Abstract
The nitric oxide (NO•) sibling, nitroxyl or nitrosyl hydride (HNO), is emerging as a molecule whose pharmacological properties include providing functional support to failing hearts. HNO also preconditions myocardial tissue, protecting it against ischemia-reperfusion injury while exerting vascular antiproliferative actions. In this review, HNO's peculiar cardiovascular assets are discussed in light of its unique chemistry that distinguish HNO from NO• as well as from reactive oxygen and nitrogen species such as the hydroxyl radical and peroxynitrite. Included here is a discussion of the possible routes of HNO formation in the myocardium and its chemical targets in the heart. HNO has been shown to have positive inotropic/lusitropic effects under normal and congestive heart failure conditions in animal models. The mechanistic intricacies of the beneficial cardiac effects of HNO are examined in cellular models. In contrast to β-receptor/cyclic adenosine monophosphate/protein kinase A-dependent enhancers of myocardial performance, HNO uses its “thiophylic” nature as a vehicle to interact with redox switches such as cysteines, which are located in key components of the cardiac electromechanical machinery ruling myocardial function. Here, we will briefly review new features of HNO's cardiovascular effects that when combined with its positive inotropic/lusitropic action may render HNO donors an attractive addition to the current therapeutic armamentarium for treating patients with acutely decompensated congestive heart failure. Antioxid. Redox Signal. 14, 1687–1698.
Introduction
Nitroxyl or nitrosyl hydride (HNO) is the one electron-reduction product of nitric oxide (NO•) (25), which displays a distinctive chemistry from NO• (25), but whose role has yet to be fully determined. HNO was the first molecule found in the interstellar clouds to contain a N=O bond (71) (where it appears to be particularly abundant). Further, it was nearly two decades ago that investigators suggested that HNO may have cardiovascular effects and may ultimately account for the mechanism of endothelium-derived relaxing factor (EDRF) (24). More recently, HNO has also been suggested to be a mechanism for endothelial-derived hyperpolarization (2). However, despite the fact that the presence of HNO may span from the heavens to the heart, research dedicated to HNO would not be one which we would call a burgeoning field. One plausible explanation for the limited attention accorded to HNO, so far, is the lack of evidence for its in vivo generation. However, this limitation is likely technical in nature and may not be a true biological limitation. Currently, there are no definitive methods available to unequivocally detect the “footprints” of HNO in a biological system. HNO is different from NO• and some oxygen-derived redox-active species, in that it is not a “radical,” thus it cannot be directly trapped by electron paramagnetic resonance/electron spin resonance–based methods. Moreover, the use of currently available HNO donors in biological buffers leads to the very short half-life (2.7 min) of this purported gasotransmitter (45), rendering HNO a rather “fleeting” species. Hence, this caveat prevents us from speculating about its physiological relevance. Nonetheless, given the strikingly different functional outcomes produced by HNO versus NO• or other reactive nitrogen species (RNS) such as nitrite (35) and peroxynitrite (32), we are free to consider HNO donors as pharmacological tools for treating several disorders, especially cardiovascular diseases. In this review, we will first describe possible routes through which HNO may be generated in the myocardium as well as its chemistry and putative targets within the contractile machinery. Then, we will describe in detail the ability of HNO to improve myocardial function, focusing on myocardial mechanics, both in vivo and in vitro, in normal and failing hearts. The intriguing vascular effects of HNO donors will not be addressed here, as these are the subject of a fully dedicated article also in this Forum Issue. Finally, we will illustrate how the distinctive redox chemistry and putative signaling pathways of HNO can represent a valid alternative or an advantageous addition to the current therapeutic mainstays for treating congestive heart failure (CHF) of ischemic or nonischemic origin.
HNO in the Heart: Possible Sources, Chemical Targets, and Ways of Detection
In vitro evidence of HNO generation
The endogenous generation of HNO remains speculative. Thus, it is currently unknown whether HNO is a gastrotransmitter akin to other endogenously synthesized small molecules such as NO•, carbon monoxide (CO), or hydrogen sulfide (H2S). Equally unknown is whether HNO has a signaling capacity similar to low levels of hydrogen peroxide. HNO was detected in interstellar space more than 30 years ago (71), and an abundance of in vitro evidence supports its possible existence in biologically relevant systems. Moreover, as we will learn later, in several settings, the actions of HNO are potent, very selective, and fast in both the onset and offset. If “nature operates in the fastest way possible” (Roger Bacon, Opus Maius), then it is plausible that HNO is one of nature's messengers; however, its endogenous generation remains hypothetical.
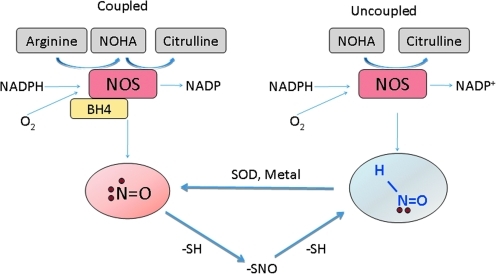
Putative pathways for the generation of HNO in vivo have been developed that are either dependent or independent of the enzyme nitric oxide synthase (NOS). NOS functions through the conversion of L-arginine to NO• and L-citrulline and in the process consumes O2 and NADPH while utilizing the cofactor tetrahydrobioterpin (BH4). However, this process also produces the intermediate compound, NG-hydroxy-L-arginine (NOHA), in the oxidation of arginine, as well as peroxide and superoxide (O2•−) when it is in its uncoupled state (induced by the absence of either arginine or BH4). One possible mechanism for the NOS-dependent synthesis of HNO is through the direct oxidation of NOHA when NOS is uncoupled (24, 59). In turn, HNO can be converted to NO• through the reaction of Fe(II) at the heme site of the enzyme or via superoxide dismutase (28, 62). HNO can also be produced by the catalysis of arginine to citrulline in the absence of BH4 (1) (Fig. 1).
FIG. 1.
Possible nitric oxide synthase (NOS)-dependent pathways leading to the in vitro formation of nitroxyl or nitrosyl hydride (HNO). In addition to the direct oxidation of NG-hydroxy-L-arginine (NOHA) when NOS is uncoupled and to the possibility of generating HNO via tetrahydrobioterpin (BH4)-free NOS, the lower part of the diagram highlights the possibility of forming HNO from the decomposition of S-nitrosothiols. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
In addition to these NOS-dependent mechanisms, there are also a number of possibilities for the NOS-independent formation of HNO. However, these mechanisms suffer from the limitation that unlike O2•− and O2, HNO cannot be formed through the direct reduction of NO• to HNO due to energetic barriers (this is also highlighted by the fact that the conversion of HNO to NO• requires the use of a metal catalyst). The mechanisms by which HNO can be formed include the decomposition of nitrosothiols (74), the dismutation of NO• into nitrosonium (NO+) and HNO (65) (catalyzed by iron sulfur complexes), or the reduction of NO• by xanthine oxidase (61). As with the enzyme nitrite reductase, there is also the possibility of a reduction of coordinated NO+ to NO• and NO−, which has been recently re-evaluated (36).
Interestingly, Yong et al. have suggested that HNO may be generated by a mechanism involving the interaction of H2S with NO•. This is based on the observation that when a H2S donor (NaHS) is combined with an NO• donor (sodium nitroprusside) in isolated rat ventriculocytes, cellular contraction was increased in a manner closely reminiscent of the actions of HNO (78). One possible mechanism by which this could occur is that HS− acts as a nucleophile, attacking the Fe nitrosyl bond of sodium nitroprusside forming an S-NO intermediate that decomposes through the reaction with SH- to give HNO.
There are additional pathways that do not implicate the generation and interconversion of nitrogen oxides in HNO formation. One is through the heme protein mediated oxidation of hydroxylamine (NH2OH) in the presence of peroxide (16). This is a particularly intriguing biomediator, as it involves the possible cyclic production of HNO involving peroxide, thiols, and NH2OH. When HNO is initially formed, it can react with thiols such as glutathione (GSH), which results in NH2OH. In the cell, there is between 2 and 5 mM GSH, making it a primary target of HNO and a primary and decomposition product of NH2OH. As just stated, NH2OH in the presence of peroxide and heme protein can generate HNO. This forms a cycle that under oxidative conditions can generate HNO, thus providing a recycled supply of this molecule. However, this mechanism would be limited if there was a breakdown in disulfide reduction. This mechanism is especially interesting, as it suggests a possible endogenous pathway by which signaling molecules such as hydrogen peroxide and the oxygenated derivative of ammonia may lead to HNO formation.
In the end, there are numerous possible mechanisms for HNO formation at least in vitro, and the recent evidence showing that endogenous molecules can act as substrates for HNO formation renders its endogenous biosynthesis even more likely. The exact connotations of each one of these routes remain to be verified in vivo.
