Abstract
In the present study, galago brains were sectioned in the coronal, sagittal or horizontal planes, and sections were processed with several different histochemical and immunohistochemical procedures to reveal the architectonic characteristics of the various cortical areas. The histochemical methods used included the traditional Nissl, cytochrome oxidase and myelin stains, as well as a zinc stain, which reveals free ionic zinc in the axon terminals of neurons. Immunohistochemical methods include parvalbumin (PV) and calbindin (CB), both calcium-binding proteins, and the vesicle glutamate transporter 2 (VGluT2). These different procedures revealed similar boundaries between areas, which suggests that functionally relevant borders were being detected. These results allowed a more precise demarcation of previously identified areas. As thalamocortical terminations lack free ionic zinc, primary cortical areas were most clearly revealed by the zinc stain, due to the poor zinc staining of layer 4. Area 17 was especially prominent, as the broad layer 4 was nearly free of zinc stain. However, this feature was less pronounced in the primary auditory and somatosensory cortex. As VGluT2 is expressed in thalamocortical terminations, layer 4 of primary sensory areas was darkly stained for VGluT2. Primary motor cortex had reduced VGluT2 staining, and increased zinc-enriched terminations in the poorly developed granular layer 4 compared to the adjacent primary somatosensory area. The middle temporal visual (MT) showed increased PV and VGluT2 staining compared to the surrounding cortical areas. The resulting architectonic maps of cortical areas in galagos can usefully guide future studies of cortical organizations and functions.
Keywords: primate, cortical areas, visual cortex, motor cortex, somatosensory cortex, auditory cortex, prosimian
Introduction
Galagos are prosimian primates that represent a branch of the primate evolution that gave rise to anthropoids about 50 million years ago (Simons and Rasmussen, 1994; Martin, 2004). The brain weights of prosimian primates are smaller relative to body weights than in anthropoid primates (Jerison, 1979; Stephan et al., 1981; Preuss and Goldman-Rakic, 1991a; 1991c). They have also retained many anatomical features of early primates, whereas these anatomical features have been modified during the course of anthropoid evolution (Martin, 1990; Fleagle, 1999). As such, it can be assumed that features of neocortical organization in early primates will be conserved to a greater degree in prosimians than in anthropoids (Preuss and Goldman-Rakic, 1991a). Here, we describe the architectonic subdivisions of neocortex in the galago, so as to establish a reliable areal cortical map that can be used to guide functional studies, and can also be compared to cortical maps of anthropoid primates, such as macaque monkeys, to identify features that are similar or different.
Galagos have often been used in studies of visual, auditory, somatosensory and motor cortex (e.g. Allman et al., 1973; Raczkowski and Diamond, 1978; Symonds and Kaas, 1978; Wall et al., 1982; Weller and Kaas, 1982; Xu et al., 2004). The retinotopic organization of V1 in galagos is similar to monkeys, and mammals in general, containing a complete representation of the visual field (Rosa et al., 1997), with the lower visual hemifield represented dorsally and upper visual hemifield represented ventrally (Weller and Kaas, 1982; Rosa et al., 1997). The temporal lobe of galagos contains several well differentiated areas (Zilles et al., 1979) and share several common areas present in the macaque monkey, including auditory associated, multisensory and visual associated areas (Preuss and Goldman-Rakic, 1991a). Area 3b(S1), the primary somatosensory area of galagos, contains a single systematic representation of the cutaneous body surface (Carlson and Welt, 1980; Sur et al., 1980; Carlson and Welt, 1981), receives topographically organized input from the ventroposterior nucleus, and projects to the ventrally located secondary somatosensory area (S2)(Kaas, 1982; Burton and Carlson, 1986). Area 3a lies rostral to area 3b(S1) and is likely homologous to area 3a of monkeys that contains a systematic representation (Kaas et al., 1979) and is responsive to muscle spindle receptor activation (Krubitzer and Kaas, 1990b).
There are several cytoarchitectural cortical maps of galagos to date, mostly based on the traditional Nissl stain for cell bodies, or myelin (von Bonin, 1942; von Bonin, 1945; von Bonin and Bailey, 1961; Kanagasuntheram et al., 1966; Zilles et al., 1979; Preuss and Goldman-Rakic, 1991a). As the repertoire of staining procedures available has increased, an updated cortical map using a battery of staining preparations to characterize the cortical areas in the galago is now timely. For the present study, in addition to the traditional Nissl, myelin and cytochrome oxidase stains, we use another histochemical preparation, the zinc stain (Danscher, 1981; Danscher, 1982; Danscher and Stoltenberg, 2005) that has been useful in revealing areal borders in the neocortex. This histochemical procedure reveals unbound ionic zinc in the synaptic vesicles of cortical neuron terminations and synaptic clefts. Thalamocortical terminations do not contain free ionic zinc and are as such distinguished from corticocortical terminations. Primary sensory areas are distinguished from secondary sensory areas due to the lack of zinc staining in layer 4 of primary sensory areas, where dense thalamocortical inputs terminate (e.g. Valente et al., 2002). In addition, three immunohistochemical stains for parvalbumin (PV) and vesicle glutamate transporter 2 (VGluT2) were employed. The PV antibody reveals a subset of GABAergic, nonpyramidal cells, such as basket and double bouquet interneurons (Celio, 1986; Conde et al., 1996; DeFelipe, 1997; Hof et al., 1999), that contain the calcium-binding PV protein. More importantly for the present study, PV is a useful marker that labels a subset of afferent GABA-ergic cortical terminals from sensory thalami nuclei (Van Brederode et al., 1990; DeFelipe and Jones, 1991; Hackett et al., 1998; de Venecia et al., 1998; Latawiec et al., 2000; Wong and Kaas, 2008; Wong and Kaas, 2009a, b). VGluT2 preparations reveal a subset of glutamatergic thalamocortical and not corticocortical terminations (Fujiyama et al., 2001; Kaneko and Fujiyama, 2002; Wong and Kaas, 2008; Hackett and de la Mothe, 2009; Wong and Kaas, 2009a, b).
The use of a battery of staining procedures, we are able to provide more detailed descriptions of the architectonic subdivisions present in the neocortex of galagos. When borders in similar locations are detected across adjacent series of sections stained with different histological and immunohistochemical stains, comprehensive cortical maps with rigorous areal borders are more reliably established.
Materials and Methods
The cortical architecture was studied in a total of six adult Otolemur garnetti. All experimental procedures were approved by the Vanderbilt Institutional Animal Care and Use Committee and followed the guidelines published by the National Institute of Health.
Tissue Preparation
The galagos were given a lethal dose of sodium pentobarbital (100mg/kg). For visualizing synaptic zinc in the cortex, galagos were given 200mg/kg body weight of sodium sulfide with 1cc of heparin in 0.1M phosphate buffer (PB), pH 7.2, intravenously. Perfusion was carried out transcardially with phosphate-buffered saline (PBS), pH 7.2, followed by 4% paraformaldehyde in 0.1M PBS and 4% paraformaldehyde and 10% sucrose in PBS. The brains were removed from the skull, bisected and post-fixed for 2 to 4 hours in 4% paraformaldehyde and 10% sucrose in PBS. The hemispheres were placed in 30% sucrose overnight for cryoprotection before cutting on a freezing microtome into 40μm thick sections in the coronal, parasagittal or horizontal sections. Brain sections were saved in four to five series. In some cases, the brains were artificially flattened, then cut tangentially to the pia at 40μm, and saved in three series.
Histochemistry
One series of sections from each hemisphere was processed for Nissl substance (with thionin) and another series was processed for myelin, using the (Gallyas, 1979) silver procedure. In some cases, a third series of sections were processed for cytochrome oxidase (CO)(Wong-Riley, 1979).
Zinc Histochemistry
In galagos that were given IV injections of sodium sulfide, a series of sections was processed using the protocol outlined by Ichinohe et al. (2003) to visualize synaptic zinc. Brain sections were washed thoroughly with 0.1M PB, followed by 0.01M PB. The zinc-enriched terminals were visualized using the IntenSE M Silver enhancement kit (Amersham International, Little Chalfont Bucks, UK). The developing reagent was a one-to-one cocktail of the IntenSE M kit solution and 50% gum Arabic solution. The development the sections was terminated when a dark brown/black signal was seen by rinsing sections in 0.01M PB. Sections were then mounted and dehydrated in an ascending series of ethanols, (70% for 20min, 95% for 10min, 100% for 10min), cleared in xylene and coverslipped using Permount.
Immunohistochemistry
Each case contains one to two series of sections that have been immunostained for parvalbumin (PV) (1:4000; Sigma-Aldrich, St. Louis, Mo), or vesicle glutamate transporter 2 (VGluT2) (1:4000; Chemicon now part of Millipore, Billerica, MA). Sections were incubated in their respective antibodies for 40 to 48 hours at 4°C. Details of the immunohistochemical procedures have been described in Wong and Kaas (2008).
Antibody characterization
The mouse monoclonal anti-parvalbumin antibody is specific for PV and does not react with other members of the EF-hand family. This anti-parvalbumin antibody specifically reacts with the Cabinding spot of PV (MW = 12,000), on a two-dimensional gel, from human, bovine, goat, pig, rabbit, canine, feline, rat, frog and fish tissue (manufacturer's technical information).
The mouse monoclonal anti-VGluT2 antibody has shown species reactivity to the mouse and rat. The antibody epitope for VGluT2 from Millipore is not known. However, preadsorption of this monoclonal antibody (MAB5504) by Wässle et al., (2006) with the C-terminal peptide (562–582) did not block staining.
Light microscopy
The architectonic borders were delineated from the brain sections that have undergone the various histochemical and immunohistochemical procedures described above. The locations of architectonic borders were determined by analysis of laminar and cell density changes in the processed sections when viewed at high power using a projection microscope. The Nissl, zinc and VGluT2 preparations were most useful in identifying primary sensory areas, while sensorimotor cortical areas were better distinguished in the Nissl preparations. Other histological preparations were used for corroborating ambiguous borders. Digital photomicrographs of sections were acquired using a Nikon DXM1200 (Nikon Inc., Melville, NY) camera mounted on a Nikon E800 (Nikon Inc., Melville, NY) microscope and adjusted for brightness and contrast using Adobe Photoshop (Adobe Systems Inc., San Jose, CA).
Anatomical reconstruction
For further details on how the anatomical reconstruction is done, please refer to Wong and Kaas (2008). In brief, architectonic borders were identified and drawn for each outlined brain sections using a Bausch and Lomb Microprojector (Bausch & Lomb, Rochester, NY). Adjacent brain sections were aligned based on blood vessels and other landmarks that were added to the section outlines. The different histological procedures revealed similarly located boundaries between areas, suggesting that functionally relevant borders were being identified. Outlines of brain sections were imported into Adobe Illustrator (Adobe Systems Inc., San Jose, CA) and aligned using the contour of the outline sections and the landmarks that were drawn. For coronal and horizontal sections, the distances of the architectonic borders from the midline were measured. Positions of sagittal sections were aligned on a dorsal view of the brain. The surface views of the brain were reconstructed by projecting cortical and areal borders of brain sections onto lines appropriate for dorsal, lateral, medial and 45-degree angle view, and spacing these lines according to the location on the brain. In general, different histological procedures revealed similarly located boundaries between areas, suggesting that functionally relevant borders were being identified. In instances where architectonic borders were not clearly defined, but functional borders from microelectrode mapping and anatomical studes were well established, the locations of these functional borders were placed on the summary illustration to better guide future studies. The two types of borders are distinguished in the results.
Results
The present results provide further evidence for the presence of several previously proposed subdivisions of neocortex in galagos, as well as providing an interpretation of cortical organization that differs somewhat from previous depictions (von Bonin, 1942; von Bonin, 1945; von Bonin and Bailey, 1961; Kanagasuntheram et al., 1966; Zilles et al., 1979; Preuss and Goldman-Rakic, 1991a). The proposed areas are outlined on views of the galago brain in figures that follow. For abbreviations, refer to Table 1.
Table 1.
Abbreviations
| 3a | Dysgranular area |
| 19d | Area 19 dorsal |
| 19v | Area 19 ventral |
| 3b(S1) | Primary somatosensory area |
| 7m | Medial area 7 |
| A | Primary auditory area |
| Ab | Auditory belt area |
| CGd | Cingulate dorsal area |
| CGv | Cingulate ventral area |
| CLI | Claustral cortex or area claustralis isocorticalis |
| CLId | Dorsal claustral area |
| CLIv | Ventral claustral area |
| CMAc | Caudal cingulate motor area |
| CMAr | Rostral cingulate motor area |
| CO | Cytochrome oxidase |
| DL | Dorsolateral visual area |
| DM | Dorsomedial visual area |
| Ent | Entorhinal cortex |
| FEF | Frontal eye field |
| FST | Fundus of the superior temporal area |
| GrA | Granular frontal anterior area |
| GrM | Granular frontal medial area |
| GrP | Granular frontal posterior area |
| IPS | Intraparietal sulcus |
| ITc | Inferior temporal caudal area |
| ITr | Inferior temporal rostral area |
| M1 | Primary motor area |
| MF | Medial frontal cortex |
| MST | Middle superior temporal area |
| MT | Middle temporal visual area |
| MTc | Middle temporal cresent |
| OFd | Orbital frontal dorsal area |
| Ofm | Orbital frontal medial area |
| OFv | Orbital frontal ventral area |
| Para | Paralimbic area |
| PB | Phosphate buffer |
| PBS | Phosphate buffer with saline |
| Pirf | Piriform cortex |
| PMD | Premotor dorsal area |
| PMV | Premotor ventral area |
| PPc | Posterior parietal caudal area |
| PPl | Posterior parietal lateral area |
| PPr | Posterior parietal rostral area |
| PRh | Perirhinal area |
| PS | Prostriata |
| PV | Parvalbumin |
| Pv | Parietal ventral area |
| R | Rostral auditory area |
| RSag | Retrosplenial agranular area |
| RSg | Retrosplenial granular area |
| S2 | Secondary somatosensory area |
| SMA | Supplementary motor area |
| STd | Superior temporal dorsal area |
| TG | Area temporopolaris |
| V1 | Primary visual area |
| V2 | Secondary visual area |
| VGluT2 | Vesicle Glutamate Transporter 2 |
An overview of cortical organization based on brain sections cut parallel to the surface of flattened cortex
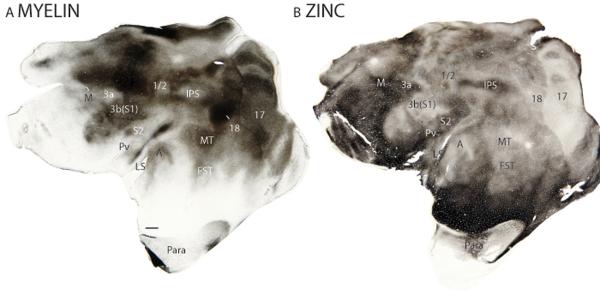
Brain sections that contain all regions of flattened cortex in single sections nicely indicate the relative positions of cortical areas, and often the extents of cortical borders, but they also need to be interpreted carefully as cortical layers stain differently, and such sections typically contain regions involving different layers. This is apparent in the myelin and zinc-stained sections of figure 1. The border of area 17 with area 18 is clear, whereas regional differences in staining within area 17 are apparent as the sections course from layer 3 to layer 4 (Fig. 1). Thus, in zinc preparations, layer 4 stains lightly over much of area 17, whereas darker regions correspond to layer 3 (Fig. 1B). In the myelin stained section, darker areas correspond to the myelin dense inner layers of area 17, while lighter regions correspond to superficial layers. Given this caution, area 3b(S1) is overall more myelinated then adjoining cortex, as is the auditory core (A). The middle temporal area, MT, is also more myelinated. In contrast, the middle layers of these areas, 17, MT, A and 3b(S1), all have reduced levels of free ionic zinc. Descriptions of cortical areas, region by region, follow.
Figure 1.
Architectonic characteristics galago cortex in flattened preparations stained for myelin (A) and for synaptic zinc (B). Scale bar in panel A = 2mm.
Occipital cortex
Area 17 (V1)
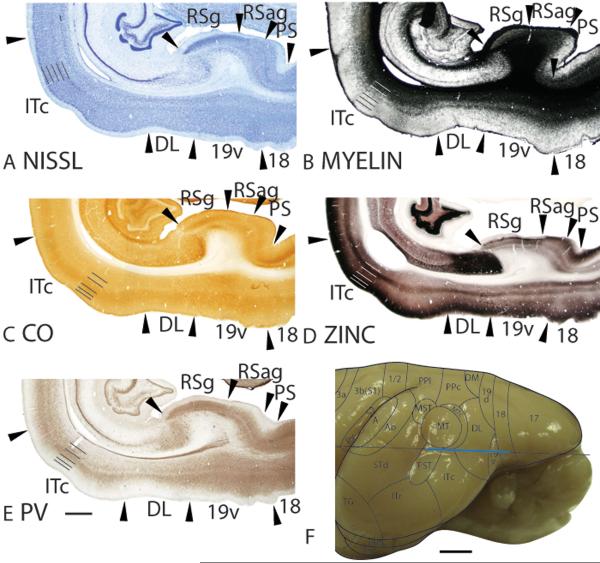
Area 17 in galagos, which is co-extensive with the primary visual area (V1), occupies the caudal most extent of the dorsolateral surface of occipital cortex and extends over the medial wall to occupy both banks of the calcrine sulcus. Area 17, with an approximate surface area of approximately 200mm2 (Rosa et al., 1997), has distinct architectonic characteristics that allow it to be easily distinguished from the rostrolaterally adjoining extrastriate area 18 (V2) and the medially bordering prostriata (PS). The cortical borders of area 17 are apparent even at low magnification in Nissl, CO, myelin, zinc, PV and VGluT2 stains, and the borders across adjacent main sections from different preparations are in similar locations (Fig. 2).
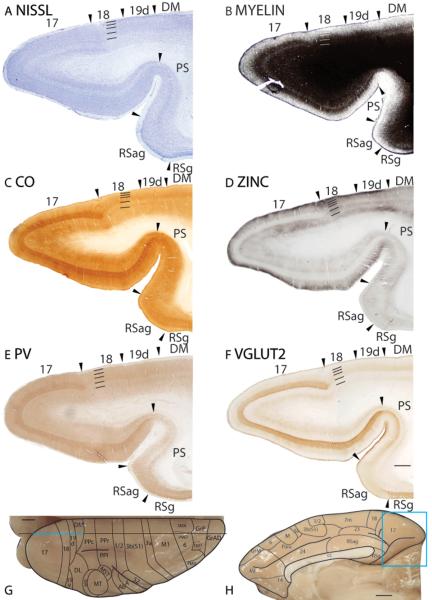
Figure 2.
Architectonic characteristics of visual areas 17, 18, 19d and DM. Sagittal sections from occipital cortex were processed for (A) Nissl substance, (B) myelin, (C) CO, (D) synaptic zinc, (F) parvalbumin (PV) and (F) vesicle glutamate transporter 2 (VGluT2). The architectonic borders of proposed cortical areas are shown on the dorsal view (G) and medial view (H) of the galago brain. The horizontal line on the brain shows the level from which the sections were taken for panels A–F. The thicker portion of the line marks the regions illustrated in panels A–F. Occipital areas 17, 18 and 19 are adopted from Brodmann (1909). DM is the dorsal medial visual area. Arrowheads on the sections illustrated here and in the following figures mark architectonic boundaries. Short lines on the sections indicate cortical layers 1 to 6. See table 1 for abbreviations for other areas. The scale bar for brain sections (panel E) = 1mm. The scale bar on the brain (panel F) = 2.5mm.
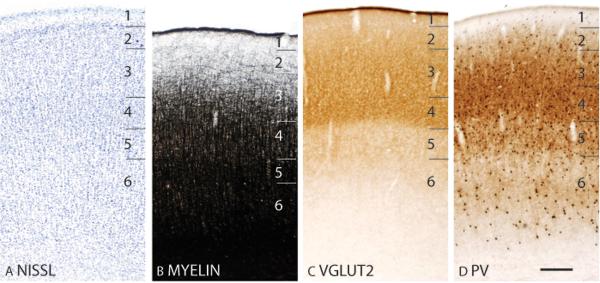
In Nissl preparations, area 17 has a banded appearance, with darkly stained layers 4 and 6, and paler stained layers 3 and 5 (Fig. 2A). Area 17 is densely myelinated (Fig. 2B; 4B). Layer 4 of area 17 is darkly stained band for CO and layers 1, 2, 5 and 6 are lighter stained (Fig. 2C). In zinc preparations, layer 4 of area 17 stands out as a white band as it expresses very little synaptic zinc (Fig. 2D), suggesting that the main projections to this cortical layer is from the thalamic nuclei rather than other cortical areas. Layers 1 to 3 and layer 5 are more darkly stained, likely because these cortical layers receive corticocortical connections that contain free ionic zinc in their terminations. Layer 4 of area 17 stains darkly for PV (Fig. 2E) and VGluT2 (Fig. 2F) immunopositive terminations, reflecting dense terminations from thalamic nuclei. A second, faint band of VGluT2 immunopositive terminations is observed in layer 6 (Fig. 2F), possibly reflecting the collaterals of axons that terminate more extensively in layer 4 (Casagrande and Kaas, 1994).
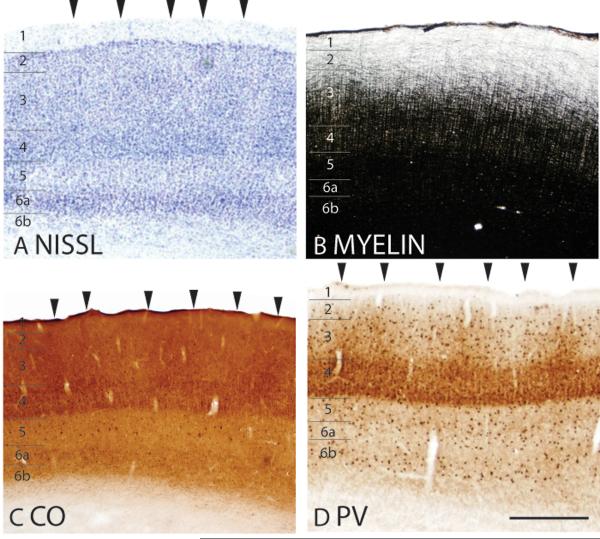
Figure 4.
The laminar characteristics of area 17 at higher magnification. The arrowheads in panels A, C and D indicate the locations of CO blobs in layer 3. Layer 6 has two sublayers, 6a and 6b, that are apparent in Nissl, and PV preparations. Scale bar = 0.5mm.
Layer 3 of area 17 is subdivided into a number of ovals that are histologically distinct from surrounding matrix, corresponding to the well-known CO rich “blobs” and CO poor “interblobs” (Casagrande and Kaas, 1994; Preuss and Kaas, 1996; Condo and Casagrande, 1990). In flattened preparations through layer 3 of area 17, a patchy pattern with myelin-poor clusters is observed within a myelin-rich background (Fig. 3B). Flattened sections of area 17 at a comparable cortical depth stained for CO showed darkly stained patches of CO-rich ovals within a CO-poor region (Fig. 3C). In zinc preparations, there are circular regions of dark staining surrounding zinc-poor patches (Fig. 3D). These results are consistent with the evidence that thalamocortical and corticocortical projections follow a modular organization in layer 3 of the galago visual cortex, with the thalamic projections from lateral geniculate K cells forming clusters in a background of corticocortical projections (Carey et al., 1979; Casagrande and De Bruyn, 1982; Diamond et al., 1985; Lachica and Casagrande, 1992). These patches of thalamocortical terminations in layer 3 stain darkly in VGluT2 (Fig. 3E) and PV (Fig. 3F) preparations.
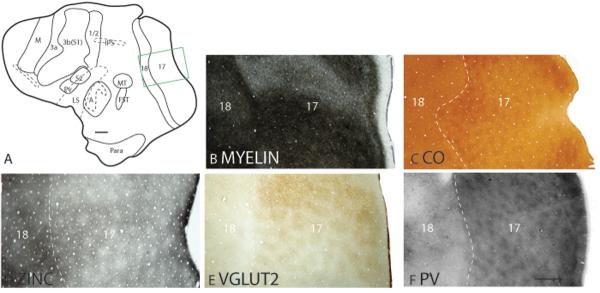
Figure 3.
Patchy staining pattern of area 17. The boxed region in A is shown in panels B to F at higher magnification. A myelin (B), CO (C), synaptic zinc (D) VGluT2 (E) and PV (F) stained section cut parallel to the surface of an artificially flattened cerebral hemisphere. Cytochrome oxidase rich regions, known as CO blobs are observed in area 17 of the galago neocortex (C). Dashed lines show the approximate location of the cortical borders. Scale bar in panel A = 4mm, in panel F = 1mm.
In coronal sections stained for Nissl bodies viewed at higher magnifications, layers 4 and 6 of area 17 are densely populated with cells, contributing to their dark appearance (Fig. 4A). Middle layer 3, primarily composed of small pyramidal cells, has clusters of darkly stained cells (Fig. 4A) that are likely to be co-extensive with the cytochrome oxidase blobs (Fig. 4C) and the patches of PV-immunopositive thalamocortical terminations (Fig. 4D). At higher magnifications, darkly CO-stained cells are present in layer 5 of area 17 (Fig. 4C). PV-immunopositive cell bodies are present in all layers, with a lower concentration in the infragranular layers 5 and 6 (Fig. 4D). Furthermore, area 17 is densely myelinated with distinct inner and outer bands of Baillarger (Fig. 2B) that tend to merge in darkly stained sections (Fig. 4B).
Area 18 (V2)
Area 18 of galagos lies along most of the lateral border of area 17 and is approximately one-third the surface area of area 17, with a maximum width of 3mm (Rosa et al., 1997). Co-extensive with the secondary visual area (V2), area 18 contains a representation of the contralateral visual hemifield, with the lower visual field represented dorsally and the upper visual field represented ventrally (Rosa et al., 1997).
The area 17/18 border is distinct due to the less conspicuous laminar pattern of area 18. In Nissl preparations, layers 4 and 6 of area 18 is less darkly stained and less densely populated with cells, resulting in a muted banded appearance compared to area 17 (Fig. 2A). Area 18 is densely myelinated, but the outer and inner bands of Baillarger that are present in area 17 are not distinct in area 18 (Fig. 2B). Layer 4 of area 18 is also less metabolically active than layer 4 of area 17, as evidenced by the reduction in staining intensity for CO (Fig. 2C). A band of CO staining is apparent in layer 4 and a faint band of CO staining is present in inner layer 5 of area 18 (Fig. 2C). Area 18 exhibits darker staining than area 17 throughout the cortical layers in zinc preparations (Fig. 2D). The increased intensity of zinc staining is especially prominent in layer 4 as area 17 transitions to area 18 (Fig. 2D). Inner layer 5 of area 18, which corresponds to the faint CO-dense band, is more lightly stained than outer layer 5 in zinc preparations (Fig. 2D). Area 18 lacks the dense PV- and VGluT2- immunopositive terminations that are present in layer 4 of area 17 (Fig. 2F). In addition, the faint band of VGluT2 staining present in layer 6 of area 17 is absent in area 18 (Fig. 2F). Thus, a thalamic input to layer 4 is absent or greatly reduced. The reduced staining intensity in VGluT2 preparations and the increased staining intensity in zinc preparations in layer 4 of area 18 suggest that corticocortical inputs dominate.
In tangential sections along layer 3, area 18 has a more homogenous myelination pattern (Fig. 3B) and lacks the CO rich patches or `blobs' that are present in area 17 (Fig. 3C). Area 18 stains darker and more evenly for the zinc stain (Fig. 3D), and lighter for the VGluT2 (Fig. 3E) and PV (Fig. 3F) stains compared to area 17.
