Abstract
Induction of phase 2 enzymes, which neutralize reactive electrophiles and act as indirect antioxidants, appears to be an effective means for achieving protection against a variety of carcinogens in animals and humans. Transcriptional control of the expression of these enzymes is mediated, at least in part, through the antioxidant response element (ARE) found in the regulatory regions of their genes. The transcription factor Nrf2, which binds to the ARE, appears to be essential for the induction of prototypical phase 2 enzymes such as glutathione S-transferases (GSTs) and NAD(P)H:quinone oxidoreductase (NQO1). Constitutive hepatic and gastric activities of GST and NQO1 were reduced by 50–80% in nrf2-deficient mice compared with wild-type mice. Moreover, the 2- to 5-fold induction of these enzymes in wild-type mice by the chemoprotective agent oltipraz, which is currently in clinical trials, was almost completely abrogated in the nrf2-deficient mice. In parallel with the enzymatic changes, nrf2-deficient mice had a significantly higher burden of gastric neoplasia after treatment with benzo[a]pyrene than did wild-type mice. Oltipraz significantly reduced multiplicity of gastric neoplasia in wild-type mice by 55%, but had no effect on tumor burden in nrf2-deficient mice. Thus, Nrf2 plays a central role in the regulation of constitutive and inducible expression of phase 2 enzymes in vivo and dramatically influences susceptibility to carcinogenesis. Moreover, the total loss of anticarcinogenic efficacy of oltipraz in the nrf2-disrupted mice highlights the prime importance of elevated phase 2 gene expression in chemoprotection by this and similar enzyme inducers.
A key component in understanding the initial events of carcinogenesis was the recognition by James and Elizabeth Miller that many chemical carcinogens are not chemically reactive per se but must undergo metabolic activation to form electrophilic reactants (1). These reactive species can interact with nucleophilic groups in DNA to impart an array of deleterious lesions. The importance of metabolism in carcinogenesis is highlighted by the fact that target organ specificity and even species susceptibilities can be determined by the presence or absence of metabolic pathways (2, 3). A variety of chemicals protect rodents against neoplastic, mutagenic, and other toxic effects of many types of carcinogens (4, 5). Some of these protective substances alter the metabolic fate of carcinogens by modulating the activities of either or both phase 1 and phase 2 drug-metabolizing enzymes.¶
The concept that modulation of carcinogen metabolism could be exploited to provide protection against carcinogenesis gained support from the conclusion of the Millers that inhibition of the hepatocarcinogenicity of certain azo dyes by methylcholanthrene (7) could be ascribed to the elevation of enzymes (subsequently identified as cytochromes P450) that destroyed the reactive functions of the carcinogenic dyes (8). However, almost no consideration was given to the possible role of phase 2 enzymes in the protective phenomenon until work from this laboratory demonstrated that glutathione S-transferases (GSTs) and NAD(P)H:quinone reductase (NQO1) were greatly elevated in cytosols from liver and extrahepatic tissues of rodents that were fed the anticarcinogenic dietary antioxidants butylated hydroxyanisole and ethoxyquin (9, 10). Such cytosols also eliminated the mutagenic activities of urines of mice treated with benzo[a]pyrene (9). These findings led to the explicit suggestion that phase 2 enzyme induction could play a major role in protection against neoplasia and toxicity.
More recently, monitoring of enzyme induction has led to the recognition or isolation of novel, potent chemoprotective agents including 1,2-dithiole-3-thiones (11), terpenoids (12), and the isothiocyanate sulforaphane (13). Oltipraz, a substituted 1,2-dithiole-3-thione originally developed as an antischistosomal agent, has chemoprotective activity against different classes of carcinogens targeting multiple organs. A double-blind, placebo-controlled clinical trial with oltipraz was recently conducted in residents of Qidong, People's Republic of China, who are commonly exposed to aflatoxin, a food-borne mycotoxin, and who are at high risk for development of hepatocellular carcinoma (14). Oltipraz significantly enhanced the excretion of a phase 2 product, aflatoxin-mercapturic acid, a derivative of the aflatoxin-glutathione conjugate, in the urine of study participants administered oltipraz daily by mouth. Such studies highlight the general feasibility of inducing phase 2 enzymes in humans, but they do not firmly establish that induction of phase 2 enzymes is a core component of the chemoprotective actions of agents like oltipraz or sulforaphane. For example, both compounds are also reported to inhibit the activities of several isoforms of cytochrome P450 gene products (15, 16).
