xylem and bottom xylem of whole and dead poplar plants and at different time points. (A) PCB 95; (B) PCB 136.
However, there was different tendency observed for root-first and root-second samples. On the one hand, EFs of PCB95 had no changes on the surface of the root (root first), which remained racemic suggesting the roots of whole poplars equally took-up and absorbed the two enantiomers of PCB95 from hydroponic solution. On the other hand, the change of EFs inside the root (root second) exhibited the reverse tendency, slightly increasing from 0.499±0.001 at day 5 to 0.511±0.001 at day 20, which meant roots of whole poplars selectively biotransformed E2-PCB95 more than E1-PCB95. It is also likely that E2-PCB95 was easier to transfer inside the root compared with other parts of whole poplars.
Although the mass of E1-PCB95 inside the root was more than that of E2-PCB95, the total mass of E1-PCB95 was less than that of E2-PCB95 in whole poplars. The total mass difference of E1-PCB95 and E2-PCB95 was 12.36±1.69 ng (Table 2), about 1% of total added mass of PCB95 in the reactor, which was a significant change during only 20 days exposure. Therefore, the total mass difference of E1-PCB95 and E2-PCB95 also suggested that PCB95 can be enantioselectively biotransformed in whole poplars.
EFs and Distribution of PCB136 in Poplar Plants
The EF of PCB136 was calculated by the concentration of (+)-PCB136 divided by the sum of (+)-PCB136 and (−)-PCB136. This was possible because their optical rotations were known on the Chirasil-Dex column (34) and the second eluting enantiomer is (+)-PCB136. No enantiomers of PCB136 were detected in blank poplar controls from the samples at day 5 to day 20, which excludes the inadvertent contamination of the reactors and the procedures during the exposure and pretreatment (data not shown). Possible background interference of signals of GC-ECD was not found at the retention times of PCB136 enantiomers (Figure S2 C and D) with clean baseline.
In contrast to PCB95, PCB136 showed no such enantioselectivity in poplar. Once again, the loss from solution was rapid and leveled-off after only 5 days in the dead plant (negative) control shown in Table S1. The mass of PCB136 in hydroponic solution decreased from 800 ng to 107±1.27 ng in the first 5 days of exposure due to absorption and diffusion. Most of the mass was transferred to the bottom bark (403±3.91 ng at day 5), and it was not much translocated by the dead tissues. However, in the live plant treatment with PCB136 of Table S2, results show there was more translocation to roots and to bottom and middle xylem in the live plant treatment. Approximately 76.5±0.5 to 77.9±0.7 % of the PCB136 mass added on day zero was recovered from the negative control (Table S1), while 78.6±4.6 to 80.9±6.3 % of the mass added was recovered from the treatment at day 5, 10 and 20.
It can be seen from Tables S1 and S2 that the masses and the concentrations of PCB136 in middle xylem were detected only in middle xylem of whole poplar at day 20 and those in bottom xylem increased from day 5 to day 20 for dead poplars and increased and then decreased for whole poplars. A lack of PCB 136 in the middle xylem was likely due to slower uptake and transloction than PCB95 because PCB136 is higher in molecular weight. However, EFs of PCB136 remain racemic or nearly racemic in the poplars (Figure 2B and Tables S1 and S2). Firstly, EFs of PCB136 in the bottom xylem of dead and whole poplars did not show the apparent difference between different time points and standard (racemic). Secondly, there was a small but significant enantioselection of (+)-PCB136 (nearly racemic) after 20 days in the middle xylem in whole poplar (Figure 2), which could represent the beginning of enantio-transformation of (+)-PCB136 in poplars.