Area 19 dorsal (V3d) and area 19 ventral (V3v)
Early studies of extra-striate areas of galagos did not include a third visual area, V3 or area 19 (Rosa et al., 1997; Beck and Kaas, 1998a; Collins et al., 2001), whereas others included other visual areas, such as DM and DL, in area 19 (e.g. Raczkowski and Diamond, 1978). Areas V3d (19d) and V3v (19v) were first differentiated from DM and DL in galagos by (Lyon and Kaas, 2002a) in studies of V1 projections. While area 19 has been used inconsistently as a term for visual regions of cortex (e.g. Brodmann, 1909), the term has been associated with V3 in cats (Hubel and Wiesel, 1965). Area 19 is used here as the architectonic term for V3.
Areas 19d and 19v have previously been described as regions that are moderately myelinated and stain dark for CO in flattened preparations of cortex (Lyon and Kaas, 2002a). As expected, areas 19d and 19v resemble each other. Here, we observe that area 19d has a thicker granular layer 4 than area 18 (Fig. 2A) in Nissl preparations and area 19d has a lighter appearance than DM (Fig. 2A). Sections stained for myelinated fibers show that area 19d is darkly myelinated, but lacks distinct inner and outer bands of Baillarger (Fig. 2B). Area 19d stains slightly darker for CO than area 18, but is lighter stained than DM (Fig. 2C). In zinc preparations, the upper cortical layers, layers 5 and inner 6 of area 19d stain darkly (Fig. 2D). Overall, area 19d is more darkly stained for free ionic zinc in the synapses than both areas 18 and DM (Fig. 2D). Area 19d stains more intensely in PV preparations than 18 and DM (Fig. 2E). Layer 4 of area 19d is more darkly stained for VGluT2 immunopositive terminations than layer 4 of area 18, and is stained at similar intensities to layer 4 of DM (Fig. 2F).
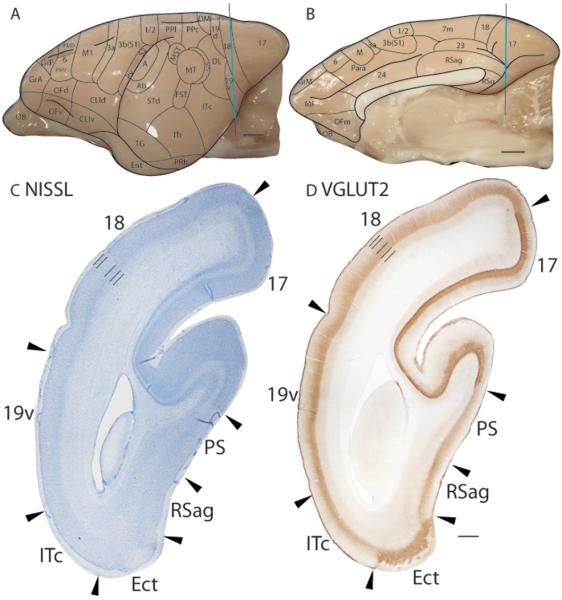
Area 19v is discontinuous with area 19d, with DL separating them. In Nissl preparations, area 19v has a thin, darkly stained layer 4 that is thicker than that of DL (Fig. 5A) and area 18 (Fig. 5A). Area 19v is less myelinated than area 18 and more myelinated than DL (Fig. 5B). In CO preparations, area 19v is moderately stained (Fig. 5C). The upper cortical layers, layers 5 and inner 6 of area 19v are darkly stained for zinc, whereas layer 4 and outer layer 6 stain lighter, giving area 19v a banded appearance in zinc preparations (Fig. 5D). Additionally, Area 19v stains as darkly as area 18, and layer 4 of area 19v is lighter stained than layer 4 of DL in the zinc stain (Fig. 5D). In PV preparations, area 19v stains moderately for PV-immunopositive terminations, with lower intensity in layer 5 (Fig. 5E). The PV staining in area 19v is not homogenous, tapering off towards the area 19v/DL border (Fig 5E). In VGluT2 preparations, a moderately stained band is present in layer 4 of area 19v and this band is darker, but thinner than that of area 18 (Fig. 6D).
Figure 5.
Architectonic characteristics of visual and temporal visual areas. The level at which the horizontal sections are taken from is indicated by the horizontal line on the lateral view of the brain in panel F. The thicker line in panel F marks the regions illustrated in panels A–E. The extent of each cortical layers 1 to 6 is indicated by the short horizontal lines on panels A–E. The scale bar for brain sections (panel E) = 1mm. The scale bar on the brain (panel F) = 2.5mm.
Figure 6.
Architectonic characteristics of visual areas and adjoining retrosplenial cortex. Coronal sections from occipital cortex were processed for (C) Nissl substance and (D) VGluT2. The level at which the coronal sections are taken from is indicated by the vertical line on the lateral and medial view of the brain in panels A and B respectively. The thicker line in panel A and B marks the regions illustrated in panels C and D. Short lines on the sections indicate the extent of each cortical layers 1 to 6. See table 1 for abbreviations for other areas. The scale bar for brain (panels A and B) = 2.5mm. The scale bar on the brain section (panel D) = 1mm.
Dorsomedial (DM) and dorsolateral (DL) visual areas
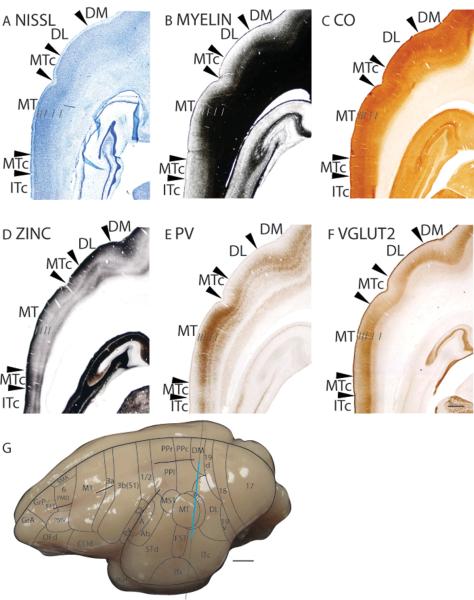
The dorsomedial visual area, DM, has a thin layer 4 that is densely packed with granule cells and is as such darkly stained in the Nissl stain (Fig. 2A; 7A). DM is moderately myelinated (Fig. 2B; 7B). Furthermore, DM is more myelinated than DL (Fig. 7B) and less myelinated than area 19d (Fig. 2B). Layer 4 of DM expresses moderate levels of CO, with two bands of CO staining, in layers 4 and 6 (Fig. 2C; 7C). In zinc preparations, DM stains with lower intensity compared to area 19d (Fig. 2D), and DL (Fig. 7D). DM stains darker in PV preparations than DL (Fig. 7E) and stains lighter than area 19d (Fig. 2E). In favorable sections, DM has two intensely stained bands of PV immunopositive terminations, one in layer 4 and another in outer layer 6 (Fig. 7E). Layer 4 of DM stains for VGluT2 immunopositive terminations at a similar level to area 19d (Fig. 2F) and DL (Fig. 7F).
Figure 7.
Architectonic characteristics of middle temporal cortex. The level at which the coronal sections are taken from is indicated by the vertical line on the dorsolateral view of the brain in panels G. The thicker line in panel G marks the regions illustrated in panels A to F. Short lines on the sections indicate the extent of each cortical layers 1 to 6. See table 1 for abbreviations for other areas. The scale bar for brain (panel G) = 1mm. The scale bar on the brain section (panel F) = 2.5mm.
The dorsolateral visual area, DL, surrounds at least the caudal portion of the middle temporal visual area (MT) in galagos and is likely to have projections to the inferior temporal area of cortex (Wall et al., 1982). In Nissl preparations, layer 4 of DL is moderately stained and densely populated with granule cells, and is thinner than layer 4 of MT (Fig. 7A) and 19v (Fig. 5A). Furthermore, the supragranular layers of DL are paler in appearance than those of MT (Fig. 7A). DL is less myelinated than the adjoining DM, MT and 19v, and has a distinct outer band of Baillarger (Fig. 5B; 7B). Both layers 4 and 6 of DL are moderately stained in CO preparations (Fig. 5C; 7C). In zinc preparations, DL expresses a moderate level of free ionic zinc, and is more darkly stained than MT, DM (Fig. 7D) and 19v (Fig. 5D). DL is lightly stained in PV preparations compared to the adjoining MT, DM and 19v, but two thin and faint bands of PV immunopositive terminations, in layers 4 and outer 6, are observed (Fig. 5E; 7E). In VGluT2 preparations, a single, lightly stained band is present in layer 4 of DL (Fig. 7F).
Temporal cortex
The temporal cortex of galagos is rather large and can be broadly divided into three regions. First are the temporal extrastriate areas, which consists of the middle temporal visual area (MT), the crescent surrounding MT (MTc), the middle superior temporal area (MST) and the fundus of the superior temporal area (FST). Second is the inferior temporal region, a large region that is further divided into the rostral (ITr) and caudal (ITc) areas. Third are the auditory association areas, which include the primary auditory cortex (A1), the rostral auditory area (R), and the auditory belt (Ab).
Temporal extrastriate areas - Middle temporal visual area (MT) and the crescent of the middle temporal visual area (MTc)
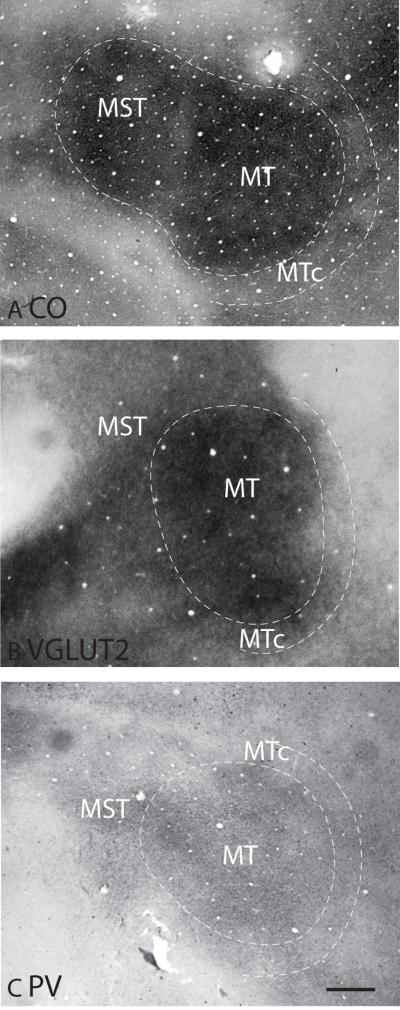
In galagos, MT is complete exposed on the surface as galagos lack a superior temporal sulcus. It has been defined as an oval region that is highly myelinated and has a surface area of approximately 18mm2 (Allman et al., 1973; Beck and Kaas, 1998a; Xu et al., 2004). MT and the adjoining areas, such as the crescent of MT (MTc) and MST are perhaps best appreciated in sections that were cut tangentially to the pia, as the full extent of these areas are present in a single section (Fig. 9). In CO preparations, both MT and MST are darkly stained, and MTc is stained as a series of CO-dense puffs along the caudal portion of MT (Fig. 9A). MT is also darkly stained in VGluT2 preparations, whereas MST and MTc are lighter stained, which makes the border of MT distinct (Fig. 9B). In PV preparations, the dense PV immunopositive terminations in MT have a somewhat patchy distribution (Fig. 9C). MST and MTc are less densely populated by PV-immunopositive terminations than MT (Fig. 9C).
Figure 9.
Architectonic characteristics of middle temporal cortex in flattened preparations stained for CO (A), VGluT2 (B) and PV (C). Dashed lines show the approximate location of the cortical borders. The scale bar on the brain section (panel C) = 1mm.
Area MT has many architectonic features of a sensory area of cortex. In coronal sections stained for Nissl bodies, MT has a layer 4 that is more darkly stained and densely populated with granule cells than layer 4 of the adjoining areas (Fig. 5A). At higher magnification, layer 5 of MT is sparsely populated by larger pyramidal cells (Fig. 10A). In myelin preparations, MT is densely myelinated (Fig. 7B; 8B; 10B). Layer 4 of MT stains darkly for CO (Kaskan and Kaas, 2007), although this is not especially evident in figures 5C and 8C. Throughout the cortical layers, MT stains lighter for synaptic zinc than the surrounding areas (Fig. 7D; 8D). However, MT is more darkly stained for free synaptic zinc, especially in layer 4, than area 17. This is consistent with the evidence that MT receives a large amount of corticocortical inputs, a major portion of which originates from area 17 (Kaskan and Kaas, 2007). The PV immunostain is perhaps one of the best markers for MT, as MT stains darkly for PV immunopositive terminations and has a tri-banded appearance (Fig. 7E; 8E). A large concentration of PV immunopositive terminations is present in layers 3 and 4, followed by a second, thinner band in inner layer 5, likely 5b, and a third, faint band is present in middle layer 6, likely 6B (Fig. 10C). In VGluT2 preparations, a thick, darkly stained band of VGluT2 immunopositive terminations is present in layers 3 and 4 of MT (Fig. 7F; 8F; 10D). The higher expression of VGluT2 immunopositive terminations by MT (Fig. 7F; 8F) suggests that MT receives more thalamic inputs than the adjoining cortical areas. Much of this input comes from the visual pulvinar (Wong et al., 2009c).
Figure 10.
The laminar characteristics of MT at higher magnification. Scale bar = 0.25mm.
Figure 8.
Architectonic characteristics of middle temporal cortex. The level at which the coronal sections are taken from is indicated by the vertical line on the dorsolateral (G) and medial (H) views of the brain. The thicker line in panels G and H marks the regions illustrated in panels A to F. Short lines on the sections indicate the extent of each cortical layers 1 to 6. See table 1 for abbreviations for other areas. The scale bar for brain (panels G and H) = 2.5mm. The scale bar on the brain section (panel F) = 1mm.
The middle temporal cresent area (MTc)
In Nissl preparations, MTc has a thinner, less densely populated layer 4 than MT (Fig. 7A; 8A) and is less densely myelinated (Fig. 7B; 8B). MTc expresses moderate amounts of CO and is less darkly stained for CO than MT (Fig. 7C; 8C). In zinc preparations, MTc stains more darkly for synaptic zinc, especially in the upper cortical layers, layer 5 and inner layer 6 (Fig. 7D; 8D). Layers 4 and outer 6 of MTc are faintly stained for PV immunopositive terminations, and overall, MTc is more faintly stained than MT in PV preparations (Fig. 7E; 8E; 9C). In VGluT2 preparations, a thin, faintly stained band is present in layer 4 of MTc (Fig. 7F; 8F), and MTc is lighter stained than MT (Fig. 9B). In sections cut tangentially to the pia, MTc contains several CO-dense puffs (Fig. 9A; Kaskan and Kaas, 2007).
The middle superior temporal area (MST) and the fundal area of the superior temporal sulcus (FST)
In sections cut tangentially to the pia, MST stains at similar levels for CO to MT (Fig. 9A). In PV and VGluT2 preparations, MST is more lightly stained than MT (Fig. 9B; 9C). In Nissl preparations, FST does not have the well-defined lamination of MT, with no distinct granular layer 4 (Fig. 8A; 11A) and FST is less densely myelinated than MT (Fig. 8B). FST stains lighter for CO than MT (Fig. 8C). In zinc preparations, FST has a banded appearance as the upper cortical layers 1 to 3, and layers 5 and innermost 6 are darkly stained, whereas layers 4 and upper 6 are lighter stained (Fig. 8D; 11D). FST is poorly stained for PV compared to MT, with a faint band of PV immunopositive terminations in layer 4 (Fig. 8E). FST expresses lower levels of VGluT2 immunopositive terminations than MT (Fig. 8F).
Figure 11.
Architectonic characteristics of inferior temporal cortex. The level at which the coronal sections are taken from is indicated by the vertical line on the lateral view of the brain in panels G. The thicker line in panel G marks the regions illustrated in panels A to F. Short lines on the sections indicate the extent of each cortical layers 1 to 6. See table 1 for abbreviations for other areas. The scale bar for brain (panel G) = 2.5mm. The scale bar on the brain section (panel F) = 1mm.
Inferior temporal rostral (ITr) and inferior temporal caudal (ITc) areas
Previous architectonic studies have identified two to three areas within the inferior temporal cortex of galagos (Zilles et al., 1979; Preuss and Goldman-Rakic, 1991a). Here, we have identified two cortical areas, the inferior temporal rostral (ITr) and caudal (ITc) areas.
In Nissl preparations, ITr has a thin, darkly stained band in layer 4 and a pale layer 5 (Fig. 11A). Throughout the cortical layers, ITr is more darkly stained than the pole of the temporal cortex, the area temporopolaris (TG) (Fig. 11A). In myelin preparations, ITr is more densely myelinated than TG and is as densely myelinated as FST (Fig. 11B). ITr stains more darkly for CO than TG, and stains at similar intensity to FST (Fig. 11C). Layers 4 and inner 6 of ITr express lower levels of free ionic zinc than the other cortical layers, giving ITr a banded appearance in the zinc stain (Fig. 11D). Compared to FST and TG, ITr expresses less synaptic zinc throughout the cortical layers, with the greatest difference in layers 4 and inner 6 of ITr (Fig. 11D). ITr stains poorly for PV immunopositive termination, and has a scattered population of PV immunopositive cell bodies in layer 4 that tapers off towards the ITr/TG border (Fig. 11E). In VGluT2 preparations, ITr has a darkly stained band in layer 4 that tapers off towards the ITr/TG border (Fig. 11F).
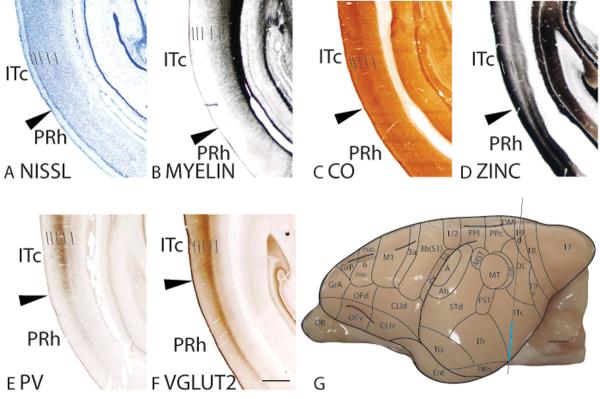
Throughout the cortical layers, ITc is more densely packed with cells than the ventrally adjoining perirhinal cortex (PRh), giving ITc a darker appearance than PRh in Nissl preparations (Fig. 12A). However, ITc lacks the thin, darkly stained band in layer 4 that is present in ITr. ITc is moderately myelinated and is more densely myelinated than PRh (Fig. 12B). In CO preparations, layer 4 of ITc is darkly stained (Fig. 12C). Layer 4 and, to a lesser extent, inner layer 6 of ITc express less free ionic zinc than the other cortical layers, giving ITc a banded appearance (Fig. 12D). Furthermore, ITc expresses less synaptic zinc than PRh (Fig. 12D). ITc has a scattering of darkly stained PV immunopositive cell bodies in layers 3 to 5 and a dark band of PV immunopositive terminations in layer 4 (Fig. 12E). The poor PV staining in PRh provides a distinct ITc/PRh border. In VGluT2 preparations, ITc has a darkly stained band in layer 4 (Fig. 12F). Additionally, layers 3 and 5 of ITc, but less so for layer 6, express a moderate amount of VGluT2 immunopositive terminations (Fig. 12F). Throughout the cortical layers, ITc expresses more VGluT2 immunopositive terminations than PRh. The presence of darkly stained bands of PV and VGluT2 immunopositive terminations, and relatively poor zinc staining in layer 4 of ITc suggest a predominance of thalamocortical over corticocortical inputs to this layer. The difference in architectonic appearances between ITr and ITc in galagos are subtle, and include a more densely populated layer 4 and slightly denser myelination in ITc than ITr. In addition, PV and VGluT2 staining is darker and zinc staining is lighter in ITc than ITr (not shown).
Figure 12.
Architectonic characteristics of inferior temporal cortex. The level at which the coronal sections are taken from is indicated by the vertical line on the dorsolateral view of the brain in panels G. The thicker line in panel G marks the regions illustrated in panels A to F. Short lines on the sections indicate the extent of each cortical layers 1 to 6. See table 1 for abbreviations for other areas. The scale bar for brain (panel G) = 2.5mm. The scale bar on the brain section (panel F) = 1mm.
Auditory associated areas – Primary auditory (A) and auditory belt (Ab) areas
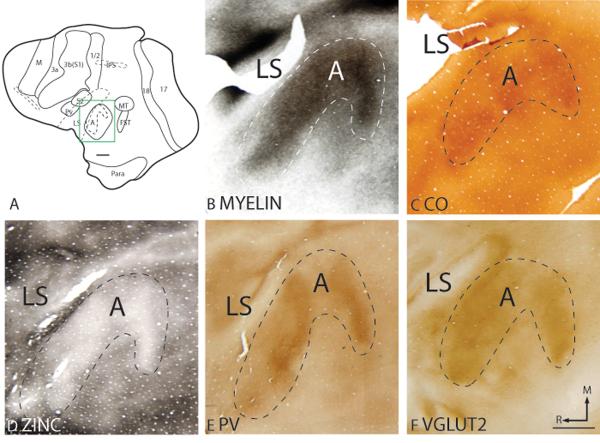
The primary auditory region, A, includes primary auditory cortex, A1, and the rostral primary area, R, of Brugge (1982). These two representations of tone frequencies were not distinguished architectonically in the present study and are included together in the auditory field, A. A portion of area A is on the surface of the temporal lobe and another portion is on the caudal bank of the lateral sulcus (Fig. 1). Sectioning artificially flattened cortex tangential to the pia is a way to appreciate much of the borders of area A (Fig. 13). This involves unfolding the lateral sulcus and it is difficult to keep all of area A on the same plane. The hooked shape of area A in the flattened sections in figure 13 is likely to be due to uneven flattening, as area A is likely to be ovalish in shape (Brugge, 1982). In flattened sections, area A is a densely myelinated region, surrounded by a myelin-poor region, the auditory belt (Fig. 13B). Area A also expresses higher levels of CO than the surrounding cortex, suggesting that area A is more highly metabolically active (Fig. 13C). Sections through layer 4 of area A stain poorly for free ionic zinc (Fig. 13D), consistent with the evidence that primary auditory cortex receives dense thalamic inputs from the medial geniculate complex and few layer 4 corticocortical terminations, at least in other primates (Luethke et al., 1989). Furthermore, area A stains darkly for PV (Fig. 13E) and VGluT2 (Fig. 13F) immunopositive terminations, indicating a higher population of thalamocortical terminations in layer 4 of area A than in surrounding cortex.
Figure 13.
Architectonic characteristics of auditory cortex in flattened preparations. The boxed region in A is shown in panels B to F at higher magnification. Dashed lines show the approximate location of the cortical borders. The scale bar on the brain (panel A) = 4mm, on brain section (panel F) = 2mm.
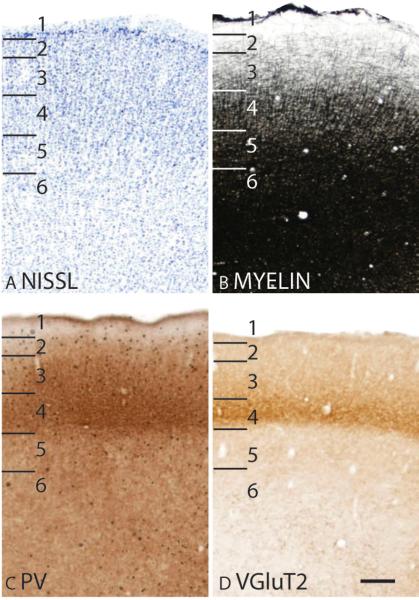
In Nissl preparations, layer 4 of area A is densely populated with granule cells, and layer 5 is more sparsely populated with larger pyramidal cells (Fig. 14A; 15A). Area A is densely myelinated (Fig. 14B; 15B) and has a layer 4 that is darkly stained in CO preparations (Fig. 14C). Layer 4 of area A stains poorly for free ionic zinc compared to layer 4 of the adjoining Ab (Fig. 14D). In PV preparations, a darkly stained band of PV immunopositive terminations is present in layer 4 of area A (Fig. 14E; 15C), with a scattering of darkly stained PV immunopositive cell bodies in the upper cortical layers (Fig. 15C). In addition, layer 4 of area A is also darkly stained for VGluT2 immunopositive terminations (Fig. 15D). The presence of dense populations of PV and VGluT2 immunopositive terminations, and the near absence of terminations containing free ionic zinc suggests that the predominating input to layer 4 of area A is from the thalamic nuclei, likely the ventral subdivision of the medial geniculate body (Luethke et al., 1989; de la Mothe et al., 2006), rather than from other cortical areas.
Figure 14.
Architectonic characteristics of auditory cortex. The level at which the horizontal sections are taken from is indicated by the horizontal line on the lateral view of the brain in F. The thicker line in panel F marks the regions illustrated in panels A to E. Short lines on the sections indicate the extent of each cortical layers 1 to 6. See table 1 for abbreviations for other areas. The scale bar for brain (panel F) = 2.5mm. The scale bar on the brain section (panel E) = 1mm.
Figure 15.
The laminar characteristics of primary auditory area at higher magnification. Scale bar = 0.25 mm.
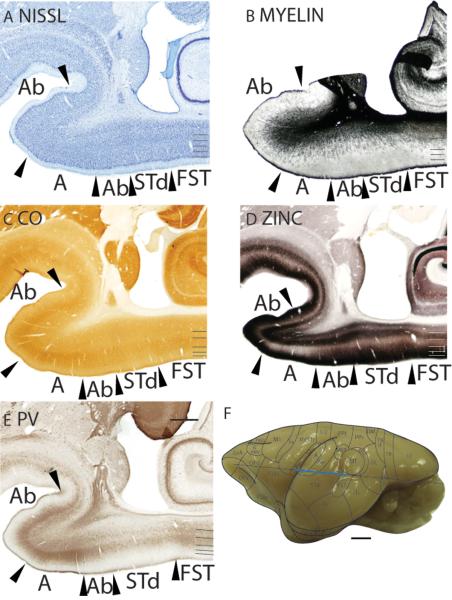
In monkeys, a narrow belt of secondary auditory areas surrounds the primary core auditory areas (Kaas and Hackett, 2000). These areas are not uniform in architectonic appearance (Hackett et al., 1998), and they can be difficult to distinguish from each other. Here, we identify a narrow, lateral auditory belt and a narrow medial auditory belt, the main divisions that have been defined in monkeys. Connectional studies with tracer injections have suggested that lateral Ab (identified as A II in Conley et al., 1991) has connections with secondary nuclei of the medial geniculate body and none with the ventral subdivision of the medial geniculate body. Architectonically, layer 4 of lateral Ab is paler and less densely packed with cell bodies than layer 4 of area A (Fig. 14A). Lateral Ab is less myelinated (Fig. 14B) and expresses lower staining for CO (Fig. 14C) than area A. Furthermore, lateral Ab stains darker in zinc preparations than area A, especially in layer 4 (Fig. 14D). In PV (Fig. 14E) and VGluT2 (not shown) preparations, lateral Ab is lighter stained for PV and VGluT2 immunopositive terminations than area A. The increased expression of synaptic zinc, and decreased expression of PV and VGluT2 immunopositive terminations in layer 4 of lateral Ab suggests that lateral Ab receives a higher proportion of corticocortical than thalamocortical inputs. It is possible that some of these inputs originate from area A.
Medial Ab has a thinner granular layer 4 in Nissl preparations (Fig. 14A) and is moderately myelinated (Fig. 14B). Additionally, medial Ab expresses lower staining for CO (Fig. 14C) than area A. The border between medial Ab and area A is distinct in zinc preparations as medial Ab stains darker in zinc preparations than area A, especially in layer 4 (Fig. 14D). In PV (Fig. 14E) and VGluT2 (not shown) preparations, medial Ab is moderately stained. Additionally, the population of PV and VGluT2 immunopositive terminations in medial Ab is less dense than in area A. This indicates that medial Ab receives a higher proportion of corticocortical inputs and a lower proportion of thalamocortical inputs than area A.