HNO targets
HNO has specific targets that do not react with either NO• nor CO. Specifically, HNO will react with higher oxidation state metals [i.e., Fe(III), Cu(II), and Mn(III)] as well as thiols (25). Utilizing the affinity of HNO for ferric heme proteins, methods using the reductive nitrosylation of Mb(III) to form the nitrosyl complex in solution and xerogels have been used to detect HNO, distinguishing it from NO• (15). As such, HNO can affect key metalloproteins by either activation (e.g., soluble guanylyl cyclase [sGC]) or inhibition (e.g., peroxidases and monoxygenases like cytochrome P450) (48). Thus, metal-based reactions carried out by HNO may be important in different signaling pathways that differ from those of NO• and CO.
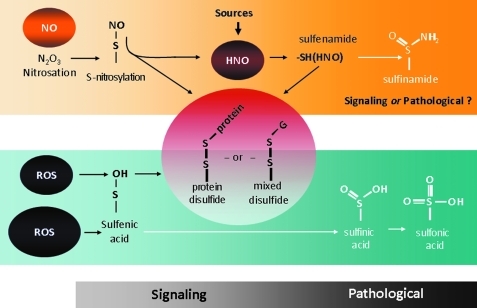
The most unique effect of HNO, and what substantially differentiates it from NO•, is its reactivity with thiols. Thiols are major targets for HNO, which can lead to reversible and potentially irreversible modifications. These are particularly important residues, as mounting evidence supports the notion that thiol(-SH)-based switches (chiefly via post-translational modification of cysteine residues) can control several cellular functions. Typically, a “redox switch” consists of two redox-sensitive cysteine residues and the disulfide bond between them. The possibility that two cysteines are oxidized to form a disulfide bond depends not only on the topology but also on the temperature- and pH-dependent redox potential of cysteine-cystine (49). As shown in Figure 2, HNO can modify cysteines in two distinct ways. The first is via its direct targeting of reactive thiols in cysteine residues to form N-hydroxysulfenamide (RSNHOH). Second, if there is an additional available thiol in the vicinity, a disulfide bond may form (36). The possible signaling role of sulfenamide formation remains to be fully ascertained, that is, whether this post-translational modification is always irreversible and coupled to pathologic structural and function changes. Conversely, it is increasingly evident that, under mild oxidizing conditions, the appearance of inter- and/or intradisulfide bonds often leads to a change in protein function, due to a conformational change that may or may not implicate an oligomer transition (49). Then, these modifications are reversed by cellular reducing factors belonging to the Trx and GSH systems, with or without transformation of the oligomeric state that accounts for the change in protein function (49). When it comes to HNO, this type of reversible post-translational modifications may have functional relevance as we will see when discussing HNO-induced modifications in isolated cardiac myocytes and muscles.
FIG. 2.
Reactive oxygen species (ROS) and HNO interaction with thiols. Thiols, and in particular reactive cysteines, are subjected to a continuum of modifications by ROS and HNO. HNO can modify cysteines in two distinct ways. The first is via its direct targeting of reactive thiols in cysteine residues to form N-hydroxysulfenamide (RSNHOH). Second, if there is an additional available thiol in the vicinity, a disulfide bond may form. Whether the formation of sulfenamide always leads to irreversible and likely pathological signaling remains to be fully established. Conversely, it is plausible that HNO acting as a mild oxidizing agent promotes the formation of reversible inter- and/or intradisulfide bonds that ultimately leads to changes in protein function (see also text). (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Interestingly, it has been widely reported that HNO may modify thiols despite the presence of high concentrations of low-molecular-weight thiols (e.g., GSH) (12, 34, 40). The affinity of HNO for GSH and formation of the adduct GSH sulfinamide has been used to determine the presence of HNO in chemical and biochemical models (17, 29, 66). Since GSH has a concentration within the millimolar range inside the intracellular compartment, it should be one of the major reactants resulting in little additional modifications (i.e., protein modification). However, HNO does have a profound effect on cellular signaling, thus suggesting that the ability of HNO to modify a given thiol depends on other biochemical reasons such as its acid dissociation constant (pKa) or location within the cell (e.g., a hydrophobic subcellular compartment). When the pKa of a particular thiol is very low, the reactivity of this site for HNO will out-compete the presence of excess GSH. One well-suited example is glyceraldehyde 3-phosphate dehydrogenase (GAPDH), which is in the cytoplasm and inhibited by HNO despite the presence of GSH (40) due to the high reactivity of its reactive cysteine residue.
As mentioned, it is interesting that HNO can function in the face of high levels of additional thiols in the form of GSH. HNO would not sail too far in cellular microdomains in which GSH is particularly abundant. This evidence leaves many questions open, such as how HNO travels in the bloodstream that is full of thiols such as GSH, cysteine, cysteinyl-glycine, and homocysteine, both at plasma and whole blood level. When it comes to intracellular compartments such as intramyocyte environment, in addition to its reactivity, the location of a cysteine containing protein in the cell is also an important consideration. HNO is hydrophobic in nature; thus, it is plausible that it can preferentially find its targets in hydrophobic regions. GSH typically does not exist in these regions and as such, HNO will be more likely to react with thiol or metal containing proteins in these hydrophobic regions than in subcellular citadels filled with GSH (36).
Therefore, the pKa of a given cysteine and the location of the molecular target are likely to “compartmentalize” the reactivity of HNO, thereby conferring to it a specificity of action. Tyrosine hydroxylase and NF-κB are additional interesting examples of possible HNO targets, because they harbor a low pH key cysteine whose modifications may drive changes in function.
In the heart, it is possible that key protein residues located in functionally relevant structures of the contractile machinery contribute to intracellular cardiac control of Ca2+ cycling/handling and myofilament response to Ca2+, positively modulating rather than countering overall myocardial performance. As will be discussed later, this eventuality is likely the outcome of “selective” targeting operated by reactive oxygen species (ROS)/RNS on critical, redox-sensitive protein residues, chiefly cysteines, containing highly reactive thiols. Hence, the biochemical ground for HNO pharmacological actions, as well as a palette of possibilities by which HNO reactivity toward thiols is seen, is ultimately different from other well-known oxidizing agents.
In Vivo Cardiovascular Action of HNO Donors
HNO donors
For biological studies, many HNO donors are currently available (47), but not all of these are amenable for in vivo or even in vitro studies. The most commonly used HNO-releasing agents for animal studies are the prototypic HNO donor Angeli's salt (AS, Na2N2O3) (45), Piloty's acid (PhSO2NHOH), and its derivatives, isopropylamine- NO• (IPA/NO) (48) and acyloxy nitroso compounds such as 1-nitrosocyclohexyl acetate (NCA, also known as the “blue compound”) (63). Without a doubt, AS remains the most widely used HNO donor despite its short half-life and the well-known downside of being a coreleaser of NO2− (which requires the use of proper controls to exclude its possible confounding effects).
HNO in normal in vivo hearts
Initially, HNO donors such as AS and Piloty's acid have attracted attention as vasodilating substances. Fukuto et al. were the first to report that, in both rabbit aorta and bovine intrapulmonary artery, AS/HNO relaxes vessels by a sGC-dependent mechanism (24). Since then, several studies have explored the modalities by which AS, Piloty's acid, and its derivatives relax vessels, both in vitro and in vivo. The total or partial sGC-dependency of HNO vasodilation and more, in general, the way by which HNO vasorelaxes both capacitance and resistance vessels is the subject of an extensive review in this forum issue (7).
In addition to its vascular effects, HNO exerts a unique in vivo action on contracting myocardium. Differently from other NO• donors, which result in a modest or clearly negative impact on contractile function [particularly when in excess (43), HNO donors promptly and markedly increase myocardial contractility (positive inotropy) while accelerating relaxation (positive lusitropy)]. Using conscious dogs instrumented for pressure–volume relationships (an approach that allows the dissection of primary direct effects on the myocardium versus cardiac changes due to modified vascular loading conditions) (32, 33), our group was the first to demonstrate that AS/HNO (10 μg/kg=0.082 μmol/kg for 10–20 min, intravenously) increases intrinsic ventricular stiffness, independent from changes in cardiac volumes. In addition, HNO hastens ventricular relaxation and unloads the heart (55). All these effects persist after both left ventricular (LV) volume repletion—to match baseline filling volumes—or after autonomic blockade with hexamethonium—to offset possible confounding effects deriving from baroreflex activation. The modalities of HNO cardiac actions neatly distinct from those of well-known inotropic agents such as β-agonists or phosphodiesterase inhibitors have been confirmed, showing that the AS/HNO effects in conscious dogs are not offset by sustained β-adrenergic blockades. The unique combination of positive inotropy and preload reduction, in conjunction with a balanced veno- and arterial dilation, cannot be mimicked by donors of NO•, nitrate, or other RNS such as peroxynitrite (32). In addition, the HNO-evoked rise in function is fully prevented by the thiol-donating compound N-acetyl-L-cysteine. L-cysteine has been used to discriminate between in vitro aortic relaxations to HNO and NO (58). This suggests that when the intracardiac environment is enriched with free floating thiols, the ability of in situ thiols to react with HNO is reduced, thereby blunting its effect.
