Abstract
A modular system for source-to-dose-to-effect modeling analysis has been developed based on the modeling environment for total risk studies (MENTOR),(1) and applied to study the impacts of hypothetical atmospheric releases of anthrax spores. The system, MENTOR-2E (MENTOR for Emergency Events), provides mechanistically consistent analysis of inhalation exposures for various release scenarios, while allowing consideration of specific susceptible subpopulations (such as the elderly) at the resolution of individual census tracts. The MENTOR-2E application presented here includes atmospheric dispersion modeling, statistically representative samples of individuals along with corresponding activity patterns, and population-based dosimetry modeling that accounts for activity and physiological variability. Two hypothetical release scenarios were simulated: a 100 g release of weaponized B. anthracis over a period of (a) one hour and (b) 10 hours, and the impact of these releases on population in the State of New Jersey was studied. Results were compared with those from simplified modeling of population dynamics (location, activities, etc.), and atmospheric dispersion of anthrax spores. The comparisons showed that in the two release scenarios simulated, each major approximation resulted in an overestimation of the number of probable infections by a factor of 5 to 10; these overestimations can have significant public health implications when preparing for and responding effectively to an actual release. This is in addition to uncertainties in dose-response modeling, which result in an additional factor of 5 to 10 variation in estimated casualties. The MENTOR-2E system has been developed in a modular fashion so that improvements in individual modules can be readily made without impacting the other modules, and provides a first step toward the development of models that can be used in supporting real-time decision making.
Keywords: Anthrax spores, dose, emergency events, exposure, MENTOR, modeling, risk
1. INTRODUCTION
During the B. anthracis attacks on the U.S. postal system in the fall of 2001, six letters, each containing 1 to 2 g of anthrax spores, caused anthrax infections in 22 individuals, and resulted in five deaths,(2) despite aggressive treatment and care.(3) In addition to the human cost, the economic costs of the B. anthracis attacks were significant. U.S. postal facilities contaminated by B. anthracis required more than two years of decontamination at a cost of more than $200 million.(4) Understandably, concerns have been expressed that small amounts of powdered B. anthracis inserted into the air intakes of subways, airports, shopping malls, sports arenas, and other public complexes could be devastating,(5) with long-term consequences potentially posing greater challenges than the short-term impact.(6)
Several studies have attempted to quantify the overall impact of large-scale releases of B. anthracis: concluding (a) an aircraft release of 50 kg of B. anthracis over an urban area would result in tens to hundreds of thousands of deaths,(7) (b) an airborne release of 100 kg of B. anthracis upwind of Washington, DC, would result in 130,000 to 3 million deaths,(8) and (c) 1.49 million people out of 11.5 million people (13.1%) would be infected downwind from a point of release of 1 kg of B. anthracis.(9) The economic costs were estimated to reach over $26 billion per 100,000 people exposed,(10) not including the cost of decontamination. However, unlike nuclear weapons, the consequences of B. anthracis attacks can be mitigated.(5)
Though the consequences of a bioterrorism event are often enormous, it is prudent to not overestimate their impact,(11) as the scenario may appear unmanageable to responding agencies, leaving both the public and responders feeling helpless. Proper planning based on more complete interpretation and evaluation of available knowledge and experience can reduce public anxiety as well as increase confidence on behalf of the professionals responding to an adverse event.(12)
Planning and responding to a bioterrorism event, including vaccinations and postexposure prophylaxis, is a challenging task. Vaccines for anthrax exist,(13,14) but in practice several obstacles are present in the form of potential adverse effects,(15,16) lengthy dose regimen requirements,(17) and widely varying perceptions of risk.(18) Furthermore, vaccination of the majority of populations in large urban areas would be impractical due to limited availability of prophylactics, and the costs associated with procuring and handling large supplies.
1.1. Planning for Emergency Response
Several public health issues need to be addressed in planning and responding to emergency events.(19,20) While postattack response to bioterrorism is vital, preattack measures, such as detection, planning, and training, are just as important.(5) Spatial and temporal patterns of detected levels of biological agents can be combined with diagnostic modeling methods and real-time meteorological data to estimate release source strength and location and, consequently, high impact areas. This in turn will allow targeted emergency response planning, including targeted prophylaxis administration in high impact areas, because timely initiation of treatment is a critical factor.(21,22) As a first step toward rapid detection and response to bioterrorism, a national monitoring network in the United States, called BioWatch, has been set up.(23,24) It is being expanded to detect the presence of a number of airborne biological agents, including anthrax spores; however, currently the results are not available in real time. Recent modeling efforts have also focused on evaluating response based on syndromic surveillance and on prioritizing threats.(25-27)
After the anthrax attacks in the United States in 2001, several modeling studies focused on estimating effects of B. anthracis releases, with varying levels of model detail and scale. Reshetin and Regens(28) modeled the release of B. anthracis inside a 50-story building. Webb and Blaser(29) modeled the transmission of B. anthracis through cross-contamination of postal letters, whereas Ho and Duncan(30) modeled the aerosol hazards from a letter containing anthrax spores. Fowler et al.(31) performed a probabilistic comparison of vaccination and antibiotic prophylaxis based on probabilities of exposure. Wein et al.(9) and Craft et al.(32) performed detailed modeling analyses linking B. anthracis dispersion over large areas with infection and disease progression mechanism, hospital response logistics, and corresponding outcomes. More recent efforts include Biowar,(33,34) an agent-based model of bioattacks that links simple dispersion models with models for social networks and behavioral attributes such as health-care-seeking behaviors and pharmaceutical purchases.
One of the limitations of existing B. anthracis modeling studies is that they have employed substantial simplifying assumptions. For example, Craft et al.(32) assumed uniform population density over a large area, uniform population demographics, Gaussian dispersion with uniform wind speed and direction, constant inhalation rates for all individuals, and personal exposure concentrations being the same as outdoor concentrations. The rationale for such simplifying assumptions is that the equations become mathematically tractable and result in analytical solutions. However, such simplifications limit the model applicability to only a small set of potential scenarios and conditions and minor changes in modeling assumptions may require rewriting underlying model equations.
2. APPROACH
This study presents a prototype modeling system for performing source-to-dose-to-effect analysis of inhalation anthrax infections from airborne weaponized anthrax spore releases. The main principle guiding the development of the present system is that prioritized exposure analysis(35,36) should be conducted in order to minimize misclassification of exposure. Consistent quantification of exposures and doses for different release scenarios, as well as for different emergency response strategies, provides a sound scientific basis for developing and then implementing plans for response strategies. The main objective of this system is to utilize available databases of distributions of demographics, human activity patterns, etc., and to provide sufficient flexibility so that this system can be useful in planning multiple emergency response scenarios for training purposes and for rapid decision making in the aftermath of emergency events.
A modular, mechanistic framework that links available models and databases for characterizing exposures and adverse impacts would improve risk assessment in terms of (a) providing consistency, (b) allowing assessments on multiple scales, and incorporating the important processes from release source to dose received by individuals, (c) optimizing the use of the most up-to-date models and databases for individual processes, and (d) allowing systematic sensitivity and uncertainty analyses to identify the important factors that affect the outcomes the most. The underlying person-oriented exposure modeling approach has been utilized and evaluated in the past for different types of contaminants.(37,38)
2.1. Important Factors Influencing the Impact of B. anthracis Releases
There are several factors that influence inhalation exposures and doses of B. anthracis to humans from airborne releases.
2.1.1. Release Source(s)
The magnitude, location of source(s), and patterns of release are the primary factors and major unknowns in performing dispersion modeling for emergency events. An attack scenario can include single or multiple sources (differing in location or time), with anthrax spores released quickly (“instantaneous”) or slowly over time (“continuous”). These are required as initial inputs to the exposure and dose modeling analysis.
2.1.2. Meteorological Conditions and Topography/Terrain
The meteorological conditions and local topography (rural, urban, etc.) influence the extent of dispersion of anthrax spores and, thus, the overall impact of a release. These include variables such as wind speed, direction, temperature, atmospheric stability, boundary layer thickness, surface topography, surface roughness, land use, etc.,(39,40) which can together contribute to over a factor of 20 variation in potential casualties.(8)
2.1.3. Population Distributions
The distribution of population in the areas affected by the releases (“downwind locations”) determines the intensity and spread of exposure and potential infections. This is a highly variable and often ignored factor, in the sense that the population distribution changes in time (e.g., commuting to major urban centers or large gatherings at major events). However, it is manageable to an extent, for example, through evacuation of people from downwind locations or enforcing shelter-in-place.