Mass Transport Model of Chiral PCBs in Whole Poplars
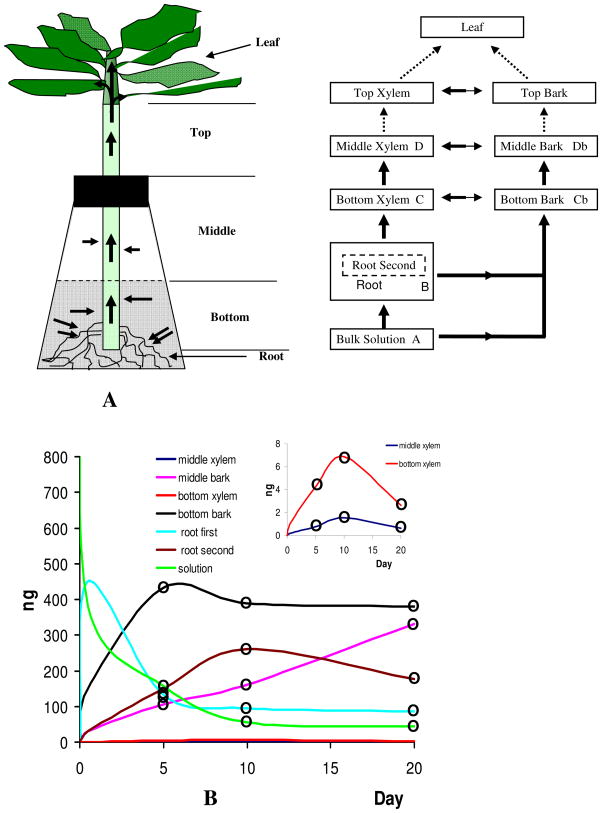
A schematic of the mass transport shown in Figure 3 is supported by the data of PCB95 in Table 2. Figure 3A is consistent with a simple linear uptake model following A → B → C → D, where A is the bulk hydroponic solution containing the racemic mixture of PCB, B is the root tissues, C is the bottom xylem, and D is the middle xylem. Diffusional processes between xylem and cork also occur in parallel with bottom bark (Cb) and with middle bark (Db). In addition, the wick effect is responsible for PCBs to move from the hydroponic solution up through the bark tissue within the reactor containment.
Figure 3.
(A) Schematic of mass transport through the hydroponic solution and plant compartments; (B) Mass change in the hydroponic solution and plant compartment supported by measurements in Table 2.
Data are shown and a schematic of the process is presented in the inset graph of Figure 3B for PCB95. The mass in bulk solution decreases rapidly as PCB is absorbed to bottom bark and uptaken by roots. Then, PCB95 enantiomers move up the bottom xylem to the middle xylem. In the case of PCB136, the EF remains racemic or nearly racemic. However, for PCB95, the first-eluting enantiomer begins to be selected by the plant tissues as it enters the bottom xylem (EF=0.449±0.012). Further enantioselection occurs as the compounds move up the plant to the middle xylem and bark, resulting in the final EF=0.307±0.051 observed in the middle xylem (Table 2). No such enantioselectivity was observed in either the dead plant controls (Tables 1 and S1), or in the reactor with PCB136 (Table S2). Only PCB95 is clearly enantioselected by poplars in these experiments.
Comparison of PCB95 and PCB136 in Whole Poplars
As mentioned above, PCB95 and PCB136 showed different results in whole poplars: PCB95 showed apparent enantioselective biotransformation and/or translocation and PCB136 kept nearly racemic and showed very slight enantioselective biotransformation and/or translocation in some tissues in whole poplars. This might be explained by the relationship of the two congener structures. Borlakoglu et al (35) summarized some rules to explain the rates of metabolism of PCBs by P450 isoenzymes as following: 1) The rates of metabolism of PCBs decrease with increasing molecular mass; 2) the rates of metabolism display no correlation with the extent of polyortho-halosubstitution of biphenyl; and 3) the rates of metabolism may increase with increasing number of meta-para hydrogen atoms. Therefore, the molecular mass differences between PCB95 and PCB136 could be one of major reasons for the EF changes of PCB95 and PCB136 in whole poplars. Rule #3 would not account for the differences in enatioselectivity between PCB95 and PCB136 because both of them have neighboring hydrogen atoms in two meta-para positions, belonging to the readily metabolizable PCBs. Another likely reason for the EF differences of PCB95 and PCB136 in whole poplars could be that the two enatiomers of these chiral PCBs are metabolized by different enzymes or have different affinity with poplar macromolecules, such as proteins and DNA. For example, differences in enantioselectivity between xylems and root-second samples (inside roots) might be due to different enzymes in the various tissues of whole poplars.