HNO in failing hearts
CHF is a chronic disease condition in which ROS and RNS are generated by multiple sources (52), possibly leading to a reduced bioavailability of NO•. In addition to decreased antioxidant defenses, the reduction in NO• can result in a further increase in ROS due to the uncoupling of NOS. The consequent tissue redox imbalance can result in key events determining or associated with the onset and progression of the CHF disease. These include altered excitation-contraction (EC) coupling, cardiomyocyte maladaptive hypertrophy, extracellular matrix remodeling, abnormal tissue energetics, loss in viable myocardium, vascular and capillary alterations, and inflammation.
Further to this, we should consider that the β-adrenergic system (which adjusts cardiac force development to increased work demand) is downregulated in CHF due to β-receptor desensitization and uncoupling to their signaling molecules (18). Increased emission of ROS/RNS may further dampen this β-agonist stimulated contractile reserve and relaxation (directly or indirectly) by either altering the β-agonists per se (32) or negatively interacting with their associated signaling pathways. For instance, endogenous NO• and nitrates are known to attenuate the response of β-adrenergic stimulation in both experimental and human CHF (43). This effect represents a major drawback when a combination of β-agonists and unloading agents is desired in aiding patients with CHF. Thus, inotropic/lusitropic agents that do not lose their efficacy in the CHF setting nor display a facile reaction toward ROS/RNS should be ideal to treat patients with acutely decompensated heart failure.
Using the approach just described (i.e., conscious dogs instrumented for studying the pressure–volume relationship), we were able to show that in dogs in whom CHF was induced by tachypacing (33), HNO donors such as AS improve both contractility and relaxation to a similar extent as in control preparations (54). Further, when HNO was administered concomitantly with β-agonist mimetics such as dobutamine, they were additive in supporting myocardial contraction as opposed to NO/nitrite that blunted dobutamine-induced enhancement in function.
From a signaling point of view, there was an initial contention that HNO inotropy could be mediated by the release of calcitonin gene-related peptide (CGRP) from nonadrenergic/noncholinergic fibers. CGRP is a neuropeptide endowed with several, important pharmacological properties. In the cardiovascular system, CGRP is a potent vasodilator and induces positive cardiac inotropy in several species including humans (3). The credence that CGRP could mediate HNO inotropy was based on the fact that HNO enhancement in contractility in vivo can be blocked by the CGRP receptor antagonist (CGRP8-37) (55) and evidence which showed that in vivo HNO infusion leads to increased systemic levels of CGRP (54). However, subsequent studies in normal and CHF dogs showed that CGRP-induced inotropy is essentially indirect, as it is mediated by the release of norepinephrine from sympathetic efferent fibers and as such is fully prevented by β-blockers such as timolol (33). That HNO and CGRP-evoked inotropes diverge in terms of signaling is further borne out by the evidence that CGRP stimulatory action is lost in CHF preparations (33), in keeping with the well-known downregulation of the β-receptor mediated signaling in this syndrome (18). Thus, although CGRP release might, in part, explain HNO-induced vasodilation (21), it definitely does not account for HNO-evoked positive inotropy-lusitropy. This realization prompted further in vitro studies that aimed at dissecting the mechanism(s) of HNO's action.
From a pharmacological point of view, HNO effects are not specific to AS, but it instead represents only one of the many HNO donors that are likely to constitute a new class of compounds. This contention has been subsequently proved by the use of IPA/NO (46) (a HNO donor that is chemically unrelated to AS) as well as of the use of the “blue compound,” NCA (63). One promise of HNO is that its cardiac action is fully preserved in CHF preparations in face of altered tissue and vascular redox conditions. In addition, there is also evidence gathered by the Kemp-Harper's group which showed that the in vitro action of HNO is resistant to both the development of tolerance and scavenging by superoxide (O2−•) (7). In essence, these in vitro findings are not “radical” and are fully consistent with the HNO chemistry and with previous in vivo studies in which it was demonstrated that repeated infusions, AS/HNO, did not lead to any loss in efficacy (55).
HNO in ischemia-reperfusion injury
One of the first in vivo studies (if not the very first) to employ AS as a HNO donor was a report from Ma et al. in which the impact of AS and the NO• donor S-nitrosoglutathione (GSNO) were compared in rabbits subjected to 45 min of regional myocardial ischemia followed by 180 min of reperfusion (41). The use of GSNO 5 min before reperfusion markedly attenuated reperfusion injury, as evidenced by an improvement in myocardial function, reduced infarct size and decreased activity of creatine kinase (CK) and myeloperoxidase. Conversely, the administration of hemodynamically equieffective doses of AS (3 μmol/kg for 20–30 min) aggravated reperfusion injury as indicated by increased necrotic size, CK release, and end-diastolic pressure. It is possible that the ability of HNO to recruit neutrophils may have had some part in the detrimental effect of AS on reperfused myocardium (72). However, the mechanistic underpinnings of this negative impact still await clarification, and equally relevant is the possibility of a dose dependency of the effects. Indeed, the authors found that 1 μmol/kg AS did not significantly affect the extent of ischemia-reperfusion injury. Although these AS concentrations widely exceed those used later in in vivo dogs (54, 55), this article also suggests the need of conducting full-scale dose-response studies, particularly in light of subsequent studies showing that AS given before ischemia is a good early preconditioning-like agent in isolated and perfused rat hearts (53). Indeed, Pagliaro et al. reported a few years later that, in rat hearts, AS/HNO intracoronary infusion (1 μM, final concentration in the heart for 19 min) before 30 min of global ischemia (and reperfusion) grants a protection similar to that afforded by classical ischemic preconditioning. The magnitude of AS-evoked protection cannot be achieved with equimolar amounts of the NO donor diethylamine/NO• complex (DEA/NO), which has a similar half-life and in vitro kinetics as AS (46). Coinfusion in the triggering phase of AS and the HNO scavenger, N-acetyl-L-cysteine (4 mM), completely reversed the beneficial effects of AS, both at 30 and 120 min of reperfusion. These data suggest that (as in other settings) HNO is sensitive to the actions of thiol-donating compounds and likely follows a signaling pathway different from that of NO•. Preliminary studies conducted by our group showed that in isolated rat hearts, as opposed to DEA/NO, HNO protection via either AS or IPA/NO cannot be prevented by the mitochondrial K(ATP) channel (mKATP) blocker 5-hydroxydecanoate (42). However, very recently Queliconi et al. have shown that in isolated mitochondria, HNO (from AS) inhibits complex II and opens mKATP, showing that HNO protects cardiomyocytes against ischemia/reperfusion injury in an mKATP-dependent manner (60). Additional studies to further dissect the protective signaling cascade(s) triggered by HNO given before ischemia are required. Moreover, it would be of interest to establish whether and how HNO interacts with other gas-transducing systems such as CO, H2S, and, of course, NO• to affect mitochondrial function and eventually protect the heart. Finally, it is worthwhile to firmly establish whether HNO is always (i.e., at any dose) detrimental at reperfusion and why this may be the case. HNO might worsen cerebral ischemia-reperfusion injury by increasing oxidative stress and decreasing brain perfusion (10). Nevertheless, it is difficult to draw definitive conclusions from these studies entailing different experimental approaches (i.e., injection vs. intravenous infusions), animal species, and possibly HNO doses.
HNO Effects on Isolated Cardiac Myocytes
Redox-dependent control of E-C coupling
In ventricular cardiac cells, the release of Ca2+ from the sarcoplasmic reticulum (SR) is a key event in the EC coupling process. For any given heart beat, a small amount of Ca2+ enters via the sarcolemmal voltage-dependent Ca2+ channels (L-type Ca2+ channels), which triggers the release of a bigger pulse of Ca2+ from the SR through specialized Ca2+-gated channels (i.e., ryanodine receptors [RyR2]). This event, known as Ca2+-induced Ca2+ release, makes Ca2+ available to the myofilaments and their regulatory proteins, resulting in myofilament sliding and ultimately sarcomere contraction. At the end of each systolic phase, Ca2+ is pumped back into the SR via the ATP-dependent SR Ca2+-ATPase (SERCA2a) or excluded from the cell by the Na+-Ca2+ exchanger. The function of SERCA2a is regulated by the reversible inhibitor phospholamban (PLN). β-adrenergic stimulation via cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA)-mediated signaling may alter the phosphorylation status of L-type Ca2+ channels, RyR2, PLN, and myofilament regulatory proteins, thus modifying the function of these key E-C components. For instance, β-agonist-induced, PKA-mediated phosphorylation of PLN leads to the removal of its inhibitory effect on SERCA2a, thus accelerating the rate of Ca2+ uptake into SR.
In the heart, both acute and chronic exposure to β-adrenergic stimulation may result in ROS production (80). This is important, because PKA, PKG, or PKC phosphorylation is not the sole modulatory mechanisms for the EC machinery (i.e., channels, pumps, transporters, and contractile and regulatory proteins). These structures also harbor critical sulfhydryl groups, mainly in the form of cysteine residues that form the basis of redox reactive “switches” which are susceptible to a continuum of chemical modifications from endogenous signaling molecules such as NO•, RNS, or ROS (75). These molecules are continuously produced during muscle contraction and modulate the mechanical properties of muscles such as contraction and relaxation (44). For example, NO• is likely a key mediator in the increase in SR-Ca2+ release after mechanical stretch (57).