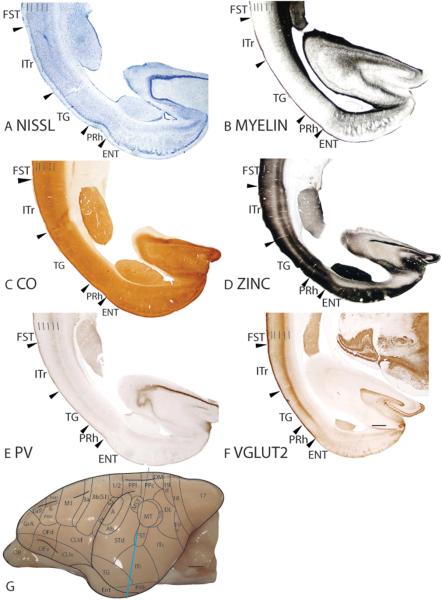
Remaining temporal areas – Area temporopolaris (TG) and superior temporal dorsal area (STd)
The temporal pole was identified as area TG by von Bonin and Bailey (1947) and as area 38 by Brodmann (1909). We have retained area TG as the nomenclature of the temporal pole in galagos to be consistent with Preuss and Goldman-Rakic (1991a). Area TG is bordered dorsally by ITr and STd, and ventrally by PRh. In Nissl preparations, TG is differentiated from ITr by the lack of a darkly stained layer 4 in TG, and from PRh by the overall paler appearance of TG compared to PRh (Fig. 11A). Furthermore, TG is less densely myelinated than ITr and more densely myelinated than PRh (Fig. 11B). Layer 4 of TG is lighter stained in CO preparations than ITr (Fig. 11C). In zinc preparations, layer 4 of TG is more lightly stained than the other cortical layers, giving TG a banded appearance (Fig. 11D). Throughout the cortical layers, TG stains darker for synaptic zinc than ITr (Fig. 11D). The TG/PRh border is distinct in zinc preparations as the lighter zinc-stained layer 4 of TG terminates at the border (Fig. 11D). TG is poorly stained in PV preparations and does not have any visible staining for PV immunopositive terminations or cell bodies (Fig. 11E). In VGluT2 preparations, TG has a darkly stained band of VGluT2 immunopositive terminations in layer 4 (Fig. 11F). This VGluT2 immunopositive band is thinner than that in ITr and thicker than that in PRh.
The superior temporal dorsal area (STd) is bordered rostrally by Ab, and caudally by ITr and FST. The dorsal border of STd with the posterior parietal cortex is not clear and is left unmarked. STd is bordered ventrally by TG. In Nissl preparations, STd has a thin layer 4, and broad layers 5 and 6 (Fig. 14A). Additionally, layers 4 and outer 6 of STd are pale in appearance (Fig. 14A). STd is moderately myelinated and layer 6 of STd is more myelinated than Ab and FST (Fig. 14B). STd expresses less CO and is as such less darkly stained in CO preparations than the adjoining Ab and FST (Fig. 14C). Layers 1 to 3, and 5 of STd are darkly stained for free ionic zinc (Fig. 14D). Throughout the cortical layers, STd is more lightly stained than Ab and more darkly stained than FST in zinc preparations (Fig. 14D). STd is lightly stained in PV (Fig. 14E) and VGluT2 (not shown) preparations. In sections stained for PV, two faint bands of PV immunopositive terminations with a scattering of darkly stained PV immunopositive cell bodies are observed in layers 4 and outer 6 of STd (Fig. 14E).
Parietal cortex
The parietal cortex of galagos can be divided into anterior, lateral and posterior regions. The anterior region consists of areas involved in early stages of cortical processing of somatosensory inputs, and includes the primary somatosensory area, 3b(S1), a rostrally adjoining strip of transition cortex, area 3a and a caudally adjoining area termed here as area 1/2. Posterior parietal cortex, identified by Brodmann (1909) in a prosimian lemur as area 7, includes all the parietal areas caudal to area 1/2, with some of the cortex buried in the intraparietal sulcus (IPS). The lateral somatosensory cortex in the lateral sulcus includes the second somatosensory area, S2, and the adjoining parietal ventral area, Pv.
Primary somatosensory area, 3b(S1)
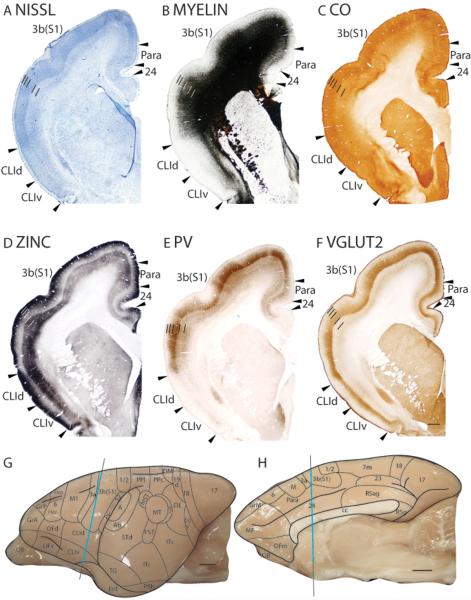
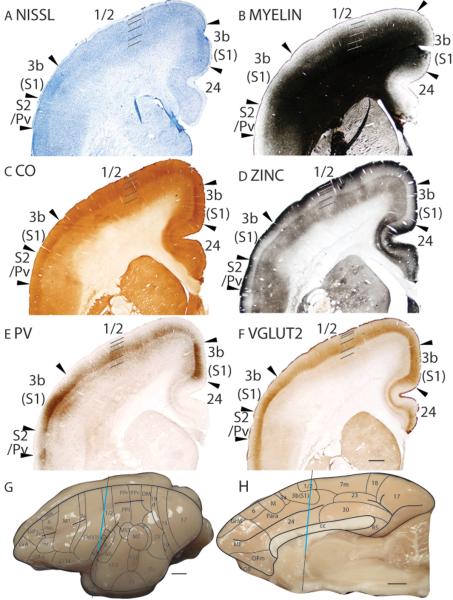
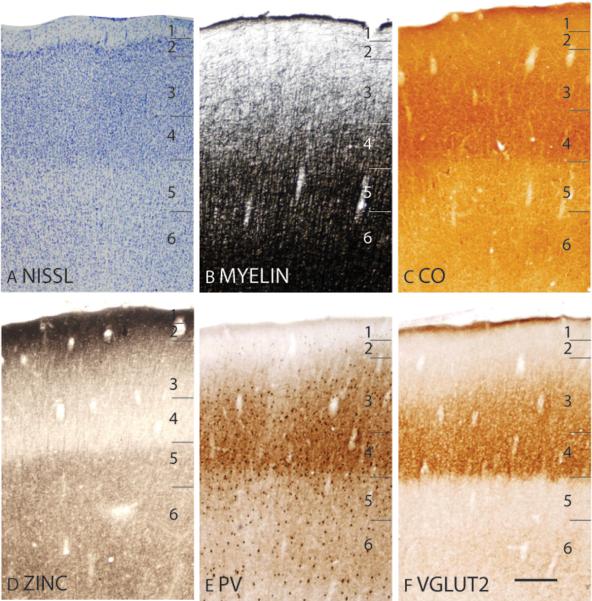
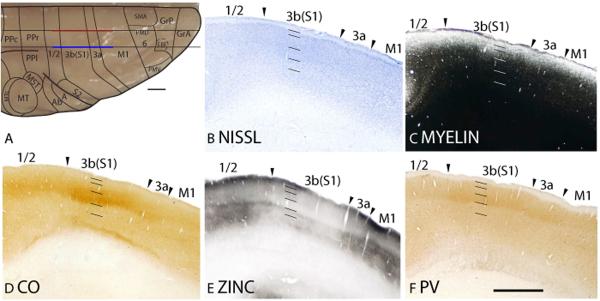
Microelectrode mapping studies (Carlson and Welt, 1980; Sur et al., 1980) have shown that the primary somatosensory area in galagos contains a complete, inverted representation of the contralateral cutaneous surface, with the oral and face representations located ventrally, followed by the hand, trunk, foot, leg then tail dorsally. This area is coextensive with the architectonically defined area 3b(S1) that has a koniocellular appearance. In Nissl preparations, area 3b(S1) has a thick, darkly stained layer 4 that is densely packed with granule cells (Fig. 16A; 19A). Furthermore, layer 4 of area 3b(S1) does not maintain a constant thickness throughout the coronal plane (Fig. 16A), being generally thicker laterally for hand and face representations than medially for trunk and foot representations. Thinner regions also correspond to discontinuities in the representation of the body surface, such as between the hand and face representations. At higher magnifications, layer 3 is densely packed with medium-sized pyramidal cells, whereas layer 5 is sparsely populated with larger pyramidal cells (Fig. 17A). Area 3b(S1) is densely myelinated (Fig. 16B; 19B), with poorly defined inner and outer bands of Baillarger (Fig. 17B). In CO preparations, layer 4 of area 3b(S1) is darkly stained, suggesting that this area is metabolically active (Fig. 16C; 19C). Fainter bands of CO staining are present in inner layer 3, likely 3b and 3c, and layer 6 (Fig. 17C). The architectonic borders of area 3b(S1) are distinct in zinc preparations as the poor staining of layer 4 terminates at the medial boundary with the paralimbic area (Para), at the ventral boundary with the claustral region (Fig. 16D), and at the caudal boundaries with areas 1/2 and S2/Pv (Fig. 19D). In PV preparations, area 3b(S1) stains darker than the surrounding paralimbic and claustral regions (Fig. 16E). A dense, discontinuous band of PV immunopositive terminations is present in layer 4 (Fig. 16E) and, to a lesser extent, layers 3, 5 and 6 of area 3b(S1) (Fig. 16E; 17E; 19E). Layers 3b, 3c and 4 of area 3b(S1) are also highly populated, whereas layers 5 and 6 are less densely populated with darkly PV stained cell bodies (Fig. 17E). In VGluT2 preparations, two stained bands are observed in area 3b(S1). A thick, darkly stained band of VGluT2 immunopositive terminations in layer 4 also extends up into inner layer 3 (Fig. 16F; 17F; 19F). A fainter VGluT2 immunostained band is present in layer 6 (Fig. 16F; 17F). The dense populations of PV and VGluT2 immunopositive terminations, and sparse population of terminations containing free ionic zinc in layer 4 of area 3b(S1) suggests that a larger proportion of inputs to layer 4 originate from the ventroposterior nucleus of the thalamus rather than from other cortical areas.
Figure 16.
Architectonic characteristics of somatosensory cortex. The level at which the coronal sections are taken from is indicated by the vertical line on the lateral (G) and medial (H) of the brain. The thicker line in panels G and H marks the regions illustrated in panels A to F. Short lines on the sections indicate the extent of each cortical layers 1 to 6. See table 1 for abbreviations for other areas. The scale bar for brain (panels G, H) = 2.5mm. The scale bar on the brain section (panel F) = 1mm.
Figure 19.
Architectonic characteristics of somatosensory cortex. The level at which the coronal sections are taken from is indicated by the vertical line on the lateral (G) and medial (H) of the brain. The thicker line in panels G and H marks the regions illustrated in panels A to F. Short lines on the sections indicate the extent of each cortical layers 1 to 6. See table 1 for abbreviations for other areas. The scale bar for brain (panels G, H) = 2.5mm. The scale bar on the brain section (panel F) = 1mm.
Figure 17.
The laminar characteristics of primary somatosensory area at higher magnification. Scale bar = 0.25mm.
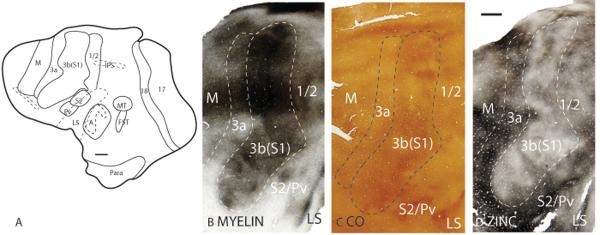
The full extent and heterogeneous appearance of area 3b(S1) can be appreciated in artificially flattened cortex that has been sectioned tangentially to the cortical surface (Fig. 18). Area 3b(S1) is more highly myelinated (Fig. 18A) and expresses more CO (Fig. 18B) than the surrounding cortical areas. In addition, sections through layer 4 show that area 3b(S1) is more poorly stained for free ionic zinc than the surrounding cortical area (Fig. 18C). However, the staining patterns in the myelin, CO and zinc preparations are patchy. The patchy staining pattern is partly due to the flattening process as the sectioning plane goes in and out of layer 4. In addition, the patchy pattern reflects the discontinuous representation of the cutaneous surface (Nelson et al., 1980; Jain et al., 2001; Kaas et al., 2006), with each patch corresponding to a particular region of the cutaneous surface.
Figure 18.
Architectonic characteristics of somatosensory cortex in flattened preparations. The boxed region in A is shown in panels B to D at higher magnification. Dashed lines show the approximate location of the cortical borders. The scale bar on the brain (panel A) = 4mm, on brain section (panel D) = 2mm.
Area 3a
In Nissl preparations, area 3a has a thinner layer 4 than area 3b(S1), and a layer 5 that is populated with larger and darker staining cells (Fig. 20B). Area 3a is less densely myelinated than area 3b(S1) with no distinct inner or outer bands of Baillarger (Fig. 20C). Layer 4 of area 3a stains less intensely for CO than layer 4 of area 3b(S1) (Fig. 20D). In zinc preparations, area 3a stains darker than area 3b(S1) (Fig. 20E), suggesting that area 3a receives more corticocortical inputs than area 3b(S1). Layer 4 of area 3a has reduced staining for PV (Fig. 20F) and VGluT2 (not shown) immunopositive terminations than area 3b(S1), providing further evidence that area 3a receives proportionately less thalamocortical inputs to layer 4 than area 3b(S1). In artificially flattened sections that were cut tangentially to the cortical surface, area 3a is a strip of cortex that lies along the length of the rostral border of area 3b(S1) (Fig. 18). In these sections, area 3a is a more lightly myelinated strip of cortex (Fig. 18A) and stains lighter for CO (Fig. 18B) than area 3b(S1). Furthermore, area 3a is more darkly stained for free zinc ions than area 3b(S1) in sections through layer 4 of the cortex (Fig. 18C).
Figure 20.
Architectonic characteristics of somatosensory cortex. The level at which the sagittal sections are taken from is indicated by the horizontal lines on the dorsal view (A) of the brain. The thicker lines in panel A marks the regions illustrated in panels B to F, with the red line indicating the regions illustrated in panel C and the blue line indicating the regions illustrated in panels B, C, D and F. Short lines on the sections indicate the extent of each cortical layers 1 to 6. See table 1 for abbreviations for other areas. The scale bar for brain (panel A) = 2.5mm. The scale bar on the brain section (panel F) = 1mm.
Area 1/2
The identity of the band of somatosensory cortex just caudal to area 3b(S1) is not established, but it is in the position of area 1 or area 1 plus 2. There is a tradition of referring to this region as area 1/2 (Sanides and Kristanamurti, 1967; see Wu and Kaas, 2003). The region of cortex that we have identified as area 1/2 corresponds to area 1/2 of Wu and Kaas (2003), and overlaps with the posterior somatosensory area (area 2–5) identified by (Preuss and Goldman-Rakic, 1991a). Connectional studies using tracer injections have shown that area 1/2 of galagos has dense connections with area 3b(S1), as well as areas S2 and Pv of lateral somatosensory cortex (Wu and Kaas, 2003).
In Nissl preparations, area 1/2 has a paler appearance than area 3b(S1) and layer 4 of area 1/2 is less densely packed with cells in comparison to area 3b(S1) (Fig. 19A; 20B). Area 1/2 is moderately myelinated and is less myelinated than area 3b(S1)(Fig. 19B; 20C). The lower myelination density of area 1/2 is also observed in sections that were cut tangentially to the pia (Fig. 18A). Layer 4 of area 1/2 has a CO-stained band that is less intensely stained than that in area 3b(S1) (Fig. 19C; 20D). The lowered CO staining of area 1/2 is observed in flattened sections as well (Fig. 18B). Area 1/2 expresses higher levels of zinc staining, especially in layer 4, than area 3b(S1) (Fig. 19D; 20E). The increased zinc staining intensity of area 1/2 is also observed in sections cut tangentially to the cortical surface, through layer 4 (Fig. 18C). Layer 4 of area 1/2 stains moderately for PV immunopositive terminations and a fainter band in layer 5 is observed (Fig. 19E; 20F). Additionally, PV immunopositive cell bodies are present in layers 3 to 5 and, to a lesser extent, layer 6. Compared to area 3b(S1), area 1/2 expresses lower levels of PV staining (Fig. 19E; 20F). There is a diffuse band of VGluT2 staining in layer 4 of area 1/2 that is less intensely stained than that in layer 4 of area 3b(S1)(Fig. 19F). The increased intensity of zinc staining and lowered intensities of PV and VGluT2 staining in area 1/2 compared to area 3b(S1), likely reflect a proportionately more corticocortical inputs, and proportionately less thalamocortical inputs than area 3b(S1).
Secondary somatosensory (S2) and parietal ventral (Pv) areas
The secondary somatosensory (S2) and parietal ventral (Pv) areas lie on the rostral bank toward the borders of the lateral sulcus, where they extend into the depths. Connectional studies using tracer injections have shown that S2 and Pv have topographic connections with area 3b(S1)(Wu and Kaas, 2003). S2 and Pv have a topographic representation of the cutaneous surface and the neurons in both areas have larger receptive fields than those in area 3b(S1) (Burton and Carlson, 1986; Garraghty et al., 1991; Wu and Kaas, 2003). Architectonically, S2 and Pv have similar characteristics. As such we did not distinguish an architectonic border between the two fields and the results presented here for S2 also apply to Pv.
In Nissl preparations, S2 has a paler stained layer 4 that is less densely populated by granule cells than area 3b(S1)(Fig. 19A). Furthermore, S2 has a paler appearance than the ventrally adjoining claustral area (CLI)(Fig. 19A). S2 is moderately myelinated (Fig. 19B). Compared to the dorsally adjacent area 3b(S1), S2 is less densely myelinated, and compared to the ventrally adjacent CLI, S2 is more densely myelinated (Fig. 19B). In CO preparations, S2 expresses less CO than the adjoining areas 3b(S1) and CLI (Fig. 19C). Throughout the cortical layers, S2 stains darker in zinc preparations than the surrounding areas 3b(S1) and CLI (Fig. 19D). In PV preparations, S2 does not have the distinct band of PV immunopositive terminations that is present in the surrounding cortical areas 3b(S1) and CLI (Fig. 19E). Furthermore, a scattering of PV immunostained cell bodies is present in S2 (Fig. 19E). S2 expresses moderate levels of VGluT2 immunopositive terminations in layer 4, which is lower than that in area 3b(S1) and thinner than that in CLI (Fig. 19F).
In artificially flattened sections that were cut tangentially to the pia, S2 has a lower level of myelination (Fig. 18A) and expression of CO (Fig. 18B) compared to area 3b(S1). Additionally, in sections through layer 4, S2 is more darkly stained for free ionic zinc than area 3b(S1)(Fig. 18C). The staining of S2 in flattened sections is patchy in appearance and this may be due to the presence of different representations of body parts in a single section.
Posterior parietal region
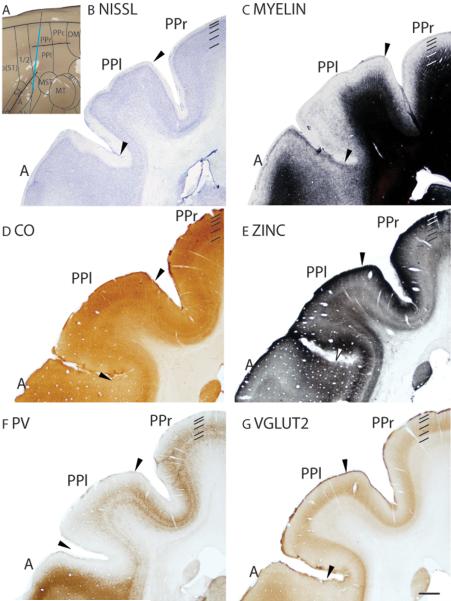
The posterior parietal region covers most of the IPS region and extends over the medial wall as area 7m. Divided into at least three areas, the posterior parietal region includes the posterior parietal rostral (PPr), lateral (PPl), and caudal (PPc) areas. The region we define as the posterior parietal cortex closely matches area 7 of Preuss and Goldman-Rakic (1991a), which is divided into six fields. The rostral portion of the posterior parietal cortex, which likely includes PPr and PPl, produces complex movements in the galagos when stimulated by microelectrode (Stepniewska et al., 2005b), whereas the caudal region, which is likely to be co-extensive with PPc, seems to receive visual inputs from areas such as V1 (Lyon and Kaas, 2002c).
The architectonic appearances of PPl and PPr are similar, with subtle differences. In Nissl preparations, PPl has a paler appearance than PPr (Fig. 21B). Layer 4 of PPl is less densely populated with granule cells and layer 4 of PPr is thin, but more densely populated with granule cells (Fig. 21B). PPl and PPr have similar myelination densities (Fig. 21C). In CO preparations, layer 4 of PPl and PPr are moderately stained, with a second faint and thin band in layer 6 (Fig. 21D). Throughout the cortical layers, PPl is more darkly stained for free ionic zinc than PPr, and both PPl and PPr are more intensely zinc-stained than the auditory core, area A (Fig. 21E). There are two PV immunopositive bands in layers 4 and outer 6 of PPl and PPr (Fig. 21F). In addition, PPc is more intensely stained than PPl, with a larger population of PV immunopositive cell bodies and terminations (Fig. 21F). PPl and PPr are moderately stained for VGluT2 immunopositive terminations, with PPr containing a more diffusely stained band in layer 4 (Fig. 21G).
Figure 21.
Architectonic characteristics of posterior parietal cortex. The level at which the coronal sections are taken from is indicated by the vertical line on the dorsolateral view of the brain (A). The thicker line in panel A marks the regions illustrated in panels B to G. Short lines on the sections indicate the extent of each cortical layers 1 to 6. See table 1 for abbreviations for other areas. The scale bar for brain (panel A) = 2.5mm. The scale bar on the brain section (panel G) = 1mm.
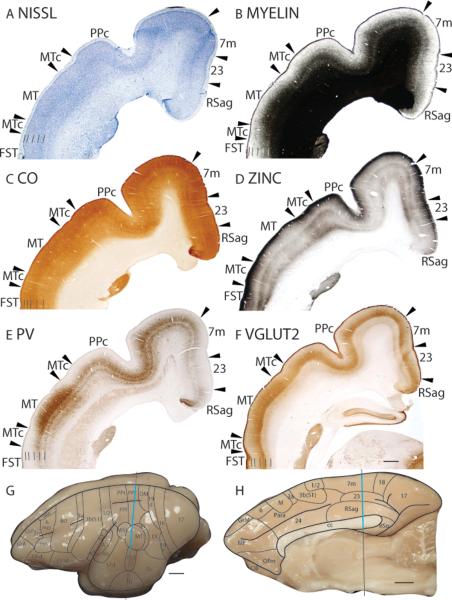
Architectonically, PPc has a moderately populated granular layer 4 that is less densely populated than layer 4 of area 7 (Fig. 8A). In myelin preparations, PPc is moderately myelinated with a distinct outer band of Baillarger, and is more highly myelinated than area 7m (Fig. 8B). PPc has two CO stained bands, in layers 4 and 6, and is less darkly CO stained than area 7m (Fig. 8C). PPc has similar CO expression levels to PPr and PPl (not shown). PPc stains less darkly for synaptic zinc than the adjoining areas MTc and 7m (Fig. 8D). In PV preparations, PPc is moderately stained for PV immunopositive terminations, with a dark, thicker band in layer 4 and a thinner band in layer 6 (Fig. 8E). PPc is more darkly PV stained than MTc and area 7m (Fig. 8E). Furthermore, PPc is more darkly stained for PV immunopositive terminations than PPr and PPl (not shown). In VGluT2 preparations, PPc is more darkly stained than MTc and area 7m (Fig. 8F), as well as PPr and PPl (not shown).
Medial area 7 (7m)
In Nissl preparations, layer 4 of area 7m is darkly stained and densely populated with granule cells (Fig. 8A). Area 7m is moderately myelinated with a distinct outer band of Baillarger (Fig. 8B). There are two CO-stained bands in area 7m, a thicker, darker staining band in layer 4 and a thinner, lighter staining band in outer layer 6 (Fig. 8C). Area 7m stains darker than area 23 and lighter than PPc in CO preparations (Fig. 8C). In zinc stained sections, area 7m has a banded appearance, with layers 1 to 3, 5 and inner 6 staining darker than layers 4 and outer 6 (Fig. 8D). Area 7m stains darker than PPc and lighter than area 23 in zinc preparations (Fig. 8D). Layers 4 and outer 6 of area 7m stains moderately for PV immunopositive terminations, and area 7m is sparsely populated with moderately stained cell bodies (Fig. 8E). Area 7m stains lighter than PPc and darker than area 23 in PV preparations (Fig. 8E). In VGluT2 preparations, layer 4 is moderately stained and is less intensely stained than layer 4 of DM (Fig. 8F). Furthermore, a thin, faintly stained band of VGluT2 immunopositive terminations is present in outer layer 6 (Fig. 8F).
Claustral cortex
The claustral cortex, or area claustralis isocorticalis (Cli) of Zilles et al. (1979) in galagos is located rostral to the lateral sulcus and ventral to area 3b(S1). This region has been also refered to as insular cortex, although this cortex is not comparable in location to insular cortex of anthropoid primates. Rather, it is more rostral and over the region of the claustrum. We have identified two areas, the dorsal claustral area (CLId) and ventral claustral area (CLIv).
In Nissl preparations, the border between area 3b(S1) and CLId is distinct as the thick granular layer 4 of area 3b(S1) terminates at the boundary with CLId (Fig. 16A). Throughout the cortical layers, CLId has a paler appearance and is more sparsely populated with cells than area 3b(S1)(Fig. 16A). The CLId/CLIv border is not distinct in Nissl preparations, although CLIv stains darker in Nissl preparations away from the border with CLId (Fig. 16A). Both CLId and CLIv are poorly myelinated (Fig. 16B). The poor myelination of CLId provides for a distinct area 3b(S1)/CLId border (Fig. 16B). Both CLId and CLIv stain moderately in CO preparations, with a diffuse CO-stained band in layer 4. However, CLIv is more darkly stained away from the CLId/CLIv border (Fig. 16C). The architectonic border between CLId and CLIv is perhaps most distinct in zinc preparations as the intense zinc staining in CLId terminates at the CLId/CLIv border (Fig. 16D). The difference in zinc staining intensities is most marked in layer 4, as layer 4 of CLIv is paler in appearance than CLId (Fig. 16D). This suggests that layer 4 of CLIv receives proportionately less corticocortical inputs using free ionic zinc than layer 4 of CLId. Both CLId and CLIv are poorly stained in PV preparations (Fig. 16E). Layers 4 of CLId and CLIv stain moderately for VGluT2 immunopositive terminations, with the band in layer 4 of CLIv being thicker and more darkly stained than that in CLId (Fig. 16F).
Frontal cortex
Frontal cortex of galagos is divided into the motor, granular frontal, orbital frontal and the medial frontal regions. The motor region consists of primary motor cortex (M1) and area 6, also known as the premotor and supplementary motor areas. There are at least three areas in the granular frontal region, the granular anterior (GrA), posterior (GrP), and medial (GrM) areas. The orbital frontal region consists of the dorsal (OFd), ventral (OFv) and medial (OFm) areas. There is a possibility of the medial frontal region consisting of more than one cortical area, but due to the lack of clear architectonic and functional evidence, we have left it as a single, medial frontal (MF) area.