Several regulatory elements that control the expression and inducibility of phase 2 genes have been characterized. The aryl hydrocarbon receptor is a subunit of a dimeric ligand-activated transcription factor that as a complex binds to the xenobiotic response element in the upstream region of Cyp1A and Cyp1B genes as well as a limited number of phase 2 genes (17). A distinct element termed the antioxidant response element (ARE) was initially identified in the 5′-flanking region of the rat GST Ya gene (18). Now, AREs have been detected in the promoters of nearly a score of phase 2 genes; all share a common RTGACnnnGC motif (19). The term bifunctional inducer has been applied to those compounds that induce gene expression through both the xenobiotic response element and ARE, whereas compounds acting only through the ARE are termed monofunctional inducers (20). Prestera et al. (21) observed that members of eight distinct chemical classes of monofunctional inducers stimulate expression of an ARE-growth hormone reporter gene construct in murine hepatoma cells. Comparisons of potency for induction of reporter gene expression and NQO1 activity in the same cells indicated a striking concordance over a 4-log concentration range for the two outcomes. Furthermore, De Long et al. (22) have observed that induction of phase 2 enzymes by oltipraz and other monofunctional inducers occurs unabated in aryl hydrocarbon receptor-defective hepatoma cells. Collectively, these results suggest that the ARE mediates most, if not all, of the phase 2 enzyme inducer activity of these monofunctional inducers.
The transcription factors that bind to the ARE consensus sequence have not been fully identified and are likely to vary among cell types. Nrf1 and Nrf2, members of the basic-leucine zipper NF-E2 family of transcription factors that regulate expression of globin genes during erythroid development, are known to bind and activate the ARE (23). Overexpression of Nrf1 and Nrf2 in human hepatoma cells enhances the basal and inducible transcriptional activity of an ARE reporter gene (24). The role of the Nrf2 transcription factor has also been directly examined by exploring the effects of disruption of the nrf2 gene in vivo on induction of phase 2 enzymes. Butylated hydroxyanisole elevated GST and NQO1 mRNA expression in livers of wild-type mice, but not in nrf2-deficient mice (23). Comparable effects have been observed with the unsubstituted congener of oltipraz, 3H-1,2-dithiole-3-thione (25).
The cancer chemoprotective efficacy of oltipraz was initially established in the mid-1980s when Wattenberg and Bueding demonstrated that this drug inhibited benzo[a]pyrene-induced gastric carcinogenesis in mice (26). Thus, we reasoned that use of this model in the current study would allow for (i) investigation of the impact of disruption of the nrf2 gene on inherent susceptibility to carcinogenesis and (ii) a direct assessment of the contribution of enzyme induction to the protective actions of oltipraz in mice with a genetically impaired capacity to induce multiple phase 2 genes. Our results establish that loss of expression of Nrf2 significantly enhances susceptibility of mice to chemical carcinogenesis and completely abolishes the chemoprotective properties of phase 2 inducers such as oltipraz. Thus, the selective induction of phase 2 proteins is a highly effective and unilaterally sufficient strategy for achieving protection against carcinogenesis.
Materials and Methods
Chemicals and Materials.
Benzo[a]pyrene and other chemicals were from Sigma. 5-(2-Pyrazinyl)-4-methyl-1,2-dithiole-3-thione (oltipraz) was provided by the Chemoprevention Branch, National Cancer Institute. [α-32P]dCTP (3,000 Ci/mmol; 1 Ci = 37 GBq) was from ICN. cDNA probes for Nrf2, GST Ya, Yp, microsomal epoxide hydrolase, glyceraldehyde 3-phosphate dehydrogenase, and albumin were isolated (23, 27). cDNA probes for UDP-glucuronosyltransferase 1A6 (≈600 bp) and NQO1 (≈900 bp) were synthesized by using reverse transcriptase reactions (25). Mouse GST Yc cDNA was kindly provided by David Eaton (University of Washington, Seattle) (28). Probe-labeling and RNA reagents were obtained from Boehringer-Mannheim, Life Technologies (Gaithersburg, MD), and Schleicher & Schuell (Keene, NH).
Animals and Treatments.
Nrf2-deficient ICR mice were generated as described by Itoh et al. (23). Genotypes of homozygous wild-type and nfr2-deficient mice (7–9 weeks old) were confirmed by PCR amplification of genomic DNA isolated from blood or liver tissue. PCR amplification was carried out by using three different primers, 5′-TGGACGGGACTATTGAAGGCTG-3′ (sense for both genotypes), 5′-CGCCTTTTCAGTAGATGGAGG-3′ (antisense for wild type), and 5′-GCGGATTGACCGTAATGGGATAGG-3′ (antisense for LacZ). To study the effects of nrf2 genotype on induction of phase 2 enzyme activities, female mice (7–9 weeks old) were fed AIN-76A diet and water ad libitum, treated by gavage (0.2 ml) with 500 mg/kg oltipraz (suspended in 1% cremophor and 25% glycerol) or vehicle only, and killed 48 h later by cervical dislocation. Similarly treated animals were killed 6 and 24 h after treatment to determine the effect of oltipraz on nuclear localization of Nrf2 and mRNA levels, respectively.
Enzyme Activity Assays.
Total GST activity was measured in cytosolic fractions (105,000 × g) in the presence of 0.1% BSA with 1-chloro-2,4-dinitrobenzene as a substrate (29), whereas NQO1 activity was determined by using menadione as substrate (30). Protein concentration was determined by the bicinchoninic acid protein assay (Pierce).
Northern Blot Analysis.