The interplay between ROS/RNS and reactive cysteine residues may span from S-nitrosylation to permanent irreversible modifications such as carbonylation. Post-translational modifications, such as S-nitrosylation, can alter a protein's function as demonstrated by the increased probability of the RyR2 channel being in the “open” state after modification [reviewed in Ref. (44)]. It has been postulated that hyper-reactive cysteine (Cys) moieties may represent biochemical components of a more complex “redox sensor” that conveys information about localized changes in redox potential produced by physiologic (NO•) and pathophysiologic (quinones, ROS) channel modulators to the Ca2+ release process (56). Nevertheless, the presence of strong oxidants or the persistence of oxidative stress results in an altered redox balance that permanently modifies these same structures. For instance, in failing hearts, the RyR2 has an increased number of oxidized thiols; and treating failing myocytes with antioxidants significantly improves intracellular Ca2+ handling in these cells (67). Likewise, L-type Ca2+ channels can be modified in a redox-dependent manner (likely through cysteine alterations), thereby increasing Ca2+ influx through the channel and increasing its intracellular levels (30). In a similar manner, abundant evidence in intact muscle or myofibrillar preparations has demonstrated that oxidation of myofilament proteins results in contractile dysfunction. For instance, Canton and coworkers have reported that oxidative modifications at tropomyosin occur in a microembolization-induced model of CHF in dogs (8). That sulfhydryl groups are critically involved in myocardial dysfunction of different etiology is also suggested by a recent study which reported the oxidation of cysteine residues in myosin light chain 1 and actin after treatment with the oxidizing agent, 2,2'-dithiodipyridine, at concentrations which cause a reduction in contractile function (27).
From all these findings, it manifests that (i) ROS/RNS are major endogenous modulators of basal and β-agonist stimulated contractility via key redox switches; (ii) the concentration of the reagent used, the length of exposure, and the nature of the chemical reaction the reagent undertakes with sulfhydryl groups are the primary factors to determine the ultimate functional outcome (56); (iii) similar to any other excitable cells, myocytes maintain their intracellular milieu under tightly controlled reducing conditions to avoid local changes in redox potential that would ultimately affect the redox status of channels harboring hyper-reactive sulfhydryl groups, thereby leading to their spontaneous opening (76).
Effects of HNO on E-C coupling
When in vitro, “the reaction of a protein with a modified cysteine residue is determined by the overall condition of the protein, including the spatial position of the cysteine residue, electrostatic interaction between the cysteine residue and other charged residues, spatial interactions between the cysteine residue and a chemical compound, electrophilicity of the chemical compound, and the pH of the solution” (50). From this statement, it is evident that as a thiophylic agent, HNO may represent the ideal candidate for a facile reaction with cysteine residues aligned with key redox active sensor sites in the myocardial contractile machinery.
Recently, we (35, 69) and others (38, 78) have shown that HNO donors, such as AS, enhance the function of isolated rodent (mouse and rat) myocytes, such that they have an increased shortening capacity and an accelerated relaxation with an increased whole Ca2+ transient. The latter effect seems to be entirely dependent on HNO-induced stimulation on RyR2, due to enhanced SR Ca2+ fractional release (69) without recruiting extracellular Ca2+ from L-type channel activity (35). Indeed, HNO increases the open probability of the Ca2+ release channels in RyR2 reconstituted in lipid bylayers and the Ca2+ spark frequency without altering other characteristics of these functional units such as coordination and termination. NO• donors (69) or nitrite (35) cannot reproduce these effects. Similar stimulatory action from HNO was also described for skeletal Ca2+ release channels (RyR1) (9). In both cases (i.e., RyR2 and RyR1), the effects were reversible on the addition of a sulfhydryl reducing agent, dithiothreitol, suggesting that HNO targets hyperactive cysteine groups in these channels.
Although there was an increase in Ca2+ release after AS/HNO treatment, the induced inotropy was sustained. This suggested that the SR should be continuously replenished with Ca2+ via a HNO-dependent pathway stimulating the activity of SERCA2a. This is indeed the case, as it has been determined that HNO activates SERCA2a in a manner that resembles β-adrenergic/PKA-dependent removal of inhibition exerted by PLN (69). However, the modality of HNO activation of SERCA2a is different from those elicited by β-agonists. Froehlich et al. found that when the three cysteines present in PLN transmembrane domain are replaced with alanine, the stimulatory action of HNO is lost (23). Hence, thiol modification is pivotal in explaining the cardiac stimulatory action of HNO.
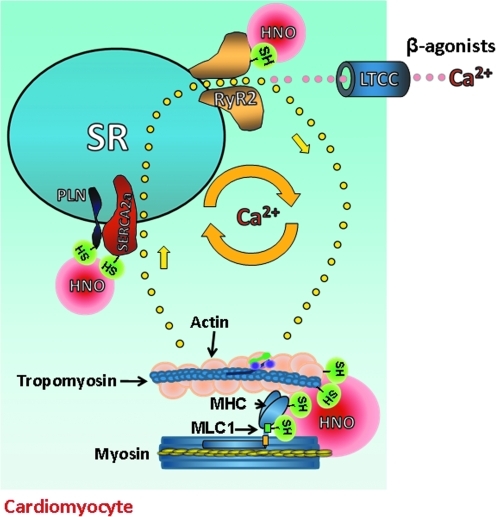
In the specific case of the PLN/SERCA2a interaction, HNO likely configures the formation of a “forbidden disulfide” between the cysteines of the PLN transmembrane domain forming a cross-link in the secondary structure where, for spatial reasons, a disulfide should not exist (in theory) (75). This hypothesis, and whether HNO cardiac action requires the presence of PLN and/or involves additional changes within the cysteine residues present in SERCA2a, as recently suggested (38), warrants further investigation. Moreover, additional studies are needed to establish whether HNO alters SERCA2a conformational flexibility in the presence of PLN. Finally, HNO functioning as a “Ca2+ cycling enhancer” (Fig. 3) could be particularly prominent in rodents whose intracellular Ca2+ availability depends almost exclusively on SR Ca2+ content, but less in species such as humans in whom extracellular Ca2+ is more important.
FIG. 3.
Effects of HNO donors on cardiomyocyte electro-contraction coupling. To increase basal contractile function, classical inotropic agents such as β-adrenergic agonists recruit extracellular Ca2+ by opening L-type Ca2+ channels (LTCC). This amount of Ca2+ triggers a larger Ca2+ release from the ryanodine receptor 2 (RyR2), making more ions available to myofilaments, thus allowing the formation of cross-bridges between actin and myosin that ultimately generates cardiac muscle contraction. This process is regulated by additional, regulatory myofilament proteins such as tropomyosin. Differently from β-agonists and other cyclic adenosine monophosphate/protein kinase A-dependent inotropic agents, HNO does not require extracellular Ca2+ to increase myocyte contractility. Rather, it enhances intracellular Ca2+ cycling, enhancing the fractional release of Ca2+ from the sarcoplasmic reticulum (SR) by opening RyR2 and by promoting a faster Ca2+ re-uptake by the SR, avoiding a rise in SR Ca2+ load that may ultimately lead to adverse effects such as arrhythmias. This finely tuned effect of HNO on SR Ca2+ balance is due to the fact that HNO stimulates a bigger Ca2+ release from RyR2 but, at the same, it also favors a faster removal of Ca2+ from the cytosol. Indeed, HNO removes the “brake-effect” exerted by phospholamban (PLN) on SR Ca2+-ATPase (SERCA2a), that is, the enzymatic activity deputed to re-uptake Ca2+ inside the SR. Likely, HNO modifies SR Ca2+ cycling via its interaction with highly-reactive thiols present in several intracellular EC structures such as RyR2, PLN, and/or SERCA2a and myofilaments with associated regulatory proteins, but not LTCC. MLC1, myosin light chain 1; MHC, myosin heavy chain. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
HNO also acts at the myofilament level to enhance cardiac function (Fig. 3). When directly applied to isolated intact cardiac muscle (right ventricular trabecuale), HNO enhances myofilament responsiveness to Ca2+, augmenting myocardial contractility more than whole Ca2+ transients (13), thus suggesting that HNO also functions as a Ca2+ sensitizing agent. Again, altering intracellular redox conditions with dithiothreitol prevented HNO-induced augmentation in muscle force development, consistent with the idea that HNO has affinity for strategically located thiols (13). Mapping these residues, either at myofilament or at the regulatory protein level, is an ongoing effort in our laboratory. Since the influence of HNO on myocyte mechanical properties is fully cyclic guanosine monophosphate (cGMP)- and cAMP-independent (69, 78), this highlights a new, redox-dependent way by which cardiac force generation can be enhanced. The fact that HNO action appears to be preserved in failing myocytes (68) or in β-adrenergic desensitized ones (20) hints to the intriguing possibility that the targets of HNO are “protected” thiol residues, not readily available to general oxidants and are still available for redox modifications in face of the overall oxidized environment that characterizes failing cardiac cells (52).