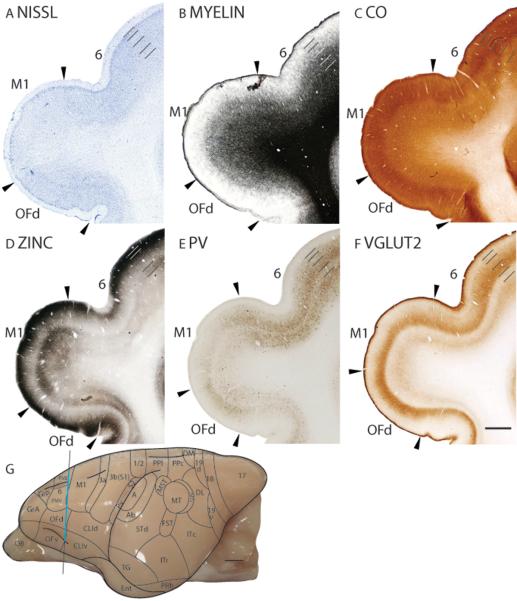
Primary motor cortex (M1)
In Nissl preparations, M1 has a paler appearance than the rostrally adjoining area 6 and ventrally adjoining claustral region (Fig. 22A). M1 lacks a distinct granular layer 4 and has a thick layer 5 that is populated with large pyramidal cells (Fig. 22A). In myelin preparations, M1 is moderately myelinated and has similar myelination levels to the dorsal claustral area (CLId) and area 6 (Fig. 22B). Layers 2 to 4 of M1 stain moderately for CO (Fig. 22C). The band of CO staining in M1 is thicker but paler than that in area 6 and OFd (Fig. 22C). In zinc preparations, M1 has a banded appearance, with layers 1 to 3 and layer 5 staining darker than layers 4 and 6 (Fig. 22D). Additionally, there are zinc stained `threads' running through layer 4 of M1 (Fig. 22D). Compared to the adjoining cortical areas, M1 stains more intensely in zinc preparations, with layers 4 and 6 expressing more free ionic zinc than the surrounding cortical areas (Fig. 22D). The increased zinc staining in M1 suggests that M1 receives proportionately more corticocortical inputs that use free ionic zinc in the synapses. M1 stains poorly in PV preparations, with a faint band of PV immunopositive terminations in layer 4 and a scattering of darkly stained cell bodies in layer 5 (Fig. 22E). In VGluT2 preparations, M1 has a moderately stained band in layer 4 and a faintly stained band in layer 6 (Fig. 22F). Compared to the surrounding cortical layers, M1 expresses less VGluT2 immunopositive terminations in layers 4 and 6 (Fig. 22F).
Figure 22.
Architectonic characteristics of frontal cortex. The level at which the coronal sections are taken from is indicated by the vertical line on the lateral view of the brain (G). The thicker line in panel G marks the regions illustrated in panels A to F. Short lines on the sections indicate the extent of each cortical layers 1 to 6. See table 1 for abbreviations for other areas. The scale bar for brain (panel G) = 2.5mm. The scale bar on the brain section (panel F) = 1mm.
Area 6
Studies of connections using tracer injections and microstimulations have identified up to four areas within area 6, including the dorsal (PMd) and ventral (PMv) premotor areas, the supplementary motor area (SMA) and the frontal eye field (FEF) (Wu et al., 2000; Fang et al., 2006; Fang et al., 2008). Although these areas have different connectional and response properties, differences in their architectonic properties are subtle. Their borders, as defined by microelectrode mapping and connectional studies are included on the cortical maps of the brain, and they are described here as a single architectonic field, area 6.
In Nissl preparations, area 6 stains darker than M1 throughout the cortical layers (Fig. 22A). The M1/area 6 border is marked by a more densely populated layer 2, the reappearance of a layer 4 that is moderately populated with granule cells, and a thinner layer 5 that is populated with smaller pyramidal cells in area 6 (Fig. 22A). Area 6 is moderately myelinated and the M1/area 6 border is not clearly demarcated in myelin preparations (Fig. 22B). There are two CO stained bands in area 6, a moderately stained band in layer 4 and a lighter stained band in layer 6 (Fig. 22C). In zinc preparations, layer 4 is the most lightly stained, then layer 6, followed by layer 5 (Fig. 22D). Layers 1 to 3 of area 6 stain darkly in zinc preparations (Fig. 22D). Compared to M1, area 6 expresses less free ionic zinc throughout the cortical layers (Fig. 22D). The M1/area 6 boundary is distinct in PV preparations as area 6 stains darker than M1 (Fig. 22E). There are two bands of PV immunopositive terminations, in layers 4 and 6, of area 6 (Fig. 22E). Darkly stained, PV immunopositive cell bodies are also concentrated in layers 4 and 6 of area 6 (Fig. 22E). Layer 4 of area 6 stains moderately for VGluT2 immunopositive terminations, with some staining extending up into inner layer 3 (Fig. 22F). Additionally, a faint band of VGluT2 immunopositive terminations is present in layer 6 of area 6 (Fig. 22F). Area 6 has increased staining for PV and VGluT2 immunopositive terminations, and decreased expression of free ionic zinc compared to M1. This suggests that area 6 receives a proportionately more thalamocortical terminations and less corticocortical terminations than M1.
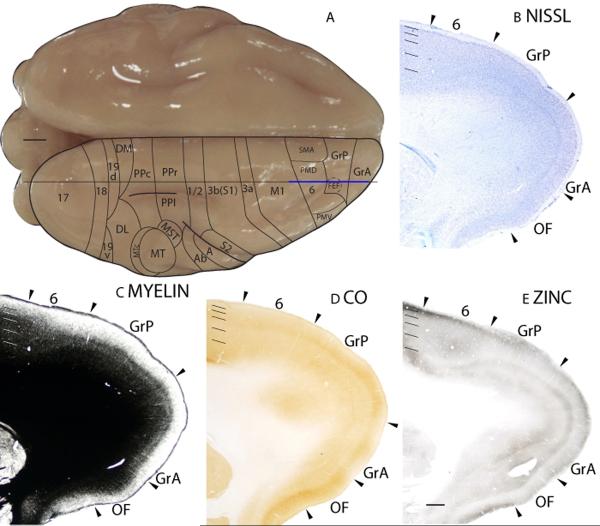
Granular frontal posterior area (GrP)
The granular frontal posterior area (GrP) is bordered caudally by area 6 and ventrally by the orbital frontal region. In Nissl preparations, a thicker granular layer, a darkly stained outer layer 5 and a paler inner layer 5 in GrP marks the area 6/GrP border, (Fig. 23B). Furthermore, GrP has a darker appearance than the dorsal orbital frontal area (OFd) in Nissl stained sections (Fig. 24A). GrP is moderately myelinated with a distinct outer band of Baillarger (Fig. 23C; 24B). In CO preparations, a moderately stained band is present in layer 4 of GrP (Fig. 23D; 24C). GrP, especially layer 4, stains lightly in zinc preparations compared to the granular frontal anterior (GrA)(Fig. 23E) and medial (GrM)(Fig. 24D) areas. In PV preparations, GrP stains moderately for PV immunopositive terminations in layers 3 and 4, with a scattering of moderately stained PV immunopositive cell bodies in layers 3 to 5 (Fig. 24E). Layer 4 is moderately stained and layer 6 is faintly stained for VGluT2 immunopositive terminations (Fig. 24F). GrP less intensely stained than GrM (Fig. 24F) and more intensely stained than GrA (not shown) in VGluT2 preparations.
Figure 23.
Architectonic characteristics of frontal cortex. The level at which the coronal sections are taken from is indicated by the horizontal line on the dorsal view of the brain (A). The thicker line in panel A marks the regions illustrated in panels B to E. Short lines on the sections indicate the extent of each cortical layers 1 to 6. See table 1 for abbreviations for other areas. The scale bar for brain (panel A) = 2.5mm. The scale bar on the brain section (panel E) = 1mm.
Figure 24.
Architectonic characteristics of frontal cortex. The level at which the coronal sections are taken from is indicated by the vertical lines on the lateral (G) and medial (H) views of the brain. The thicker lines in panels G and H marks the regions illustrated in panels A to F with the red line indicating the regions illustrated in panel D and the blue line indicating the regions illustrated in panel A, B, C, E and F. Short lines on the sections indicate the extent of each cortical layers 1 to 6. See table 1 for abbreviations for other areas. The scale bar for brain (panel G, H) = 2.5mm. The scale bar on the brain section (panel F) = 1mm.
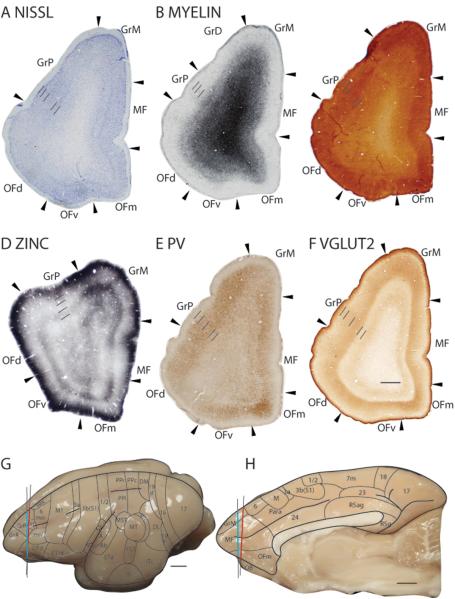
Granular frontal anterior area (GrA)
The granular frontal anterior area (GrA) lies rostral to GrP and extends under to the ventral cortex to border the ventral orbital frontal area (OFv). GrA has a thinner, more sparsely populated granular layer 4 and a thin, darkly stained layer 5 in Nissl preparations (Fig. 23B). In myelin preparations, GrA has similar myelination levels to GrP and lower myelination levels than OFv, with a less distinct outer band of Baillarger than GrP (Fig. 23C). GrA stains less intensely for CO (Fig. 23D) and more intensely for free ionic zinc (Fig. 23E) than both GrP and OFv. In PV preparations, GrA stains more lightly than GrP and similarly to OFv (not shown). Layer 4 of GrA is faintly stained in VGluT2 preparations, and GrA expresses less VGluT2 immunopositive terminations than both GrP and OFv (not shown).
Granular frontal medial area (GrM)
The granular frontal medial area is bordered dorsally by GrP and ventrally by the medial frontal area (MF). In Nissl preparations, GrM has a less densely populated layer 4 than GrP and a layer 5 that is populated by medium-sized, darkly stained cells (Fig. 24A). GrM is moderately myelinated and is not densely populated with vertically running myelinated fibers, unlike GrP and MF (Fig. 24B). Layers 3 and 4 of GrM stain darkly for CO and overall, GrM stains lighter than MF (Fig. 24C). In zinc preparations, GrM is darker stained throughout the cortical layers than GrP and MF (Fig. 24D). Layers 3, 4 and 6 of GrM are less intensely stained for free ionic zinc than layers 1, 2 and 5 (Fig. 24D). PV staining in GrM is not homogenous (Fig. 24E). The dorsal portion being poorly stained for PV immunopositive terminations and cell bodies, whereas the ventral portion being moderately stained in layer 4 for PV immunopositive terminations and populated by darkly stained PV immunopositive cell bodies (Fig. 24E). Compared to GrP and MF, GrM stains less intensely in PV preparations (Fig. 24E). A moderately stained band of VGluT2 immunopositive terminations is present in layer 4 of GrM and extends up to layer 3 (Fig. 24F). GrM expresses more VGluT2 immunopositive terminations than GrP and MF (Fig. 24F).
Orbital frontal dorsal area (OFd)
The orbital frontal dorsal area is bordered dorsally by M1 and GrP, and ventrally by the orbital frontal ventral area (OFv). In Nissl preparations, OFd has a darker appearance than M1 (Fig.22A) and a lighter appearance than GrP (Fig. 24A). OFd has a moderately thick layer 4 that is populated by granule cells and a thinner layer 5 than M1 (Fig. 22A; 24A). OFd is moderately myelinated and has no distinct bands of Baillarger (Fig. 22B; 24B). Layer 4 of OFd stains darker for CO than M1 (Fig. 22C) and GrP (Fig. 24C). In zinc preparations, OFd, especially layers 4 to 6, is less intense stained than M1 (Fig. 22D) and more intensely stained than GrP (Fig. 24D). OFd stains at similar levels to OFv in zinc preparations (Fig. 24D). OFd is lightly stained in PV preparations. Layer 4 has a thin, faint band of PV immunopositive terminations, and layers 4, 5 and 6 are sparsely populated with darkly stained PV immunopositive cell bodies (Fig. 22E; 24E). In VGluT2 preparations, Layer 4 of OFd is moderately stained and layer 6 is faintly stained. OFd stains more intensely than M1 (Fig. 22F) and similarly to GrP (Fig. 24F) in VGluT2 preparations.
Orbital frontal ventral area (OFv)
The orbital frontal ventral area (OFv) is bordered dorsally by OFv and medially by the orbital frontal medial area (OFm). In Nissl preparations, OFv is darkly stained compared to the adjoining cortical areas OFv and OFm. The upper cortical layers of OFv is populated with medium-sized, darkly stained cells, layer 4 is thin and layer 5 is populated with darkly stained cells (Fig. 23B; 24A). OFv has a higher myelination density than GrA (Fig. 23C) and has similar myelination density to OFd (Fig. 24B). In CO preparations, OFv stains more intensely than GrA (Fig. 23D) and OFd (Fig. 24C). OFv stains lighter than GrA in zinc preparations (Fig. 23E) and at similar intensities to OFd, although layer 4 of OFv is darker than layer of OFd (Fig. 24D). In PV preparations, OFv stains moderately for PV immunopositive terminations, with a scattering of moderately stained PV immunopositive cell bodies in layers 3 and 4 (Fig. 24E). OFv stains darker in PV preparations than OFd, and lighter than OFm (Fig. 24E). In VGluT2 preparations, a moderately stained band is present in layer 4 and a lightly stained band is present in layer 6 of OFv (Fig. 24F). The band of staining in layer 4 of OFv is thinner than that of OFd and less intense than that of OFm (Fig. 24F).
Orbital frontal medial area (OFm)
The orbital frontal medial area (OFm) is bordered laterally by OFv and dorsally by the medial frontal area (MF). OFm has a banded appearance in Nissl preparations, due to moderately stained upper cortical layers, a thin and pale granular layer 4, a thin and moderately stained layer 5, and a pale layer 6 (Fig. 24A). Layer 3 of OFm is populated with medium-sized cells. Of the three orbital frontal areas, OFm is the least densely myelinated and contains more horizontal fibers (Fig. 24B). Layers 3 and 4 of OFm stain darkly for CO (Fig. 24C). In zinc preparations, layers 1, 2, outer 3 and 5 are darkly stained, whereas layers 4 and 6 are lighter stained (Fig. 24D). Throughout the cortical layers, OFm stains darker than the adjacent OFv and MF in zinc preparations (Fig. 24D). OFm stains darkly for PV immunopositive terminations in layer 4 that extends to inner layer 3 (Fig. 24E). Moderately PV stained cell bodies are present in inner layer 3 and layer 4, and lightly PV stained cell bodies are present in layer 6 (Fig. 24E). A moderately stained band of VGluT2 terminations is present in layer 4 of OFm and extends up to inner layer 3 (Fig. 24F). OFm expresses higher levels of VGluT2 terminations than OFv and MF (Fig 24F). As OFm is darkly stained in zinc, PV and VGluT2 preparations, it suggests that OFm receives proportionately more corticocortical terminations that contain free ionic zinc, as well as more PV and VGluT2 immunopositive thalamocortical terminations than the adjoining OFv and MF.
Medial frontal area (MF)
The medial frontal area (MF) is bordered dorsally by GrM and ventrally by OFm. In Nissl preparations, MF does not have a distinct granular layer 4 and has a thin layer 5 that is darkly stained and densely populated with cells (Fig. 24A). MF is moderately myelinated with a heterogeneous myelination pattern (Fig. 24B). At higher magnifications, nearer to the GrM/MF border, MF is populated by myelination pattern is in a vertical orientation, whereas nearer to the MF/OFm border, the myelination pattern that is in a horizontal orientation (not shown). This suggests that there may be further subdivisions in MF. In CO preparations, layer 4 of MF is moderately stained (Fig. 24C). The varying staining pattern of MF is present in zinc preparations as well (Fig. 24D). MF stains darker nearer to the GrM/MF border and lighter nearer to the MF/OFm border (Fig. 24D). In PV preparations, MF stains less intensely for PV immunopositive terminations than OFm and more intensely than GrM (Fig. 24E). Moderately PV stained cell bodies are present in layers 4 and 6 of MF (Fig. 24E). In VGluT2 preparations, a thin and moderately stained band is present in layer 4 and a thin and lightly stained band is present in layer 6 of MF (Fig. 24F). Throughout the cortical layers, MF expresses less VGluT2 immunopositive terminations than GrM and OFm (Fig. 24F).
Medial cortex
The medial wall of the galago neocortex can be divided into the rostral, middle and caudal regions. GrM, MF and OFm make up the rostral region of the medial cortex. The dorsal medial cortex includes parts of area 6, M1, area 3a, area 3b(S1) and area 1/2, which have been described on previous pages. Ventral medial cortex consists of the paralimbic area (Para) and anterior cingulate, area 24. The caudal region of the medial cortex is largely occupied by area 17 at the caudal pole. Additionally, area 18, prostriata (PS), the medial area of the posterior parietal cortex (area 7m), the posterior cingulate (area 23), and the retrosplenial areas are located within the caudal region of the medial cortex. Areas 23, 24 and 30 follow the description of Brodmann (1909) for prosimian lemur.
Paralimbic area (Para)
The paralimbic area (Para) largely corresponds to the rostral (CMAr) and caudal (CMAc) cingulate motor area (Wu et al., 2000). Para is bordered by the motor, premotor and somatosensory areas dorsally, and by area 24 ventrally. In Nissl preparations, layer 4 of Para is not distinct and seems to merge with layer 3 to form a thick band populated with medium sized cells (Fig. 16A). Layer 5 of Para has a pale appearance and is more sparsely populated with cells than layer 5 of area 3b(S1)(Fig. 16A). Para is less densely myelinated than area 3b(S1) and more densely myelinated than area 24 (Fig. 16B). Furthermore, Para has a distinct outer band of Baillarger (Fig. 16B). In CO preparations, Para is less darkly stained than area 3b(S1) and stains at similar intensities to area 24 (Fig. 16C). Darkly CO stained cells are present in layer 5 of Para (Fig. 16C). In zinc preparations, Para is more darkly stained throughout the cortical layers than the adjoining areas 3b(S1) and 24 (Fig. 16D). A moderately stained band of PV immunopositive terminations is present in layer 4 of Para, and a second, fainter band is present in layer 6 (Fig. 16E). Para is also populated by moderately stained PV immunopositive cells (Fig. 16E). In VGluT2 preparations, layer 4 is moderately stained and layer 6 is faintly stained (Fig. 16F). Para expresses less VGluT2 immunopositive terminations in layers 4 and 6 than the corresponding layers in area 3b(S1)(Fig. 16F).
Area 24
In Nissl preparations, area 24 has a pale appearance with no obvious granular layer 4 (Fig. 16A; 19A). Area 24 is poorly myelinated with no distinct bands of Baillarger (Fig. 16B; 19B). A thin, moderately stained band in layer 4 of area 24 is present in CO preparations (Fig. 16C; 19C). The more rostral portions of area 24 stain paler in zinc preparations, especially in layers 4 and 5 (Fig. 16D). However, towards the caudal end, area 24 stains darker in zinc preparations (Fig. 19D). This hints at the possibility of subdivisions within area 24. This variation in staining pattern is present in PV preparations as well. There are two moderately PV-stained bands in layers 4 and 6 of the rostral portion of area 24 (Fig. 16E) that become faintly stained in the caudal portion (Fig. 19E). A scattered population of moderately stained PV immunopositive cell bodies is present in area 24 (Fig. 16E; 19E). Area 24 has two VGluT2 stained bands; a thin, moderately stained band in layer 4 and a fainter band in layer 6 (Fig. 16F; 19F). Compared to area 3b(S1)(Fig. 19F) and Para (Fig. 16F), area 24 expresses lower level of VGluT2 staining.
Area 23
Area 23, the posterior cingulate area, is bordered dorsally by area 7m and ventrally by area 30. In Nissl preparations, the upper cortical layers of area 23 is paler than area 7m and the granular layer 4 of area 24 is thinner than that in area 7m. Furthermore, area 23 has a darker appearance than area 30 in Nissl stained sections. In myelin preparations, area 23 is less densely myelinated than the adjoining areas 7m and 30 (Fig. 8B). Area 23 is moderately stained in CO preparations, with a single stained band in layer 4 (Fig. 8C). Compared to areas 7m and 30, area 23 is more lightly CO stained (Fig. 8C). Area 23 is more darkly stained, especially inner layer 6, than area 7m in sections stained for free ionic zinc (Fig. 8D). Additionally, area 23 is more darkly stained throughout the cortical layers than area 30 (Fig. 8D). In PV preparations, area 23 is less intensely stained than both areas 7m and 30 (Fig. 8E). Layer 4 of area 23 stains less intensely for VGluT2 immunopositive terminations than layer 4 of area 7m and stains at similar intensity to layer 4 of area 30 (Fig. 8F). A pale, thin band of VGluT2 immunopositive terminations is present in layer 6 of area 23 as well (Fig. 8F).
Retrosplenial agranular area (RSag)
The retrosplenial agranular area (RSag) in galagos is mostly buried within the calcarine sulcus. In Nissl preparations, RSag has a darkly stained layer 2, but otherwise is not well laminated. It has a paler appearance than the adjoining prostriata area (PS) due to a lowered cell packing density (Fig. 2A; 6A; 8A). RSag is moderately myelinated, and is more densely myelinated than PS and more sparsely myelinated than the retrosplenial granular area (RSg)(Fig. 2B; 6B; 8B). Layers 2 to 5 of RSag stain lightly for CO (Fig. 8C), and there is no distinct boundary between RSag and PS in CO preparations (Fig. 2C; 6C). In zinc preparations, RSag stains less intensely for corticocortical terminations that contain free ionic zinc compared to PS (Fig. 2D; 6D) and more intensely compared to RSg, especially in layer 2/3 (Fig. 2D; 6D). Layers 2/3, 4 and 6 of RSag stain with moderate intensity for PV immunopositive terminations, and layer 5 is lightly stained (Fig. 2E; 6E; 8E). Furthermore, RSag is populated by lightly stained PV immunopositive cell bodies (Fig. 2E; 6E; 8E). RSag stains darker than PS and lighter than RSg in PV preparations (Fig. 2E; 6E). In VGluT2 preparations, RSag is moderately stained (Fig. 8F), and is more lightly stained than both PS and RSg (Fig. 2F).
Retrosplenial granular area (RSg)
The retrosplenial granular region in galagos is bordered dorsally by RSag, and is buried within the calcarine sulcus. RSg, like RSag, does not have well defined laminar properties. In Nissl preparations, layer 2/3 of RSg is darkly stained, marking the RSag/RSg boundary (Fig. 2A; 6A). In myelin preparations, RSg is more densely myelinated than RSag and has a distinct outer band of Baillarger (Fig. 2B). RSg stains darker than RSag in CO preparations, with a moderately stained band in layer 2/3 (Fig. 2C; 6C). Layer 2/3 of RSg stains poorly, whereas upper layer 2, layer 5 and inner layer 6 stain darkly, as such RSg has a banded appearance in sections stained for free ionic zinc (Fig. 2D; 6D). In PV preparations, RSg stains darker than RSag, and has a band of PV immunopositive terminations in layer 2/3 and another in layer 5/6 (Fig. 2E; 6E). RSg stains darkly for VGluT2 immunopositive terminations, making a distinct RSag/RSg border (Fig. 2F).
Prostriata area (PS)
The prostriata area (PS) is a more recently defined visual area (Rosa et al., 1997) that appears to be present in most mammals (Rosa and Krubitzer, 1999). PS runs along the ventromedial boundary of area 17 and is bordered rostrally by the retrosplenial areas. In Nissl preparations, the border between area 17 and PS is marked by the reduction in thickness of layer 4 in PS (Fig. 2A; 6A). The PS/RSag border is clearly demarcated as the thin and densely populated granular layer 4 is present in PS, and is absent in RSag (Fig. 2A; 6A). In myelin preparations, PS is poorly myelinated (Fig. 2B; 6B). PS stains moderately for CO, with a darker band in layer 4 and a lighter band in layer 6 (Fig. 2C; 6C). In CO preparations, PS is more lightly stained than area 17 and is more darkly stained than RSag (Fig. 2C; 6C). In zinc preparations, the almost white band in layer 4 of area 17 terminates at the area 17/PS border (Fig. 2D). PS stains darker than RSag in sections stained for free ionic zinc (Fig. 2D; 6D). Layers 3/4 and 6 of PS stain moderately for PV immunopositive terminations and a scattering of lightly stained PV immunopositive cell bodies are present (Fig. 2E; 6E). In VGluT2 preparations, PS expresses two bands of VGluT2 immunopositive terminations, in layers 4 and 6 (Fig. 2F). PS is less intensely stained than area 17 and more intensely stained than RSag in sections stained for VGluT2 immunopositive terminations (Fig. 2F).
Remaining cortical areas – Perirhinal area (PRh)
In Nissl preparations, the perirhinal area (PRh) has a darkly stained layer 2 and does not have a well developed granular layer 4. Overall, PRh has a darker appearance than the adjoining entorhinal cortex (Ent)(Fig. 11A; 12A). PRh is poorly myelinated compared to the surrounding cortical areas, such as TG (Fig. 11B) and ITc (Fig. 12B), and stains poorly in CO preparations (Fig. 11C; 12C). In zinc preparations, PRh is darkly stained throughout the cortical layers (Fig. 11D; 12D). The architectonic boundaries of PRh are clearly demarcated in zinc preparations as PRh stains darker than the surrounding cortical areas (Fig. 11D). PRh has low immunoreactivity for both PV (Fig. 11E; 12E) and VGluT2 (Fig. 11F; 12F).
Discussion
Galagos belong to the prosimian suborder, one of the three major branches of the primate order. They are of special interest as they have retained many anatomical features of early primates (Radinsky, 1977; Jerison, 1979), and as such their brains may have changed the least in primate evolution (Martin, 1990; Fleagle, 1999). Past architectonic studies of galagos (e.g. von Bonin, 1942; von Bonin, 1945; von Bonin and Bailey, 1961; Kanagasuntheram et al., 1966; Zilles et al., 1979; Preuss and Goldman-Rakic, 1991a) utilized a limited range of histological stains to characterize the cortical areas of galagos. In this study, we have used a battery of histological and immunohistochemical procedures to reveal and characterize the architectonic subdivisions of galago neocortex that have known or presumed functional significance. Some of these procedures give hint to the functional properties of a cortical area. For example, cortical areas with a layer 4 that is densely populated withVGluT2 and PV terminations, and few terminations containing free ionic zinc would suggest that the layer is dominated by thalamic rather than cortical inputs (Van Brederode et al., 1990; DeFelipe and Jones, 1991; Hackett et al., 1998; de Venecia et al., 1998; Latawiec et al., 2000; Fujiyama et al., 2001; Kaneko and Fujiyama, 2002; Wong and Kaas, 2008; Hackett and de la Mothe, 2009; Wong and Kaas, 2009a, 2009b). Furthermore, areas that stain darkly for CO are typically sensory areas that have high metabolic activity (Wong-Riley et al., 1978; Wong-Riley, 1979). These procedures will provide an improved overview of how the neocortex is organized in galagos. Our broader goal is to understand what features of cortical organizations are shared between prosimians and anthropoid primates, and features that may be different. Different features in cortical organizations between prosimian galagos and anthropoid primates may reflect functional specializations As such, the present results are discussed in relation to previous architectonic studies of both prosimians and anthropoid primates.