Total RNA was isolated from tissues by the procedure of Chomczynski and Sacchi (31). RNA samples were subjected to electrophoresis on 1% agarose gels containing 2.2 M formaldehyde and transferred to nylon membranes. Membranes were UV-crosslinked and placed in prehybridization solution for 2–4 h at 42°C. cDNAs were labeled with [α-32P]dCTP by use of a random prime labeling kit (Amersham Pharmacia Biotech), hybridized, and washed. Labeled membranes were exposed to x-ray film for varying lengths of time at −80°C with intensifying screens and developed using a Konica film processor (Shinjuku-ku, Tokyo). Levels of RNA were quantified and normalized for RNA loading by stripping and reprobing the blots with a cDNA probe for either rat albumin or glyceraldehyde 3-phosphate dehydrogenase.
Preparation of Nuclear Extracts and Immunoblot Analysis of Nrf2 Levels.
Nuclear extracts from murine forestomach and liver tissues were prepared according to Dignam et al. (32). Crude nuclear fractions were collected by centrifugation and extracted by using a buffer containing 20 mM Hepes (pH 7.9), 1.5 mM MgCl2, 420 mM NaCl, 10% glycerol, 0.2 mM EDTA, and 0.5 mM phenylmethylsulfonyl fluoride. SDS/PAGE of nuclear extracts and immunoblotting of Nrf2 were carried out as described (25).
Carcinogenesis Study.
Female wild-type and nrf2-disrupted mice (7–9 weeks of age) were randomized into groups of 20 mice and fed purified diet of the AIN-76A formulation lacking 0.02% ethoxyquin. Animals were given oltipraz at 500 mg/kg of body weight or solvent only, by gavage. Benzo[a]pyrene (120 mg/kg in 0.2 ml corn oil) was given 48 h later by oral intubation. This sequence of oltipraz and benzo[a]pyrene administrations was repeated once a week for a total of 4 weeks. Animals were weighed weekly, killed by CO2 asphyxiation followed by cervical dislocation, and autopsied 30 weeks after the first oltipraz treatment. Forestomach tissues were removed and fixed in 10%-buffered formalin. Tumors of the forestomach were counted grossly as described by Wattenberg (33).
Statistical Analyses.
Statistical significance was determined by one-way ANOVA followed by Tukey's multiple comparison. Experimental values are given as means ± SE.
Results
Effect of nrf2 Genotype and Oltipraz Treatment on Phase 2 Enzyme Activities.
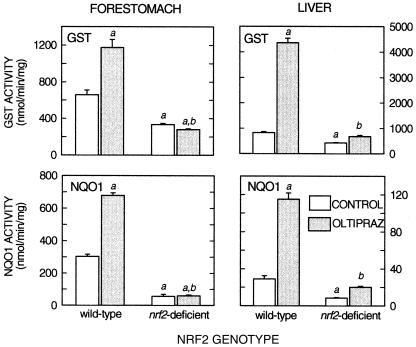
As shown in Fig. 1, GST and NQO1 activities were measured 48 h after a single administration of oltipraz (500 mg/kg) or vehicle to female wild-type and nrf2-disrupted mice. Hepatic GST activity was increased 5.3-fold in wild-type mice by oltipraz but was increased only marginally in nrf2-deficient mice. Moreover, basal hepatic activity of GST was reduced by half in nrf2-disrupted mice. Hepatic NQO1 activity was increased 4-fold in wild-type mice by oltipraz, and the induction was largely attenuated in nrf2-deficient mice. Similar to activity of hepatic GST activity, basal activity of NQO1 was 70% lower in nrf2-disrupted mice than in wild-type mice. Comparable effects of nrf2 genotype and oltipraz treatment were seen on the activities of GST and NQO1 in forestomach. Although both enzymes were only induced 2-fold by oltipraz in wild-type mice, this induction was completely abrogated in the forestomachs of nrf2-deficient mice. Moreover, basal activities of GST and NQO1 were reduced 50% and 80%, respectively, in the stomachs of the knockout mice.
Figure 1.
Effect of nrf2 genotype on gastric and hepatic activities of GST and NQO1. GST (1-chloro-2,4-dinitrobenzene) and NQO1 (menadione) activities were measured in gastric and hepatic cytosols prepared from wild-type and nrf2-disrupted mice treated with vehicle or oltipraz (500 mg/kg, p.o.) 48 h before being killed. a, P < 0.05 compared with wild-type vehicle-treated control; b, P < 0.05 compared with wild-type mice treated with oltipraz. Values are mean ± SE for three to four animals in each group.
Effect of nrf2 Genotype and Oltipraz on Gene Expression.
To evaluate the role of Nrf2 genotype on gene expression, relative mRNA levels of GST Ya, Yp, Yc, NQO1, UDP-glucuronosyltransferase 1A6, microsomal epoxide hydrolase, and NRF2 were analyzed 24 h after a single dose of oltipraz in wild-type and nrf2-deficient mouse liver and, in some cases, forestomach. In wild-type mice, hepatic levels of mRNA for all of the genes analyzed were significantly increased after oltipraz treatment, with the highest increase (treated/control) for NQO1 mRNA levels (7.6-fold) (Table 1). The Northern blot analyses demonstrated that the observed increases in GST and NQO1 activities by oltipraz in wild-type mice were preceded by significant elevations in RNA expression. Interestingly, mRNA levels of Nrf2 itself were increased more than 3-fold by oltipraz treatment.