Of interest is the possibility that HNO's interaction with structures in the EC coupling machinery suggest a possible interrelationship between HNO and neuronal NOS (nNOS). As discussed earlier, HNO can be generated by uncoupled or BH4-free nNOS (1). Since nNOS has been shown to be contiguous to RyR (77), this is a plausible mechanism by which nNOS generated HNO may modify the RyR in vivo. Given the increasingly appreciated role of nNOS in regulating SR Ca2+ handling, one may wonder whether under conditions of increased oxidative stress HNO could be the leading product of nNOS, tonically influencing the function of the neighboring RyR2. Again, verifying this intriguing idea is subordinated to the possibility of effectively detecting HNO emission from nNOS in intact myocytes.
Other Actions of HNO in the Circulatory System
In concourse with its peculiar cardiac functional endowments, HNO donors possess properties that may positively affect failing hearts or may be helpful in the treatment of other chronic cardiovascular disorders. First, one effect of AS that was appreciated even before it was recognized as a HNO and not a NO• donor was that HNO donors are good inhibitors of platelet aggregation (4). Further, although a cGMP-dependent or redox-mediated mechanism is yet to be defined, HNO donors may also serve as antithrombotic agents. In addition to this, Fukuto's group have shown that HNO may act as an antioxidant via facile hydrogen-atom donation to oxidizing radical species (an event similar to what happens with tocopherols) and subsequent generation of NO• (39), a well-established quencher of ROS. The latter finding goes hand in hand with studies from Naughton et al., which showed that AS/HNO is able to induce the expression of mRNA for heme-oxygenase-1(HO-1) in a concentration- and time-dependent manner, which translated into increased HO-1 activity in rat H9c2 cells (51). HO-1 controls heme and iron distribution, as such, playing a crucial physiological role in cell survival (14). HO-1 as well as its products, CO and bilirubin, also have major implications in countering endothelial cell damage, atherogenesis, and oxidation of low-density lipoproteins (14). All of these effects are desirable in patients suffering from acute or chronic coronary artery disease, eventually leading to CHF.
HNO donors, such as IPA/NO, have also been reported to inhibit neointimal hyperplasia by inhibiting vascular smooth muscle cell proliferation and macrophage infiltration (70). Concomitant with possible anti-inflammatory actions of HNO donors are preliminary data which show that HNO donors such as NCA (63) induce a twofold increase in active ERK1/2 and inhibit leukemia-inhibiting factor-induced IL-6 type cytokine activation of JAK-STAT signaling (79) [which was previously shown to be redox-sensitive (37)]. However, in vascular settings, certain doses of IPA/NO may counter endothelial cell proliferation and increase animal mortality (70). This comes as no surprise, as we and others have demonstrated that high levels of HNO may have pro-oxidant effects (73), as demonstrated by its exacerbating effects on ischemia-reperfusion injury in the heart (41) or in the brain (10). These findings reiterate the same mainstay that different levels of ROS/RNS elicit different thiol chemistry and ultimately functional outcomes. In the end, at low levels, HNO is a mild oxidizing agent, and its interaction with highly reactive groups unlikely exceeds the formation of reversible disulfide bonds. This is in contrast to stronger oxidants (or high fluxes of ROS/RNS) that may lead to an overriding oxidative chemistry, including the formation of sulfinic and sulfonic acid-Cys (5) and/or the modification of additional protein residues, such as tyrosine.
A yet poorly covered, but very relevant territory of, investigation is the possibility that HNO donors may have an antihypertrophic effect, particularly in settings such as postischemic LV remodeling. Preliminary data obtained by Ritchie's group in neonatal rat cardiac myocytes hints to the intriguing possibility that AS/HNO may counter angiotensin II-induced hypertrophy in a cGMP-dependent manner (31). While these data remain to fully validated and extended to adult cardiac cells, the current lack of long lasting HNO donors prevents us from assessing whether (and through which mechanisms) HNO donors may prevent or retard the shipwreck of structure and function of hearts subjected to chronic volume or pressure overload.
Therapeutical Potential of HNO Donors to Treat Acute and Chronic Cardiac Disorders
For several reasons, CHF represents a “therapeutic challenge.” First, signaling pathways such as the β-adrenergic receptors, which are essential for the heart to cope with increased physiological demand or decreased cardiac function, are downregulated in heart failure. Moreover, although NO• and other nitrogen-derived species such as nitrite/nitrate are useful for unloading failing hearts, their presence may dampen β-evoked increases in contractile reserve (43). Second, inotropic agents that use intracellular cAMP levels to leverage myocardial performance (i.e., β-agonists and phosphodiesterase inhibitors) are deleterious in the long term. When given chronically, these agents alter intracellular Ca2+ homeostasis, thus making the heart more prone to potentially fatal arrhythmias. Accordingly, their use in patients with CHF is currently limited to palliative care or a bridge to transplantation or mechanical assist device implantation. However, the failing heart still requires functional support. Unfortunately, recent clinical trials employing the most promising Ca2+ sensitizer, levosimendan, were accompanied by increased mortality (26). Hence, efforts are currently ongoing to convert a “Paradise Lost” [the status of currently available inotropes (11)] into a “Paradise Regained,” because poor contractile function is a major functional impediment in patients with CHF and is, in part, at the foundation of their symptoms of congestion. A plethora of animal studies have shown the ability of supplement antioxidants to prevent LV remodeling and improve function in several models of CHF. However, when translated to human CHF settings, these interventions invariably fail or even result in increased mortality, unless the antioxidant effect is combined with another pharmacological activity, as is the case of the β-blocker carvedilol (22), which is also an antioxidant. As recently stated:
“Since the RyR protein complex plays a central role in Ca2+ signaling, it is an ideal therapeutic target in heart disease. In situations where enhanced RyR activity is desirable, a RyR2-based therapy might include a cocktail of drugs to maintain the correct balance between Ca2+ uptake into the SR and Ca2+ release from the SR. In other situations where the RyR activity is enhanced, a reduction in activity alone might be sufficient to help rectify the imbalances in Ca2+ homeostasis. Indeed this has already been demonstrated with the drug JTV519. The proven cardioprotective action of this drug has established the RyR as a viable therapeutic target and has opened the door for the development of a new classes of drugs directed towards the calcium release channel” (19).
On closer examination, HNO donors may reunite all these favorable effects in one single molecule. Combined with its unloading action, HNO's role on SR Ca2+ cycling and myofilament function consign it to the status of an “inodilator.” This should be ideal in the treatment of decompensated patients with CHF, at least acutely or in subchronic terms. Conversely, for a thorough exploration of the effects of HNO on a chronic setting, that is, prevention of LV remodeling, we should wait until reliable long-lasting HNO donors are available to be used in animal models of CHF or other forms of chronic cardiac and noncardiac diseases.
Future Directions in HNO Research in the Cardiovascular Arena
There are many aspects of the HNO pharmacological profile that warrant further, more in-depth investigation. The most striking gap in the HNO field is that the functional impact of HNO on systems other than the cardiovascular one, or additional cellular components, is almost completely unknown. Using a proteomics approach, an extensive modification of mitochondrial protein thiols by AS/HNO has been reported (64). This study suggests that HNO interacts with mitochondria through a mechanism distinct from those of either NO• or peroxynitrite (ONOO−). However, similar to NO• (6), HNO appears to inhibit mitochondrial respiration through the inhibition of complexes I and II, most probably via modification of specific cysteine residues in the proteins. The meaning of these findings remains to be contextualized in relevant pathophysiological settings such as ischemia-reperfusion and CHF where HNO may exert important influences on energy production or ROS emission. Equally important will be delineating the functional impact of HNO on several aspects of the pulmonary and the renal systems, as both of these are crucially involved in the onset and progression of acute and chronic cardiac diseases. Finally, although HNO is capable of inducing oxidative stress, it can also act as an antioxidant via facile hydrogen-atom donation to oxidizing radical species (akin to tocopherol) and subsequent generation of NO•. Significantly, the studies demonstrated that the pro-oxidant effects were performed at high levels of HNO, whereas the antioxidant properties were observed at much lower concentrations. Future studies should address whether the presence of endogenous or exogenously applied HNO donors can result in beneficial effects when high tissue oxidative stress is present in the context of pathophysiologically relevant conditions in which pro-inflammatory signaling pathways are at play.
Concluding Remarks
Among all possible redox-sensitive keys harbored by structures belonging to the EC coupling machinery, HNO is likely able to interact and modify those that have a specific spatial location and pKa, thus enlisting a biological response. Therefore, to fully understand the effect of HNO on cardiovascular mechanics, it will be necessary to fully unearth where all these “keys” are situated. Further, we also have to establish whether (and to what extent) HNO signaling interact, or perhaps interfere, with other thiol-based ROS/RNS-induced modifications such as S-nitrosylation. Equally relevant would be to fully dissect the mechanisms whereby HNO action is terminated, for example, through GSH, thioredoxin system, etc. This is another important avenue for future studies that will presumably teach us more about when HNO effects cease being beneficial and start becoming detrimental.
From a practical point of view, ongoing clinical trials are evaluating whether a redox-based approach to improve deteriorated myocardial function, similar to the one granted by HNO, is a valid alternative or a useful addition to what are currently known as cardiac inotropes.