Occipital cortex
Area 17
The occipital cortex of galagos consists of a large primary visual cortex (V1 or area 17) that occupies most of the caudal pole of the cerebral hemisphere. Extrastriate areas in the occipital lobe of galagos follow that of the primate visual system organization, sharing common cortical areas such as secondary visual cortex (V2 or area 18) that contains a second-order representation of the visual field (Rosa et al., 1997), and the third visual area, V3 (Lyon and Kaas, 2002a). Other proposed visual areas include the middle temporal visual area (MT)(Allman et al., 1973; Rosa et al., 1997; Collins et al., 2001; Kaskan and Kaas, 2007), the dorsomedial visual area (DM)(Rosa et al., 1997; Beck and Kaas, 1998a; Beck and Kaas, 1998b; Collins et al., 2001) and the dorsolateral visual area (DL)(Collins et al., 2001).
In galagos, the surface area of V1 is about 200mm2, and is two to three times the surface area occupied by V1 of nocturnal nonprimates with similar body mass (Rosa et al., 1997), such as ferrets (Law et al., 1988) and hedgehogs (Kaas et al., 1970). Microelectrode recording studies have shown that V1 is retinotopically organized, with central vision represented on the anterolateral surface and peripheral vision represented on the medial wall, covering the banks of the calcarine sulcus (Rosa et al., 1997). V1 is co-extensive with the architectonically defined area 17 of Brodmann (1909). Area 17 has pronounced lamination pattern compared to adjoining area 18 that allowed it to be easily identified in early architectonic studies (e.g. von Bonin, 1942; von Bonin, 1945; von Bonin and Bailey, 1961; Kanagasuntheram et al., 1966; Zilles et al., 1979; Preuss and Goldman-Rakic, 1991a). Similar to primates and other mammals, layer 4 of area 17 is densely populated with granule cells in galagos. However, layer 4 of area 17 in galagos corresponds to layers 4Cα and 4Cβ as identified in anthropoid primates by Brodmann (1909), and in most studies today. Hassler (1967) was one of the first to stress that Brodmann (1909) had misidentified layers in area 17 of anthropoid primates, and that sublayers 4A and 4B of Brodmann were sublayers of layer 3. The laminar designations used here and previously for prosimian primates apply to all primates (e.g. Weller and Kaas, 1982; Diamond et al., 1985; Florence and Casagrande, 1987). Unlike area 17 in tree shrews (Lund et al., 1985; Wong and Kaas, 2009a), a cell poor cleft does not divide layer 4 into sublayers 4a and 4b in galagos. Layer 4 has been identified in previous architectonic studies of area 17 in galagos as in the present study (e.g. von Bonin, 1942; von Bonin, 1945; von Bonin and Bailey, 1961; Kanagasuntheram et al., 1966; Zilles et al., 1979; Preuss and Goldman-Rakic, 1991a). In terms of connections with the lateral geniculate nucleus, layer 4 of area 17 in galagos has similar connection patterns with that of monkeys (e.g. Hubel and Wiesel, 1972; Tigges et al., 1977; Hendrickson et al., 1978). Layer 4a of area 17 in galagos can be defined as the sublayer that receives projections from the magnocellular layers 1 and 2 of the lateral geniculate nucleus, whereas layer 4b is the sublayer that receives projections from the parvocellular layers 3 and 6 of the lateral geniculate nucleus (Glendenning et al., 1976; Diamond et al., 1985). In our preparations, layer 4 of area 17 in galagos is also characterized by dense expressions of PV and VGluT2 immunopositive terminations, reflecting dense inputs from the lateral geniculate nucleus of the sensory thalamus (Glendenning et al., 1976; Diamond et al., 1985). Furthermore, the poor zinc staining of layer 4 suggests that this layer is dominated by thalamic rather than cortical inputs.
Layer 3 of area 17 in galagos does not have the clear sublayers of many anthropoid primates. This layer is broad and has a heterogeneous architectonic appearance. This layer consists mainly of small pyramidal cells, with clusters of darkly stained cells that appear to co-localize with patches of PV-immunopositive terminations and CO rich patches known as CO blobs. In anthropoid primates, CO blobs are regions where processing of chromatic information takes place (Livingstone and Hubel, 1984; Hendrickson, 1985; Livingstone and Hubel, 1987; Tootell et al., 1988; Ts'o and Gilbert, 1988). Galagos do not have color vision (Petry and Hárosi, 1990). However, the distribution of CO blobs in galagos (Condo and Casagrande, 1990) is similar to that of diurnal anthropoid primates (Carroll and Wong-Riley, 1984; Livingstone and Hubel, 1984; Livingstone and Hubel, 1987). The CO blobs in galagos, as with the CO blobs in the nocturnal owl monkey (Ding and Casagrande, 1997), have been shown to be the target of projections from the koniocellular layers of the lateral geniculate nucleus (Lachica and Casagrande, 1992). Additionally, layer 3 of area 17 in galagos receives inputs from sublayers 4A and 4B (Casagrande et al., 1989) that converge within the CO blobs (Lachica et al., 1993). This has been observed in the diurnal anthropoid primates, macaques (Lachica et al., 1992) and squirrel monkeys (Lachica et al., 1993). The CO blobs in layer 3 of area 17 in galagos are also co-extensive with patchy callosal input to much of area 17 (Cusick et al., 1984). In addition to the intrinsic, callosal and thalamic projections to layer 3 of area 17 in galagos, layer 3 is the projection target of other extrastriate visual areas as well (Diamond et al., 1985; Cusick and Kaas, 1988; Krubitzer and Kaas, 1988; Krubitzer and Kaas, 1989; Beck and Kaas, 1998a; Collins et al., 2001; Lyon and Kaas, 2002c). The cortical projections to layer 3 of area 17 in galagos may terminate in a patchy manner, as evidenced by the patchy distribution of synaptic zinc, which is found in corticocortical terminations (Danscher, 1982; Frederickson and Moncrieff, 1994; Frederickson et al., 2000; Ichinohe et al., 2003; Ichinohe and Rockland, 2004), in sections cut parallel to the artificially flattened cortex (Fig. 3). The patchy distributions of the zinc and VGluT2 stains may reflect a pattern of VGluT2-rich terminals of the lateral geniculate nucleus inputs, likely from the koniocellular layers 4 and 5, that are surrounded by walls of zinc-enriched terminals of projections from other visual cortical areas (Carey et al., 1979; Casagrande and De Bruyn, 1982; Diamond et al., 1985).
Area 18
Area 18 of this study corresponds to the second visual area, V2 (Rosa et al., 1997). V2 of galagos is a homologue of V2 that has been described in anthropoid primates (Allman and Kaas, 1974; Gattass et al., 1981; Rosa et al., 1988) and other mammals. The visuotopic organization of V2 in galagos (Rosa et al., 1997) is similar to that of anthropoid primates (e.g. Allman and Kaas, 1974; Gattass et al., 1981), with a complete representation of the visual field. However, galagos differ from anthropoid primates (Allman and Kaas, 1971a; Rosa et al., 1988) in that V2 of galagos does not completely surround V1 as V1 is bordered by area prostriata in the calcrine sulcus (Allman and Kaas, 1971a; Sanides, 1972; Gattass et al., 1987; Rosa et al., 1997). Furthermore, V2 of galagos is small and narrow, with a surface area of about 65mm2 and a maximum width of about 3mm (Rosa et al., 1997). Area 18 of galagos has also been identified in previous architectonic studies (von Bonin, 1942; von Bonin, 1945; von Bonin and Bailey, 1961; Kanagasuntheram et al., 1966; Zilles et al., 1979; Preuss and Goldman-Rakic, 1991) and closely corresponds to area occipitalis 2 of Zilles et al. (1979). Architectonically, area 18 of galagos has lowered cell packing density in layers 4 and 6 than area 17 and the overall laminar pattern of area 18 is less distinct. Furthermore, area 18 expresses less PV and VGluT2 immunopositive terminations and more synaptic zinc in layers 4 and 6 than area 17. These observations suggest that area 18 receives proportionately less thalamocortical and more corticocortical inputs, including dense, topographically organized inputs from area 17 (Symonds and Kaas, 1978; Cusick and Kaas, 1988), and other extrastriate areas such as MT (Wall et a., 1982; Krubitzer and Kaas, 1990a) and DM (Krubitzer and Kaas, 1993; Beck and Kaas, 1998a). In anthropoid primates, area 18 shows a distinct, regular periodic pattern of CO staining, with thick and thin CO stripes (Wong-Riley and Carroll, 1984; Tootell et al., 1985; Hendrickson, 1985; Livingstone and Hubel, 1987). Area 18 of galagos, however, only shows a weak pattern of patches or bands CO preparations (Condo and Casagrande, 1990; Kaskan and Kaas, 2007), and these were not apparent in the present preparations.
Area 19
Area 19 is used here to refer to the third visual area, V3, that was first defined in macaque monkeys based on the pattern of connections with V1 (Cragg, 1969; Zeki, 1969). More recently, V3 has been defined in several New World and Old World monkeys (Gattass et al., 1988; Sousa et al., 1991; Lyon and Kaas, 2001; Lyon and Kaas, 2002b; Lyon and Kaas, 2002c), and consists of the dorsal and ventral portions and a mirror reversal of the retinotopy of V2. In galagos, the dorsal and ventral portions of area 19 or V3 have not always been differentiated from the dorsomedial (DM) and dorsolateral (DL) visual areas (e.g. Rosa et al., 1997; Beck and Kaas, 1998a; Collins et al., 2001) because microelectrode mapping studies failed to provide compelling evidence for the presence of V3 (Allman and Kaas, 1975; Allman et al., 1979; Rosa et al., 1997) and V3 did not have distinct enough staining characteristics to allow it to be differentiated from DM, DL or area 18 (von Bonin, 1942; von Bonin, 1945; von Bonin and Bailey, 1961; Kanagasuntheram et al., 1966; Zilles et al., 1979; Preuss and Goldman-Rakic, 1991a). As a result, the lack of a distinct architectonic area 19 in galagos may have affected the interpretation of the results in studies of connections. Some foci of label observed in previous studies of connections were within the dorsal and ventral portions of V3, but they were not differentiated from DM and DL. By placing injections of multiple tracers along the upper and lower field representations of V1, Lyon and Kaas (2002a) were able to provide clear connectional evidence for the existence of V3 in galagos. In sections from flattened cortex, V3 expressed more CO than V2 and was more myelinated, but these characteristics were similar to those in DM. V3 in galagos is narrower than V2 and likely to be discontinuous in the middle, such that the dorsal portion, containing the lower visual field representation, and the ventral portion, containing the upper visual field representation, are separate (Lyon and Kaas, 2002a). Further evidence for the existence of V3 comes from placements of tracers into MT of galagos that labeled neurons in the dorsal and ventral portions of V3 (Kaskan and Kaas, 2007).
The lack of distinct architectonic characteristics of area 19 has also lead to varying definitions of area 19. An early architectonic study of galago neocortex by von Bonin (1945) has an area 19 that is rather large and likely includes portions, if not all, of DM and DL. Using the cresyl-fast-violet stain for cell bodies, Zilles and colleagues (1979) identified a second visual area (Oc2) and rostrally adjoining parietal area 2 and temporal area 4, with no area 19, DM, nor DL. We observe that area 19 has a thin granular layer 4, moderate myelination density and CO expression. In zinc preparations, area 19 expresses high levels of synaptic zinc compared to the adjoining area 18, DM and DL. Area 19 expresses moderate levels of PV and VGluT2 immunopositive terminations, and compared to the adjoining area 18, stains more darkly in PV and VGluT2 preparations. This suggests that area 19 receives proportionately more corticortical and thalamocortical terminations than area 18.
DM and DL
DM is a visual area first recognized in owl monkeys (Allman and Kaas, 1975), and it is at least roughly co-extensive with the area sometimes referred to as V3A (Van Essen and Zeki, 1978). DM of monkeys has connections with from V1, V2, MT and posterior parietal cortex (Wagor et al., 1975; Zeki, 1980; Burkhalter et al., 1986; Stepniewska and Kaas, 1996; Felleman et al., 1997; Gattass et al., 1997; Beck and Kaas, 1998a; Lyon and Kaas, 2002a). Similarly, DM of galagos has connections with V1, V2, MT and posterior parietal cortex (Rosa et al., 1997; Beck and Kaas, 1998a; Collins et al., 2001; Lyon and Kaas, 2002a). The cortical area defined as DM in earlier studies likely included the dorsal portion of V3, and as such, the findings that were reported for DM may also apply to the dorsal portion of V3 (for review see Kaas and Lyon, 2001; Lyon and Kaas, 2002a). DM as defined here is similar in location to area D of Preuss and Goldman-Rakic (1991a) and the caudal portion of Pa2 of Zilles and colleagues (1979). The rostral portion of cortex rostral defined as area 19 by von Bonin (1945) would include DM. Architectonically, DM has a thin, densely packed granular layer 4 and is moderately myelinated. In zinc preparations, DM expresses less synaptic zinc than the adjoining DL and area 19d. This indicates that DM receives proportionately less corticortical inputs than DL and area 19d. Compared to DL, DM also expresses more PV immunopositive terminations and similar levels of VGluT2 immunopositive terminations, indicating that DM receives proportionately more thalamocortical inputs than DL.
DL, first described in owl monkeys (Allman and Kaas, 1974) and subsequently reduced in extent next to MT (Kaas and Morel, 1993) roughly corresponds to V4 (Stepniewska et al., 2005a), and is strongly interconnected with V2 and portions of the superior temporal lobe in monkeys (Tigges et al., 1974; Weller and Kaas, 1985; Cusick and Kaas, 1988; Steele et al., 1991; Stepniewska and Kaas, 1996; Gattass et al., 1997). In galagos, similar connection patterns are observed (Collins et al., 2001). As in monkeys, DL in galagos also has connections with V1 (Lyon and Kaas, 2002a), MT and MTc (Kaskan and Kaas, 2007). In previous architectonic studies, the region of DL was included within the rostroventral portion of area 19 of von Bonin (1945), the temporal areas 4 and 5 of Zilles et al. (1979) and the rostral portion of V4 or area D of Preuss and Goldman-Rakic (1991a). Architectonically, DL has a thin, moderately stained granular layer 4 and is less densely myelinated than the adjoining DM, MT and area 19. DL expresses more synaptic zinc, and less PV and VGluT2 immunopositive terminations than the surrounding cortical areas. This indicates that DL receives proportionately more corticocortical and less thalamocortical terminations than the surrounding cortical areas.
Temporal cortex
MT
Visual associated areas in the temporal lobe of galagos include the middle temporal visual area (MT), the crescent surrounding MT (MTc), the middle superior temporal area (MST) and the fundus of the superior temporal area (FST). First described in owl monkeys (Allman and Kaas, 1971a), MT is now considered a visual area common to all primates (Kaas, 1997). MT is involved in visual motion processing and contains neurons that are selective for the direction of moving visual stimuli (Allman and Kaas, 1971a; Zeki, 1974; Van Essen et al., 1981; Rosa and Elston, 1998). There is a complete retinotopic representation of the visual hemifield in MT (Allman et al., 1973; Rosa et al., 1997; Collins et al., 2001; Kaskan and Kaas, 2007). In monkeys, MT receives direct corticocortical inputs from V1 and V2 (Allman et al., 1973; Rosa et al., 1997; Orban, 1997; Collins et al., 2001; Britten, 2003; Born and Bradley, 2005; Kaskan and Kaas, 2007), and some thalamocortical inputs from the lateral geniculate nucleus (Stepniewska et al., 1999; Sincich et al., 2004). The medial subdivision of the inferior visual pulvinar provides the major inputs (Lin and Kaas, 1980; O'Brien et al., 2001). Architectonically, MT of monkeys is characterized by dense myelination and dark staining in CO preparations (e.g. (Allman and Kaas, 1971b; Spatz and Tigges, 1972; Spatz, 1977; Van Essen et al., 1981; Tootell et al., 1985). Furthermore, MT of marmoset monkeys stains darkly in PV preparations (Bourne et al., 2007).
MT of galagos was first identified by Allman and colleagues (1973) as an oval region that has a surface area of approximately 18mm2 (Xu et al., 2004; Beck and Kaas, 1998a; Allman et al., 1973) and has similar corticocortical connection patterns to MT of monkeys (Cusick and Kaas, 1988; Krubitzer and Kaas, 1990a; Collins et al., 2001; Kaskan and Kaas, 2007). MT in galagos has a somewhat different connections with the inferior pulvinar compared to monkeys. In addition to receiving inputs from the medial nucleus of the inferior pulvinar like in monkeys, MT in galagos receives projections from the rest of the inferior pulvinar, the posterior and caudal subdivisions, as well (Wong et al., 2009c). The cortical area that we identify as MT corresponds to that of Preuss and Goldman-Rakic (1991a) and is in a similar location, albeit smaller, to that identified by Zilles and colleagues (1979). von Bonin did not identify an area that may be in MT in the cortical map of galagos (1945). Architectonically, MT of galagos has a similar appearance to MT of monkeys, with dense myelination and high CO expression (Allman et al., 1973; Wall et al., 1982; Krubitzer and Kaas, 1990a; Collins et al., 2001; Kaskan and Kaas, 2007; Wong et al., 2009c). These previous findings are congruent with our current observations. In addition, the reduced zinc staining compared to surrounding cortex likely reflects the presence of dense thalamocortical inputs from the inferior pulvinar, while zinc staining that is present corresponds to corticocortical projections from cortical areas, such as V1 and V2 (Wall et al., 1982; Cusick and Kaas, 1988; Krubitzer and Kaas, 1990a; Collins et al., 2001; Kaskan and Kaas, 2007). The dark staining for PV and VGluT2 immunopositive terminations in layers 3 and 4 also reflects presence of strong projections from thalamic nuclei, such as the inferior pulvinar (Weller and Kaas, 1982).
MTc
Much of MT is bordered by a thin strip of cortex, MTc, first identified in New World owl monkeys (Kaas and Morel, 1993) and more recently in marmosets (Palmer and Rosa, 2006). In Old World macaques, part of this region had been previously identified as V4t (Ungerleider and Desimone, 1986). In monkeys (Ungerleider and Desimone, 1986; Kaas and Morel, 1993; Palmer and Rosa, 2006) and galagos (Kaskan and Kaas, 2007), MTc has connections with MT, the adjacent MST and FST areas, as well as with V1, V2, V3, DL, and portions of the posterior parietal and inferior temporal cortex. Architectonically, MTc in galagos has a heterogenous appearance and is probably best observed in sections from artificially flattened cortex that was cut tangential to the pia. In these sections stained for CO, MTc consists of a series of CO-dense puffs and CO-sparse surrounds (Kaskan and Kaas, 2007). Furthermore, MTc stains lighter for PV and VGluT2 immunopositive thalamocortical terminations and darker for synaptic zinc in corticocortical terminations compared to MT. This is likely to be due to the proportionately larger population of inputs from other cortical areas, such as MT, V1 and V2 (Kaskan and Kaas, 2007), and lower population of inputs from thalamic nuclei.
FST and MST
The fundal area of the superior temporal sulcus (FST) was first defined in macaque monkeys with a deep superior temporal sulcus (Desimone and Ungerleider, 1986). FST in monkeys has strong connections with both MT (Boussaoud et al., 1990; Krubitzer and Kaas, 1990a; Kaas and Morel, 1993; Palmer and Rosa, 2006) and MTc (Kaas and Morel, 1993). The medial superior temporal area (MST) was named as a projection target of MT in macaque monkeys by Maunsell and Van Essen (1983). Across primates, MST receives projections from MT (Weller et al., 1984; Krubitzer and Kaas, 1990a; Rosa et al., 1993). FST of galagos is highly interconnected with MT as well (Krubitzer and Kaas, 1990a; Kaskan and Kaas, 2007). MST of galagos is a darkly CO stained oval that is located rostrally to MT (Kaskan and Kaas, 2007; present study), and is highly interconnected with MT (Kaskan and Kaas, 2007). Both FST and MST express proportionately more synaptic zinc, and less PV and VGluT2 thalamocortical immunopositive terminations than MT. This indicates that a large proportion of inputs to FST and MST of galagos originate from surrounding cortical areas, such as MT (Krubitzer and Kaas, 1990a; Kaskan and Kaas, 2007).
Inferior temporal and remaining temporal areas
The inferior temporal cortex of galagos has previously been divided into at least two (von Bonin, 1945), to as many as five (Preuss and Goldman-Rakic, 1991a) areas. Here, we have defined four areas in the inferior temporal cortex, the interior temporal rostral (ITr), inferior temporal caudal (ITc), temporopolaris (TG) and superior temporal dorsal (STd) areas. ITr overlaps with TEm and perhaps a portion of LMZ of Preuss and Goldman-Rakic (1991a), and the caudal portion of Te2.2 and rostral portion of Te5 of Zilles and colleagues (1979). Architectonically, TEm has a well defined layer 4 (Preuss and Goldman-Rakic, 1991a). This is congruent with our observations on ITr. Additionally, ITr appears to have both a moderate population of corticocortical terminations, identified by the expression of synaptic zinc, and a moderate population of VGluT2 immunopositive thalamocortical terminations. ITc overlaps with TEc of Preuss and Goldman-Rakic (1991a) and Te5 of Zilles and colleagues (1979). This caudal portion of the inferior temporal cortex in galagos is moderately myelinated, as in macaques and owl monkeys (Preuss and Goldman-Rakic, 1991a; Fig. 12C). Additionally, ITc may receive proportionately more PV and VGluT2 immunopositive thalamocortical terminations than synaptic zinc containing corticocortical terminations. In monkeys, a major input to ITc is from DL(V4)(see Stepniewska et al., 2005 for review), but there is only limited evidence for this from the cortical injections that involves DL in galagos (Wall et al., 1982; Collins et al., 2001; Kaskan and Kaas, 2007). In addition, injections in MTc labeled cells in ITr and ITc (Kaskan and Kaas, 2007). Furthermore, ITc has projections to V2 (Collins et al., 2001). The present TG is in a similar location to TG, and perhaps a portion of TEr, of Preuss and Goldman-Rakic (1991a), and Te6 of Zilles et al. (1979). Architectonically, TG is sparsely myelinated (Preuss and Goldman-Rakic, 1991a; present study). Additionally, there is evidence from the higher levels of synaptic zinc and lower levels of VGluT2 expression that TG receives proportionately more corticocortical terminations and proportionately less thalamocortical terminations than ITr. Our STd is in a similar location to STd of Preuss and Goldman-Rakic (1991a) and overlaps the rostral portion of Te2.2 of Zilles et al. (1979). Architectonically, STd has a thin layer 4 and broad layers 5 and 6, and is moderately myelinated (Preuss and Goldman-Rakic, 1991a; present study). Furthermore, STd expresses proportionately more synaptic zinc positive corticocortical terminations and proportionately less PV and VGluT2 immunopositive thalamocortical terminations than ITr and ITc.
Auditory areas
The auditory cortex of galagos contains at least two core sensory areas and a surrounding belt region (Kanagasuntheram et al., 1966; Brugge, 1982; Preuss and Goldman-Rakic, 1991a). Primary auditory cortex of galagos is located on the caudal bank of the lateral sulcus within the temporal lobe (Kanagasuntheram et al., 1966; Zilles et al., 1979; Brugge, 1982; Conley et al., 1991; Preuss and Goldman-Rakic, 1991a). In monkeys, two major core auditory fields, A1 and a rostral area (R) have been identified (Allman and Kaas, 1971b; Merzenich and Brugge, 1973; Imig et al., 1977) and receive inputs from the ventral subdivision of the medial geniculate nucleus (Morel et al., 1993; Kaas et al., 1999; Kaas and Hackett, 2000; de la Mothe et al., 2006). Two primary-like auditory areas have been identified in galagos as well, the koniocellular primary auditory cortex (A1), which receives projections from the ventral subdivisions of the medial geniculate nucleus in the thalamus (Conley et al., 1991), and the rostral auditory area (R) with similar architecture and likely similar thalamic inputs. An auditory responsive lateral belt, possibly consisting of at least two areas, was also identified (Brugge, 1982). The lateral auditory belt has connections with the secondary, but not primary, subdivisions of the medial geniculate body (Conley et al., 1991).
The primary-like auditory fields A1 and R have similar architectonic appearances, and as such were described in this study as a single field, A. Area A has a koniocellular appearance with a densely packed layer 4 and is densely myelinated (Preuss and Goldman-Rakic, 1991a). These observations are congruent with our results. Furthermore, area A expresses more PV and VGluT2 immunopositive terminations, and less synaptic zinc than the surrounding areas. This suggests that area A receives denser inputs from the sensory thalamus, likely the ventral subdivision of the medial geniculate nucleus (Conley et al., 1991), and sparser inputs from other cortical areas compared to the adjoining areas. The lateral and medial belt areas that surround area A are less densely packed with cells and less myelinated than area A. The darker staining in zinc preparations suggests that both the lateral and medial Ab receives more corticocortical projections than area A. PV and VGluT2 immunopositive thalamic terminations are present in layer 4 of both lateral and medial Ab, although to a lesser extent than area A. Some of these thalamic projections are likely to originate from the secondary subdivisions of the medial geniculate body (Conley et al., 1991).
Parietal cortex
Area 3b(S1)
In prosimian galagos, there are at least three cortical areas in the anterior parietal cortex, the primary somatosensory area, 3b(S1), 3a, and 1/2 (Sur et al., 1980; Carlson and Welt, 1980; 1981; Wu and Kaas, 2003). Area 3b(S1) has a topographic representation of the contralateral body surface that begins with the oral cavity and face representations ventrally and progresses medially to the hand, arm, trunk, and on the medial wall is the hindlimb representation (Carlson and Welt, 1980; Sur et al., 1980; Wu and Kaas, 2003), as for monkeys (Kaas, 1983; Kaas and Pons, 1988; Kaas, 2007) and other mammals (for review, see Kaas, 1983). Area 3b(S1) of galagos corresponds to Pa1 of Zilles et al., (1979). As expected of primary sensory areas, area 3b(S1) of galagos has a koniocellular appearance with a layer 4 that is densely packed with granule cells (Kanagasuntheram et al., 1966; Preuss and Goldman-Rakic, 1991a; present study), is densely myelinated and metabolically active. Furthermore, layer 5 of area 3b(S1) in galagos contains large, elongated PV immunopositive pyramidal cells (Preuss and Kaas, 1996). Area 3b(S1) of galagos also expresses low levels of free zinc ions, and is densely populated with PV and VGluT2 immunopositive thalamocortical terminations. This suggests that layer 4 receives proportionately more inputs from the thalamus than from other cortical areas. Area 3b(S1) of galagos receives thalamic inputs from the ventroposterior nucleus, as in other mammals (Kaas, 1982; Burton and Carlson, 1986; see Kaas, 1983 for review). As in squirrels (Wong and Kaas, 2008) and tree shrews (Wong and Kaas, 2009a), architectonic features of area 3b(S1) are not uniform throughout, especially in layer 4, where the thickness varies throughout the extent of the cortical area (Fig. 16). The variable thickness of layer 4 in area 3b(S1) of galagos is likely to be related to discontinuities in the cortical representations of the cutaneous surface.