Table 1.
Effects of nrf2 genotype on changes in mRNA levels of phase 2 enzymes after treatment with oltipraz
| Nrf2 genotype
|
||||
|---|---|---|---|---|
| +/+ | −/− | +/+ | −/− | |
| Relative
constitutive mRNA levels (vehicle/vehicle)
|
Relative
inducible mRNA levels (treated/vehicle)
|
|||
| Liver | ||||
| GST Ya | 1 | 0.61 | 4.53* | 1.25 |
| GST Yp | 1 | 0.50 | 2.66* | 1.61† |
| GST Yc | 1 | 0.92 | 1.97* | 1.10 |
| NQO1 | 1 | 0.80 | 7.64* | 1.39 |
| UDP-glucuronosyltransferase 1A6 | 1 | 1.03 | 1.34* | 1.25 |
| Microsomal epoxide hydrolase | 1 | 0.54 | 3.64* | 0.72 |
| NRF2 | 1 | ND | 3.69* | ND |
| Forestomach | ||||
| GST Ya | 1 | 0.55 | 2.04* | 0.62 |
| GST Yp | 1 | 0.31 | 1.67* | 0.26 |
| NQO1 | 1 | 0.08 | 1.68* | 0.10 |
| NRF2 | 1 | ND | 7.62* | ND |
Values are the mean of determinations on three to four mice. Levels of liver and forestomach mRNA for each gene were normalized to albumin and GAPDH mRNA levels, respectively, and expressed as a ratio to vehicle-treated, wild-type control. Transcript levels were measured 24 h after oltipraz treatment (500 mg/kg). ND, not detectable. *, P < 0.05, compared with vehicle-treated wild-type mice. †, P < 0.05, compared with vehicle-treated nrf2-deficient mice.
Both constitutive and inducible expression of these genes was affected in the nrf2-deficient mice. For example, constitutive levels of mRNA for GST Ya, Yp, and microsomal epoxide hydrolase were 40–50% lower in nrf2-deficient mice. Negligible inducibility was observed, as most of the genes analyzed failed to respond after oltipraz treatment. Only the GstP gene retained its inducibility in liver by oltipraz in the absence of Nrf2 (P < 0.05), although levels were lower than those seen in wild-type mice. As expected, no Nrf2 mRNA was detected in nrf2-deficient mice after either vehicle or oltipraz treatment.
Approximately 2-fold elevations in mRNA levels for GST Ya, GST Yp, and NQO1 were seen in forestomach after treatment with oltipraz. Basal expression of these genes was reduced 60–90% in the nrf2-deficient mice, and no elevation was observed with oltipraz.
Nuclear Accumulation of Nrf2.
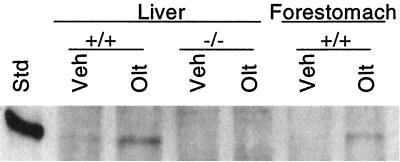
As shown in Fig. 2, Nrf2 protein was barely detectable in extracts of hepatic nuclei prepared from wild-type mice. However, 3- to 4-fold increases were observed 6 h after treatment with oltipraz. Somewhat smaller increases were seen in gastric nuclei. Although not shown, time-course experiments indicate that nuclear accumulation of Nrf2 is detectable within 20 min, reaches a maximum around 6 h, and is largely dissipated by 24 h after inducer treatment. No Nrf2 was detectable in hepatic nuclear extracts prepared from either vehicle- or oltipraz-treated nrf2-deficient mice.
Figure 2.
Nuclear Nrf2 levels after treatment of mice with oltipraz. Hepatic and gastric extracts were prepared from livers of mice treated for 6 h with either vehicle (Veh) or 500 mg/kg oltipraz (Olt). Std, purified recombinant Nrf2.
Increased Susceptibility to Benzo[a]pyrene-Induced Neoplasia in nrf2-Deficient Mice.
Although nrf2-deficient mice were initially slightly lighter (≈2.5 g) than their wild-type counterparts, no significant differences in body weight gain were observed among the four groups during the experimental period. Several animals from each group did not survive until the end of the experiment. The cause of premature death for all but one mouse was respiratory distress. Upon autopsy, the thymus was typically found to be enlarged, occupying most of the thoracic space and compressing the lungs against the posterior wall. In the wild-type, vehicle-treated group, six mice died between the 16th and 27th weeks, each with between six and eight gastric tumors. By contrast, only two mice in the wild-type, oltipraz-treated group died prematurely on weeks 13 and 27, each with no gastric tumors. In nrf2-deficient vehicle- and oltipraz-treated groups, six and four mice died during the experimental period, between weeks 10 and 26, with 8–18 gastric tumors and between weeks 12 and 27, with 7–11 tumors, respectively. All animals in both nrf2-deficient vehicle- and oltipraz-treated groups bore tumors. The one tumor-related early death occurred in an nrf2-deficient, oltipraz-treated mouse. The animal had a massive forestomach neoplasm, involving virtually the entire mucosal surface, filling and obstructing the lumen.