2.1.4. Human Activity Patterns
The location of the individual at any given time (e.g., outdoors versus indoors), and the activity performed (e.g., running versus sleeping or resting), determine the exposure concentrations, the breathing rates, the efficiency of particle uptake, and thus the number of inhaled anthrax spores. These factors are also important in characterizing the potential contact or lack of contact with a contaminant. However, they are often overlooked or poorly characterized in emergency event analyses.(9,28,29,31,32,41)
2.1.5. Physiological Characteristics of Individuals
The base inhalation rates are dependent on the age, gender, and physical characteristics such as body weight and life style patterns. Furthermore, the infection potential of B. anthracis is dependent on the age of the individual.(29,32)
Other factors that are important in determining exposures are socioeconomic attributes, such as housing characteristics (age, size, ventilation, etc.), which determine the fraction of outdoor B. anthracis that gets entrained indoors.
2.2. Steps and Resources in the Estimation of Exposures, Doses, and Infections
Based on the approach introduced by Georgopoulos et al.,(37) several modeling steps (or components, as some of them do not have to be performed in sequence) are needed in assessing the impacts of airborne releases of B. anthracis. In general, the following eight steps are needed, as shown in Fig. 1:
- Estimation of outdoor concentration levels of airborne anthrax spores through one of the following:
- Numerical modeling of the atmospheric dispersion of B. anthracis. This involves application of atmospheric dispersion models such as California Puff Model (CALPUFF),(44) Hazard Prediction and Assessment Capability (HPAC),(45) or Hybrid Particle and Concentration Transport (HYPACT).(46) These models use dynamic meteorological profiles as inputs (either user-provided or from meteorological data sources) and provide contaminant concentration profiles at different spatial and temporal resolutions. Dispersion modeling at finer scales, for example, within a building or within the vicinity of the release location, can be accomplished through detailed subgrid modeling approaches such as Computational Fluid Dynamics (CFD) based models.(28)
- Estimation of local B. anthracis levels at the scale of interest (such as a census tract) or a conveniently defined grid through one of the following:
- Spatiotemporal statistical interpolation of monitor data or outputs of a “coarse-scale” model, using the techniques mentioned in Step 1a.
- Aggregation of the outputs of a “fine-scale” model. This is important when the atmospheric dispersion model provides concentration profiles at a finer scale than the resolution of other model components, such as population distributions, which have a typical resolution of a census tract or a census block.
-
Characterization of attributes of populations (geographic density, age, gender, race, income, etc.) through one of the following:
- Selection of a fixed-size sample population (“virtual individuals”) that statistically reproduces essential census demographics.
- Division of the population of interest into an exhaustive set of cohorts based on different relevant population attributes.
The population attributes, such as the distributions of age, gender, employment, and housing, can be developed from available census data (see, e.g., USCB47). Sometimes, relevant databases are available as components of other modeling systems, as in the case of the Air Pollution Exposure Model (APEX),(48) which provides databases for housing as well as for commuting profiles. Depending on the emergency response scenario, relevant adjustments to the distributions of population profiles may be necessary (e.g., changes in the population distribution during special events).
-
Development of activity event (or exposure event) sequences for each member of the sampled population or for each cohort for the exposure period through one of the following:
- Existing databases from composites of past studies (for baseline assessment).
- Hypothetical scenario-based or “simulated” activity patterns based on options such as “shelter-in-place” versus different evacuation options.
For baseline assessments, the Consolidated Human Activity Database (CHAD)(49) can be used. It contains over 22,000 person days (diary records) of activity patterns developed from preexisting human activity studies. Each diary record provides a basis for simulating the movement of the “virtual individual” through geographic locations and microenvironments during the simulation period. Each event is defined by geographic location, start time, duration, microenvironment visited, and an activity performed. The attributes of CHAD records include age, gender, employment status, and smoking status of each individual, which can be used for matching the demographic characteristics of each sampled individual.(50) For planning and training purposes, several additional options can be considered for protective action, including evacuation, “shelter-in-place,” or a combination.(51) The corresponding activity profiles can be either synthesized independently or through scenario-specific modifications to existing CHAD diaries.
-
Estimation of personal exposure levels and temporal profiles of B. anthracis concentrations in various microenvironments (residences, offices, restaurants, vehicles, etc.) through either one or more of the following methods:
- Simple linear, steady-state mass balance.
- Nonlinear, dynamic models.
- Detailed computational fluid dynamics models.
Several modeling studies of indoor/outdoor relationships of fine particles have been presented in the literature, addressing issues such as contaminant penetration of indoor environment and corresponding particle size dependence.(52-54) The Stochastic Human Exposure and Dose Simulation (SHEDS) application(55) provides distributions of air exchange rates for different types of residential microenvironments, while other models and databases provide distributions for air exchange rates for general nonresidential microenvironments(56) and vehicle microenvironments.(57)
Calculation of appropriate inhalation rates for the members of the sample population by combining physiological attributes of study subjects and activities pursued during the individual exposure events. The CHAD diary records also provide information on energy expenditure, which can be used directly to estimate inhalation rates, as discussed in Step 6 of Section 4. Alternatively, probability distributions or tables describing age-specific inhalation rates of humans can also be used.(58-60) The inhalation rates, along with personal exposure levels, provide intake rates of anthrax spores.
Calculation of target tissue dose through physiologically-based respiratory deposition modeling by estimating the amount of inhaled B. anthracis that is deposited in the lungs. The International Commission on Radiological Protection (ICRP)(61,62) provides deposition fractions of fine particles in different regions of the human lungs. These fractions are age and gender dependent, and are also dependent on the size of the particles.
Estimation of probability of infection for each simulated individual based on calculated dose and physiological attributes, and summation of these probabilities to estimate the total number of potential infections for each local area in the simulation domain (e.g., each census tract in the modeling domain). Sparse data are available for describing the dose-response relationships of B. anthracis in humans,(29) and several dose-response models have been proposed in the literature, as described in Step 8 in Section 4.
Fig. 1.
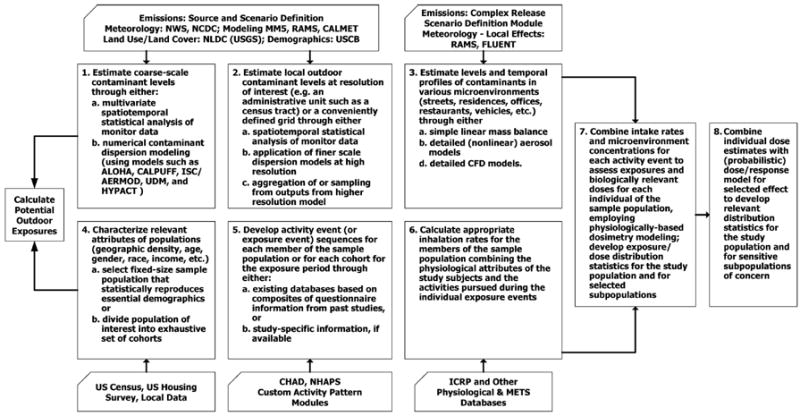
A generalized eight-step flowchart describing the processes involved in assessing risk using a source-to-dose-to-effect framework, adapted from Georgopoulos et al.(37) this is also referred to as person-oriented population-based exposure modeling (POM/PBEM). This flowchart reflects the structure of the MENTOR approach and provides a general “template” for comparing the application of risk assessment systems. A subset of the models appearing in this flowchart has been used in this study.
3. MENTOR-2E IMPLEMENTATION
The steps outlined above were implemented in a modular manner as part of the Modeling Environment for Total Risk studies (MENTOR).(1,37) The general approach of MENTOR is to utilize existing models when available and to provide new modules to “fill gaps” in the source-to-dose-to-effect sequence. In that sense, MENTOR is not a “new model”; it can be viewed as a computational toolbox intended to facilitate consistent multiscale risk assessment. In this particular study, several existing models and approaches relevant to exposure estimation have been used. For example, various concepts from the SHEDS approach, which has been applied for studying exposures to particulate matter(55) and pesticides,(63) have been adapted and incorporated into the formulation of different MENTOR modules.