Area 3a
Area 3b(S1) of galagos is bordered rostrally by area 3a, a narrow strip of cortex where neurons are activated by taps to the body and noncutaneous stimuli, which suggests the activation of deep receptors in muscles and joints (Sanides and Krishnamurti, 1967; Kaas, 1983; Wu and Kaas, 2003). Movements can be evoked by microstimulation of neurons in area 3a (Stepniewska et al., 1993; Wu and Kaas, 1999), and microelectrode stimulation studies have revealed a motor map in area 3a of galagos that is organized in a manner parallel to the somatotopic map in area 3b(S1) (Wu and Kaas, 2003). As in monkeys (Huerta and Pons, 1990; Huffman and Krubitzer, 2001), area 3a of galagos has connections to area 3b(S1), S2 and Pv (Wu and Kaas, 2003). Architectonically, area 3a of galagos has a thin layer 4 and larger pyramidal cells in layer 5 (Preuss and Goldman-Rakic, 1991a; Wu et al., 2000; present study). A subpopulation of the larger pyramidal cells in layer 5 of area 3a is PV immunopositive (Preuss and Kaas, 1996). Area 3a of galagos is also less densely myelinated and expresses less CO than area 3b(S1). Furthermore, area 3a has more synaptic free zinc ions, and less PV and VGluT2 immunopositive terminations. This suggests that area 3a receives proportionately more corticocortical than thalamocortical projections. Area 3a in galagos is likely to be homologous to Poc2 of Zilles et al. (1979). The cortical connections with area 3b(S1), S2 and Pv (Wu and Kaas, 2003) likely contribute to the dark staining of area 3a in zinc preparations. Area 3a in galagos is also likely to receive projections from the ventroposterior superior nucleus, as in monkeys (Cusick et al., 1985).
Area 1/2
The cortical area caudal to area 3b(S1), area 1/2, is an area that has topographic connections with area 3b(S1) and is likely to receive inputs from the ventral thalamus representing receptors mediating proprioception (Kaas and Pons, 1988; Wu and Kaas, 2003). Architectonically, area 1/2, which extends onto the medial cortical surface, is characterized by a moderately populated layer 4 and larger pyramidal cells in layer 5 compared to area 3b(S1)(Preuss and Goldman-Rakic, 1991a; Wu and Kaas, 2003; present study), and reduced myelination with no distinct bands of Baillarger. In layer 4, area 1/2 expresses higher levels of free synaptic zinc and lower levels of PV and VGluT2 immunopositive thalamocortical terminations than area 3b(S1). This suggests an increase in proportion of corticocortical over thalamocortical inputs. Some of these cortical inputs may be from area 3b(S1) (Wu and Kaas, 2003). Area 1/2 of galagos overlaps area 5 of Brodmann (1909), area 2–5 of Preuss and Goldman-Rakic (1991a), and the rostral portion of Pa2 of Zilles et al. (1979). Area 1/2 of galagos may be related to areas 1 and 2 of monkeys (Wu and Kaas, 2003). By location, architectonic and some connectional properties, area 1/2 of galagos is similar to the caudal somatosensory area, SC, of tree shrews (Remple et al., 2006; Remple et al., 2007; Wong and Kaas, 2009a), and the parietal medial area of squirrels (Krubitzer et al., 1986; Slutsky et al., 2000; Wong and Kaas, 2008) and rats (Donoghue and Parham, 1983; Reep et al., 1990; Reep et al., 1994; Wang and Kurata, 1998).
S2 and Pv
Areas S2 and Pv lie posterior and ventral to area 3b(S1) and extend onto the upper bank of the lateral sulcus. These two areas have been identified in some species of monkeys (Cusick et al., 1989; Krubitzer et al., 1995; Qi et al., 2002) and other mammals (see Disbrow et al., 2000 for review) such as squirrels (Nelson et al., 1979; Wong and Kaas, 2008), rats (Walker and Sinha, 1972; Remple et al., 2003) and tree shrews (Remple et al., 2006; Wong and Kaas, 2009a). In galagos, S2 (Burton and Carlson, 1986; Wu and Kaas, 2003) and Pv (Wu and Kaas, 2003) have separate somatotopic representations that are organized such that the face representations of both S2 and Pv adjoin the face presentation in area 3b(S1)(Wu and Kaas, 2003). The neurons in S2 and Pv have a larger receptive field than the neurons in area 3b(S1)(Wu and Kaas, 2003). Both S2 and Pv of galagos, like most other mammals, have topographic connections with area 3b(S1)(Wu and Kaas, 2003). Furthermore, S2 and Pv are densely interconnected (Wu and Kaas, 2003). S2 of galagos has differing thalamocortical connections compared to other monkeys. In galagos, S2 receives dense inputs from the ventroposterior nucleus (Burton and Carlson, 1986; Garraghty et al., 1991), whereas in monkeys, S2 receives dense inputs from the ventroposterior inferior rather than ventroposterior proper nucleus (Garraghty et al., 1990; Krubitzer and Kaas, 1992). Architectonically, S2 and Pv have similar appearances (Wu and Kaas, 2003; present study). Both S2 and Pv have thinner granular layer 4, and are less densely myelinated than area 3b(S1). The increased expression of synaptic zinc in the corticocortical terminations in S2 and Pv compared to area 3b(S1) suggests that S2 and Pv receive proportionately more corticocortical inputs than area 3b(S1). Furthermore, S2 and Pv express lower levels of PV immunopositive thalamocortical terminations than area 3b(S1), suggesting a reduced population of thalamic projections to S2 and Pv. However, S2 and Pv express moderate levels of VGluT2 immunopositive thalamocortical terminations. These thalamocortical terminations are likely to originate from the ventroposterior nucleus (Burton and Carlson, 1986; Garraghty et al., 1991).
Posterior parietal cortex
In galagos, the posterior parietal cortex, which extends onto the medial cortical surface, is also known as Brodmann's area 7 (Brodmann, 1909) and overlaps with Pa2 of Zilles and colleagues (1979). We defined three areas, PPr, PPc and PPl, within the posterior parietal cortex, which has been divided into as many as six areas (Preuss and Goldman-Rakic, 1991a). The rostral portion of the posterior parietal cortex, area 7b in monkeys, is involved in higher-order processing of somatosensory information (Kaas, 2004). In macaques, area 7b contains neurons that are responsive to visual and somatosensory stimulation (Hyvärinen and Shelepin, 1979; Robinson and Burton, 1980a; 1980b). Furthermore, area 7b of monkeys has connections with the premotor, prefrontal, caudal posterior parietal and superior temporal sulcus areas (Cavada and Goldman-Rakic, 1989; Lewis and Van Essen, 2000). The region defined as area 7b of galagos by Wu and Kaas (2003) corresponds in location to area 7b of macaques (Wu and Kaas, 2003). In galagos, the rostral portion of the posterior parietal cortex, PPr and PPl, or area 7b (Preuss and Goldman-Rakic, 1991a) has a crude map of complex behaviors that can be evoked by microstimulation (Stepniewska et al., 2005; 2009a). Furthermore, this rostral posterior parietal cortex region has connections with S2, Pv, M1 and premotor areas (Wu and Kaas, 2003; Fang et al., 2005; Stepniewska et al., 2005; 2009b). Architectonically, PPr and PPl have similar appearances and possess the characteristics of association cortex. Both PPr and PPl of galagos have a thin layer 4 and are moderately myelinated. In zinc preparations, PPr and PPl express high levels of synaptic free zinc ions, with PPl staining more darkly than PPr. PPr expresses more PV immunopositive thalamocortical terminations than PPl and similar levels of VGluT2 thalamocortical terminations to PPl. Compared to the surrounding cortical areas, the dominant inputs to layer 4 of PPr and PPl are from other cortical areas. Thalamocortical inputs to layer 4 of PPr and PPl may originate from the ventral lateral nucleus and the anterior pulvinar, as in monkeys (Kaas, 2004). Between PPl and PPr, the darker staining in zinc preparations and lighter staining PV preparations indicates that PPl receives proportionately more corticortical and less thalamocortical inputs than PPr.
PPc, within the caudal portion of the posterior parietal cortex overlaps with area 7a of Preuss and Goldman-Rakic (1991a). PPc has both visual and visuomotor functions (Kaas, 2004). Visual inputs to PPc of area 7a of monkeys include those from the superior temporal cortex (Maunsell and van Essen, 1983) and dorsal regions of the prelunate gyrus (May and Andersen, 1986). In galagos, PPc has connections with V2, V3, DM, DL, and MT (Beck and Kaas, 1998a; Collins et al., 2001; Lyon and Kaas, 2002; Kaskan and Kaas, 2007). Architectonically, PPc of galagos has a moderately populated granular layer 4. Compared to the surrounding cortical areas, PPc stains lighter for free ionic zinc, and darker for PV and VGluT2 immunopositive terminations. This suggests that layer 4 of PPc in galagos receives proportionately less corticocortical and more thalamocortical projections than the adjoining cortical areas MTc and area 7m. PPc may receive projections from the medial pulvinar, as in monkeys (Kaas, 2004).
Claustral cortex
We have retained the nomenclature from Zilles et al. (1979) and identified claustral cortex in galagos as the cortical areas that overlie the claustrum (Fig. 16). Claustral cortex in galagos is ventral to the somatosensory areas, and caudal to orbital frontal cortex. The claustral cortex of this study includes the insular and frontal parietal opercular areas of Preuss and Goldman-Rakic (1991a; 1991c). Claustral cortex in galagos is in a similar location to insular allocortex in another prosimian, the slow loris (Sanides and Krishnamurti, 1966), and to insular cortex in rodents (e.g. Zilles, 1990; Wong and Kaas, 2008) and in tree shrews (Zilles, 1978; Wong and Kaas, 2009a). Compared to monkeys, claustral cortex in galagos is in a similar location to precentral opercular (PrCO) area (Preuss and Goldman-Rakic, 1991a) or proisocortical motor area (Cipolloni and Pandya, 1999) in Old World macaques. In macaques, PrCO or the proisocortical motor area may be responsible for initiating motor movements related to orofacial, head and neck structures (Cipolloni and Pandya, 1999). Architectonically, PrCO or proisocortical motor area in macaques has thin, granular layers 2 and 4 (Mesulam and Mufson, 1982). Studies of connections in macaques showed that proisocortical motor area has widespread cortical connections, including connections with the ventral granular frontal cortex (Preuss and Goldman-Rakic, 1989), area 3b(S1), and the secondary somatosensory, insula, premotor and cingulate areas (Cipolloni and Pandya, 1999; Disbrow et al., 2003). In galagos, claustral cortex, also identified as area 13 to 16 or insular cortex by Pritzel and Markowitsch (1982), has connections with the orbital frontal areas 9, 10 and 11 of Brodmann (1909) and area 6. There are two architectonically distinct areas within the claustral cortex, the dorsal and ventral areas. Architectonically, both areas, CLId and CLIv have a thin layer 4, as in PrCO of macaques (Mesulam and Mufson, 1982) and are sparsely myelinated. The border between CLId and CLIv are distinct in zinc and VGluT2 preparations. CLId stains darker for synaptic zinc and lighter for VGluT2 immunopositive terminations compared to CLIv. This suggests that CLId receives proportionately more corticocortical inputs and less VGluT2 immunopositive thalamocortical inputs than CLIv. It is possible that claustral cortex of galagos has similar connectivity to that in PrCO or prosiocortical motor area in macaques (Cipolloni and Pandya, 1999; Disbrow et al., 2003), in addition to having connections with the orbital frontal cortex and area 6 (Pritzel and Markowitsch, 1982).
Frontal cortex
Primary motor cortex
Primary motor cortex (M1) is about 2 to 3mm wide in galagos (Wu et al., 2000) and extends from the dorsal surface on to the upper portion of the medial surface (Zuckerman and Fulton, 1941). Electrical stimulation studies with microelectrodes have shown that M1 contains an complete body and orofacial map, with the orofacial regions represented laterally, the trunk centrally and hindlimb represented dorsally (Zuckerman and Fulton, 1941; Kanagasuntheram et al., 1966; Fogassi et al., 1994; Wu et al., 2000). This region is coextensive with the architectonically defined M1, also known as area 4 of Brodmann (1909), area F posterior of Fogassi et al. (1994) and is contained within the area praecentralis 1 of Zilles et al. (1979). M1 of galagos has strong connections with the ventrolateral nucleus of the thalamus (Fang et al., 2006) and with premotor areas (Fang et al., 2005). M1 of galagos has the general architectonic features of motor cortices, such as a poorly developed granular layer 4, a thick layer 5 that is populated with large pyramidal cells, and moderate myelination. Furthermore, the large pyramidal neurons in layer 5 of M1 in galagos are also SMI-32 (Wu et al., 2000) and PV (Preuss and Kaas, 1996) immunopositive. M1 stains darkly for synaptic zinc, poorly for PV immunopositive terminations and moderately for VGluT2 immunopositive terminations. From the dark zinc staining, it is likely that M1 receives proportionately more corticortical projections than the adjoining cortical areas. The presence of VGluT2, although not PV, immunopositive terminations in M1 are consistent with the evidence that M1 receive thalamic inputs (Fang et al., 2006) although proportionately less than the surrounding cortical areas.
Area 6
In galagos, area 6 consists of the premotor and supplementary motor areas (Preuss and Goldman-Rakic, 1991a, 1991b, 1991c; Wu et al, 2000; Fang et al., 2005), and is within the area identified as Prc1 by Zilles and colleagues (1979). The premotor area has been further divided into the dorsal and ventral subdivisions (Wu et al., 2000; Fang et al., 2005), which respectively corresponds to area 6D and 6V of Preuss and Goldman-Rakic (1992). The supplementary motor area (Wu et al., 2000; Fang et al., 2005) is in a similar location to area 6m of Preuss and Goldman-Rakic (1991a, 1991c). These premotor areas typically contain neurons that require higher levels of current to evoke movements than those in M1 (Wu et al., 2000). The premotor areas have strong connections with M1, the prefrontal cortex and the spinal cord (Wu et al., 2000), and connections with the ventrolateral nucleus of the motor thalamus (Fang et al., 2006). Furthermore, a frontal eye field (FEF) has been described in galagos, where eye movements are evoked by when electrical stimulation with microelectrodes (Wu et al., 2000). These areas, defined by their neuron response properties and connection patterns, are included in the cortical maps in this study. However, the architectonic differences between these areas are subtle, and are described as a single field, area 6. Architectonically, area 6 have smaller pyramidal cells than M1 (Wu et al., 2000), and a moderately populated, thin layer 4. Area 6 stains lighter for synaptic zinc, and darker for PV and VGluT2 immunopositive terminations compared to M1. This indicates that area 6 receives proportionately less corticocortical and more thalamocortical inputs than M1.
Remaining frontal areas
We have divided the frontal pole of galagos into three main regions, the granular frontal, orbital frontal and medial frontal regions. The granular frontal area is further divided into three areas, the granular frontal anterior (GrA), posterior (GrP), and medial (GrM) areas. These three areas are similar to GrA, GrP and GrM of Preuss and Goldman-Rakic (1991a, 1991c). GrA and GrP may contain more than one cortical area, but we have not further subdivided GrA and GrP as the architectonic evidence for doing so is not strong in our material. In comparison with macaque monkeys, GrP in galagos has similar architectonic appearances to area 8 in monkeys (Barbas and Pandya, 1989; Preuss and Goldman-Rakic, 1991c), as both areas have well-defined granular layer and moderate myelination with distinct bands of Baillarger. However, GrA in galagos do not have a clear homologous area in macaque monkeys (Preuss and Goldman-Rakic, 1991c). The granular frontal areas are likely to have widespread connections with the rest of cortex, including the cingulate, insular, parietal, posterior parietal and inferior temporal cortices (Pritzel and Markowitsch, 1982; Preuss and Goldman-Rakic, 1991a). Furthermore, thalamocortical projections to the granular frontal areas may originate from the ventrolateral and ventroanterior nuclei (Pritzel and Markowitsch, 1982). Architectonically, the granular frontal areas have a distinct layer 4 and a layer 5 that is populated with darkly stained cells. All three areas are moderately myelinated, with GrP showing a distinct outer band of Baillarger. GrP stains lighter, whereas GrA and GrM stain darker in zinc preparations, suggesting that GrP receives proportionately less corticocortical inputs than GrA and GrM. Furthermore, GrA receives proportionately less thalamocortical inputs than GrP, as GrA expresses less PV and VGluT2 immunopositive terminations. GrM does not have a homogenous appearance in PV preparations, hinting at the presence of a dorsal and ventral subdivision. Additionally, GrM receives proportionately more thalamocortical projections than GrP, as GrM stains darker for VGluT2 immunopositive terminations than GrP.
The orbital frontal region is further divided into three areas, the dorsal (OFd), ventral (OFv) and medial (OFm) areas. OFd is similar to area 13, OFv to area 14L and OFm to area 14M of Preuss and Goldman-Rakic (1991a, 1991c). These three areas are architectonically distinct from each other in the Nissl stain, where OFd has a distinct, moderately thick granular layer 4, OFv has a thin layer 4, and OFm has a thin and pale layer 4. Of the three orbital frontal areas, OFm is the least densely myelinated and stains the darkest for synaptic zinc. OFv has the lowest and OFd has the highest expression of PV and VGluT2 immunopositive terminations of the three orbital frontal areas. These architectonic characteristics suggest that OFd receives the highest proportion of PV and VGluT2 immunopositive thalamocortical terminations, followed by OFm, and OFv receives the lowest proportion. OFm receives the highest proportion of corticocortical inputs, followed by OFv, and OFd receives the lowest proportion. Thalamocortical projections to the orbital frontal areas in galagos originate from the mediodorsal nuclei, and corticocortial projections to these areas originate from the insular cortex (Pritzel and Markowitsch, 1982).
The medial frontal (MF) area in galagos has been subdivided into the rostral and caudal areas (Preuss and Goldman-Rakic, 1991a, 1991c). We have left it as a single area, as architectonic subdivisions could not be reliably established in our material. Yet, a heterogeneous myelination pattern and variations in zinc staining within MF suggests the presence of subdivisions. The intense staining in zinc preparations and poor staining for PV and VGluT2 immunopositive terminations suggests that MF receives a higher proportion of corticocortical than thalamocortical projections. These corticocortical projections may originate from the cingulate and retrosplenial cortices (Pritzel and Markowitsch, 1982).
Medial cortex
Medial area 7
Medial area 7, 7m, in galagos approximate corresponds to area 7dm and 7vm of Preuss and Goldman-Rakic (1991a) and Pa2 of Zilles and colleagues (1979). By location, it has been suggested that area 7m in galagos may have some homology to the medial parietal areas in macaque monkeys as both areas are located above the posterior cingulate area 23 (Preuss and Goldman-Rakic, 1991a). Architectonically, area 7m in galagos has a well developed granular layer 4 and is moderately myelinated. Area 7m of galagos stains darkly for synaptic zinc, moderately for PV and VGluT2 immunopositive terminations. This indicates that inputs to area 7m originate from other areas of cortex, as well as from nuclei in the thalamus.
Cingulate areas
In most mammals, the cingulate cortex surrounds the anterior portion of the corpus callosum and consists of at least the rostral, dorsal and ventral cingulate areas (e.g. Zilles, 1990; Vogt et al., 1992; Vogt et al., 2004; Wong and Kaas, 2008, 2009a, 2009b). The cingulate cortex is part of the limbic system and the Papex circuit (Vogt et al., 1992) and has architectonic features of both isocortex and allocortex (Zilles, 1990). The primate cingulate areas are involved in attention and memory, as evidenced by the presence of connections with the hippocampal cortex (Kobayashi and Amaral, 2007) and the frontal cortex (Kobayashi and Amaral, 2003). In addition, the primate cingulate areas mediate somatic and autonomic motor responses, and pain responses (Smith, 1945; Ward, 1948; Kaada, 1951; Barris and Schuman, 1953; Foltz and White, 1962; Foltz and White, 1968). In Old World macaque monkeys, the anterior cingulate (area 24) receives auditory inputs from areas around the superior temporal gyrus and multimodal inputs from the orbital frontal areas (Vogt and Pandya, 1987). Thalamic inputs to the anterior cingulate (area 24) in macaque monkeys include projections from the centrodensocellular, parafasicular, ventroanterior and mediodorsal nuclei (Vogt et al., 1987). The posterior cingulate (area 23) of macaque monkeys has connections with area 19 (Vogt and Pandya, 1987), and with the anteromedial, lateroposterior and medial pulvinar nuclei of the thalamus (Baleydier and Mauguiere, 1985; Vogt et al., 1987).
We have retained the nomenclature of Preuss and Goldman-Rakic (1991a) for the cingulate areas in galagos, where cingulate cortex in galagos is divided into the paralimbic area (Para) that is part of the dorsal anterior cingulate cortex, area 24 that is part of the ventral anterior cingulate cortex, and area 23 that is part of the posterior cingulate cortex. These three cingulate areas are within the cingulate region defined by Zilles and colleagues (1979). Area 24 as identified here overlaps with a portion of the cingulate motor area (CMAc) that has connections with the spinal cord and M1 and contains neurons that causes movements of the forelimb, trunk and hindlimb with electrically stimulated by microelectrodes (Wu et al., 2000).
Architectonically, all three cingulate areas have poorly developed granular layer 4 and are poorly myelinated. In galagos, both Para, which approximately corresponds to area 24a of macaques by location (Vogt and Pandya, 1987; Vogt et al., 1987), and area 24, which is similar to area 24b and c of macaques by location (Vogt and Pandya, 1987; Vogt et al., 1987), express high levels of synaptic zinc, and lower levels of PV and VGluT2 immunopositive terminations. These staining patterns suggest that Para and area 24 of galagos receive proportionately high levels of corticortical inputs, possibly originating from the auditory and orbital frontal cortex as in macaques (Vogt and Pandya, 1987), and moderate levels of thalamocortical inputs, possibly originating from the centrodensocellular, parafasicular, ventroanterior and mediodorsal nuclei as in macaques (Vogt et al., 1987). The posterior cingulate area in galagos, area 23, has increased staining for synaptic zinc and reduced staining for PV and VGluT2 immunopositive terminations, with the reduction being most pronounced for PV immunopositive terminations. This indicates that area 23 receives proportionately larger amounts of corticocortical projections and lower amounts of thalamocortical projections. Area 23 in galagos may have connections with areas 9 and 46 in the frontal cortex, the posterior parietal cortex (Kobayashi and Amaral, 2003) and areas around the superior temporal cortex (Seltzer and Pandya, 2009), as in macaques. Thalamocortical projections to area 23 in galagos may originate from anteromedial, lateroposterior and medial pulvinar nuclei, as in macaques (Vogt et al., 1987; Baleydier and Mauguiere, 1985).
Retrosplenial areas
In most mammals, the retrosplenial cortex surrounds the posterior portion of the corpus callosum and consists of at least the granular and agranular areas (e.g. Zilles, 1978; Palomero-Gallagher and Zilles, 2004, Wong and Kaas, 2008, 2009a, 2009b). The retrosplenial cortex plays a role in the processes of learning and memory (van Groen and Wyss, 1990), as evidenced by the major inputs area 29 receives from the hippocampal and prefrontal cortices in monkeys (Kobayashi and Amaral, 2003). Area 30 of retrosplenial cortex in macaques have connections with the adjoining area 23 (Morris et al., 1999a; Morris et al., 1999b), as well as extrastriate visual areas, the middorsolateral prefrontal cortex, areas in the superior temporal sulcus and the parahippocampal cortex (Morecraft et al., 2003; 2004; Seltzer and Pandya, 2009). Thalamic connections of area 30 in macaques include those with the lateroposterior, laterodorsal and anteroventral limbic nuclei (Morris et al., 1999a).
We have retained the nomenclature of Zilles et al., (1979) for galagos, where retrosplenial cortex is divided into the granular (RSg) and agranular (RSag) areas, which respectively corresponds to areas 29 and 30 of Preuss and Goldman-Rakic (1991). Architectonically, Both RSg and RSag of galagos do not have well defined lamination patterns. In galagos, RSg is characterized by a layer 2/3 that is densely packed with cells and by moderate myelination. RSag is characterized by low cell packing density and is more sparsely myelinated. RSg expresses less synaptic zinc than RSag, suggesting that RSg receives proportionately less corticocortical terminations than RSag. Furthermore, RSg expresses more PV and VGluT2 immunopositive terminations than RSag, suggesting that a larger proportion of inputs to RSg originates from nuclei in the thalamus than RSag. The parvalbumin distribution in the retrosplenial areas of galagos is similar to that of rhesus monkeys (Vogt et al., 2005).
Prostriata area
Area prostriata (PS), identified in primates by Sanides (1970), is part of the limbic cortex lying along the posteriomedial border of area 17 that is visual in function and contains a representation of the peripheral vision of the contralateral visual hemifield. PS has been identified in a number of mammals, including short-tailed opossums (Wong and Kaas, 2009b), grey squirrels (Wong and Kaas, 2008), tree shrews (Wong and Kaas, 2009a), and monkeys (Allman and Kaas, 1971a). In rats, the comparable region is known as the posteromedial visual area (Wang and Burkhalter, 2007), medial area 18b (Krieg, 1946; Caviness, 1975) and Oc2MM (Zilles and Wree, 1995). In cats, this area is known as the splenial visual area (see Rosa and Krubitzer, 1999, for review). PS in nonprimate mammals receives inputs from V1 and is responsive to visual stimuli (Kalia and Whitteridge, 1973; Tiao and Blakemore, 1976; Wagor et al., 1980; Olavarria and Montero, 1984; Law et al., 1988; Olavarria and Montero, 1990; Montero, 1993). In primates, PS has connections with visual areas such as V1, MST and the cingulate motor cortex (Sousa et al., 1991; Rosa et al., 1993; Morecraft et al., 2000). Additionally, PS in nonprimates mammals is more expansive than primates (Rosa et al., 1997). In galagos, PS contains neurons that are responsive to visual stimuli (Rosa et al., 1997). Architectonically, PS of galagos resembles that of other mammals, including poor myelination, high expression of synaptic zinc, and moderate expression of PV and VGluT2 immunopositive terminations. The architectonic characteristics of PS in galagos suggest that this area is likely to have a larger proportion of corticocortical, rather than thalamocortical inputs. PS in galagos is relatively larger than in monkeys, which may be reflective of the ancestral state (Rosa et al., 1997).
Perirhinal area
The perirhinal area (PRh) in galagos is a narrow strip of cortex along the dorsal bank of the rhinal fissure and ventral to the temporal lobe. This area corresponds to area 35 of Brodmann (1909) and to area 35 in galagos identified by Preuss and Goldman-Rakic (1991). As with most mammals, such as grey squirrels (Wong and Kaas, 2008), rats (Burwell, 2001; Palomero-Gallagher and Zilles, 2004), and tree shrews (Wong and Kaas, 2009a), PRh of galagos is poorly myelinated and lacks a well defined lamination pattern. Furthermore, PRh stains darkly for synaptic zinc and poorly for PV and VGluT2 immunopositive terminations. This suggests that PRh receives a large proportion of corticortical inouts and few thalamocortical inputs. In rats, PRh has connections with the hippocampal formation (Burwell and Amaral, 1998; Palomero-Gallagher and Zilles, 2004; Furtak et al., 2007) and is as such implicated in memory processes. Furthermore, PRh of rats receives projections from the anterior thalamic nuclei (Palomero-Gallagher, and Zilles, 2004), the piriform, frontal, temporal, and insular cortices (Furtak et al., 2007). In macaque monkeys, PRh receives projections from visual areas in the temporal cortex, the parahippocampal cortex, and the insular cortex (Suzuki and Amaral, 1994) and has reciprocal connections with areas within the orbitofrontal cortex (Suzuki and Amaral, 1994; Kondo et al., 2005). It is probable that PRh in galagos, like most other mammals, has a role in memory processes, and has similar connection patterns to that in macaque monkeys.
Acknowledgements
We thank Laura Trice for help with the histological procedures. This research was supported by a grant from the National Eye Institute, EY 02686 to J.H.K.
This research was supported by a grant from the National Eye Institute, EY 02686 to J.H.K.