Due to the lack of available studies of chiral PCBs in other plants, some animal species were selected to show how species-dependent and congener-dependent characteristics affect the enantioselectivity and ability to biotransform chiral PCBs in the literature. Generally, PCB95 and PCB136 exhibited all three possible metabolic results in different animal species: easy biotransformation of the first eluting enantiomer, easy biotransformation of the second eluting enantiomer, or almost no selective biotransformation with racemic or nearly racemic PCBs in other species. For PCB95 in whole poplar of this research, E1-PCB95 was revealed to be easily biotransformed in most tissues except for the root-second sample. This finding was consistent with reports of nonracemic PCB95 in mysids (22), dolphins (14) and mice (23). But it contrasts with reports of E2-PCB95 with lower ratios in porpoises (36), rat (24) and human livers (17). Interestingly, similar species, mice and rat, exhibited contrary enantioselectivity to PCB95. Furthermore, PCB95 remained racemic or nearly racemic in fish species: rainbow trout (21) and grouper livers (13). PCB 136 was racemic or nearly racemic in most tissues of whole poplars in this work, which is consistent with reports of racemic or nearly racemic mixtures of PCB136 in grouper livers (13) and dolphins (14). But (+)-PCB 136 was easily biotransformed in rainbow trout (21), while (−)-PCB 136 was easily biotransformed in mice (23). Other congener-dependent characteristics were reported in the results of PCB95 and PCB136 in dolphins (14) and mice (23) showed different enantioselective biotransformation in the same species. Therefore, plants may also have species-dependent characteristics in the biotransformation of chiral PCBs in different species, and also congener-dependent characteristics for some chiral PCBs by different enzymes in the same plant such as demonstrated in this report.
Potentially, one of reasons that PCB136 did not exhibit apparently enatioselective biotransformation in whole poplars was the relatively short exposure time in this work. A twenty day exposure period might not be sufficient to show the clear concentration differences of the two enantiomers of PCB136. However, whole poplars have displayed clearly enantioselectivity to PCB95 during the same 20 day exposure. All in all, considering the huge biomass of plants on the earth, plants likely play an important role in the enantioselective biotransformation and/or translocation of chiral PCBs. More plant species and more chiral congeners of PCBs should be investigated to further confirm the species-dependent and congener-dependent enantioselective biotransformation of chiral PCBs. Further literatures on metabolic mechanism of chiral PCBs in animals and whole poplars are provided in Supporting Information (SI). To summarize, our results showed that whole poplars can clearly enantioselectively biotransform PCB95, but remain nearly racemic for PCB136 during a 20 day exposure. To the best of our knowledge, this is the first report of the enantioselective biotransformation of chiral PCBs in whole plants.
Supplementary Material
Acknowledgments
This work was supported by the Iowa Superfund Basic Research Program (SBRP), National Institute of Environmental Health Science, Grant Number P42ES013661. We thank Collin Just, Civil and Environmental Engineering, University of Iowa, for supporting this experiment and also the Center for Global and Regional Environmental Research (CGRER) for financial support. This paper is a contribution from the W. M. Keck Phytotechnology Laboratory at the University of Iowa.
Footnotes
Supporting Information Available
The figures, including the experiment setup, background signals of blank poplar tissues at the retention times of PCB95 and PCB136 on GC-ECD, some selected typical chromatograms of PCB95, are shown in figures. Tables with the data of PCB136 and metabolic mechanisms of chiral PCBs in animals potentially applicable to poplars are put into the SI. This material is available free of charge via the Internet at http://pubs.acs/org/.
Literature Cited
- 1.Safe SH. Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol. 1994;24:87–149. doi: 10.3109/10408449409049308. [DOI] [PubMed] [Google Scholar]