The MENTOR-2E system has been coded in Matlab(64) (www.mathworks.com), while various relevant programs such as the CALPUFF model(44) have been linked in a pipeline manner. The model code and the underlying data files are available upon request. The computational time for the CALPUFF simulation is dependent on the extent of the modeling domain, the grid resolution, and the duration of the simulated period. For the case of 250 m resolution, and an area covering the entire State of New Jersey, the CALPUFF simulation required about 2 CPU hours on a 3 GHz Pentium Processor. The computational time for calculation of exposures, doses, and effects of B. anthracis release is dependent mainly on the number of virtual individuals simulated per census tract, the number of census tracts, and the time period considered in the simulation. This study used 500 virtual individuals per census tract, and the exposure and dose calculations required about 4 minutes of CPU time per census tract for population sampling, exposures, and dose calculations. However, the calculations involving activity patterns, exposures, and doses are run in a distributed manner on a computer cluster, as these calculations for each census tract are independent of those for other census tracts.
4. CASE STUDY APPLICATION
The location and timing of the hypothetical scenarios used in this study to demonstrate the MENTOR-2E system were assumed to represent potential variations of the anthrax attacks of 2001 through the postal system in the State of New Jersey. The main difference is in the amount and release characteristics. B. anthracis was assumed to be released in the air and the release was assumed to occur starting at 08:00 hours on September 18, 2001 in the vicinity of the Hamilton Post Office (the site and the day of the mailing of letters containing anthrax spores in 2001), as shown in Fig. 2. A hypothetical release of 100 g (1 trillion spherical spores per gram) of weaponized anthrax spores was assumed, similar to the release characteristics assumed by the modeling study of Craft et al.(32) Two types of releases were considered in the simulation: a “quick” release, where the spores were assumed to be released over a period of one hour (release scenario A), and a “continuous” release, where the spores were assumed to be released over a period of 10 hours (release scenario B). The study focused on the impact of these releases on the general population of the State of New Jersey, and exposures during the day of September 18, 2001 were simulated. Only the census tracts in New Jersey (totaling 1944 census tracts) were considered here, instead of the entire region of potential impact, to illustrate the application of the system to targeted administrative areas by responding agencies. The inclusion of other census tracts in other states is straightforward.
Fig. 2.
The location of the hypothetical release of anthrax spores and the distribution of the census tracts within the State of New Jersey. The hypothetical release location corresponds to the Hamilton Post Office, the place from which envelopes containing B. anthracis were mailed on September 18, 2001.
The specific MENTOR-2E application for this case study used the following eight steps (Fig. 1) for assessing exposures, dose, and potential infections.
Step 1
The ambient B. anthracis concentrations were calculated using the CALPUFF model,(44) which is a generalized nonsteady-state air quality model that simulates the transport, transformation, and dispersion processes of “puffs” of material from emission sources, and provides hourly average estimates of concentrations. CALPUFF has been adopted by the USEPA in its Guideline on Air Quality Models(65) as a preferred model for assessing long-range transport of pollutants, and on a case-by-case basis for certain near-field applications involving complex meteorological conditions. Meteorological data were retrieved from the National Oceanic and Atmospheric Administration (NOAA)(66) and converted into CALPUFF input format. In this study, it was assumed that the hourly averages provided by the CALPUFF model at a resolution of 250 m are adequate for characterizing B. anthracis exposures and doses, based on the general guidance on CALPUFF modeling.(67) In this study, a 1,000 × 1,000 grid at 250 m resolution was used.
In general, the selection of a particular dispersion model and modeling options depends heavily on the type of release and the response options, including the scale of attack, detection time, complexity of the geography, and population densities in the downwind regions. Some emergency events need to be modeled at high temporal and spatial resolutions. Examples include large releases of chemicals such as chlorine, where high-resolution concentration profiles are important in order to capture peak concentrations. However, in the case of B. anthracis releases, the main metric of concern is the total uptake of anthrax spores, so hourly averages were considered adequate. In general, a tradeoff can be made by considering model resolution, model complexity, and setup and simulation time.
In order to perform comparative evaluation, a simplified dispersion equation was also used(32) to characterize ambient concentrations of anthrax spores. A constant wind speed of 1.5 m/s was assumed blowing toward the northeast, at 45 degrees, corresponding to the general direction and speed of the wind during September 18, 2001, as part of this simplified application.
Step 2
Concentration estimates from CALPUFF at regularly spaced grid points at 250 m × 250 m resolution were aggregated at the level of a census tract; the average of concentrations at all grid points within a census tract was assumed to represent the concentration at the geometrical centroid of the census tract. The spatial averaging approach can sometimes result in artificial “discontinuities” in the census tract level concentrations, as is the case of Fig. 3, where two census tracts close to the plume show a zero concentration, whereas the surrounding census tracts show nonzero concentrations; however, this approach follows mass balance. The spatial averaging approach was used here because detailed allocation of concentrations can be computationally very demanding. It should be noted that for finer-scale spatial resolution (e.g., exposures studies focusing on census block level resolution or on regularly spaced grids), the CALPUFF model outputs can be aggregated or interpolated depending on the resolution. In case of the simplified dispersion modeling, the concentrations were estimated directly at the geometric centroid of each census tract.
Fig. 3.
Outdoor number concentrations of anthrax spores (1/m3) during 08:00–09:00 calculated by CALPUFF for a release of 100 g of B. anthracis over a period of one hour (left) (release scenario A) and 10 hours (right) (release scenario B), for the day of September 18, 2001, with the release starting at 08:00 hours (values less than 1 indicate the probability of finding one spore per m3).
Step 3
The attributes of the population under study were retrieved from the 2000 U.S. Census Survey.(47) Due to the variability of the urban population, in order to statistically reproduce essential demographic distributions of age, gender, housing type, and employment status, a rather large statistical sample of 500 “virtual individuals” was sampled for each of the 1944 census tracts under study.(37,55) In this study, the effect of commuting on population distributions was not considered, and the residential distributions were assumed to represent the population distributions throughout.
Step 4
A 24-hour activity diary for each “virtual individual” was selected from the CHAD diaries using the approach described earlier. In this study, the 113 microenvironments in the CHAD diaries were grouped into four categories: home, other indoor, outdoor, and vehicle.
Step 5
The outdoor concentration levels of B. anthracis, aggregated at the census-tract level, were used as inputs to the MENTOR modules for estimating microenvironmental concentrations. The estimation of B. anthracis levels in the various microenvironments in this study was based on the simple mass balance equation:
| (1) |
where Cin is the indoor B. anthracis concentration (number/volume), f p the penetration factor (dimensionless fraction, indicating the amount of B. anthracis that can penetrate indoors), A the air exchange rate between outdoor and indoor (1/time), Cout the outdoor B. anthracis concentration (number/volume), S the indoor B. anthracis source rate (mass/time), V the indoor volume (volume), and Fd the decay rate of B. anthracis indoors (via deposition, absorption to walls, etc.; 1/time). This equation was further simplified on the basis of the following assumptions: (1) steady-state approximation (which can provide a reasonable approximation for time durations of hours), (2) S = 0 (i.e., no indoor sources), thus resulting in Cin = A f p.Cout/(A + Fd). Furthermore, the anthrax spores were assumed to behave similar to fine particulate matter in the estimation of penetration and deposition. The resuspension of anthrax spores was assumed to be negligible at the modeling time scale in this study. However, it will be a critical factor in the decontamination process.
For each microenvironment corresponding to the activity event, the parameters in the mass balance equation were generated through random samples. The residential parameters include day- and nighttime air exchange rates, house volumes, and penetration and deposition factors; these were sampled once for each individual from the corresponding distributions (see Table 2 from Burke et al.55), and were held constant throughout the simulation. Parameters for other microenvironments, such as the air exchange rates in the vehicles, stores, etc., were sampled separately throughout the simulation from two distributions: one for the general nonresidential microenvironments(56) and one for vehicles References 37 and 68. In the simplified application, all virtual individuals were assumed to be outdoors, and anthrax spore concentration at the census tract centroid is assumed to be the exposure concentration.
Table II.