In those animals that survived until the end of the 30-week experimental period, many of the forestomachs were enlarged. The lumens were narrowed by the papillomas; the mucosa was excessively folded, rough, and corrugated. The papillomas varied from small pinpoint excrescences to large, branching structures or cauliflower growths.
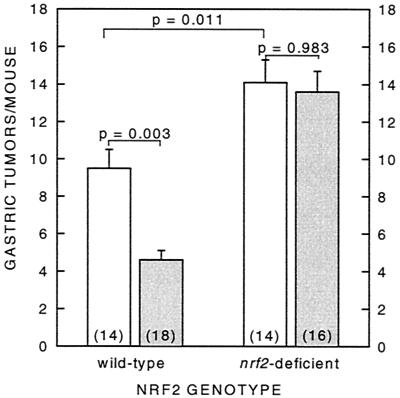
As shown in Fig. 3, oltipraz treatment reduced significantly (P = 0.003) the number of neoplasms of the forestomach by 52% in the wild-type mice. Wild-type, vehicle-treated mice had 9.5 ± 1.0 tumors per mouse compared with 4.6 ± 0.5 tumors per mouse in the oltipraz-treated group. However, oltipraz completely lost its efficacy in nrf2-deficient mice (P = 0.983). Moreover, both nrf2-deficient vehicle- and oltipraz-treated mice were more susceptible to benzo[a]pyrene-induced neoplasia of the forestomach than wild-type mice (P = 0.011), with 14.1 ± 1.2 and 13.6 ± 1.1 tumors per mouse, respectively.
Figure 3.
Effect of oltipraz on benzo[a]pyrene-induced neoplasia of the forestomach in female wild-type and nrf2-deficient mice. Female mice (7–9 weeks of age) were treated weekly with 500 mg/kg oltipraz 48 h before dosing with benzo[a]pyrene (120 mg/kg in corn oil, p.o.) for 4 consecutive weeks and killed 30 weeks after the initial treatment. Gastric tumors are reported as number of gastric tumors in the entire group/number of mice at risk at termination of experiment (number in parentheses). Open bar, vehicle-treated; shaded bar, oltipraz-treated.
Discussion
Gene-disrupted animals provide elegant models for identifying metabolic pathways in carcinogen activation and detoxication as well as for assessing the molecular mechanisms underlying the pharmacodynamic actions of chemoprotective agents (34, 35). Benzo[a]pyrene, an environmental pollutant and major component of cigarette smoke, provides several examples of the former point. This carcinogen undergoes sequential metabolism by CYP1A1, epoxide hydrolase, and CYP1A1 or 3A4 to yield a reactive diol-epoxide metabolite that alkylates DNA. Although alternate pathways have been described for the activation of benzo[a]pyrene to reactive intermediates (36), the importance of the diol-epoxide activation pathway has been asserted by the observation that benzo[a]pyrene carcinogenicity is completely lost in mice lacking the aryl hydrocarbon receptor and subsequent expression of cyp1A genes (37). Similarly, targeted disruption of the epoxide hydrolase gene diminishes the carcinogenicity and toxicity of a related polycyclic aromatic hydrocarbon, dimethylbenz[a]anthracene (38). Selective disruption of phase 2 genes, by contrast, can enhance sensitivity to the carcinogenicity of polycyclic aromatic hydrocarbons. Skin tumorigenesis induced by dimethylbenz[a]anthracene or benzo[a]pyrene is increased in mice lacking pi class GST (39) or NQO1 (40), respectively. Thus, our finding that nrf2-deficient mice develop more gastric tumors agrees with the general concept that these phase 2 enzymes are significant protectors. Comparison of expression patterns of phase 2 genes in knockout and wild-type mice indicates that basal expression of phase 2 genes is typically repressed in the nrf2-deficient animals (Fig. 1; refs. 23 and 25). Specific activities of GST and NQO1 in both liver and forestomach were at least 50% lower in the knockout compared with wild-type mice. Moreover, the elevated sensitivity of the nrf2-deficient mice to chemical carcinogenesis is consistent with recent observations documenting their enhanced sensitivity to the acute pneumotoxicity of butylated hydroxytoluene (41) and the hepatotoxicity of acetaminophen (42). In both cases, these outcomes can be postulated to reflect diminished expression of detoxication pathways known to be regulated (at least in part) by Nrf2.