- 2.Evans MS, Noguchi GE, Rice CP. The biomagnifications of polychlorinated biphenyls, toxaphene, and DDT compounds in a Lake Michigan offshore food web. Arch Environ Contam Toxicol. 1991;20:87–93. doi: 10.1007/BF01065333. [DOI] [PubMed] [Google Scholar]
- 3.Kaiser K. On the optical activity of polychlorinated biphenyls. Environ Pollut. 1974;7:93–101. [Google Scholar]
- 4.Lehmler HJ, Harrad SJ, Hunerfuss H, Kania-Korwel I, Lee CM, Lu Z, Wong CS. Chiral polychlorinated biphenyl transport, metabolism, and distribution: a review. Environ Sci Technol. 2010;44:2757–2766. doi: 10.1021/es902208u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puttmann M, Mannschreck A, Oesch F, Robertson LW. Chiral effects in the induction of drug-metabolizing enzymes using synthetic atropisomers of polychlorinated biphenyls (PCBs) Biochem Pharmacol. 1989;38:1345–1352. doi: 10.1016/0006-2952(89)90342-0. [DOI] [PubMed] [Google Scholar]
- 6.Rodman LE, Shedlofsky SI, Mannschrek A, Puttmann M, Swim AT, Robertson LW. Differential potency of atropisomers of polychlorinated biphenyls on cytochrome P450 induction and uroporphyrin accumulation in the chick embryo hepatocyte culture. Biochem Pharmacol. 1991;41:915–922. doi: 10.1016/0006-2952(91)90196-c. [DOI] [PubMed] [Google Scholar]
- 7.Smith SW. Chiral toxicology: It’s the same thing…only different. Toxicol Sci. 2009;110:4–30. doi: 10.1093/toxsci/kfp097. [DOI] [PubMed] [Google Scholar]
- 8.Robson M, Harrad S. Chiral PCB signatures in air and soil: Implications for atmospheric source apportionment. Environ Sci Technol. 2004;38:1662–1666. doi: 10.1021/es0349002. [DOI] [PubMed] [Google Scholar]
- 9.Wong F, Robson M, Diamond M, Harrad S, Truong J. Concentrations and chiral signatures of POPs in soil and sediments: A comparative urban versus rural study in Canada and UK. Chemosphere. 2009;74:404–411. doi: 10.1016/j.chemosphere.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 10.Wong CS, Garrison AW, Foreman WT. Enantiomeric composition of chiral polychlorinated biphenyl atropisomers in aquatic bed sediment. Environ Sci Technol. 2001;35:33–39. doi: 10.1021/es0012570. [DOI] [PubMed] [Google Scholar]
- 11.Wong CS, Garrison AW, Smith PD, Foreman WT. Enantiomeric composition of chiral polychlorinated biphenyl atropisomers in aquatic and riparian biota. Environ Sci Technol. 2001;35:2448–2454. doi: 10.1021/es0018872. [DOI] [PubMed] [Google Scholar]
- 12.Ross MS, Verreault J, Letcher RJ, Gabrielsen GW, Wong CS. Chiral organochlorine contaminants in blood and eggs of glaucous gulls (Larus hyperboreus) from the Norwegian Arctic. Environ Sci Technol. 2008;42:7181–7186. doi: 10.1021/es8000147. [DOI] [PubMed] [Google Scholar]
- 13.Serrano R, Fernandez M, Rabanal R, Hernandez LM, Gonzalez MJ. Congener-specific determination of polychlorinated biphenyls in shark and grouper livers from the Northwest African Atlantic Ocean. Arch Environ Contam Toxicol. 2000;38:217–224. doi: 10.1007/s002449910029. [DOI] [PubMed] [Google Scholar]
- 14.Reich S, Jimenez B, Marsili L, Hernández LM, Schurig V, González MJ. Congener specific determination and enantiomeric ratios of chiral polychlorinated biphenyls in striped dolphins (Stenella coeruleoalba) from the Mediterranean Sea. Environ Sci Technol. 1999;33:1787–1793. [Google Scholar]
- 15.Hoekstra PF, Wong CS, O’Hara TM, Solomon KR, Mabury SA, Muir DCG. Enantiomer-specific accumulation of PCB atropisomers in the bowhead whale (Balaena mysticetus) Environ Sci Technol. 2002;36:1419–1425. doi: 10.1021/es015763g. [DOI] [PubMed] [Google Scholar]
- 16.Harrad S, Ren J, Hazrati S, Robson M. Chiral signatures of PCB#s 95 and 149 in indoor air, grass, duplicate diets and human faeces. Chemosphere. 2006;63:1368–1376. doi: 10.1016/j.chemosphere.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 17.Chu S, Covaci A, Schepens P. Levels and chiral signatures of persistent organochlorine pollutants in human tissues from Belgium. Environ Res. 2003;93:167–176. doi: 10.1016/s0013-9351(03)00016-1. [DOI] [PubMed] [Google Scholar]
- 18.Asher BJ, Wong CS, Rodenburg LA. Chiral source apportionment of polychlorinated biphenyls to the Hudson River estuary atmosphere and food web. Environ Sci Technol. 2007;41:6163–6169. doi: 10.1021/es070763n. [DOI] [PubMed] [Google Scholar]