A Comparison of the Estimates of Probable Infections Derived Through Different Approaches for Exposure and Dose-Response Modeling
| Dose-Response Model |
||||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
| Scenario A; Craft et al. (simplifying assumption regarding breathing rates and location) | 166,750 | 119,910 | 719,160 | 161,930 | 172,230 | 141,310 |
| Scenario B; Craft et al. (simplifying assumption regarding breathing rates and location) | 140,660 | 109,940 | 525,920 | 140,910 | 145,650 | 124,870 |
| Scenario A; EPA EFH | 85,669 | 56,020 | 485,770 | 75,747 | 88,549 | 70,466 |
| Scenario B; EPA EFH | 71,954 | 52,118 | 355,560 | 70,046 | 74,994 | 61,247 |
| Scenario A; MENTOR-2E | 25,013 | 15,631 | 187,840 | 21,059 | 25,908 | 20,472 |
| Scenario B; MENTOR-2E | 19,747 | 12,950 | 129,420 | 17,416 | 20,247 | 16,458 |
| Scenario A/B; Craft et al. (simplifying assumptions regarding breathing rates, location, and plume dispersion) | 1,180,932 | 1,090,121 | 1,076,816 | 1,049,390 | 1,144,563 | 1,187,637 |
Note: Total number of probable infections in study area are shown for the two release scenarios (100 g of anthrax released over (A) 1 h and (B) 10 h). The columns correspond to the different dose-response models used, and the rows correspond to different exposure modeling approaches used.
Step 6
In this study, exposure to B. anthracis was assumed to occur solely through the inhalation of contaminated air. Thus, one of the main factors in B. anthracis exposure is a person’s inhalation rate. For each activity event of a virtual individual, inhalation rates were calculated using a combination of age- and gender-dependent ideal body mass, basal metabolic rate, and activity-specific energy expenditure and METs (Metabolic Equivalent of Tasks; described in detail in References 37 and 68). In the simplified application, the breathing rates were assumed to be constant across all individuals.
Step 7
The population-based lung dosimetry model employed by Georgopoulos et al.,(37) based on the HUMTRN model,(69) was used to calculate the delivered doses for individuals of both genders and of different ages. The calculated inhalation rates were combined with the corresponding microenvironmental concentrations to estimate the inhaled dose delivered to the lung for each virtual individual. Lung deposition of anthrax spores was calculated for three regions of the lungs: nasal-pharyngeal (NP), tracheobronchial (TB), and pulmonary (P), using empirical values of deposition fractions from the International Commission on Radiological Protection (ICRP) databases.(61,62) Total uptake of B. anthracis for each virtual individual was estimated for the day of September 18, 2001, from the sum of event-based doses inhaled by the individual during the exposure event sequence. In the simplified application, all inhaled anthrax spores were assumed to be initially deposited in the lung.
Step 8
Calculation of the probability of infection for each sampled individual in a census tract through the application of a B. anthracis dose-response model, and scaling that to the total population of the census tract. Several empirical dose-response models have been proposed for B. anthracis,(9,29,32,41) and the range of potential infections spans an order of magnitude, solely based on the choice of the dose-response relationship.(41) Six different dose-response models were used in this study to estimate the probability P(s, a) that an individual of age a would be infected from a dose of s spores. Here, the dose is assumed to represent the number of spores “initially deposited” into the lung, whereas the dose-response models are assumed to account for the subsequent, time-dependent cilial clearance.
, based on Craft et al.,(32) where c1 = 38,000 and c2 = 450, a1 = min (a, Acut), and Acut is the cut-off age of 80 years, beyond which the dose response is assumed to plateau with age.
P(s, a) = Φ (α + β · log(s) + γ · a + δ · a2), an age-dependent probit model based on Wein et al.(9) where Φ is the cdf of the normal distribution, and parameters α = −9.733; β = 1.025; γ = −0.016/year; and δ = 0.0006/year.(2)
P(s, a) = Φ (α + β · log(s)), an age-independent probit model from Wilkening,(41) where α = −2.6361, and β = 0.291, corresponding to an ID50 of 8,600 spores, and a probit slope of 0.67.
Another age-independent probit model from Wilkening,(41) of the form P(s, a) = Φ (α + βlog(s)), with α = 5.6263, and β = 0.621, corresponding to an ID50 of 8,600 spores, and a probit slope of 1.43.
, an exponential model that accounts for probability of spore destruction and spore germination in the lungs,(41, 70) where θ = 0.109/day, and λ = 8.8 × 10−8.
, an age-dependent logit model from Webb and Blaser,(29) where α and β are derived from age-dependent values for ID50 and ID10, classified into four age groups.
In principle, the uncertainties in the dose-response relationship modeling directly translate into the corresponding uncertainties in the number of probable infections. However, the age-dependence of dose-response and activity patterns warrant explicit characterization of these uncertainties.
The total number of potential infections in each census tract was obtained by summing the probabilities of infections across all the virtual individuals in that census tract, and scaling that value to the population of the census tract. The summation is possible because infections of different individuals represent independent events.
| (2) |
where Xj is the number of potential infections in census tract j, Nsample,j the number of sampled virtual individuals in census tract j, sij the dose of inhaled B. anthracis for sampled virtual individual i in census tract j, aij the age of sampled virtual individual i in census tract j, and Nj the total population of census tract j.
The impact of B. anthracis releases on sensitive population subgroups, such as the elderly, can also be studied easily using MENTOR-2E. Since the virtual individuals are described via several attributes that include age, gender, body weight, employment status, etc., the potential infections within subgroups of interest can be obtained by selecting the virtual individuals that belong to that subgroup. In general, for a subgroup M, the corresponding potential infections can be calculated through
| (3) |
5. RESULTS
Fig. 3 shows the average outdoor B. anthracis concentrations during 08:00–09:00 hours for both release scenarios. Fig. 4 shows the average outdoor concentrations during 14:00–15:00 hours; this corresponds to six and a half hours after the start of the B. anthracis release, and five and half hours after the end of the one-hour release. As the maps show, the pattern of B. anthracis concentrations varies significantly depending on the pattern of the release. Though the initial concentrations are high in the quick-release case (scenario A), high-airborne concentrations of B. anthracis remain in the slow-release case (scenario B).
Fig. 4.
Outdoor number concentrations of anthrax spores (1/m3) during 14:00–15:00 calculated by CALPUFF for release scenario A (left) and release scenario B (right). Values less than 1 indicate the probability of finding one spore per m3. In the case of scenario A (left), the release has already been completed and no spores were released during the previous five and a half hours.
Median and 95th percentile values for individual biological doses per census tract for the general population are shown in Fig. 5 (for release scenario A) and in Fig. 6 (for release scenario B). The MENTOR-2E system also allows focusing on susceptible subpopulations; the 95th percentile doses for the elderly (65 years and older) are shown for both release scenarios in Fig. 7.
Fig. 5.
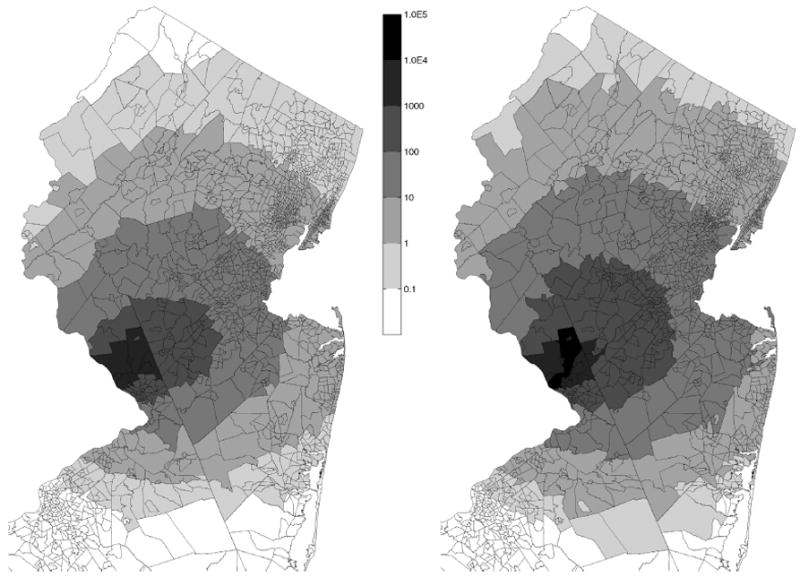
Estimated individual biological doses (spores/day) of B. anthracis in each census tract due to a release scenario A: median (left) and 95th percentiles (right) of doses for the simulated population in each census tract. Values less than 1 indicate the probability of inhaling one spore.
Fig. 6.
Estimated individual biological doses (spores/day) of B. anthracis in each census tract due to a release scenario B: median (left) and 95th percentiles (right) of doses for the simulated population in each census tract.
Fig. 7.