Of greater importance is the observation that loss of expression of Nrf2 completely abrogates the chemoprotective activity of oltipraz. As with most pharmacologic agents, multiple mechanisms of action may account for the anticarcinogenic actions of this drug. Because oltipraz was predicted to be a chemoprotective agent on the basis of its potent induction of phase 2 enzymes (11), it has been assumed that this effect is indeed the primary mode of action for oltipraz (43). There are now substantial correlative data in animal bioassays to substantiate this view (43, 44). However, enzyme kinetic studies on heterologously expressed human CYP1A2 indicate that oltipraz is also a competitive inhibitor of this cytochrome P450, with an apparent Ki of 10 μM, a pharmacologically achievable concentration (45). CYP3A4 can also be inhibited, but with an 8-fold higher Ki (45). Inhibition of the bioactivation of various environmental carcinogens could be envisioned. For example, observations that administration of oltipraz to rats or humans results in lower urinary excretion rates of a CYP1A2-derived oxidation metabolite (aflatoxin M1) of the hepatocarcinogen aflatoxin B1 (14, 46) indicate the potential importance of such a mechanism. Studies on the effects of dose and scheduling of oltipraz on its chemoprotective efficacy have suggested that intermittent (i.e., weekly) dosing protocols are nearly equi-effective to daily administration in the inhibition of tumorigenesis, even in the face of daily exposure to carcinogen (44). Thus, it is attractive to speculate that a mechanism incorporating a persistent pharmacodynamic action, such as elevated expression of phase 2 genes, is important. Although the plasma half-life of oltipraz in mammals is <6 h (47), the inductive effects on some phase 2 enzymes in the liver of rats persist for more than 1 week (44). The current study finally provides direct evidence that enhanced expression of protective genes is of central importance to the anticarcinogenic action of oltipraz, and presumably, many other similarly acting natural and synthetic agents (21). There are two major practical implications of such a finding. First, the mechanism of induction provides a long biological half-life of the enzyme inductive response. The use of intermittent dosing schedules in humans may offer advantages (fewer side effects, greater compliance, lower cost) while maintaining efficacy. By contrast, a mechanism with a short pharmacodynamic half-life, such as P450 inhibition, will presumably require nearly constant agent administration to sustain efficacy. Second, these findings provide a clear-cut strategy for identification of critical pharmacophores in inducer molecules and sites for interactions of these molecules with their molecular targets for signaling gene expression.
Little is known about the regulatory mechanisms of nrf2 except the identification of Keap1, a cytoplasmic chaperone that suppresses Nrf2 transcriptional activity by specific binding to the amino-terminal regulatory domain of Nrf2 (48). Transient cotransfections of a reporter for monitoring Nrf2 transactivation in cells in the presence of increasing concentrations of a Keap1 expression plasmid indicated that Keap1 could completely repress the activity of Nrf2 (48). Concordant with these in vitro results, keap1-deficient mice exhibit substantially elevated constitutive expression of phase 2 gene products (N. Wakabayashi, K.I., M.Y., unpublished observations). Interestingly, administration of sulfhydryl reactive reagents like diethylmaleate, which are also phase 2 inducers, abrogate Keap1 repression of Nrf2 activity in cells and facilitate the nuclear accumulation of Nrf2 (48). Similarly, oltipraz was observed to increase the amount of Nrf2 in hepatic nuclei isolated from wild-type mice (Fig. 2). Although dithiolethiones, together with the eight other known classes of monofunctional inducers, display little structural homology, all are chemically reactive, and with few exceptions, are electrophiles (49). All can react with sulfhydryl groups by alkylation or redox reactions. The possibility that Keap1, a protein rich in basic cysteine residues, represents a target or sensor molecule for activating Nrf2 signaling to the nucleus is attractive. Identification of the factors regulating the expression of Nrf2 and its cognate binding partners should provide considerable insight into the design or isolation of effective enzyme inducers and, hence, chemoprotective agents.
Finally, it is clear that genetic polymorphism is probably the single most important determinant of enzyme multiplicity in man and considerable interindividual variation in drug oxidation and conjugation has long been recognized. Polymorphisms in many, but not all, phase 1 and phase 2 enzymes have been described. Polymorphisms in phase 2 enzymes may influence cancer risk. For example, risk for smoking-related cancers can increase in individuals deficient in GSTM1 (50). whereas allelic variations in NQO1 confer higher risk of benzene hematotoxicity (51). Thus, it is tempting to speculate that polymorphisms in genes that regulate the expression of phase 2 genes, such as Nrf2, may also be critical determinants of individual susceptibility to carcinogenesis. Identification of such polymorphisms in transcription factors is a potentially important area of endeavor. In support of this view, Cho et al. [Cho, H., Reddy, S.P.M., Reddy, L., Zhang, L.-Y., Jedlicka, A. E. & Kleeberger, S. R. (2000) Toxicologist 54, 254 (abstr.).] used quantitative trait analysis of hyperoxic lung injury to identify nrf2 as a primary susceptibility gene. Diminished levels of expression of Nrf2 in a series of mouse strains were correspondingly associated with increased sensitivity to hyperoxia. Such an association of Nrf2 expression with cancer risk in humans also appears plausible.
Acknowledgments
This work was supported by National Institutes of Health Grants CA39416 and CA44530. M.R.-G. was partially supported by the Consejo Nacional de Ciencia y Tecnologia (CONACYT-Mexico) and the Universidad Autonoma de Queretaro.
Abbreviations
- GST
glutathione S-transferase
- NQO1
NAD(P)H:quinone oxidoreductase
- ARE
antioxidant response element
- CYP
cytochrome P450
Footnotes
See commentary on page 2941.