Abbreviations Used
- AS
Angeli's salt
- BH4
tetrahydrobiopterin
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- CGRP
calcitonin gene-related peptide
- CHF
congestive heart failure
- CK
creatine kinase
- CO
carbon monoxide
- DEA/NO
diethylamine/NO complex
- EC
excitation-contraction
- EDRF
endothelium-derived relaxing factor
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GSH
glutathione
- GSNO
s-nitrosoglutathione
- HNO
nitroxyl or nitrosyl hydride
- HO-1
heme-oxygenase-1
- H2S
hydrogen sulfide
- IPA
isopropylamine
- LTCC
L-type Ca2+ channels
- LV
left ventricular
- MHC
myosin heavy chain
- mKATP
mitochondrial K(ATP) channel
- MLC1
myosin light chain 1
- NCA
1-nitrosocyclohexyl acetate
- NH2OH
hydroxylamine
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NOHA
NG-hydroxy-L-arginine
- NOS
nitric oxide synthase
- pKa
acid dissociation constant
- PKA
protein kinase A
- PLN
phospholamban
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RyR2
ryanodine receptor 2
- SERCA2a
Ca2+-ATPase
- sGC
soluble guanylyl cyclase
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
- SR
sarcoplasmic reticulum
Acknowledgments
This work is dedicated to the memory of Dr. Jeffrey P. Froehlich (1943–2009), who pioneered studies on HNO effects on SERCA2a/PLN interaction. This work was supported by the ISHR-ES/Servier to C.G.T., the Marie Curie Intra European Fellowship within the 7th European Community Framework Programme (PIEF-GA-2008-221666 to S.D.), the American Heart Association (GIA to N.P., 10POST414001 to B.A.S., Pre-Doctoral Grant to 0815145E and 0815217E to V.S.); the NIH grants P01-HL081427 and PO1-HL077180 to J.V.E., HL-077180 to D.A.K., and HL075265 and HL091923 to N.P.
Author Disclosure Statement
Nazareno Paolocci and David A. Kass are scientific founders and stock owners of Cardioxyl Pharmaceuticals.
References
- 1.Adak S. Wang Q. Stuehr DJ. Arginine conversion to nitroxide by tetrahydrobiopterin-free neuronal nitric-oxide synthase. Implications for mechanism. J Biol Chem. 2000;275:33554–33561. doi: 10.1074/jbc.M004337200. [DOI] [PubMed] [Google Scholar]
- 2.Andrews KL. Irvine JC. Tare M. Apostolopoulos J. Favaloro JL. Triggle CR. Kemp-Harper BK. A role for nitroxyl (HNO) as an endothelium-derived relaxing and hyperpolarizing factor in resistance arteries. Br J Pharmacol. 2009;157:540–550. doi: 10.1111/j.1476-5381.2009.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell D. McDermott BJ. Calcitonin gene-related peptide in the cardiovascular system: characterization of receptor populations and their (patho)physiological significance. Pharmacol Rev. 1996;48:253–288. [PubMed] [Google Scholar]
- 4.Bermejo E. Saenz DA. Alberto F. Rosenstein RE. Bari SE. Lazzari MA. Effect of nitroxyl on human platelets function. Thromb Haemost. 2005;94:578–584. doi: 10.1160/TH05-01-0062. [DOI] [PubMed] [Google Scholar]
- 5.Bindoli A. Fukuto JM. Forman HJ. Thiol chemistry in peroxidase catalysis and redox signaling. Antioxid Redox Signal. 2008;10:1549–1564. doi: 10.1089/ars.2008.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown GC. Borutaite V. Nitric oxide and mitochondrial respiration in the heart. Cardiovasc Res. 2007;75:283–290. doi: 10.1016/j.cardiores.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Bullen ML. Miller AA. Andrews KL. Irvine JC. Ritchie RH. Sobey CG. Kemp-Harper BK. Nitroxyl (HNO) as a vasoprotective signaling molecule. Antioxid Redox Signal. 2011;14:1675–1686. doi: 10.1089/ars.2010.3327. . This issue. [DOI] [PubMed] [Google Scholar]
- 8.Canton M. Skyschally A. Menabo R. Boengler K. Gres P. Schulz R. Haude M. Erbel R. Di LF. Heusch G. Oxidative modification of tropomyosin and myocardial dysfunction following coronary microembolization. Eur Heart J. 2006;27:875–881. doi: 10.1093/eurheartj/ehi751. [DOI] [PubMed] [Google Scholar]
- 9.Cheong E. Tumbev V. Abramson J. Salama G. Stoyanovsky DA. Nitroxyl triggers Ca2+ release from skeletal and cardiac sarcoplasmic reticulum by oxidizing ryanodine receptors. Cell Calcium. 2005;37:87–96. doi: 10.1016/j.ceca.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Choe CU. Lewerenz J. Fischer G. Uliasz TF. Espey MG. Hummel FC. King SB. Schwedhelm E. Boger RH. Gerloff C. Hewett SJ. Magnus T. Donzelli S. Nitroxyl exacerbates ischemic cerebral injury and oxidative neurotoxicity. J Neurochem. 2009;110:1766–1773. doi: 10.1111/j.1471-4159.2009.06266.x. [DOI] [PubMed] [Google Scholar]
- 11.Cohn JN. Inotropic therapy for heart failure: paradise lost. Eur Heart J. 2009;30:2965–2966. doi: 10.1093/eurheartj/ehp370. [DOI] [PubMed] [Google Scholar]
- 12.Cook NM. Shinyashiki M. Jackson MI. Leal FA. Fukuto JM. Nitroxyl-mediated disruption of thiol proteins: inhibition of the yeast transcription factor Ace1. Arch Biochem Biophys. 2003;410:89–95. doi: 10.1016/s0003-9861(02)00656-2. [DOI] [PubMed] [Google Scholar]
- 13.Dai T. Tian Y. Tocchetti CG. Katori T. Murphy AM. Kass DA. Paolocci N. Gao WD. Nitroxyl increases force development in rat cardiac muscle. J Physiol. 2007;580:951–960. doi: 10.1113/jphysiol.2007.129254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desmard M. Boczkowski J. Poderoso J. Motterlini R. Mitochondrial and cellular heme-dependent proteins as targets for the bioactive function of the heme oxygenase/carbon monoxide system. Antioxid Redox Signal. 2007;9:2139–2155. doi: 10.1089/ars.2007.1803. [DOI] [PubMed] [Google Scholar]
- 15.Dobmeier KP. Riccio DA. Schoenfisch MH. Xerogel optical sensor films for quantitative detection of nitroxyl. Anal Chem. 2008;80:1247–1254. doi: 10.1021/ac702024t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donzelli S. Espey MG. Flores-Santana W. Switzer CH. Yeh GC. Huang J. Stuehr DJ. King SB. Miranda KM. Wink DA. Generation of nitroxyl by heme protein-mediated peroxidation of hydroxylamine but not N-hydroxy-L-arginine. Free Radic Biol Med. 2008;45:578–584. doi: 10.1016/j.freeradbiomed.2008.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donzelli S. Espey MG. Thomas DD. Mancardi D. Tocchetti CG. Ridnour LA. Paolocci N. King SB. Miranda KM. Lazzarino G. Fukuto JM. Wink DA. Discriminating formation of HNO from other reactive nitrogen oxide species. Free Radic Biol Med. 2006;40:1056–1066. doi: 10.1016/j.freeradbiomed.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 18.Dorn GW. Adrenergic signaling polymorphisms and their impact on cardiovascular disease. Physiol Rev. 2010;90:1013–1062. doi: 10.1152/physrev.00001.2010. [DOI] [PubMed] [Google Scholar]
- 19.Dulhunty AF. Beard NA. Pouliquin P. Casarotto MG. Agonists and antagonists of the cardiac ryanodine receptor: potential therapeutic agents? Pharmacol Ther. 2007;113:247–263. doi: 10.1016/j.pharmthera.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 20.El-Armouche A. Wahab A. Wittkopper K. Schulze T. Bottcher F. Pohlmann L. King SB. Dumond JF. Gerloff C. Boger RH. Eschenhagen T. Carrier L. Donzelli S. The new HNO donor, 1-nitrosocyclohexyl acetate, increases contractile force in normal and beta-adrenergically desensitized ventricular myocytes. Biochem Biophys Res Commun. 2010;402:340–344. doi: 10.1016/j.bbrc.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 21.Favaloro JL. Kemp-Harper BK. The nitroxyl anion (HNO) is a potent dilator of rat coronary vasculature. Cardiovasc Res. 2007;73:587–596. doi: 10.1016/j.cardiores.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Fonarow GC. Role of carvedilol controlled-release in cardiovascular disease. Expert Rev Cardiovasc Ther. 2009;7:483–498. doi: 10.1586/erc.09.15. [DOI] [PubMed] [Google Scholar]