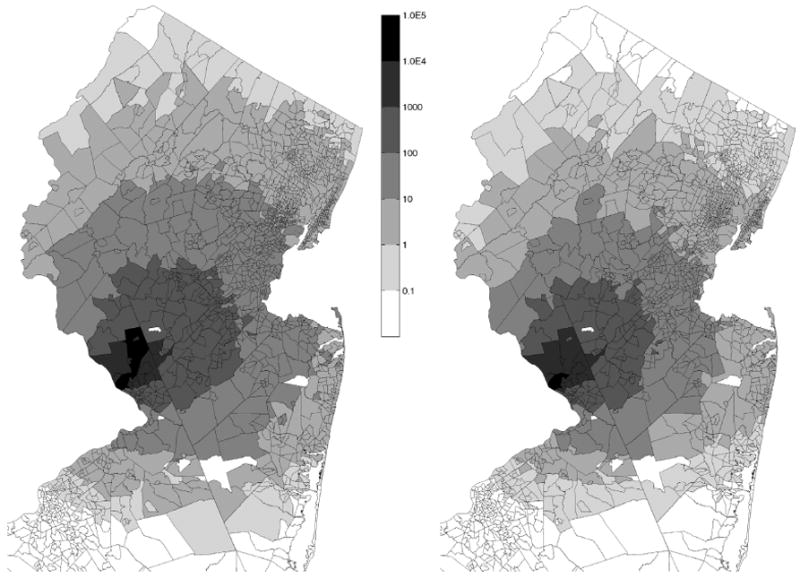
Estimated 95th percentile biological doses of B. anthracis for elderly individuals (over 65) in each census tract due to release scenarios A (left) and B (right).
5.1. Effect of Simplifying Approximations Used
In order to illustrate the differences in estimates arising out of differences in model assumptions, the MENTOR-2E results were compared with those obtained using two approximations: (a) the constant inhalation rate of 5 × 10−4 m3/s (1.944 m3/h) used by Craft et al.,(32) and (b) a reference constant inhalation rate of 0.78 m3/h from the USEPA exposure factors handbook (EFH).(71) The corresponding average breathing rate for the study population in the MENTOR-2E simulation was about 0.71 m3/h, which is approximately equal to the inhalation rate from the EFH. In employing the Craft et al. and EFH approximations, the outdoor concentration values were assumed to represent the personal exposure concentrations, and the corresponding doses and probabilities of infection were estimated.
The estimated percentiles of biological doses of B. anthracis (i.e., number of deposited anthrax spores) for the individuals in the entire study population are shown in Fig. 8 (release scenario A) and Fig. 9 (scenario B). The corresponding percentiles of probabilities of infection shown in Fig. 10 (release scenario A) and Fig. 11 (release scenario B). The total numbers of probable infections for the entire study region were also estimated, as shown in Table I. In both release scenarios, the infection estimates derived using the constant inhalation approaches are about five to ten times higher than those derived through MENTOR-2E. The dependence of the potential infection estimates on the choice of the dose response is also strong, as shown in Table II. The estimates span an order of magnitude, solely based on the choice of the dose-response model. This, coupled with the order of magnitude change in estimates based on inhalation rates and microenvironmental calculations, results in a range of two orders of magnitude for the estimates. Further approximations, such as constant wind speed, are likely to overestimate the impacts in some areas, while underestimating the impacts in others. In fact, as shown in Table II, the estimates using a constant wind speed and simplified dispersion equation employed by Craft et al.(32) are substantially higher. In fact, the corresponding doses are high enough that the differences due to various dose-response formulations become negligible. As noted earlier, such overestimations and underestimations can have significant adverse impact on response to emergency events.
Fig. 8.
Comparison of individual biological doses of B. anthracis estimated via different approaches. Percentiles of individual doses are shown for the entire study population for release scenario A (100 grams B. anthracis released over one hour).
Fig. 9.
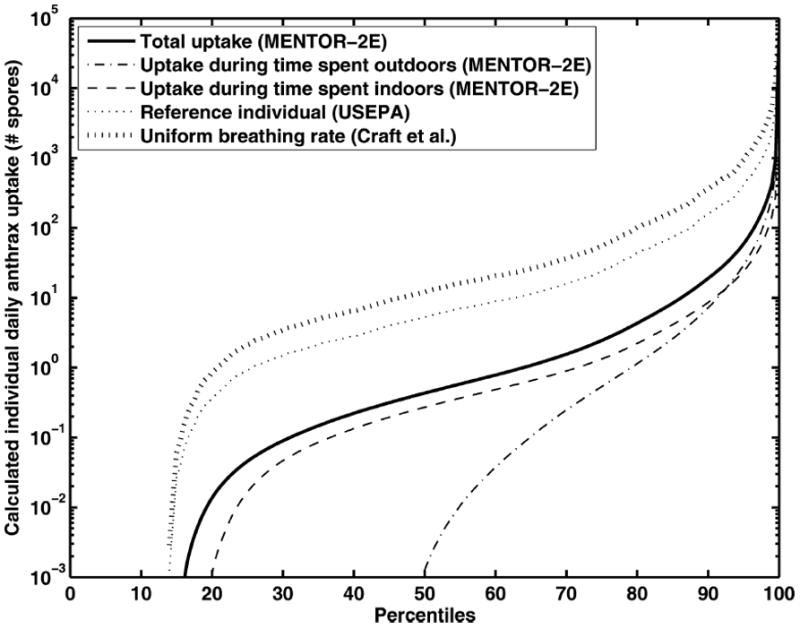
Comparison of individual biological doses of B. anthracis estimated via different approaches. Percentiles of individual doses are shown for the entire study population for release scenario B (100 grams B. anthracis released over 10 hours).
Fig. 10.
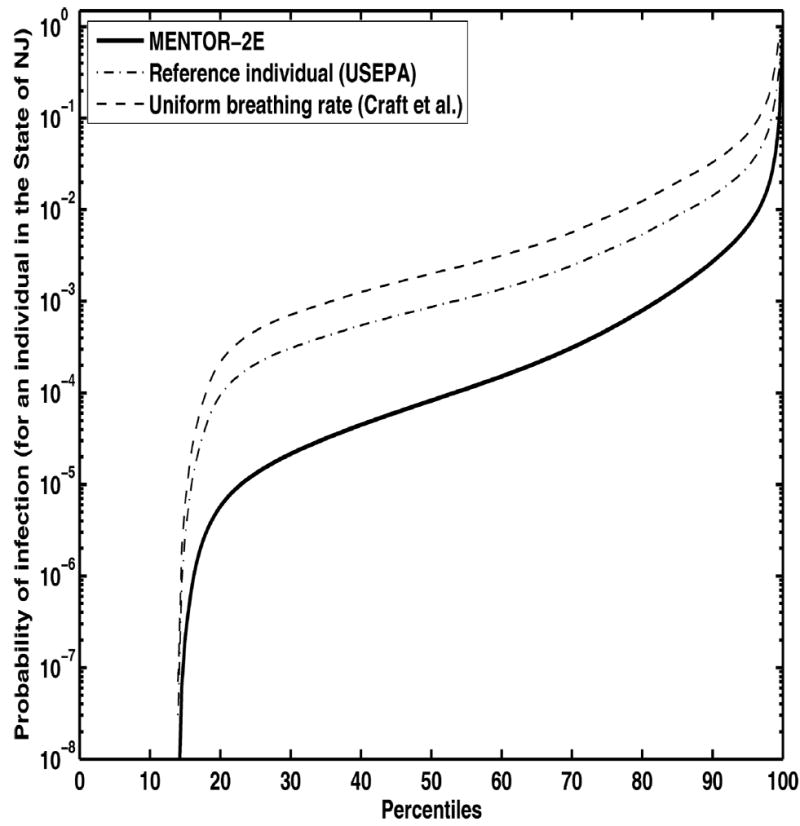
Comparison of probabilities of anthrax infection estimated via different approaches. Percentiles of probabilities of infection are shown for the entire study population for release scenario A (100 grams B. anthracis released over one hour).
Fig. 11.
Comparison of probabilities of anthrax infection estimated via different approaches. Percentiles of probabilities of infection are shown for the entire study population for release scenario B (100 grams B. anthracis released over 10 hours).
Table I.