References
- Allman JM, Kaas JH. Representation of the visual field in striate and adjoining cortex of the owl monkey (Aotus trivirgatus) Brain Res. 1971a;35(1):89–106. doi: 10.1016/0006-8993(71)90596-8. [DOI] [PubMed] [Google Scholar]
- Allman JM, Kaas JH. A representation of the visual field in the caudal third of the middle tempral gyrus of the owl monkey (Aotus trivirgatus) Brain Res. 1971b;31(1):85–105. doi: 10.1016/0006-8993(71)90635-4. [DOI] [PubMed] [Google Scholar]
- Allman JM, Kaas JH. The organization of the second visual area (V II) in the owl monkey: a second order transformation of the visual hemifield. Brain Res. 1974;76(2):247–265. doi: 10.1016/0006-8993(74)90458-2. [DOI] [PubMed] [Google Scholar]
- Allman JM, Kaas JH. The dorsomedial cortical visual area: a third tier area in the occipital lobe of the owl monkey (Aotus trivirgatus) Brain Res. 1975;100(3):473–487. doi: 10.1016/0006-8993(75)90153-5. [DOI] [PubMed] [Google Scholar]
- Allman JM, Campbell CB, McGuinness E. The dorsal third tier area in Galago senegalensis. Brain Res. 1979;179(2):355–361. doi: 10.1016/0006-8993(79)90450-5. [DOI] [PubMed] [Google Scholar]
- Allman JM, Kaas JH, Lane RH. The middle temporal visual area(MT)in the bushbaby, Galago senegalensis. Brain Res. 1973;57(1):197–202. doi: 10.1016/0006-8993(73)90576-3. [DOI] [PubMed] [Google Scholar]
- Baleydier C, Mauguiere F. Anatomical evidence for medial pulvinar connections with the posterior cingulate cortex, the retrosplenial area, and the posterior parahippocampal gyrus in monkeys. J Comp Neurol. 1985;232(2):219–228. doi: 10.1002/cne.902320207. [DOI] [PubMed] [Google Scholar]
- Barbas H, Bandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Barris RW, Schuman HR. Bilateral anterior cingulate gyrus lesions; syndrome of the anterior cingulate gyri. Neurology. 1953;3(1):44–52. doi: 10.1212/wnl.3.1.44. [DOI] [PubMed] [Google Scholar]
- Beck PD, Kaas JH. Cortical connections of the dorsomedial visual area in prosimian primates. J Comp Neurol. 1998a;398(2):162–178. [PubMed] [Google Scholar]
- Beck PD, Kaas JH. Thalamic connections of the dorsomedial visual area in primates. J Comp Neurol. 1998b;396(3):381–398. [PubMed] [Google Scholar]
- Born RT, Bradley DC. Structure and function of visual area MT. Annu Rev Neurosci. 2005;28:157–189. doi: 10.1146/annurev.neuro.26.041002.131052. [DOI] [PubMed] [Google Scholar]
- Bourne JA, Warner CE, Upton DJ, Rosa MG. Chemoarchitecture of the middle temporal visual area in the marmoset monkey (Callithrix jacchus): laminar distribution of calcium-binding proteins (calbindin, parvalbumin) and nonphosphorylated neurofilament. J Comp Neurol. 2007;500(5):832–849. doi: 10.1002/cne.21190. [DOI] [PubMed] [Google Scholar]
- Boussaoud D, Ungerleider LG, Desimone R. Pathways for motion analysis: cortical connections of the medial superior temporal and fundus of the superior temporal visual areas in the macaque. J Comp Neurol. 1990;296(3):462–495. doi: 10.1002/cne.902960311. [DOI] [PubMed] [Google Scholar]
- Brugge JF. Auditory cortical areas in primates. In cortical sensory organization: Vol 3. In: Woolsey C.Nn., editor. Multiple auditory areas. Raven Press; New York: 1981. pp. 97–111. [Google Scholar]
- Britten KH. The middle temporal area: Motion processing and the link to perception. In: Chalupa LM, Werner JS, editors. The visual neurosciences. MIT Press; Cambridge, MA: 2003. pp. 1203–1216. [Google Scholar]
- Burkhalter A, Felleman DJ, Newsome WT, Van Essen DC. Anatomical and physiological asymmetries related to visual areas V3 and VP in macaque extrastriate cortex. Vision Res. 1986;26(1):63–80. doi: 10.1016/0042-6989(86)90071-4. [DOI] [PubMed] [Google Scholar]
- Burton H, Carlson M. Second somatic sensory cortical area (SII) in a prosimian primate, Galago crassicaudatus. J Comp Neurol. 1986;247(2):200–220. doi: 10.1002/cne.902470206. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol. 1998;398(2):179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Burwell RD. Borders and cytoarchitecture of the perirhinal and postrhinal cortices in the rat. J Comp Neurol. 2001;437(1):17–41. doi: 10.1002/cne.1267. [DOI] [PubMed] [Google Scholar]
- Carey RG, Fitzpatrick D, Diamond IT. Layer I of striate cortex of Tupaia glis and Galago senegalensis: projections from thalamus and claustrum revealed by retrograde transport of horseradish peroxidase. J Comp Neurol. 1979;186(3):393–437. doi: 10.1002/cne.901860306. [DOI] [PubMed] [Google Scholar]
- Carlson M, Welt C. Somatic sensory cortex (SmI) of the prosimian primate Galago crassicaudatus: organization of mechanoreceptive input from the hand in relation to cytoarchitecture. J Comp Neurol. 1980;189(2):249–271. doi: 10.1002/cne.901890204. [DOI] [PubMed] [Google Scholar]
- Carlson M, Welt C. The somatosensory cortex SmI in prosimian primates. In: Woolsey CN, editor. Cortical sensory organisation. Humana Press; Clifton, NJ: 1981. pp. 1–27. [Google Scholar]
- Carroll EW, Wong-Riley MT. Quantitative light and electron microscopic analysis of cytochrome oxidase-rich zones in the striate cortex of the squirrel monkey. J Comp Neurol. 1984;222(1):1–17. doi: 10.1002/cne.902220102. [DOI] [PubMed] [Google Scholar]
- Casagrande VA, De Bruyn EJ. The galago visual system: Aspects of normal organization and developmental plasticity. In: Haines D, editor. The lesser busybaby (Galago) as an animal model: Selected topics. CRC Press; Boca Raton, Florida: 1982. pp. 107–135. [Google Scholar]
- Casagrande VA, Kaas JH. The afferent, intrinsic and efferent connections of primary visual cortex in primates. In: Rockland KS, Peters A, editors. Cerebral Cortex: Primary Visual Cortex in Primates. Plenum; New York: 1994. pp. 201–259. [Google Scholar]
- Casagrande VA, Back PD, Lachia EA. Intrinsic connections of cytochrome oxidase (CO) blob and nonblob regions in area 17 of nocturnal primate. Soc. Neurosci. Abstr. 1989;15:1398. [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 1989;287(4):422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Caviness VSJ. Architectonic map of neocortex of the normal mouse. J Comp Neurol. 1975;164(2):247–263. doi: 10.1002/cne.901640207. [DOI] [PubMed] [Google Scholar]
- Celio MR. Parvalbumin in most gamma-aminobutyric acid-containing neurons of the rat cerebral cortex. Science. 1986;231(4741):995–97. doi: 10.1126/science.3945815. [DOI] [PubMed] [Google Scholar]
- Cipolloni PB, Pandya DN. Cortical connections of the frontoparietal opercular areas in the rhesus monkey. J Comp Neurol. 1999;403(4):431–458. [PubMed] [Google Scholar]
- Collins CE, Stepniewska I, Kaas JH. Topographic patterns of v2 cortical connections in a prosimian primate (Galago garnetti) J Comp Neurol. 2001;431(2):155–167. [PubMed] [Google Scholar]
- Conde F, Lund JS, Lewis DA. The hierarchical development of monkey visual cortical regions as revealed by the maturation of parvalbumin-immunoreactive neurons. Brain Res Dev Brain Res. 1996;96(1–2):261–276. doi: 10.1016/0165-3806(96)00126-5. [DOI] [PubMed] [Google Scholar]
- Condo GJ, Casagrande VA. Organization of cytochrome oxidase staining in the visual cortex of nocturnal primates (Galago crassicaudatus and Galago senegalensis): I. Adult patterns. J Comp Neurol. 1990;293(4):632–645. doi: 10.1002/cne.902930408. [DOI] [PubMed] [Google Scholar]
- Conley M, Kupersmith AC, Diamond IT. The organization of projections from subdivisions of the auditory cortex and thalamus to the auditory sector of the thalamic reticular nucleus in Galago. Eur J Neurosci. 1991;3(11):1089–1103. doi: 10.1111/j.1460-9568.1991.tb00044.x. [DOI] [PubMed] [Google Scholar]
- Cragg BG. The topography of the afferent projections in the circumstriate visual cortex of the monkey studied by the Nauta method. Vision Res. 1969;9(7):733–747. doi: 10.1016/0042-6989(69)90011-x. [DOI] [PubMed] [Google Scholar]
- Cusick CG, Kaas JH. Surface view patterns of intrinsic and extrinsic cortical connections of area 17 in a prosimian primate. Brain Res. 1988;458(2):383–88. doi: 10.1016/0006-8993(88)90483-0. [DOI] [PubMed] [Google Scholar]
- Cusick CG, Steindler DA, Kaas JH. Corticocortical and collateral thalamocortical connections of postcentral somatosensory cortical areas in squirrel monkeys: a double-labeling study with radiolabeled wheatgerm agglutinin and wheatgerm agglutinin conjugated to horseradish peroxidase. Somatosens Res. 1985;3(1):1–31. doi: 10.3109/07367228509144574. [DOI] [PubMed] [Google Scholar]
- Cusick CG, Wall JT, Felleman DJ, Kaas JH. Somatotopic organization of the lateral sulcus of owl monkeys: area 3b, S-II and a ventral somatosensory area. J Comp Neurol. 1989;282(2):169–190. doi: 10.1002/cne.902820203. [DOI] [PubMed] [Google Scholar]
- Danscher G, Stoltenberg M. Zinc-specific autometallographic in vivo selenium methods: tracing of zinc-enriched (ZEN) terminals, ZEN pathways and pools of zinc ions in a multitude of other ZEN cells. J Histochem Cytochem. 2005;53(2):141–153. doi: 10.1369/jhc.4R6460.2005. [DOI] [PubMed] [Google Scholar]
- Danscher G. Exogenous selenium in the brain. A histochemical technique for light and electron microscopical localization of catalytic selenium bonds. Histochemistry. 1982;76(3):281–293. doi: 10.1007/BF00543951. [DOI] [PubMed] [Google Scholar]
- Danscher G. Histochemical demonstration of heavy metals. A revised version of the sulphide silver method suitable for both light and electronmicroscopy. Histochemistry. 1981;71(1):1–16. doi: 10.1007/BF00592566. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Jones EG. Parvalbumin immunoreactivity reveals layer IV of monkey cerebral cortex as a mosaic of microzones of thalamic afferent terminations. Brain Res. 1991;562(1):39–47. doi: 10.1016/0006-8993(91)91184-3. [DOI] [PubMed] [Google Scholar]
- DeFelipe J. Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat. 1997;14(1):1–19. doi: 10.1016/s0891-0618(97)10013-8. [DOI] [PubMed] [Google Scholar]
- Diamond IT, Conley M, Itoh K, Fitzpatrick D. Laminar organization of geniculocortical projections in Galago senegalensis and Aotus trivirgatus. J Comp Neurol. 1985;242(4):584–610. doi: 10.1002/cne.902420408. [DOI] [PubMed] [Google Scholar]
- Ding Y, Casagrande VA. The distribution and morphology of LGN K pathway axons within the layers and CO blobs of owl monkey V1. Vis Neurosci. 1997;14(4):691–704. doi: 10.1017/s0952523800012657. [DOI] [PubMed] [Google Scholar]
- Disbrow E, Litinas E, Recanzone GH, Padberg J, Krubitzer L. Cortical connections of the second somatosensory area and the parietal ventral area in macaque monkeys. J Comp Neurol. 2003;462(4):382–399. doi: 10.1002/cne.10731. [DOI] [PubMed] [Google Scholar]
- Disbrow E, Roberts T, Krubitzer L. Somatotopic organization of cortical fields in the lateral sulcus of Homo sapiens: evidence for SII and PV. J Comp Neurol. 2000;418(1):1–21. doi: 10.1002/(sici)1096-9861(20000228)418:1<1::aid-cne1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Parham C. Afferent connections of the lateral agranular field of the rat motor cortex. J Comp Neurol. 1983;217(4):390–404. doi: 10.1002/cne.902170404. [DOI] [PubMed] [Google Scholar]
- Fang PC, Stepniewska I, Kaas JH. Corpus callosum connections of subdivisions of motor and premotor cortex and frontal eye field in a prosimian primate, Otolemur garnetti. The Journal of Comparative Neurology. 2008;508(4):565–578. doi: 10.1002/cne.21706. [DOI] [PubMed] [Google Scholar]
- Fang PC, Stepniewska I, Kaas JH. Ipsilateral cortical connections of motor, premotor, frontal eye and posterior parietal fields in a prosimian primate, Otolemur garnetti. J Comp Neurol. 2005;490(3):305–333. doi: 10.1002/cne.20665. [DOI] [PubMed] [Google Scholar]
- Fang PC, Stepniewska I, Kaas JH. The thalamic connections of motor, premotor and prefrontal areas of cortex in a prosimian primate (Otolemur garnetti) Neuroscience. 2006;143(4):987–1020. doi: 10.1016/j.neuroscience.2006.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Burkhalter A, Van Essen DC. Cortical connections of areas V3 and VP of macaque monkey extrastriate visual cortex. J Comp Neurol. 1997;379(1):21–47. doi: 10.1002/(sici)1096-9861(19970303)379:1<21::aid-cne3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Fleagle JG. Primate adaptation and Evolution. Academic Press; San Diego: 1999. [Google Scholar]
- Florence SL, Casagrande VA. Organization of individual afferent axons in layer IV of striate cortex in a primate. J Neurosci. 1987;7(12):3850–868. doi: 10.1523/JNEUROSCI.07-12-03850.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Gentilucci M, Luppino G, Matelli M, Rizzolatti G. The fronto-parietal cortex of the prosimian Galago: patterns of cytochrome oxidase activity and motor maps. Behav Brain Res. 1994;60(1):91–113. doi: 10.1016/0166-4328(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Foltz EL, White LE. Pain “relief” by frontal cingulumotomy. J Neurosurg. 1962:1989–100. doi: 10.3171/jns.1962.19.2.0089. [DOI] [PubMed] [Google Scholar]
- Foltz EL, White LE. The role of rostral cingulumotomy in “pain” relief. Int J Neurol. 1968;6(3–4):353–373. [PubMed] [Google Scholar]
- Frederickson CJ, Moncrieff DW. Zinc-containing neurons. Biol Signals. 1994;3(3):127–139. doi: 10.1159/000109536. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Suh SW, Silva D, Thompson RB. Importance of zinc in the central nervous system: the zinc-containing neuron. J Nutr. 2000;130(5S Suppl):1471S–483S. doi: 10.1093/jn/130.5.1471S. [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Furuta T, Kaneko T. Immunocytochemical localization of candidates for vesicular glutamate transporters in the rat cerebral cortex. J Comp Neurol. 2001;435(3):379–387. doi: 10.1002/cne.1037. [DOI] [PubMed] [Google Scholar]
- Furtak SC, Wei SM, Agster KL, Burwell RD. Functional neuroanatomy of the parahippocampal region in the rat: the perirhinal and postrhinal cortices. Hippocampus. 2007;17(9):709–722. doi: 10.1002/hipo.20314. [DOI] [PubMed] [Google Scholar]
- Gallyas F. Silver staining of myelin by means of physical development. Neurol Res. 1979;1(2):203–09. doi: 10.1080/01616412.1979.11739553. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Florence SL, Tenhula WN, Kaas JH. Parallel thalamic activation of the first and second somatosensory areas in prosimian primates and tree shrews. J Comp Neurol. 1991;311(2):289–299. doi: 10.1002/cne.903110209. [DOI] [PubMed] [Google Scholar]
- Gattass R, Gross CG, Sandell JH. Visual topography of V2 in the macaque. J Comp Neurol. 1981;201(4):519–539. doi: 10.1002/cne.902010405. [DOI] [PubMed] [Google Scholar]
- Gattass R, Sousa AP, Rosa MG. Visual topography of V1 in the Cebus monkey. J Comp Neurol. 1987;259(4):529–548. doi: 10.1002/cne.902590404. [DOI] [PubMed] [Google Scholar]
- Gattass R, Sousa AP, Gross CG. Visuotopic organization and extent of V3 and V4 of the macaque. J Neurosci. 1988;8(6):1831–845. doi: 10.1523/JNEUROSCI.08-06-01831.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattass R, Sousa AP, Mishkin M, Ungerleider LG. Cortical projections of area V2 in the macaque. Cereb Cortex. 1997;7(2):110–129. doi: 10.1093/cercor/7.2.110. [DOI] [PubMed] [Google Scholar]
- Glendenning KK, Kofron EA, Diamond IT. Laminar organization of projections of the lateral geniculate nucleus to the striate crotex in Galago. Brain Res. 1976;105(3):538–546. doi: 10.1016/0006-8993(76)90600-4. [DOI] [PubMed] [Google Scholar]
- Hackett TA, de la Mothe LA. Regional and laminar distribution of the vesicular glutamate transporter, VGluT2, in the macaque monkey auditory cortex. J Chem Neuroanat. 2009 doi: 10.1016/j.jchemneu.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, Stepniewska I, Kaas JH. Thalamocortical connections of the parabelt auditory cortex in macaque monkeys. J Comp Neurol. 1998;400(2):271–286. doi: 10.1002/(sici)1096-9861(19981019)400:2<271::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Hendrickson AE. Dots, stripes and columns in monkey visual cortex. Trends Neurosci. 1985;8(4):404–410. [Google Scholar]
- Hendrickson AE, Wilson JR, Ogren MP. The neuroanatomical organization of pathways between the dorsal lateral geniculate nucleus and visual cortex in Old World and New World primates. J Comp Neurol. 1978;182(1):123–136. doi: 10.1002/cne.901820108. [DOI] [PubMed] [Google Scholar]
- Hof PR, Glezer I, Conde F, Flagg RA, Rubin MB, Nimchinsky EA, Vogt Weisenhorn DM. Cellular distribution of the calcium-binding proteins parvalbumin, calbindin and calretinin in the neocortex of mammals: phylogenetic and developmental patterns. J Chem Neuroanat. 1999;16(2):77–116. doi: 10.1016/s0891-0618(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture in two nonstriate visual areas (18 and 19) of the cat. J Neurophysiol. 1965;28:229–89. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Laminar and columnar distribution of geniculo-cortical fibers in the macaque monkey. J Comp Neurol. 1972;146(4):421–450. doi: 10.1002/cne.901460402. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Pons TP. Primary motor cortex receives input from area 3a in macaques. Brain Res. 1990;537(1–2):367–371. doi: 10.1016/0006-8993(90)90388-r. [DOI] [PubMed] [Google Scholar]
- Huffman KJ, Krubitzer L. Area 3a: topographic organization and cortical connections in marmoset monkeys. Cereb Cortex. 2001;11(9):849–867. doi: 10.1093/cercor/11.9.849. [DOI] [PubMed] [Google Scholar]
- Hyvärinen J, Shelepin Y. Distribution of visual and somatic functions in the parietal associative area 7 of the monkey. Brain Res. 1979;169(3):561–64. doi: 10.1016/0006-8993(79)90404-9. [DOI] [PubMed] [Google Scholar]
- Ichinohe N, Rockland KS. Region specific micromodularity in the uppermost layers in primate cerebral cortex. Cereb Cortex. 2004;14(11):1173–184. doi: 10.1093/cercor/bhh077. [DOI] [PubMed] [Google Scholar]
- Ichinohe N, Fujiyama F, Kaneko T, Rockland KS. Honeycomb-like mosaic at the border of layers 1 and 2 in the cerebral cortex. J Neurosci. 2003;23(4):1372–382. doi: 10.1523/JNEUROSCI.23-04-01372.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig TJ, Ruggero MA, Kitzes LM, Javel E, Brugge JF. Organization of auditory cortex in the owl monkey (Aotus trivirgatus) J Comp Neurol. 1977;171(1):111–128. doi: 10.1002/cne.901710108. [DOI] [PubMed] [Google Scholar]
- Jerison HJ. Brain, body and encephalization in early primates. J Hum Evol. 1979;8:615–635. [Google Scholar]
- Kaada BR. Somato-motor, autonomic and electrocorticographic responses to electrical stimulation of rhinencephalic and other structures in primates, cat and dog; a study of responses from the limbic, subcallosal, orbito-insular, piriform and temporal cortex, hippocampus-fornix and amygdala. Acta Physiol Scand Suppl. 1951;24(83):1–262. [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci U S A. 2000;97(22):11793–99. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Lyon DC. Visual cortex organization in primates: theories of V3 and adjoining visual areas. Prog Brain Res. 2001;134:285–295. doi: 10.1016/s0079-6123(01)34019-0. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Morel A. Connections of visual areas of the upper temporal lobe of owl monkeys: the MT crescent and dorsal and ventral subdivisions of FST. J Neurosci. 1993;13(2):534–546. doi: 10.1523/JNEUROSCI.13-02-00534.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. The evolution of somatosensory and motor systems in primates. In: Kaas JH, Preuss TM, editors. Evolution of primate nervous systems. Elsevier; London: 2007. [Google Scholar]
- Kaas JH. The somatosensory cortex and thalamus in Galago. In: Haines DE, editor. The lesser busybaby (Galago) as an animal model: Selected topics. CRC Press; Boca Raton, Florida: 1982. pp. 169–181. [Google Scholar]
- Kaas JH. Somatosensory system. In: Paxinos G, Mai JK, editors. The human nervous system. Elsevier Academic Press; New York: 2004. pp. 1059–1092. [Google Scholar]
- Kaas JH. Theories of visual cortex organization in primates. In: Kaas JH, Rockland KS, editors. Cerebral cortex. Plenum Press; New York: 1997. pp. 91–126. [Google Scholar]
- Kaas JH. What, if anything, is SI? Organization of first somatosensory area of cortex. Physiol Rev. 1983;63(1):206–231. doi: 10.1152/physrev.1983.63.1.206. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Pons TP. The somatosensory system of primates. In: Steklis HD, Erwin J, editors. Comparative primate biology. Alan R. Liss; New York: 1988. pp. 421–468. [Google Scholar]
- Kaas JH, Hackett TA, Tramo MJ. Auditory processing in primate cerebral cortex. Curr Opin Neurobiol. 1999;9(2):164–170. doi: 10.1016/s0959-4388(99)80022-1. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hall WC, Diamond IT. Cortical visual areas I and II in the hedgehog: relation between evoked potential maps and architectonic subdivisions. J Neurophysiol. 1970;33(5):595–615. doi: 10.1152/jn.1970.33.5.595. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Nelson RJ, Sur M, Lin CS, Merzenich MM. Multiple representations of the body within the primary somatosensory cortex of primates. Science. 1979;204(4392):521–23. doi: 10.1126/science.107591. [DOI] [PubMed] [Google Scholar]
- Kalia M, Whitteridge D. The visual areas in the splenial sulcus of the cat. J Physiol. 1973;232(2):275–283. doi: 10.1113/jphysiol.1973.sp010269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagasuntheram R, Leong CH, Mahran ZY. Observations on some cortical areas of the lesser busy baby (Galago senegalensis) J Anat. 1966;100:317–333. [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F. Complementary distribution of vesicular glutamate transporters in the central nervous system. Neurosci Res. 2002;42(4):243–250. doi: 10.1016/s0168-0102(02)00009-3. [DOI] [PubMed] [Google Scholar]
- Kaskan PM, Kaas JH. Cortical connections of the middle temporal and the middle temporal crescent visual areas in prosimian galagos (Otolemur garnetti) Anat Rec (Hoboken) 2007;290(3):349–366. doi: 10.1002/ar.20440. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. Cortical afferents. J Comp Neurol. 2003;466(1):48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: III. Cortical efferents. J Comp Neurol. 2007;502(5):810–833. doi: 10.1002/cne.21346. [DOI] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL. Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol. 2005;493(4):479–509. doi: 10.1002/cne.20796. [DOI] [PubMed] [Google Scholar]
- Krieg WJS. Connections of the cerebral cortex. I. The albino rat. A. Topography of the cortical areas. J Comp Neurol. 1946;84:221–275. doi: 10.1002/cne.900840205. [DOI] [PubMed] [Google Scholar]
- Krubitzer LA, Kaas JH. Cortical connections of MT and DL in the prosimian galago: Evidence that modular segregation of parallel pathways is a primitive feature in primates. Soc. Neurosci. Abstr. 1988;14:602. [Google Scholar]
- Krubitzer LA, Kaas JH. Cortical integration of parallel pathways in the visual system of primates. Brain Res. 1989;478(1):161–65. doi: 10.1016/0006-8993(89)91490-x. [DOI] [PubMed] [Google Scholar]
- Krubitzer LA, Kaas JH. Cortical connections of MT in four species of primates: areal, modular and retinotopic patterns. Vis Neurosci. 1990a;5(2):165–204. doi: 10.1017/s0952523800000213. [DOI] [PubMed] [Google Scholar]
- Krubitzer LA, Kaas JH. The organization and connections of somatosensory cortex in marmosets. J Neurosci. 1990b;10(3):952–974. doi: 10.1523/JNEUROSCI.10-03-00952.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer LA, Kaas JH. The dorsomedial visual area of owl monkeys: connections, myeloarchitecture and homologies in other primates. J Comp Neurol. 1993;334(4):497–528. doi: 10.1002/cne.903340402. [DOI] [PubMed] [Google Scholar]
- Krubitzer LA, Kaas JH. The somatosensory thalamus of monkeys: cortical connections and a redefinition of nuclei in marmosets. J Comp Neurol. 1992;319(1):123–110. doi: 10.1002/cne.903190111. [DOI] [PubMed] [Google Scholar]
- Krubitzer LA, Clarey J, Tweedale R, Elston G, Calford M. A redefinition of somatosensory areas in the lateral sulcus of macaque monkeys. J Neurosci. 1995;15(5 Pt 2):3821–839. doi: 10.1523/JNEUROSCI.15-05-03821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer LA, Sesma MA, Kaas JH. Microelectrode maps, myeloarchitecture and cortical connections of three somatotopically organized representations of the body surface in the parietal cortex of squirrels. J Comp Neurol. 1986;250(4):403–430. doi: 10.1002/cne.902500402. [DOI] [PubMed] [Google Scholar]
- de la Mothe LA, Blumell S, Kajikawa Y, Hackett TA. Thalamic connections of the auditory cortex in marmoset monkeys: core and medial belt regions. J Comp Neurol. 2006;496(1):72–96. doi: 10.1002/cne.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachica EA, Casagrande VA. Direct W-like geniculate projections to the cytochrome oxidase (CO) blobs in primate visual cortex: axon morphology. J Comp Neurol. 1992;319(1):141–158. doi: 10.1002/cne.903190112. [DOI] [PubMed] [Google Scholar]
- Lachica EA, Beck PD, Casagrande VA. Intrinsic connections of layer III of striate cortex in squirrel monkey and bush baby: correlations with patterns of cytochrome oxidase. J Comp Neurol. 1993;329(2):163–187. doi: 10.1002/cne.903290203. [DOI] [PubMed] [Google Scholar]
- Lachica EA, Beck PD, Casagrande VA. Parallel pathways in macaque monkey striate cortex: anatomically defined columns in layer III. Proc Natl Acad Sci U S A. 1992;89(8):3566–3570. doi: 10.1073/pnas.89.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latawiec D, Martin KA, Meskenaite V. Termination of the geniculocortical projection in the striate cortex of macaque monkey: a quantitative immunoelectron microscopic study. J Comp Neurol. 2000;419(3):306–319. doi: 10.1002/(sici)1096-9861(20000410)419:3<306::aid-cne4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Law MI, Zahs KR, Stryker MP. Organization of primary visual cortex (area 17) in the ferret. J Comp Neurol. 1988;278(2):157–180. doi: 10.1002/cne.902780202. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol. 2000;428(1):112–137. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Lin CS, Kaas JH. Projections from the medial nucleus of the inferior pulvinar complex to the middle temporal area of the visual cortex. Neuroscience. 1980;5(12):2219–2228. doi: 10.1016/0306-4522(80)90138-4. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Anatomy and physiology of a color system in the primate visual cortex. J Neurosci. 1984;4(1):309–356. doi: 10.1523/JNEUROSCI.04-01-00309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Psychophysical evidence for separate channels for the perception of form, color, movement, and depth. J Neurosci. 1987;7(11):3416–3468. doi: 10.1523/JNEUROSCI.07-11-03416.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luethke LE, Krubitzer LA, Kaas JH. Connections of primary auditory cortex in the New World monkey, Saguinus. J Comp Neurol. 1989;285(4):487–513. doi: 10.1002/cne.902850406. [DOI] [PubMed] [Google Scholar]