The metabolism of many carcinogens and other xenobiotics may be considered as resulting from the activities of two families of ubiquitous, versatile, and inducible mammalian enzymes. Phase 1 enzymes (principally cytochromes P450) introduce functional groups by oxidations and reductions and generally lead to nonelectrophilic metabolites, but they may also produce highly reactive electrophiles that avidly bind to nucleophilic centers of DNA and initiate carcinogenesis. The products of phase 1 metabolism are efficiently detoxified by phase 2 enzymes that promote a broad range of reactions, including conjugation with endogenous ligands, e.g., glutathione (glutathione transferases) or glucuronic acid (glucuronosyltransferases) or by disabling reactive functional groups (e.g., reduction of quinones to hydroquinones by NQO1 or hydrolysis of epoxides to diols by epoxide hydrolase). Thus, many carcinogens are innocuous until activated by phase 1 enzymes, and the balance of phase 1 to phase 2 enzymes controls the fate and toxicological effects of carcinogens. Although in the past much emphasis has been placed on the importance of phase 2 enzymes for disarming electrophiles, many phase 2 enzymes are also efficient antioxidants. Thus, glutathione transferases reduce a wide variety of products of oxidative stress and reactive oxygen species. Inducers of phase 2 enzymes are appropriately regarded as “indirect” antioxidants (6) and thereby play major protective roles.
References
- 1.Miller E C, Miller J A. Pharmacol Rev. 1966;18:805–838. [PubMed] [Google Scholar]
- 2.Lubet R A, Connolly G M, Nebert D W, Kouri R E. Carcinogenesis. 1983;4:513–517. doi: 10.1093/carcin/4.5.513. [DOI] [PubMed] [Google Scholar]
- 3.Boberg E W, Miller E C, Miller J A, Poland A, Liem M. Cancer Res. 1983;43:5163–5173. [PubMed] [Google Scholar]
- 4.Wattenberg L W. Cancer Res. 1985;45:1–8. [PubMed] [Google Scholar]
- 5.Kelloff G J, Boone C W, Crowell J A, Steele V E, Lubet R A, Doody L A, Malone W F, Hawk E T, Sigman C C. J Cell Biochem Suppl. 1996;26:1–28. doi: 10.1002/jcb.240630703. [DOI] [PubMed] [Google Scholar]
- 6.Fahey J W, Talalay P. Food Chem Toxicol. 1999;37:973–979. doi: 10.1016/s0278-6915(99)00082-4. [DOI] [PubMed] [Google Scholar]
- 7.Richardson H L, Cunningham L. Cancer Res. 1951;11:274. [Google Scholar]
- 8.Conney A H, Mueller G C, Miller J A. Cancer Res. 1956;16:450–459. [PubMed] [Google Scholar]
- 9.Benson A M, Batzinger R P, Ou S-Y L, Bueding E, Cha Y-N, Talalay P. Cancer Res. 1978;38:4486–4495. [PubMed] [Google Scholar]
- 10.Benson A M, Hunkeler M J, Talalay P. Proc Natl Acad Sci USA. 1980;77:5216–5220. doi: 10.1073/pnas.77.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ansher S S, Dolan P, Bueding E. Food Chem Toxicol. 1986;24:405–415. doi: 10.1016/0278-6915(86)90205-x. [DOI] [PubMed] [Google Scholar]
- 12.Lam L K T, Sparnins V L, Wattenberg L. Cancer Res. 1982;42:1193–1198. [PubMed] [Google Scholar]
- 13.Zhang Y, Cho C G, Posner G H, Talalay P. Proc Natl Acad Sci USA. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J-S, Shen X, He X, Zhu Y-R, Zhang B-C, Wang J-B, Qiang G-S, Kuang S-Y, Zarba A, Egner P A, et al. J Natl Cancer Inst. 1999;91:347–354. doi: 10.1093/jnci/91.4.347. [DOI] [PubMed] [Google Scholar]
- 15.Maheo K, Morel F, Langouët S, Kramer H, Le Ferrec E, Ketterer B, Guillouzo A. Cancer Res. 1997;57:3649–3652. [PubMed] [Google Scholar]
- 16.Langouët S, Furge L L, Kerriguy N, Nakamura K, Guillouzo A, Guengerich F P. Chem Res Toxicol. 2000;13:245–252. doi: 10.1021/tx990189w. [DOI] [PubMed] [Google Scholar]
- 17.Whitlock J P., Jr Annu Rev Pharmacol Toxicol. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- 18.Rushmore T H, King R G, Paulson K E, Pickett C B. Proc Natl Acad Sci USA. 1990;87:3826–3830. doi: 10.1073/pnas.87.10.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wasserman W W, Fahl W E. Arch Biochem Biophys. 1997;344:387–396. doi: 10.1006/abbi.1997.0215. [DOI] [PubMed] [Google Scholar]
- 20.Prochaska H J, Talalay P. Cancer Res. 1988;48:4776–4782. [PubMed] [Google Scholar]