Sources and Values of Parameters Used in the MENTOR-EE Application for Simulating Impact of Hypothetic Releases of Anthrax Spores
| Parameter | Value and/or Source |
|---|---|
| Number of anthrax spores released (hypothetical releases over 1 h and 10 h) | 1015 [32] |
| Start time for the simulation (coinciding with mailing time of anthrax letters) | 9/18/2001 08:00 h [2] |
| Location of the release (coordinates calculated using information from Reference 2) | Lat: 40.277; Lon: −74.813 [2] |
| Variable wind speed (obtained for Mercer County airport for 9/18/2001); the simplified dispersion case used a constant 1.5 m/s blowing toward north-east 0–3 m/s (Reference 66) | 0–3 m/s [66] |
| CALPUFF grid resolution (general guidance from Reference 67) | 250 m × 250 m [67] |
| Number of census tracts modeled (for application to the State of New Jersey) | 1944 [47] |
| Census tract level population distribution (age; gender; occupation) | [47] |
| Virtual individuals per census tract | 500 [37] |
| Activity patterns/metabolic equivalents (consolidated human activity database) | [49] |
| Distributions of parameters for residential microenvironments (air exchange rates; housing volumes; penetration and deposition factors; a total of 10 parameters) | Table II in [55] |
| Distributions of air exchange rates for general nonresidential microenvironments | [56] |
| Distributions of air exchange rates for vehicle microenvironments | [57] |
| Distributions for body weights (function of age and gender) | [37] |
| Empirical factors for deposition of anthrax spores in the lung (“uptake”) | [37,61,62,69] |
| Parameters for empirical dose-response models (details in Step 8 in Section 4) | [9,29,32,41] |
Note: Further details on the exposure parameters are available in Reference 68.
6. DISCUSSION AND CONCLUSIONS
This study presents a demonstration application of the integrated MENTOR-2E system for assessing the impact of airborne B. anthracis releases, with a main focus on mechanistically consistent linkage of modules in the source-to-dose-to-effect sequence. Future work can focus on characterization of commuting patterns, population gathering at different events, and custom response scenarios, such as “shelter-in-place” versus evacuation.
The difference in dose estimates between the release scenarios, shown in Fig. 5 (release scenario A) and in Fig. 6 (release scenario B), is solely due to the difference in the concentrations profiles, which in this case are influenced by the B. anthracis release patterns. However, variations in meteorological conditions can result in significantly different concentration profiles. Similarly, different forms of release, for example, releases from multiple locations, either simultaneously, or in a staggered manner, influence the concentration profiles. Therefore, for emergency response modeling, improved estimates of concentration profiles are very important. It must be noted that the estimates of infections are “probalistic” expected values, and do not take into account the time for incubation between the exposure and the actual infection; therefore, the results do not reflect the estimates of possible number of potential hospital admissions within days of the B. anthracis release. Furthermore, the exposures modeled here included only those occurring during the day of September 18, 2001, and subsequent exposures were assumed to be negligible as the B. anthracis plume expands and becomes significantly diluted.
The simplifying assumptions have a strong impact on estimates of probable infections, as shown in Table II. In both release scenarios considered, the same concentration profiles were used for the three exposure and dose calculation approaches (Table II, Rows 2–7). Furthermore, even higher estimates of infections are possible if simplifying assumptions are used for calculating the concentration profiles (e.g., Table II, Row 8, which shows results for the case where simplified dispersion modeling and simplified population characteristics are assumed, both used by Craft et al.(32)).
Wilkening(41) discusses the uncertainty associated with the selection of the dose-response model for inhalation anthrax, and concludes that a factor of 10 uncertainty exists based on the choice of the dose-response model. However, as shown in Fig. 10 and Fig. 11, the impact of simplifying assumptions with respect to microenvironments and activity patterns spans about a factor of 7–10. Therefore, all components of the source-to-dose-to-effect modeling, including dispersion, exposures, and dose response, need to be improved in order to use such models in planning, intervention design, and training.
Integrated modeling applications such as the one presented here need to be considered in the context of multiple sources and types of uncertainties, including natural uncertainty/variability (atmospheric turbulence, population demographics and variability, etc.), model/structural uncertainties (selection of the population units, alternative dose-response models, etc.), input/parameter uncertainties (estimates of source strength, wind speed, direction, etc.), and evaluation data uncertainties (data used in parameter estimation for the underlying models). This study focused on the population variability model uncertainty in exposure calculation approaches, and model uncertainty in dose-response relationships. However, there is a need for further systematic uncertainty analyses focusing on identifying major uncertainties in emergency response planning, and characterizing the contributions of these. Such characterization will further improve the confidence in simulation models used for planning, training, and decision support.
Ongoing work focuses on improving the performance of MENTOR-2E by performing some of the modeling steps beforehand and using those outputs toward achieving a real-time impact assessment and real-time evaluation of alternative response strategies. The approach as presented here can be easily parallelized (e.g., representative activity pattern generation, sampling of microenvironmental factors, housing characteristics, physiological parameters, etc., can be performed beforehand, and the “concentration profiles” can then be used to calculate the exposures). Furthermore, since each administrative unit is modeled independently (e.g., each census tract level), they can be run in parallel on a distributed cluster of computers.
A comprehensive planning scheme for detecting and responding to a bioterrorism event should consist of effective and efficient monitoring, ability to characterize the release sources based on detected values, and ability to accurately estimate the potential impact, not only in terms of the overall magnitude, but also in the spatial distribution. It should provide means to assess alternative response strategies, e.g., flexibility to define custom activity patterns, modification of population distributions and activity patterns (e.g., during population evacuation from affected areas), as it can be used for training of emergency responders. Furthermore, such a system should be tuned for near real-time application for maximum benefit.
Acknowledgments
Support for this work has been provided by the USEPA-funded Center for Exposure and Risk Modeling (CERM) under Cooperative Agreement CR-83162501, and the Environmental Bioinformatics and Computational Toxicology Center (EBCTC) under STAR Grant GAD R 832721-010. Additional funding was provided by a subcontract from Quantum Leap Innovations as part of a project for the U.S. Department of Defense (DOD), Office of Naval Research.
References
- 1.Georgopoulos PG, Lioy PJ. From theoretical aspects of human exposure and dose assessment to computational model implementation: The modeling environment for total risk studies (MENTOR) Journal of Toxicology and Environmental Health — Part B, Critical Reviews. 2006;9(6):457–483. doi: 10.1080/10937400600755929. [DOI] [PubMed] [Google Scholar]