- 23.Froehlich JP. Mahaney JE. Keceli G. Pavlos CM. Goldstein R. Redwood AJ. Sumbilla C. Lee DI. Tocchetti CG. Kass DA. Paolocci N. Toscano JP. Phospholamban thiols play a central role in activation of the cardiac muscle sarcoplasmic reticulum calcium pump by nitroxyl. Biochemistry. 2008;47:13150–13152. doi: 10.1021/bi801925p. [DOI] [PubMed] [Google Scholar]
- 24.Fukuto JM. Chiang K. Hszieh R. Wong P. Chaudhuri G. The pharmacological activity of nitroxyl: a potent vasodilator with activity similar to nitric oxide and/or endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1992;263:546–551. [PubMed] [Google Scholar]
- 25.Fukuto JM. Jackson MI. Kaludercic N. Paolocci N. Examining nitroxyl in biological systems. Methods Enzymol. 2008;440:411–431. doi: 10.1016/S0076-6879(07)00826-9. [DOI] [PubMed] [Google Scholar]
- 26.Goldhaber JI. Hamilton MA. Role of inotropic agents in the treatment of heart failure. Circulation. 2010;121:1655–1660. doi: 10.1161/CIRCULATIONAHA.109.899294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hertelendi Z. Toth A. Borbely A. Galajda Z. van d V. Stienen GJ. Edes I. Papp Z. Oxidation of myofilament protein sulfhydryl groups reduces the contractile force and its Ca2+ sensitivity in human cardiomyocytes. Antioxid Redox Signal. 2008;10:1175–1184. doi: 10.1089/ars.2007.2014. [DOI] [PubMed] [Google Scholar]
- 28.Hobbs AJ. Fukuto JM. Ignarro LJ. Formation of free nitric oxide from l-arginine by nitric oxide synthase: direct enhancement of generation by superoxide dismutase. Proc Natl Acad Sci U S A. 1994;91:10992–10996. doi: 10.1073/pnas.91.23.10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman MD. Walsh GM. Rogalski JC. Kast J. Identification of nitroxyl-induced modifications in human platelet proteins using a novel mass spectrometric detection method. Mol Cell Proteomics. 2009;8:887–903. doi: 10.1074/mcp.M800230-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hool LC. The L-type Ca(2+) channel as a potential mediator of pathology during alterations in cellular redox state. Heart Lung Circ. 2009;18:3–10. doi: 10.1016/j.hlc.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Irvine JC. Gossain S. Love JE. Kaye DM. Kemp-Harper BK. Ritchie RH. The protective role of nitroxyl (HNO) against cardiomyocyte hypertrophy via cGMP Signaling. Hypertension. 2009;53:1109. [Google Scholar]
- 32.Katori T. Donzelli S. Tocchetti CG. Miranda KM. Cormaci G. Thomas DD. Ketner EA. Lee MJ. Mancardi D. Wink DA. Kass DA. Paolocci N. Peroxynitrite and myocardial contractility: in vivo versus in vitro effects. Free Radic Biol Med. 2006;41:1606–1618. doi: 10.1016/j.freeradbiomed.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 33.Katori T. Hoover DB. Ardell JL. Helm RH. Belardi DF. Tocchetti CG. Forfia PR. Kass DA. Paolocci N. Calcitonin gene-related peptide in vivo positive inotropy is attributable to regional sympatho-stimulation and is blunted in congestive heart failure. Circ Res. 2005;96:234–243. doi: 10.1161/01.RES.0000152969.42117.ca. [DOI] [PubMed] [Google Scholar]
- 34.Kim WK. Choi YB. Rayudu PV. Das P. Asaad W. Arnelle DR. Stamler JS. Lipton SA. Attenuation of NMDA receptor activity and neurotoxicity by nitroxyl anion, NO- Neuron. 1999;24:461–469. doi: 10.1016/s0896-6273(00)80859-4. [DOI] [PubMed] [Google Scholar]
- 35.Kohr MJ. Kaludercic N. Tocchetti CG. Dong GW. Kass DA. Janssen PM. Paolocci N. Ziolo MT. Nitroxyl enhances myocyte Ca2+ transients by exclusively targeting SR Ca2+ -cycling. Front Biosci (Elite Ed) 2010;2:614–626. doi: 10.2741/e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar MR. Fukuto JM. Miranda KM. Farmer PJ. Reactions of HNO with heme proteins: new routes to HNO-heme complexes and insight into physiological effects. Inorg Chem. 2010;49:6283–6292. doi: 10.1021/ic902319d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurdi M. Booz GW. JAK redux: a second look at the regulation and role of JAKs in the heart. Am J Physiol Heart Circ Physiol. 2009;297:H1545–H1556. doi: 10.1152/ajpheart.00032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lancel S. Zhang J. Evangelista A. Trucillo MP. Tong X. Siwik DA. Cohen RA. Colucci WS. Nitroxyl activates SERCA in cardiac myocytes via glutathiolation of cysteine 674. Circ Res. 2009;104:720–723. doi: 10.1161/CIRCRESAHA.108.188441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez BE. Shinyashiki M. Han TH. Fukuto JM. Antioxidant actions of nitroxyl (HNO) Free Radic Biol Med. 2007;42:482–491. doi: 10.1016/j.freeradbiomed.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Lopez BE. Wink DA. Fukuto JM. The inhibition of glyceraldehyde-3-phosphate dehydrogenase by nitroxyl (HNO) Arch Biochem Biophys. 2007;465:430–436. doi: 10.1016/j.abb.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 41.Ma XL. Gao F. Liu GL. Lopez BL. Christopher TA. Fukuto JM. Wink DA. Feelisch M. Opposite effects of nitric oxide and nitroxyl on postischemic myocardial injury. Proc Natl Acad Sci U S A. 1999;96:14617–14622. doi: 10.1073/pnas.96.25.14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mancardi D. Ridnour L. Donzelli S. Miranda KM. Thomas DD. Katori T. Espey MG. Paolocci N. Wink DA. The nitroxyl donors Angeli's salt and IPA/NO afford equal cardiac early preconditioning-like effect that is independent of mitochondrial K-ATP channel activation. Free Radic Biol Med. 2004;37(Suppl. 1):S86–S87. [Google Scholar]
- 43.Massion PB. Pelat M. Belge C. Balligand JL. Regulation of the mammalian heart function by nitric oxide. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:144–150. doi: 10.1016/j.cbpb.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 44.Meissner G. Regulation of ryanodine receptor ion channels through posttranslational modifications. In: Serysheva I, editor. Current Topics in Membranes. Vol. 66. Burlington: Academic Press; 2010. pp. 91–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miranda KM. Dutton AS. Ridnour LA. Foreman CA. Ford E. Paolocci N. Katori T. Tocchetti CG. Mancardi D. Thomas DD. Espey MG. Houk KN. Fukuto JM. Wink DA. Mechanism of aerobic decomposition of Angeli's salt (sodium trioxodinitrate) at physiological pH. J Am Chem Soc. 2005;127:722–731. doi: 10.1021/ja045480z. [DOI] [PubMed] [Google Scholar]
- 46.Miranda KM. Katori T. Torres de Holding CL. Thomas L. Ridnour LA. McLendon WJ. Cologna SM. Dutton AS. Champion HC. Mancardi D. Tocchetti CG. Saavedra JE. Keefer LK. Houk KN. Fukuto JM. Kass DA. Paolocci N. Wink DA. Comparison of the NO and HNO donating properties of diazeniumdiolates: primary amine adducts release HNO in vivo. J Med Chem. 2005;48:8220–8228. doi: 10.1021/jm050151i. [DOI] [PubMed] [Google Scholar]
- 47.Miranda KM. Nagasawa HT. Toscano JP. Donors of HNO. Curr Top Med Chem. 2005;5:649–664. doi: 10.2174/1568026054679290. [DOI] [PubMed] [Google Scholar]
- 48.Miranda KM. Nims RW. Thomas DD. Espey MG. Citrin D. Bartberger MD. Paolocci N. Fukuto JM. Feelisch M. Wink DA. Comparison of the reactivity of nitric oxide and nitroxyl with heme proteins. A chemical discussion of the differential biological effects of these redox related products of NOS. J Inorg Biochem. 2003;93:52–60. doi: 10.1016/s0162-0134(02)00498-1. [DOI] [PubMed] [Google Scholar]
- 49.Nagahara N. Intermolecular disulfide bond to modulate protein function as a redox-sensing switch. Amino Acids. 2010. [Epub ahead of print]; PMID: 20177947. [DOI] [PubMed]
- 50.Nagahara N. Matsumura T. Okamoto R. Kajihara Y. Protein cysteine modifications: (2) reactivity specificity and topics of medicinal chemistry and protein engineering. Curr Med Chem. 2009;16:4490–4501. doi: 10.2174/092986709789760643. [DOI] [PubMed] [Google Scholar]