- 19.Singer AC, Wong CS, Crowley DE. Differential enantioselective transformation of atropisomeric polychlorinated biphenyls by multiple bacterial strains with different inducing compounds. Appl Environ Microbiol. 2002;68:5756–5759. doi: 10.1128/AEM.68.11.5756-5759.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pakdeesusuk U, Jones WJ, Lee CM, Garrison AW, O’Niell WL, Freedman DL, Coates JT, Wong CS. Changes in enantiomeric fractions (EF) during microbial reductive dechlorination of PCB 132, PCB 149, and Aroclor 1254 in Lake Hartwell sediment microcosms. Environ Sci Technol. 2003;37:1100–1107. doi: 10.1021/es026039g. [DOI] [PubMed] [Google Scholar]
- 21.Wong CS, Lau F, Clark M, Mabury SA, Muir DCG. Rainbow trout (Oncorhynchus mykiss) can eliminate chiral organochlorine compound enantioselectively. Environ Sci Technol. 2002;36:1257–1262. doi: 10.1021/es0156791. [DOI] [PubMed] [Google Scholar]
- 22.Warner NA, Wong CS. The freshwater invertebrate Mysis relicta can eliminate chiral organochlorine compounds enantioselectively. Environ Sci Technol. 2006;40:4158–4164. doi: 10.1021/es052166b. [DOI] [PubMed] [Google Scholar]
- 23.Kania-Korwel I, El-Komy M, Veng-Pedersen P, Lehmler H-J. Clearance of polychlorinated biphenyl atropisomers is enantioselective in female C57Bl/6 mice. Environ Sci Technol. 2010;44:2828–2835. doi: 10.1021/es901781p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kania-Korwel I, Garrison AW, Avants JK, Hornbuckle KC, Robertson LW, Sulkowski WW, Lehmler HJ. Distribution of chiral PCBs in selected tissues in the laboratory rat. Environ Sci Technol. 2006;40:3704–3710. doi: 10.1021/es0602086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coleman J, Blake-Kalff M, Davies E. Detoxification of xenobiotics by plants: chemical modification and vacuolar compartmentation. Trends Plant Sci. 1997;2:144–151. [Google Scholar]
- 26.Sandermann H. Higher-plant metabolism of xenobiotics - the green liver concept. Pharmacogenet Genomics. 1994;4:225–241. doi: 10.1097/00008571-199410000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Wong CS, Mabury SA, Whittle DM, Backus SM, Teixeira C, DeVault DS, Bronte CR, Muir DCG. Organochlorine compounds in Lake Superior: chiral polychlorinated biphenyls and biotransformation in the aquatic food web. Environ Sci Technol. 2004;38:84–92. doi: 10.1021/es0346983. [DOI] [PubMed] [Google Scholar]
- 28.Asher BJ, Wong CS, Rodenburg LA. Chiral source apportionment of polychlorinated biphenyls to the Hudson River estuary atmosphere and food web. Environ Sci Technol. 2007;41:6163–6169. doi: 10.1021/es070763n. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Schnoor JL. Uptake and translocation of lesser-chlorinated polychlorinated biphenyls (PCBs) in the whole hybrid poplar plants after hydroponic exposure. Chemosphere. 2008;73:1608–1616. doi: 10.1016/j.chemosphere.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhai G, Lehmler HJ, Schnoor JL. Hydroxylated metabolites of 4-monochlorobiphenyl and its metabolic pathway in whole poplar plants. Environ Sci Technol. 2010;44:3901–3907. doi: 10.1021/es100230m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Hu D, Jiang G, Schnoor JL. In vivo biotransformation of 3,3′,4,4′-tetrachlorobiphenyl by whole plants-poplars and switchgrass. Environ Sci Technol. 2009;43:7503–7509. doi: 10.1021/es901244h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schantz SL, Widholm JJ, Rice DC. Effects of PCB exposure on neuropsychological function in children. Environ Health Perspect. 2003;111:357–376. doi: 10.1289/ehp.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harner T, Wiberg K, Norstrom R. Enantiomer fractions are preferred to enantiomer ratios for describing chiral signatures in environmental analysis. Environ Sci Technol. 2000;34:218–220. [Google Scholar]
- 34.Haglund P, Wiberg K. Determination of the gas chromatographic elution sequences of the (+) and (−) enantiomers of stable enantiomeric PCBs on Chirasil-Dex. J High Resol Chromatogr. 1996;19:373–376. [Google Scholar]
- 35.Borlakoglu JT, Wilkins JPG. Correlations between the molecular structures of polyhalogenated biphenyls and their metabolism by hepatic microsomal monooxygenases. Comp Biochem Physiol. 1993;105C:113–117. doi: 10.1016/0742-8413(93)90066-t. [DOI] [PubMed] [Google Scholar]
- 36.Chu S, Covaci A, Van de Vijver K, De Coen W, Blust R, Schepens P. Enantiomeric signatures of chiral polychlorinated biphenyl atropisomers in livers of harbour porpoises (Phocoena phocoena) from the southern North Sea. J Environ Monit. 2003;5:521–526. doi: 10.1039/b300949a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.