- 2.Jernigan DB, Raghunathan PL, Bell BP, Brechner R, Bresnitz EA, Butler JC, Cetron M, Cohen M, Doyle T, Fischer M, Greene C, Griffith KS, Guarner J, Hadler JL, Hayslett JA, Meyer R, Petersen LR, Phillips M, Pinner R, Popovic T, Quinn CP, Reefhuis J, Reissman D, Rosenstein N, Schuchat A, Shieh WJ, Siegal L, Swerdlow DL, Tenover FC, Traeger M, Ward JW, Weisfuse I, Wiersma S, Yeskey K, Zaki S, Ashford DA, Perkins BA, Ostroff S, Hughes J, Fleming D, Koplan JP, Gerberding JL. Investigation of bioterrorism-related anthrax, United States, 2001: Epidemiologic findings. Emerging Infectious Diseases. 2002;8(10):1019–1028. doi: 10.3201/eid0810.020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jernigan JA, Stephens DS, Ashford DA, Omenaca C, Topiel MS, Galbraith M, Tapper M, Fisk TL, Zaki S, Popovic T, Meyer RF, Quinn CP, Harper SA, Fridkin SK, Sejvar JJ, Shepard CW, McConnell M, Guarner J, Shieh WJ, Malecki JM, Gerberding JL, Hughes JM, Perkins BA. Bioterrorism-related inhalational anthrax: The first 10 cases reported in the United States. Emerging Infectious Diseases. 2001;7(6):933–944. doi: 10.3201/eid0706.010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webb GF. Being prepared: Modeling the response to an anthrax attack. Annals of Internal Medicine. 2005;142(8):667–668. doi: 10.7326/0003-4819-142-8-200504190-00016. [DOI] [PubMed] [Google Scholar]
- 5.Webb GF. A silent bomb: The risk of anthrax as a weapon of mass destruction. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(8):4355–4356. doi: 10.1073/pnas.0830963100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyams KC, Murphy FM, Wessely S. Responding to chemical, biological, or nuclear terrorism: The indirect and long-term health effects may present the greatest challenge. Journal of Health Politics, Policy and Law. 2002;27(2):273–291. doi: 10.1215/03616878-27-2-273. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Health Aspects of Chemical and Biological Weapons—Report of a WHO Group of Consultants. Geneva, Switzerland: World Health Organization; 1970. [Google Scholar]
- 8.OTA, U.S. Congress, Office of Technology Assessment. Technologies Underlying Weapons of Mass Destruction. OTA-BP-ISC-115 1993 [Google Scholar]
- 9.Wein LM, Craft DL, Kaplan EH. Emergency response to an anthrax attack. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(7):4346–4351. doi: 10.1073/pnas.0636861100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufmann AF, Meltzer MI, Schmid GP. The economic impact of a bioterrorist attack: Are prevention and postattack intervention programs justifiable? Emerging Infectious Diseases. 1997;3(2):83–94. doi: 10.3201/eid0302.970201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Royal Society. Measures for Controlling the Threat from Biological Weapons. London, UK: Royal Society; 2000. [Google Scholar]
- 12.Alexander DA, Klein S. Biochemical terrorism: Too awful to contemplate, too serious to ignore: Subjective literature review. British Journal of Psychiatry. 2003;183(6):491–497. doi: 10.1192/bjp.183.6.491. [DOI] [PubMed] [Google Scholar]
- 13.Grabenstein JD. Anthrax vaccine: A review. Immunology and Allergy Clinics of North America. 2003;23(4):713–730. doi: 10.1016/s0889-8561(03)00093-6. [DOI] [PubMed] [Google Scholar]
- 14.Vietri NJ, Purcell BK, Lawler JV, Leffel EK, Rico P, Gamble CS, Twenhafel NA, Ivins BE, Heine HS, Sheeler R, Wright ME, Friedlander AM. Short-course postexposure antibiotic prophylaxis combined with vaccination protects against experimental inhalational anthrax. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(20):7813–7816. doi: 10.1073/pnas.0602748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tierney BC, Martin SW, Franzke LH, Marano N, Reissman DB, Louchart RD, Goff JA, Rosenstein NE, Sever JL, McNeil MM. Serious adverse events among participants in the Centers for Disease Control and Prevention’s Anthrax Vaccine and antimicrobial availability program for persons at risk for bioterrorism-related inhalational anthrax. Clinical Infectious Diseases. 2003;37(7):905–911. doi: 10.1086/377738. [DOI] [PubMed] [Google Scholar]
- 16.Sever JL, Brenner AI, Gale AD, Lyle JM, Moulton LH, West DJ. Safety of anthrax vaccine: A review by the Anthrax Vaccine Expert Committee (AVEC) of adverse events reported to the Vaccine Adverse Event Reporting System (VAERS) Pharmacoepidemiology and Drug Safety. 2002;11(3):189–202. doi: 10.1002/pds.712. [DOI] [PubMed] [Google Scholar]
- 17.MMWR. Notice to readers: Evaluation of postexposure antibiotic prophylaxis to prevent anthrax. Morbidity and Mortality Weekly Report. 2002;51(3):59. [Google Scholar]
- 18.Shepard CW, Soriano-Gabarro M, Zell ER, Hayslett J, Lukacs S, Goldstein S, Factor S, Jones J, Ridzon R, Williams I, Rosenstein N. Antimicrobial postexposure prophylaxis for anthrax: Adverse events and adherence. Emerging Infectious Diseases. 2002;8(10):1124–1132. doi: 10.3201/eid0810.020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noji EK. Disasters: Introduction and state of the art. Epidemiologic Reviews. 2005;27(1):3–8. doi: 10.1093/epirev/mxi007. [DOI] [PubMed] [Google Scholar]
- 20.Noji EK. Public health issues in disasters. Critical Care Medicine. 2005;33(Suppl 2):S29–33. doi: 10.1097/01.ccm.0000151064.98207.9c. [DOI] [PubMed] [Google Scholar]
- 21.Holty JEC, Bravata DM, Liu H, Olshen RA, Mc-Donald KM, Owens DK. Systematic review: A century of inhalational anthrax cases from 1900 to 2005. Annals of Internal Medicine. 2006;144(4):270–280. doi: 10.7326/0003-4819-144-4-200602210-00009. [DOI] [PubMed] [Google Scholar]
- 22.Bravata DM, Zaric GS, Holty JEC, Brandeau ML, Wilhelm ER, McDonald KM, Owens DK. Reducing mortality from anthrax bioterrorism: Strategies for stockpiling and dispensing medical and pharmaceutical supplies. Biosecurity and Bioterrorism-Biodefense Strategy Practice and Science. 2006;4(3):244–262. doi: 10.1089/bsp.2006.4.244. [DOI] [PubMed] [Google Scholar]
- 23.ASTHO. Association of State and Territorial Health Officials; Washington, DC: 2004. Issue Brief: Role of State Public Health Agencies in BioWatch. Available at http://www.astho.org/pubs/RoleofSHAinBioWatchFinal.pdf. [Google Scholar]
- 24.U. DHS. Fact Sheet: Biowatch—Early detection, early response. U S Department of Homeland Security, 2005. 2005 Available at http://www.milnet.com/wh/DoHS/BioWatchFactSheetFINAL.pdf.
- 25.Buckeridge DL, Owens DK, Switzer P, Frank J, Musen MA. Evaluating detection of an inhalational anthrax outbreak. Emerging Infectious Diseases. 2006;12(12):1942–1949. doi: 10.3201/eid1212.060331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nordin JD, Goodman MJ, Kulldorff M, Ritzwoller DP, Abrams AM, Kleinman K, Levitt MJ, Donahue J, Platt R. Simulated anthrax attacks and syndromic surveillance. Emerging Infectious Diseases. 2005;11(9):1394–1398. doi: 10.3201/eid1109.050223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tegnell A, Van Loock F, Baka A, Wallyn S, Hendriks J, Werner A, Gouvras G. Development of a matrix to evaluate the threat of biological agents used for bioterrorism. Cellular and Molecular Life Sciences. 2006;63(19–20):2223–2228. doi: 10.1007/s00018-006-6310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reshetin VP, Regens JL. Simulation modeling of anthrax spore dispersion in a bioterrorism incident. Risk Analysis. 2003;23(6):1135–1145. doi: 10.1111/j.0272-4332.2003.00387.x. [DOI] [PubMed] [Google Scholar]
- 29.Webb GF, Blaser MJ. Mailborne transmission of anthrax: Modeling and implications. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(10):7027–7032. doi: 10.1073/pnas.102691499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho J, Duncan S. Estimating aerosol hazards from an anthrax letter. Journal of Aerosol Science. 2005;36(5–6):701–719. [Google Scholar]
- 31.Fowler RA, Sanders GD, Bravata DM, Nouri B, Gastwirth JM, Peterson D, Broker AG, Garber AM, Owens DK. Cost-effectiveness of defending against bioterrorism: A comparison of vaccination and antibiotic prophylaxis against anthrax. Annals of Internal Medicine. 2005;142(8):601–610. doi: 10.7326/0003-4819-142-8-200504190-00008. [DOI] [PubMed] [Google Scholar]
- 32.Craft DL, Wein LM, Wilkins AH. Analyzing bioterror response logistics: The case of anthrax. Management Science. 2005;51(5):679–694. [Google Scholar]
- 33.Chen LC, Carley KM, Fridsma D, Kaminsky B, Yahja A. Model alignment of anthrax attack simulations. Decision Support Systems. 2006;41(3):654–668. [Google Scholar]
- 34.Carley KM, Fridsma DB, Casman E, Yahja A, Altman N, Chen LC, Kaminsky B, Nave D. BioWar: Scalable agent-based model of bioattacks. IEEE Transactions on Systems Man and Cybernetics Part A—Systems and Humans. 2006;36(2):252–265. [Google Scholar]