- 51.Naughton P. Foresti R. Bains SK. Hoque M. Green CJ. Motterlini R. Induction of heme oxygenase 1 by nitrosative stress. A role for nitroxyl anion. J Biol Chem. 2002;277:40666–40674. doi: 10.1074/jbc.M203863200. [DOI] [PubMed] [Google Scholar]
- 52.Nediani C. Raimondi L. Borchi E. Cerbai E. Nitric oxide/reactive oxygen species generation and nitroso/redox imbalance in heart failure: from molecular mechanisms to therapeutic implications. Antioxid Redox Signal. 2011;14:289–331. doi: 10.1089/ars.2010.3198. [DOI] [PubMed] [Google Scholar]
- 53.Pagliaro P. Mancardi D. Rastaldo R. Penna C. Gattullo D. Miranda KM. Feelisch M. Wink DA. Kass DA. Paolocci N. Nitroxyl affords thiol-sensitive myocardial protective effects akin to early preconditioning. Free Radic Biol Med. 2003;34:33–43. doi: 10.1016/s0891-5849(02)01179-6. [DOI] [PubMed] [Google Scholar]
- 54.Paolocci N. Katori T. Champion HC. St. John ME. Miranda KM. Fukuto JM. Wink DA. Kass DA. Positive inotropic and lusitropic effects of HNO/NO- in failing hearts: independence from beta-adrenergic signaling. Proc Natl Acad Sci U S A. 2003;100:5537–5542. doi: 10.1073/pnas.0937302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paolocci N. Saavedra WF. Miranda KM. Martignani C. Isoda T. Hare JM. Espey MG. Fukuto JM. Feelisch M. Wink DA. Kass DA. Nitroxyl anion exerts redox-sensitive positive cardiac inotropy in vivo by calcitonin gene-related peptide signaling. Proc Natl Acad Sci U S A. 2001;98:10463–10468. doi: 10.1073/pnas.181191198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pessah IN. Feng W. Functional role of hyperreactive sulfhydryl moieties within the ryanodine receptor complex. Antioxid Redox Signal. 2000;2:17–25. doi: 10.1089/ars.2000.2.1-17. [DOI] [PubMed] [Google Scholar]
- 57.Petroff MG. Kim SH. Pepe S. Dessy C. Marban E. Balligand JL. Sollott SJ. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat Cell Biol. 2001;3:867–873. doi: 10.1038/ncb1001-867. [DOI] [PubMed] [Google Scholar]
- 58.Pino RZ. Feelisch M. Bioassay discrimination between nitric oxide (NO.) and nitroxyl (NO-) using L-cysteine. Biochem Biophys Res Commun. 1994;201:54–62. doi: 10.1006/bbrc.1994.1668. [DOI] [PubMed] [Google Scholar]
- 59.Pufahl RA. Wishnok JS. Marletta MA. Hydrogen peroxide-supported oxidation of NG-hydroxy-L-arginine by nitric oxide synthase. Biochemistry. 1995;34:1930–1941. doi: 10.1021/bi00006a014. [DOI] [PubMed] [Google Scholar]
- 60.Queliconi BB. Wojtovich AP. Nadtochiy SM. Kowaltowski AJ. Brookes PS. Redox regulation of the mitochondrial K(ATP) channel in cardioprotection. Biochim Biophys Acta. 2010. [Epub ahead of print]; PMID: 21094666. [DOI] [PMC free article] [PubMed]
- 61.Saleem M. Ohshima H. Xanthine oxidase converts nitric oxide to nitroxyl that inactivates the enzyme. Biochem Biophys Res Commun. 2004;315:455–462. doi: 10.1016/j.bbrc.2004.01.081. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt HH. Hofmann H. Schindler U. Shutenko ZS. Cunningham DD. Feelisch M. No. NO from NO synthase. Proc Natl Acad Sci U S A. 1996;93:14492–14497. doi: 10.1073/pnas.93.25.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sha X. Isbell TS. Patel RP. Day CS. King SB. Hydrolysis of acyloxy nitroso compounds yields nitroxyl (HNO) J Am Chem Soc. 2006;128:9687–9692. doi: 10.1021/ja062365a. [DOI] [PubMed] [Google Scholar]
- 64.Shiva S. Crawford JH. Ramachandran A. Ceaser EK. Hillson T. Brookes PS. Patel RP. Darley-Usmar VM. Mechanisms of the interaction of nitroxyl with mitochondria. Biochem J. 2004;379:359–366. doi: 10.1042/BJ20031758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stojanovic S. Stanic D. Nikolic M. Spasic M. Niketic V. Iron catalyzed conversion of NO into nitrosonium (NO+) and nitroxyl (HNO/NO-) species. Nitric Oxide. 2004;11:256–262. doi: 10.1016/j.niox.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 66.Tao L. English AM. Protein S-glutathiolation triggered by decomposed S-nitrosoglutathione. Biochemistry. 2004;43:4028–4038. doi: 10.1021/bi035924o. [DOI] [PubMed] [Google Scholar]
- 67.Terentyev D. Gyorke I. Belevych AE. Terentyeva R. Sridhar A. Nishijima Y. de Blanco EC. Khanna S. Sen CK. Cardounel AJ. Carnes CA. Gyorke S. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res. 2008;103:1466–1472. doi: 10.1161/CIRCRESAHA.108.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tocchetti CG. Mazhari R. Takimoto E. Koitabashi N. Bedja D. Gabrielson KL. Aon MA. Kass DA. Paolocci N. Nitroxyl enhances contractility in failing isolated mouse cardiomyocytes. Circ Res. 2009;105:E60. [Google Scholar]
- 69.Tocchetti CG. Wang W. Froehlich JP. Huke S. Aon MA. Wilson GM. Di BG. O'Rourke B. Gao WD. Wink DA. Toscano JP. Zaccolo M. Bers DM. Valdivia HH. Cheng H. Kass DA. Paolocci N. Nitroxyl improves cellular heart function by directly enhancing cardiac sarcoplasmic reticulum Ca2+ cycling. Circ Res. 2007;100:96–104. doi: 10.1161/01.RES.0000253904.53601.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsihlis ND. Murar J. Kapadia MR. Ahanchi SS. Oustwani CS. Saavedra JE. Keefer LK. Kibbe MR. Isopropylamine NONOate (IPA/NO) moderates neointimal hyperplasia following vascular injury. J Vasc Surg. 2010;51:1248–1259. doi: 10.1016/j.jvs.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ulich BL. Hollis JM. Snyder LE. Radio detection of nitroxyl (HNO)—1st interstellar NO bond. Astrophys J. 1977;217:L105–L108. [Google Scholar]
- 72.Vanuffelen BE. Van Der Zee J. De Koster BM. Vansteveninck J. Elferink JG. Intracellular but not extracellular conversion of nitroxyl anion into nitric oxide leads to stimulation of human neutrophil migration. Biochem J. 1998;330(Pt 2):719–722. doi: 10.1042/bj3300719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wink DA. Feelisch M. Fukuto J. Chistodoulou D. Jourd'heuil D. Grisham MB. Vodovotz Y. Cook JA. Krishna M. DeGraff WG. Kim S. Gamson J. Mitchell JB. The cytotoxicity of nitroxyl: possible implications for the pathophysiological role of NO. Arch Biochem Biophys. 1998;351:66–74. doi: 10.1006/abbi.1997.0565. [DOI] [PubMed] [Google Scholar]
- 74.Wong PS. Hyun J. Fukuto JM. Shirota FN. DeMaster EG. Shoeman DW. Nagasawa HT. Reaction between S-nitrosothiols and thiols: generation of nitroxyl (HNO) and subsequent chemistry. Biochemistry. 1998;37:5362–5371. doi: 10.1021/bi973153g. [DOI] [PubMed] [Google Scholar]
- 75.Wouters MA. Fan SW. Haworth NL. Disulfides as redox switches: from molecular mechanisms to functional significance. Antioxid Redox Signal. 2010;12:53–91. doi: 10.1089/ars.2009.2510. [DOI] [PubMed] [Google Scholar]
- 76.Xia R. Stangler T. Abramson JJ. Skeletal muscle ryanodine receptor is a redox sensor with a well defined redox potential that is sensitive to channel modulators. J Biol Chem. 2000;275:36556–36561. doi: 10.1074/jbc.M007613200. [DOI] [PubMed] [Google Scholar]
- 77.Xu KY. Huso DL. Dawson TM. Bredt DS. Becker LC. Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1999;96:657–662. doi: 10.1073/pnas.96.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yong QC. Hu LF. Wang S. Huang D. Bian JS. Hydrogen sulfide interacts with nitric oxide in the heart: possible involvement of nitroxyl. Cardiovasc Res. 2010;88:482–491. doi: 10.1093/cvr/cvq248. [DOI] [PubMed] [Google Scholar]
- 79.Zgheib C. Sebastian T. Tocchetti CG. Paolocci N. King SB. Kurdi M. Booz G. W. Nitroxyl activates redox-sensitive stress signaling in endothelial cells and has anti-inflammatory actions. Hypertension. 2010;56:e128. [Google Scholar]
- 80.Zhang GX. Kimura S. Nishiyama A. Shokoji T. Rahman M. Yao L. Nagai Y. Fujisawa Y. Miyatake A. Abe Y. Cardiac oxidative stress in acute and chronic isoproterenol-infused rats. Cardiovasc Res. 2005;65:230–238. doi: 10.1016/j.cardiores.2004.08.013. [DOI] [PubMed] [Google Scholar]