- Lund JS, Fitzpatrick D, Humphrey AL. The striate visual cortex of the tree shrew. In: Jones EG, Peters A, editors. Visual cortex. Plenum Press; New York: 1985. pp. 157–205. [Google Scholar]
- Lyon DC, Kaas JH. Connectional and architectonic evidence for dorsal and ventral V3, and dorsomedial area in marmoset monkeys. J Neurosci. 2001;21(1):249–261. doi: 10.1523/JNEUROSCI.21-01-00249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon DC, Kaas JH. Connectional evidence for dorsal and ventral V3, and other extrastriate areas in the prosimian primate, Galago garnetti. Brain Behav Evol. 2002a;59(3):114–129. doi: 10.1159/000064159. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Kaas JH. Evidence for a modified V3 with dorsal and ventral halves in macaque monkeys. Neuron. 2002b;33(3):453–461. doi: 10.1016/s0896-6273(02)00580-9. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Kaas JH. Evidence from V1 connections for both dorsal and ventral subdivisions of V3 in three species of New World monkeys. J Comp Neurol. 2002c;449(3):281–297. doi: 10.1002/cne.10297. [DOI] [PubMed] [Google Scholar]
- Martin RD. Palaeontology: Chinese lantern for early primates. Nature. 2004;427:22–23. doi: 10.1038/427022a. [DOI] [PubMed] [Google Scholar]
- Martin RD. Primate origins and evolution. Chapman and Hall; London: 1990. [Google Scholar]
- Maunsell JH, van Essen DC. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci. 1983;3(12):2563–586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JG, Andersen RA. Different patterns of corticopontine projections from separate cortical fields within the inferior parietal lobule and dorsal prelunate gyrus of the macaque. Exp Brain Res. 1986;63(2):265–278. doi: 10.1007/BF00236844. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Brugge JF. Representation of the cochlear partition of the superior temporal plane of the macaque monkey. Brain Res. 1973;50(2):275–296. doi: 10.1016/0006-8993(73)90731-2. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insuloorbito-temporal component of the paralimbic brain. J Comp Neurol. 1982;212(1):1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- Montero VM. Retinotopy of cortical connections between the striate cortex and extrastriate visual areas in the rat. Exp Brain Res. 1993;94(1):1–15. doi: 10.1007/BF00230466. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Cipolloni PB, Stilwell-Morecraft KS, Gedney MT, Pandya DN. Cytoarchitecture and cortical connections of the posterior cingulate and adjacent somatosensory fields in the rhesus monkey. J Comp Neurol. 2004;469(1):37–69. doi: 10.1002/cne.10980. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Rockland KS, Van Hoesen GW. Localization of area prostriata and its projection to the cingulate motor cortex in the rhesus monkey. Cereb Cortex. 2000;10(2):192–203. doi: 10.1093/cercor/10.2.192. [DOI] [PubMed] [Google Scholar]
- Morel A, Garraghty PE, Kaas JH. Tonotopic organization, architectonic fields, and connections of auditory cortex in macaque monkeys. J Comp Neurol. 1993;335(3):437–459. doi: 10.1002/cne.903350312. [DOI] [PubMed] [Google Scholar]
- Morris R, Pandya DN, Petrides M. Fiber system linking the mid-dorsolateral frontal cortex with the retrosplenial/presubicular region in the rhesus monkey. J Comp Neurol. 1999a;407(2):183–192. doi: 10.1002/(sici)1096-9861(19990503)407:2<183::aid-cne3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Morris R, Petrides M, Pandya DN. Architecture and connections of retrosplenial area 30 in the rhesus monkey (Macaca mulatta) Eur J Neurosci. 1999b;11(7):2506–518. doi: 10.1046/j.1460-9568.1999.00672.x. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Sur M, Kaas JH. The organization of the second somatosensory area (SmII) of the grey squirrel. J Comp Neurol. 1979;184(3):473–489. doi: 10.1002/cne.901840304. [DOI] [PubMed] [Google Scholar]
- O'Brien BJ, Abel PL, Olavarria JF. The retinal input to calbindin-D28k-defined subdivisions in macaque inferior pulvinar. Neurosci Lett. 2001;312(3):145–48. doi: 10.1016/s0304-3940(01)02220-0. [DOI] [PubMed] [Google Scholar]
- Olavarria J, Montero V. Elaborate organization of visual cortex in the hamster. Neurosci Res. 1990;8(1):40–47. doi: 10.1016/0168-0102(90)90055-j. [DOI] [PubMed] [Google Scholar]
- Olavarria J, Montero VM. Relation of callosal and striate-extrastriate cortical connections in the rat: morphological definition of extrastriate visual areas. Exp Brain Res. 1984;54(2):240–252. doi: 10.1007/BF00236223. [DOI] [PubMed] [Google Scholar]
- Orban GA. Visual processing in macaque area MT/V5 and its satellites. In: Rockland KS, Kaas JH, editors. Cerebral cortex: extrastriate cortex in primates. Plenum Press; New York: 1997. [Google Scholar]
- Palmer SM, Rosa MG. Quantitative analysis of the corticocortical projections to the middle temporal area in the marmoset monkey: evolutionary and functional implications. Cereb Cortex. 2006;16(9):1361–375. doi: 10.1093/cercor/bhj078. [DOI] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Zilles K. Isocortex. In: Paxinos G, editor. The Rat Nervous System. Elsevier; London: 2004. pp. 729–757. [Google Scholar]
- Petry HM, Hárosi FI. Visual pigments of the tree shrew (Tupaia belangeri) and greater galago (Galago crassicaudatus): a microspectrophotometric investigation. Vision Res. 1990;30(6):839–851. doi: 10.1016/0042-6989(90)90053-n. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Connections of the ventral granular frontal cortex of macaques with perisylvian premotor and somatosensory areas: anatomical evidence for somatic representation in primate frontal association cortex. J Comp Neurol. 1989;282(2):293–316. doi: 10.1002/cne.902820210. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Architectonics of the parietal and temporal association cortex in the strepsirhine primate Galago compared to the anthropoid primate Macaca. J Comp Neurol. 1991a;310(4):475–506. doi: 10.1002/cne.903100403. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Ipsilateral cortical connections of granular frontal cortex in the strepsirhine primate Galago, with comparative comments on anthropoid primates. J Comp Neurol. 1991b;310(4):507–549. doi: 10.1002/cne.903100404. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Myelo- and cytoarchitecture of the granular frontal cortex and surrounding regions in the strepsirhine primate Galago and the anthropoid primate Macaca. J Comp Neurol. 1991c;310(4):429–474. doi: 10.1002/cne.903100402. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Kaas JH. Parvalbumin-like immunoreactivity of layer V pyramidal cells in the motor and somatosensory cortex of adult primates. Brain Res. 1996;712(2):353–57. doi: 10.1016/0006-8993(95)01531-0. [DOI] [PubMed] [Google Scholar]
- Pritzel M, Markowitsch HJ. Organization of cortical afferents to the prefrontal cortex in the bush baby (Galago senegalensis) Brain Behav Evol. 1982;20(1–2):43–56. doi: 10.1159/000121580. [DOI] [PubMed] [Google Scholar]
- Qi HX, Lyon DC, Kaas JH. Cortical and thalamic connections of the parietal ventral somatosensory area in marmoset monkeys (Callithrix jacchus) J Comp Neurol. 2002;443(2):168–182. doi: 10.1002/cne.10113. [DOI] [PubMed] [Google Scholar]
- Raczkowski D, Diamond IT. Connections of the striate cortex in Galago senegalensis. Brain Res. 1978;144(2):383–88. doi: 10.1016/0006-8993(78)90165-8. [DOI] [PubMed] [Google Scholar]
- Radinsky LB. Early primate brains: facts and fiction. J Hum Evol. 1977;6:79–86. [Google Scholar]
- Reep RL, Chandler HC, King V, Corwin JV. Rat posterior parietal cortex: topography of corticocortical and thalamic connections. Exp Brain Res. 1994;100(1):67–84. doi: 10.1007/BF00227280. [DOI] [PubMed] [Google Scholar]
- Reep RL, Goodwin GS, Corwin JV. Topographic organization in the corticocortical connections of medial agranular cortex in rats. J Comp Neurol. 1990;294(2):262–280. doi: 10.1002/cne.902940210. [DOI] [PubMed] [Google Scholar]
- Remple MS, Henry EC, Catania KC. Organization of somatosensory cortex in the laboratory rat (Rattus norvegicus): Evidence for two lateral areas joined at the representation of the teeth. J Comp Neurol. 2003;467(1):105–118. doi: 10.1002/cne.10909. [DOI] [PubMed] [Google Scholar]
- Remple MS, Reed JL, Stepniewska I, Kaas JH. Organization of frontoparietal cortex in the tree shrew (Tupaia belangeri). I. Architecture, microelectrode maps, and corticospinal connections. J Comp Neurol. 2006;497(1):133–154. doi: 10.1002/cne.20975. [DOI] [PubMed] [Google Scholar]
- Remple MS, Reed JL, Stepniewska I, Lyon DC, Kaas JH. The organization of frontoparietal cortex in the tree shrew (Tupaia belangeri): II. Connectional evidence for a frontal-posterior parietal network. J Comp Neurol. 2007;501(1):121–149. doi: 10.1002/cne.21226. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Burton H. Organization of somatosensory receptive fields in cortical areas 7b, retroinsula, postauditory and granular insula of M. fascicularis. J Comp Neurol. 1980a;192(1):69–92. doi: 10.1002/cne.901920105. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Burton H. Somatic submodality distribution within the second somatosensory (SII), 7b, retroinsular, postauditory, and granular insular cortical areas of M. fascicularis. J Comp Neurol. 1980b;192(1):93–108. doi: 10.1002/cne.901920106. [DOI] [PubMed] [Google Scholar]
- Rosa MG, Elston GN. Visuotopic organisation and neuronal response selectivity for direction of motion in visual areas of the caudal temporal lobe of the marmoset monkey (Callithrix jacchus): middle temporal area, middle temporal crescent, and surrounding cortex. J Comp Neurol. 1998;393(4):505–527. [PubMed] [Google Scholar]
- Rosa MG, Krubitzer LA. The evolution of visual cortex: where is V2? Trends Neurosci. 1999;22(6):242–48. doi: 10.1016/s0166-2236(99)01398-3. [DOI] [PubMed] [Google Scholar]
- Rosa MG, Casagrande VA, Preuss T, Kaas JH. Visual field representation in striate and prestriate cortices of a prosimian primate (Galago garnetti) J Neurophysiol. 1997;77(6):3193–3217. doi: 10.1152/jn.1997.77.6.3193. [DOI] [PubMed] [Google Scholar]
- Rosa MG, Soares JG, Fiorani M, Gattass R. Cortical afferents of visual area MT in the Cebus monkey: possible homologies between New and Old World monkeys. Vis Neurosci. 1993;10(5):827–855. doi: 10.1017/s0952523800006064. [DOI] [PubMed] [Google Scholar]
- Rosa MG, Sousa AP, Gattass R. Representation of the visual field in the second visual area in the Cebus monkey. J Comp Neurol. 1988;275(3):326–345. doi: 10.1002/cne.902750303. [DOI] [PubMed] [Google Scholar]
- Sanides F, Krishnamurti A. Cytoarchitectonic subdivisions of sensorimotor and prefrontal regions and of bordering insular and limbic fields in slow loris (Nycticebus coucang coucang) J Hirnforsch. 1967;9(3):225–252. [PubMed] [Google Scholar]
- Sanides F. Functional architecture of motor and sensory cortices in primates in the light of a new concept of neocortex evolution. In: Montagna W, editor. The primate brain. Appleton-Century-Crofts; New York: 1970. pp. 137–208. [Google Scholar]
- Sanides F. Representation in the cerebral cortex and its areal lamination patterns. In: Boune GH, editor. Structure and function of the nervous system. Academic Press; New York: 1972. pp. 329–453. [Google Scholar]
- Seltzer B, Pandya DN. Posterior cingulate and retrosplenial cortex connections of the caudal superior temporal region in the rhesus monkey. Exp Brain Res. 2009;195(2):325–334. doi: 10.1007/s00221-009-1795-4. [DOI] [PubMed] [Google Scholar]
- Simons EL, Rasmussen T. A whole new world of ancestors: eocene anthropoids from Africa. Evol Anthropol. 1994:3128–139. [Google Scholar]
- Sincich LC, Park KF, Wohlgemuth MJ, Horton JC. Bypassing V1: a direct geniculate input to area MT. Nat Neurosci. 2004;7(10):1123–28. doi: 10.1038/nn1318. [DOI] [PubMed] [Google Scholar]
- Slutsky DA, Manger PR, Krubitzer L. Multiple somatosensory areas in the anterior parietal cortex of the California ground squirrel (Spermophilus beecheyii) J Comp Neurol. 2000;416(4):521–539. doi: 10.1002/(sici)1096-9861(20000124)416:4<521::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Smith WK. The functional significance of the rostral cingular cortex as revealed by its responses to electrical excitation. J Neurophysiol. 1945:8214–255. [Google Scholar]
- Sousa AP, Piñon MC, Gattass R, Rosa MG. Topographic organization of cortical input to striate cortex in the Cebus monkey: a fluorescent tracer study. J Comp Neurol. 1991;308(4):665–682. doi: 10.1002/cne.903080411. [DOI] [PubMed] [Google Scholar]
- Spatz WB, Tigges J. Experimental-anatomical studies on the “middle temporal visual area (MT)” in primates. I. Efferent cortico-cortical connections in the marmoset Callithrix jacchus. J Comp Neurol. 1972;146(4):451–464. doi: 10.1002/cne.901460403. [DOI] [PubMed] [Google Scholar]
- Spatz WB. Topographically organized reciprocal connections between areas 17 and MT (visual area of superior temporal sulcus) in the marmoset Callithrix jacchus. Exp Brain Res. 1977;27(5):559–572. doi: 10.1007/BF00239044. [DOI] [PubMed] [Google Scholar]
- Steele GE, Weller RE, Cusick CG. Cortical connections of the caudal subdivision of the dorsolateral area (V4) in monkeys. J Comp Neurol. 1991;306(3):495–520. doi: 10.1002/cne.903060312. [DOI] [PubMed] [Google Scholar]
- Stephan H, Frahm H, Baron G. New and revised data on volume of brain structures in insectivores and primates. Folia primatol. 1981:351–29. doi: 10.1159/000155963. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Kaas JH. Topographic patterns of V2 cortical connections in macaque monkeys. J Comp Neurol. 1996;371(1):129–152. doi: 10.1002/(SICI)1096-9861(19960715)371:1<129::AID-CNE8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Cerkevich CM, Fang PC, Kaas JH. Organization of the posterior parietal cortex in galagos: II. Ipsilateral cortical connections of physiologically identified zones within anterior sensorimotor region. J Comp Neurol. 2009a;517(6):783–807. doi: 10.1002/cne.22190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Fang PC, Kaas JH. Organization of the posterior parietal cortex in galagos: I. Functional zones identified by microstimulation. J Comp Neurol. 2009b;517(6):765–827. doi: 10.1002/cne.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Collins CE, Kaas JH. Reappraisal of DL/V4 boundaries based on connectivity patterns of dorsolateral visual cortex in macaques. Cereb Cortex. 2005a;15(6):809–822. doi: 10.1093/cercor/bhh182. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Fang PC, Kaas JH. Microstimulation reveals specialized subregions for different complex movements in posterior parietal cortex of prosimian galagos. Proc Natl Acad Sci U S A. 2005b;102(13):4878–883. doi: 10.1073/pnas.0501048102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Preuss TM, Kaas JH. Architectonics, somatotopic organization, and ipsilateral cortical connections of the primary motor area (M1) of owl monkeys. J Comp Neurol. 1993;330(2):238–271. doi: 10.1002/cne.903300207. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Qi HX, Kaas JH. Do superior colliculus projection zones in the inferior pulvinar project to MT in primates? Eur J Neurosci. 1999;11(2):469–480. doi: 10.1046/j.1460-9568.1999.00461.x. [DOI] [PubMed] [Google Scholar]
- Sur M, Nelson RJ, Kaas JH. Representation of the body surface in somatic koniocortex in the prosimian Galago. J Comp Neurol. 1980;189(2):381–402. doi: 10.1002/cne.901890211. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994;350(4):497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Symonds LL, Kaas JH. Connections of striate cortex in the prosimian, Galago senegalensis. J Comp Neurol. 1978;181(3):477–512. doi: 10.1002/cne.901810304. [DOI] [PubMed] [Google Scholar]
- Tiao YC, Blakemore C. Functional organization in the visual cortex of the golden hamster. J Comp Neurol. 1976;168(4):459–481. doi: 10.1002/cne.901680403. [DOI] [PubMed] [Google Scholar]
- Tigges J, Spatz WB, Tigges M. Efferent cortico-cortical fiber connections of area 18 in the squirrel monkey (Saimiri) J Comp Neurol. 1974;158(2):219–235. doi: 10.1002/cne.901580208. [DOI] [PubMed] [Google Scholar]
- Tigges J, Tigges M, Perachio AA. Complementary laminar terminations of afferents to area 17 originating in area 18 and in the lateral geniculate nucleus in squirrel monkey. J Comp Neurol. 1977;176(1):87–100. doi: 10.1002/cne.901760106. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Hamilton SL, Silverman MS. Topography of cytochrome oxidase activity in owl monkey cortex. J Neurosci. 1985;5(10):2786–2800. doi: 10.1523/JNEUROSCI.05-10-02786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RB, Switkes E, Silverman MS, Hamilton SL. Functional anatomy of macaque striate cortex. II. Retinotopic organization. J Neurosci. 1988;8(5):1531–568. doi: 10.1523/JNEUROSCI.08-05-01531.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ts'o DY, Gilbert CD. The organization of chromatic and spatial interactions in the primate striate cortex. J Neurosci. 1988;8(5):1712–727. doi: 10.1523/JNEUROSCI.08-05-01712.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Desimone R. Cortical connections of visual area MT in the macaque. J Comp Neurol. 1986;248(2):190–222. doi: 10.1002/cne.902480204. [DOI] [PubMed] [Google Scholar]
- Valente T, Auladell C, Perez-Clausell J. Postnatal development of zinc-rich terminal fields in the brain of the rat. Exp Neurol. 2002;174(2):215–229. doi: 10.1006/exnr.2002.7876. [DOI] [PubMed] [Google Scholar]
- Van Brederode JF, Mulligan KA, Hendrickson AE. Calcium-binding proteins as markers for subpopulations of GABAergic neurons in monkey striate cortex. J Comp Neurol. 1990;298(1):1–22. doi: 10.1002/cne.902980102. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Maunsell JH, Bixby JL. The middle temporal visual area in the macaque: myeloarchitecture, connections, functional properties and topographic organization. J Comp Neurol. 1981;199(3):293–326. doi: 10.1002/cne.901990302. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial granular a cortex in the rat. J Comp Neurol. 1990;300(4):593–606. doi: 10.1002/cne.903000412. [DOI] [PubMed] [Google Scholar]
- de Venecia RK, Smelser CB, McMullen NT. Parvalbumin is expressed in a reciprocal circuit linking the medial geniculate body and auditory neocortex in the rabbit. J Comp Neurol. 1998;400(3):349–362. doi: 10.1002/(sici)1096-9861(19981026)400:3<349::aid-cne5>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J Comp Neurol. 1987;262(2):271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2(6):435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN, Rosene DL. Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J Comp Neurol. 1987;262(2):256–270. doi: 10.1002/cne.902620207. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Farber NB. Cingulate Cortex and Disease Models. In: Paxinos G, editor. The Rat Nervous System. Elsevier; London: 2004. pp. 704–727. [Google Scholar]
- Vogt BA, Vogt L, Farber NB, Bush G. Architecture and neurocytology of monkey cingulate gyrus. J Comp Neurol. 2005;485(3):218–239. doi: 10.1002/cne.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bonin G. The atriate area of primates. J Comp Neurol. 1942;77:405–429. [Google Scholar]
- von Bonin G. Its relation to the pattern of the primate cortex. University of Illinois Pr; Urbana: 1945. The cortex of galago. [Google Scholar]
- von Bonin G, Bailey P. Pattern of the cerebral isocortex. In: Schultz AH, Hofer H, Starck D, editors. Primatologia II/2. Karger; Basel und New York: 1961. [Google Scholar]
- Wagor E, Lin CS, Kaas JH. Some cortical projections of the dorsomedial visual area (DM) of association cortex in the owl monkey, Aotus trivirgatus. J Comp Neurol. 1975;163(2):227–250. doi: 10.1002/cne.901630208. [DOI] [PubMed] [Google Scholar]
- Wagor E, Mangini NJ, Pearlman AL. Retinotopic organization of striate and extrastriate visual cortex in the mouse. J Comp Neurol. 1980;193(1):187–202. doi: 10.1002/cne.901930113. [DOI] [PubMed] [Google Scholar]
- Walker C, Sinha MM. Somatotopic organization of Smll cerebral neocortex in albino rat. Brain Res. 1972;37(1):132–36. doi: 10.1016/0006-8993(72)90354-x. [DOI] [PubMed] [Google Scholar]
- Wall JT, Symonds LL, Kaas JH. Cortical and subcortical projections of the middle temporal area (MT) and adjacent cortex in galagos. J Comp Neurol. 1982;211(2):193–214. doi: 10.1002/cne.902110208. [DOI] [PubMed] [Google Scholar]
- Wang Q, Burkhalter A. Area map of mouse visual cortex. J Comp Neurol. 2007;502(3):339–357. doi: 10.1002/cne.21286. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kurata K. Quantitative analyses of thalamic and cortical origins of neurons projecting to the rostral and caudal forelimb motor areas in the cerebral cortex of rats. Brain Res. 1998;781(1–2):135–147. doi: 10.1016/s0006-8993(97)01223-7. [DOI] [PubMed] [Google Scholar]
- Ward AA. The cingular gyrus. J Neurophysiol. 1948;23:1003–07. doi: 10.1152/jn.1948.11.1.13. [DOI] [PubMed] [Google Scholar]
- Weller RE, Kaas JH. Cortical projections of the dorsolateral visual area in owl monkeys: the prestriate relay to inferior temporal cortex. J Comp Neurol. 1985;234(1):35–59. doi: 10.1002/cne.902340104. [DOI] [PubMed] [Google Scholar]
- Weller RE, Kaas JH. The organization of the visual system in galago: Comparisons with monkeys. In: Haines DE, editor. The lesser busybaby (Galago) as an animal model: Selected topics. CRC Press; Boca Raton, Florida: 1982. pp. 108–135. [Google Scholar]
- Weller RE, Wall JT, Kaas JH. Cortical connections of the middle temporal visual area (MT) and the superior temporal cortex in owl monkeys. J Comp Neurol. 1984;228(1):81–104. doi: 10.1002/cne.902280109. [DOI] [PubMed] [Google Scholar]
- Wong P, Kaas JH. Architectonic subdivisions of neocortex in the gray squirrel (Sciurus carolinensis) Anat Rec (Hoboken) 2008;291(10):1301–333. doi: 10.1002/ar.20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Kaas JH. Architectonic subdivisions of neocortex in the tree shrew (Tupaia belangeri) Anat Rec (Hoboken) 2009a;292(7):994–1027. doi: 10.1002/ar.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Kaas JH. An architectonic study of the neocortex of the short-tailed opossum (Monodelphis domestica) Brain Behav Evol. 2009b;73(3):206–228. doi: 10.1159/000225381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Collins CE, Baldwin MKL, Kaas JH. Cortical connections of the visual pulvinar complex in prosimian galagos (Otolemur garnetti) J Comp Neurol. 2009c;517(4):493–511. doi: 10.1002/cne.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171(1):11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT, Carroll EW. Quantitative light and electron microscopic analysis of cytochrome oxidase-rich zones in V II prestriate cortex of the squirrel monkey. J Comp Neurol. 1984;222(1):18–37. doi: 10.1002/cne.902220103. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT, Merzenich MM, Leake PA. Changes in endogenous enzymatic reactivity to DAB induced by neuronal inactivity. Brain Res. 1978;141(1):185–192. doi: 10.1016/0006-8993(78)90629-7. [DOI] [PubMed] [Google Scholar]
- Wu CW, Kaas JH. Reorganization in primary motor cortex of primates with long-standing therapeutic amputations. J Neurosci. 1999;19(17):7679–697. doi: 10.1523/JNEUROSCI.19-17-07679.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CW, Kaas JH. Somatosensory cortex of prosimian Galagos: physiological recording, cytoarchitecture, and corticocortical connections of anterior parietal cortex and cortex of the lateral sulcus. J Comp Neurol. 2003;457(3):263–292. doi: 10.1002/cne.10542. [DOI] [PubMed] [Google Scholar]
- Wu CW, Bichot NP, Kaas JH. Converging evidence from microstimulation, architecture, and connections for multiple motor areas in the frontal and cingulate cortex of prosimian primates. J Comp Neurol. 2000;423(1):140–177. doi: 10.1002/1096-9861(20000717)423:1<140::aid-cne12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Xu X, Collins CE, Kaskan PM, Khaytin I, Kaas JH, Casagrande VA. Optical imaging of visually evoked responses in prosimian primates reveals conserved features of the middle temporal visual area. Proc Natl Acad Sci U S A. 2004;101(8):2566–571. doi: 10.1073/pnas.0308745101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki SM. Representation of central visual fields in prestriate cortex of monkey. Brain Res. 1969;14(2):271–291. doi: 10.1016/0006-8993(69)90110-3. [DOI] [PubMed] [Google Scholar]
- Zeki SM. Functional organization of a visual area in the posterior bank of the superior temporal sulcus of the rhesus monkey. J Physiol. 1974;236(3):549–573. doi: 10.1113/jphysiol.1974.sp010452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki SM. The response properties of cells in the middle temporal area (area MT) of owl monkey visual cortex. Proc R Soc Lond B Biol Sci. 1980;207(1167):239–248. doi: 10.1098/rspb.1980.0022. [DOI] [PubMed] [Google Scholar]
- Zilles K. Organization of the Neocortex of the rat. In: Tees B, Kolb RC, editors. The Cerebral Cortex of the Rat. MIT Press; 1990. pp. 21–34. [Google Scholar]
- Zilles K, Wree A. In: Cortex: Areal and laminar structure. Paxinos G, editor. Academic Press; Sydney: 1995. pp. 649–685. [Google Scholar]
- Zilles K, Rehkämper G, Stephan H, Schleicher A. A quantitative approach to cytoarchitectonics. IV. The areal pattern of the cortex of Galago demidovii (e. Geoffroy, 1796), (lorisidae, primates) Anat Embryol (Berl) 1979;157(1):81–103. doi: 10.1007/BF00315642. [DOI] [PubMed] [Google Scholar]
- Zuckerman S, Fulton JF. The motor cortex in Galago and Perodicticus. J Anat. 1941;75(Pt 4):447–456. [PMC free article] [PubMed] [Google Scholar]