- 35.Lioy PJ. Exposure analysis and assessment for low-risk cancer agents. International Journal of Epidemiology. 1990;19(Suppl 1):S53–61. doi: 10.1093/ije/19.supplement_1.s53. [DOI] [PubMed] [Google Scholar]
- 36.Lioy PJ. Exposure analysis and assessment in the 21st century. Inhalation Toxicology. 1999;11(6–7):623–636. doi: 10.1080/089583799197032. [DOI] [PubMed] [Google Scholar]
- 37.Georgopoulos PG, Wang SW, Vyas VM, Sun Q, Burke J, Vedantham R, McCurdy T, Özkaynak H. A source-to-dose assessment of population exposures to fine pm and ozone in Philadelphia, PA, during a summer 1999 episode. Journal of Exposure Analysis and Environmental Epidemiology. 2005;15:439–457. doi: 10.1038/sj.jea.7500422. [DOI] [PubMed] [Google Scholar]
- 38.Georgopoulos PG, Wang SW, Yang YC, Xue J, Zartanian VG, McCurdy T, Ozkaynak H. Biologically-based modeling of multimedia, multipathway, multiroute population exposures to arsenic. Journal of Exposure Science and Environmental Epidemiology. 2008 doi: 10.1038/sj.jes.7500637. Advance Online Publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pielke RA. Mesoscale Meteorological Modeling. 2. San Diego: Academic Press; 2002. [Google Scholar]
- 40.NRC. National Research Council (U S) Committee on the Atmospheric Dispersion of Hazardous Material Releases Tracking and Predicting the Atmospheric Dispersion of Hazardous Material Releases: Implications for Homeland Security. Washington, DC: National Academies Press; 2003. [Google Scholar]
- 41.Wilkening DA. Sverdlovsk revisited: Modeling human inhalation anthrax. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(20):7589–7594. doi: 10.1073/pnas.0509551103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vyas VM, Christakos G. Spatiotemporal analysis and mapping of sulfate deposition data over eastern USA. Atmospheric Environment. 1997;31(21):3623–3633. [Google Scholar]
- 43.Serre M, Christakos G. Modern geostatistics: Computational BME in the light of uncertain physical knowledge—The equus beds study. Stochastic Environmental Research and Risk Assessment. 1999;13(1):1–26. [Google Scholar]
- 44.Scire JS, Strimaitis DG, Yamartino RJ. A User’s Guide for the CALPUFF Dispersion Model. Concord, MA: Earth Tech; 2000. [Google Scholar]
- 45.DTRA. Hazard Prediction and Assessment Capability (HPAC) User Guide Version 4.0.3. Prepared for Defense Threat Reduction Agency (DTRA) by Science Applications International Corporation; San Diego, CA: 2003. HPAC-UGUIDE-01-RBC1. [Google Scholar]
- 46.Walko RL, Tremback CJ, Bell MJ. ASTER Division, Mission Research Corporation. Fort Collins, CO: 2001. HYPACT: Hybrid Particle and Concentration Transport Model Version 1.2.0 User’s Guide. [Google Scholar]
- 47.USCB. U.S. Census Bureau Population Information Website. US Census Bureau; 2007. Available at http://www.census.gov/ [Google Scholar]
- 48.Glen G. User’s Guide for the Apex3 Model. ManTech Environmental Technology Inc. (prepared for U.S. EPA), Research Triangle Park; NC: 2002. [Google Scholar]
- 49.McCurdy T, Glen G, Smith L, Lakkadi Y. The national exposure research laboratory’s consolidated human activity database. Journal of Exposure Analysis and Environmental Epidemiology. 2000;10(6 Pt 1):566–578. doi: 10.1038/sj.jea.7500114. [DOI] [PubMed] [Google Scholar]
- 50.Graham SE, McCurdy T. Developing meaningful cohorts for human exposure models. Journal of Exposure Analysis and Environmental Epidemiology. 2004;14(1):23–43. doi: 10.1038/sj.jea.7500293. [DOI] [PubMed] [Google Scholar]
- 51.Sorensen JH, Shumpert BL, Vogt BM. Planning for protective action decision making: Evacuate or shelter-in-place. Journal of Hazardous Materials. 2004;109(1–3):1–11. doi: 10.1016/j.jhazmat.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Liu DL, Nazaroff WW. Modeling pollutant penetration across building envelopes. Atmospheric Environment. 2001;35(26):4451–4462. [Google Scholar]
- 53.Liao CM, Huang SJ, Yu H. Size-dependent particulate matter indoor/outdoor relationships for a wind-induced naturally ventilated airspace. Building and Environment. 2004;39(4):411–420. [Google Scholar]
- 54.Asmi AJ, Pirjola LH, Kulmala M. A sectional aerosol model for submicron particles in indoor air. Scandinavian Journal of Work Environment and Health. 2004;30:63–72. [PubMed] [Google Scholar]
- 55.Burke JM, Zufall MJ, Ozkaynak H. A population exposure model for particulate matter: Case study results for PM2.5 in Philadelphia, PA. Journal of Exposure Analysis and Environmental Epidemiology. 2001;11(6):470–489. doi: 10.1038/sj.jea.7500188. [DOI] [PubMed] [Google Scholar]
- 56.Turk BH, Grimsrud DT, Brown JT, Geisling-Sobotka KL, Harrison J, Prill RJ. Commercial building ventilation rates and particle concentrations. ASHRAE Transactions. 1989;95(Part 1):422–433. [Google Scholar]
- 57.Hayes SR. Use of an indoor air quality model (IAQM) to estimate indoor ozone levels. Journal of the Air and Waste Management Association. 1991;41:161–170. doi: 10.1080/10473289.1991.10466833. [DOI] [PubMed] [Google Scholar]
- 58.Allan M, Richardson GM. Probability density functions describing 24-hour inhalation rates for use in human health risk assessments. Human and Ecological Risk Assessment. 1998;4(2):379–408. [Google Scholar]
- 59.Brochu P, Ducre-Robitaille JF, Brodeur J. Physiological daily inhalation rates for free-living individuals aged 1 month to 96 years, using data from doubly labeled water measurements: A proposal for air quality criteria, standard calculations and health risk assessment. Human and Ecological Risk Assessment. 2006;12(4):675–701. [Google Scholar]
- 60.Brochu P, Ducre-Robitaille JF, Brodeur J. Physiological daily inhalation rates for free-living individuals aged 2.6 months to 96 years based on doubly labeled water measurements: Comparison with time-activity-ventilation and metabolic energy conversion estimates. Human and Ecological Risk Assessment. 2006;12(4):736–761. [Google Scholar]
- 61.ICRP. Publication 66 Human Respiratory Tract Model for Radiological Protection. Vol. 24. Oxford, UK: Annals of the ICRP; 1994. pp. 1–3. [PubMed] [Google Scholar]
- 62.ICRP. Publication 72 Age-Dependent Doses to the Members of the Public from Intake of Radionuclides: Part 5, Compilation of Ingestion and Inhalation Coefficients. Vol. 26. Oxford, UK: Annals of the ICRP; 1996. p. 1. [DOI] [PubMed] [Google Scholar]
- 63.Zartarian VG, Ozkaynak H, Burke JM, Zufall MJ, Rigas ML, Furtaw EJ. A modeling framework for estimating children’s residential exposure and dose to chlorpyrifos via dermal residue contact and nondietary ingestion. Environmental Health Perspectives. 2000;108(6):505–514. doi: 10.1289/ehp.00108505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chapman SJ. MATLAB Programming for Engineers. 2. Pacific Grove, CA: Brooks/Cole-Thomson Learning; 2002. [Google Scholar]
- 65.USEPA. EPA Federal Register: Guideline on Air Quality Models. Appendix W (July 2003) of 40 CFR Part 51 2003 [Google Scholar]
- 66.NOAA. National Climatic Data Center [November 25 2007] 2007 Available at http://www.ncdc.noaa.gov/oa/ncdc.html.
- 67.TRC Companies, Inc. CALPUFF Frequently Asked Questions (FAQs) TRC, Inc. [November 25 2007]; 2007. Available at http://www.src.com/calpuff/FAQ-questions.htm. [Google Scholar]
- 68.Georgopoulos PG, Wang SW, Vyas VM, Sun Q, Chandrasekar A, Shade P, Vedantham R, Burke J, McCurdy T, Özkaynak H. A Source-to-Dose Assessment of Population Exposures to Fine PM, Ozone and Air Toxics in Philadelphia, PA, During a 1999 Summer Episode. A Case Study Employing MENTOR/SHEDS-1A Prepared for the US Environmental Protection Agency Technical Report CERM:2005-02. 2005 Available at http://ccl.rutgers.edu/reports/cerm/CCL-CERM_Report-2005-02_philadelphia.pdf.
- 69.Gallegos AF, Wenzel WJ. HUMTRN: Documentation and Verification for an ICRP-Based Age- and Sex-Specific Human Simulation Model for Radionuclide Dose Assessment. Los Alamos, NM: Los Alamos National Laboratory; 1984. [Google Scholar]
- 70.Brookmeyer R, Johnson E, Barry S. Modelling the incubation period of anthrax. Statistics in Medicine. 2005;24(4):531–542. doi: 10.1002/sim.2033. [DOI] [PubMed] [Google Scholar]
- 71.USEPA. Exposure Factors Handbook. Cincinnati OH: US EPA, National Center for Environmental Assessment; 1997. [Google Scholar]