- 21.Prestera T, Holtzclaw W D, Zhang Y, Talalay P. Proc Natl Acad Sci USA. 1993;90:2965–2969. doi: 10.1073/pnas.90.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Long M J, Dolan P, Santamaria A B, Bueding E. Carcinogenesis. 1986;7:977–980. doi: 10.1093/carcin/7.6.977. [DOI] [PubMed] [Google Scholar]
- 23.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et al. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 24.Venugopal R, Jaiswal A K. Proc Natl Acad Sci USA. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwak, M.-K., Itoh, K., Yamamoto, M., Sutter, T. R. & Kensler, T. W. (2001) Mol. Med., in press. [PMC free article] [PubMed]
- 26.Wattenberg L W, Bueding E. Carcinogenesis. 1986;7:1379–1381. doi: 10.1093/carcin/7.8.1379. [DOI] [PubMed] [Google Scholar]
- 27.Primiano T, Gastel J A, Kensler T W, Sutter T R. Carcinogenesis. 1996;17:2291–2296. doi: 10.1093/carcin/17.11.2291. [DOI] [PubMed] [Google Scholar]
- 28.Buetler T, Eaton D L. Cancer Res. 1992;52:314–318. [PubMed] [Google Scholar]
- 29.Habig W H, Pabst M J, Jakoby W B. J Biol Chem. 1974;219:7130–7139. [PubMed] [Google Scholar]
- 30.Prochaska H J, Santamaria A B. Anal Biochem. 1988;169:328–336. doi: 10.1016/0003-2697(88)90292-8. [DOI] [PubMed] [Google Scholar]
- 31.Chomczynski P, Sacchi N. Anal Biochem. 1987;164:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 32.Dignam J D, Lebovitz R M, Roeder R G. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wattenberg L W. J Natl Cancer Inst. 1978;60:11–18. doi: 10.1093/jnci/60.1.11. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez F J, Kimura S. Cancer Lett. 1999;143:199–204. doi: 10.1016/s0304-3835(99)00125-1. [DOI] [PubMed] [Google Scholar]
- 35.Taketo M M. Prog Exp Tumor Res. 1999;35:109–119. doi: 10.1159/000062007. [DOI] [PubMed] [Google Scholar]
- 36.Cavalieri E L, Rogan E G. Xenobiotica. 1995;25:677–688. doi: 10.3109/00498259509061885. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu Y, Nakatsuru Y, Ichinose M, Takahashi Y, Kume H, Mimura J, Fujii-Kuriyama Y, Ishikawa T. Proc Natl Acad Sci USA. 2000;97:779–782. doi: 10.1073/pnas.97.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyata M, Kudo G, Lee Y-H, Yang T J, Gelboin H V, Fernández-Salguero P, Kimura S, González F J. J Biol Chem. 1999;274:23963–23968. doi: 10.1074/jbc.274.34.23963. [DOI] [PubMed] [Google Scholar]
- 39.Henderson C J, Smith A G, Ure J, Brown K, Bacon E J, Wolf C R. Proc Natl Acad Sci USA. 1998;95:5275–5280. doi: 10.1073/pnas.95.9.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Long D J, II, Waikel R L, Wang X-J, Perlaky L, Roop D R, Jaiswal A K. Cancer Res. 2000;60:5913–5915. [PubMed] [Google Scholar]
- 41.Chan K, Kan Y W. Proc Natl Acad Sci USA. 1999;96:12731–12736. doi: 10.1073/pnas.96.22.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O'Conner T, Harada T, Yamamoto M. Toxic Sci. 2001;59:169–177. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- 43.Kensler T W, Groopman J D, Sutter T R, Curphey T J, Roebuck B D. Chem Res Toxicol. 1999;12:113–126. doi: 10.1021/tx980185b. [DOI] [PubMed] [Google Scholar]
- 44.Primiano T, Egner P A, Sutter T R, Kelloff G J, Roebuck B D, Kensler T W. Cancer Res. 1995;55:4319–4324. [PubMed] [Google Scholar]
- 45.Langouët S, Coles B, Morel F, Becquemont L, Beaune P, Guengerich F P, Ketterer B, Guillouzo A. Cancer Res. 1995;55:5574–5579. [PubMed] [Google Scholar]
- 46.Scholl P, Musser S M, Kensler T W, Groopman J D. Carcinogenesis. 1996;17:1385–1388. doi: 10.1093/carcin/17.6.1385. [DOI] [PubMed] [Google Scholar]
- 47.Gupta E, Olopade O L, Ratain M J, Mick R, Baker T M, Berezin F K, Benson A B., III Clin Cancer Res. 1995;1:1133–1138. [PubMed] [Google Scholar]
- 48.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel J D, Yamamoto M. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Talalay P, De Long M J, Prochaska H S. Proc Natl Acad Sci USA. 1988;85:8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Houlston R S. Cancer Epidemiol Biomarkers Prev. 1999;8:675–682. [PubMed] [Google Scholar]
- 51.Smith M T. Proc Natl Acad Sci USA. 1999;96:7624–7626. doi: 10.1073/pnas.96.14.7624. [DOI] [PMC free article] [PubMed] [Google Scholar]