Abstract
Objective: Stroke is a major health problem, yet no studies on stroke have been reported from Palestine. This one-year, hospital-based study was conducted to determine the prevalence of risk factors and the in-hospital mortality rate in patients with ischemic stroke. Method: All patients admitted to Al-Watani government hospital and diagnosed with ischemic stroke between September 2006 and August 2007 were included in the study. Data were obtained by retrospective review of medical charts. Pearson Chi-square and independent t test were used in the univariate analysis. Multiple logistic regression analysis was used to determine the independent predictors of in-hospital mortality rates among the patients. Statistical testing and graphics were carried out using SPSS 15. Results: We identified 153 ischemic stroke patients (83 females and 70 males) of whom 92 were having a first-ever stroke (FES). Patients had several prevalent modifiable risk factors such as hypertension (HTN) (66%), diabetes mellitus (DM) (45.8%), and renal reduced renal function (crcl < 60 ml/ min) (33.9%). Twenty-six (17%) of the patients died during hospitalization. Four variables were significantly associated with in-hospital mortality: history of previous stroke (P= 0.004), crcl at admission (P = 0.004), number of post-stroke complications (P = 0.001), and age (P = 0.043). Multiple logistic regression analysis indicated that the number of post-stroke complications (P= 0.001) and previous stroke (P = 0.03) were significant independent predictors of in-hospital mortality. Conclusion: Screening and better control of risk factors, especially HTN, DM and renal dysfunction, are required to decrease the incidence and in-hospital mortality among patients with ischemic stroke.
Keywords: Ischemic stroke, Risk factors, In-hospital mortality, Palestine
Introduction
Studies have demonstrated that different ethnic groups may have different predisposing risk factors, epidemiologic patterns, and outcomes of stroke. This has been shown in African Americans, Caucasians, Hispanics, Arabs, and Asians [1–6]. These differences could be due to differences in demographic or socioeconomic factors or in lifestyle. Although epidemiologic studies on stroke were carried out in different parts of the world, including some Arab countries [7–11], there are no published data about Palestine. Data on stroke epidemiology are important for diagnostic, therapeutic and preventive purposes. Furthermore, knowledge of the prevalence of stroke-related risk factors can help health decision makers to direct efforts toward reducing stroke-related morbidity and mortality.
In Palestine, the Ministry of Health is the major provider of medical services. The private sector plays a minor role due to its high cost compared to the average income in Palestine. Currently, the Palestinian National Authority is in charge of West-bank and Gaza strip, with a total population of 4,151,668 inhabitants. The West Bank is divided into 10 districts. Nablus district is the second largest district with a population of 362,159 native Palestinian inhabitants. This study was conducted to identify the risk factors, in-hospital mortality, and discharge medications for patients with ischemic stroke admitted to Al-Watani government hospital, Nablus, Palestine.
Methods and patients
This one-year, retrospective, hospital-based study was conducted between September 01, 2006 and August 31, 2007. All patients admitted to the hospital with acute ischemic stroke were included in the study. Diagnosis of ischemic stroke had been confirmed by computerized tomography (CT) scan. Each diagnosed patient had been followed up until either death at the hospital or discharge. Data collection was approved by the health authorities at the hospital.
The data were collected by retrospective review of medical charts included age, gender, medications, serum creatinine (scr) level, risk factors, and post-stroke medical complications. Then following were considered risk factors: age, hypertension (HTN), diabetes mellitus (DM), congestive heart failure (CHF), atrial fibrillation (AF), ischemic heart disease (IHD), smoking, previous stroke, and obesity [12–15]. Hyperlipidemia is a risk factor for stroke, but we did not include it because it had not been done routinely at the hospital. Obesity was defined as a body mass index (BMI) >30 for both genders. Creatinine clearance (crcl) was calculated by using Cokcroft-Gault equations, and values for females were obtained by multiplying the result by 0.85. The number of post-stroke complications was counted for each patient. The post-stroke complications considered were the presence of one or more of the following: constipation, seizure, depression, infection, limb pain, and gastrointestinal upset.
Statistical analysis
Analysis of data was carried out using the "Statistical Package for Social Sciences" (SPSS) program for Windows version 15.0 (SPSS Inc., Chicago, IL). Univariate analysis was carried out using Pearson Chi-square for categorical variables and student's t test for continuous variables. P <0.05 was used as a significance level. Multiple logistic regression analysis was used to find independent predictors of in-hospital mortality. Variables included in the regression model were those that had a significant P value (P <0.05) in the univariate analysis.
Results
We found that 186 stroke patients were admitted to the hospital during the study period; of these, we studied 153 who were diagnosed with ischemic stroke. All patients were admitted within 48 hours of the attack. The average age of the patients was 69.08± 11.15 years (range 41–90). Less than 2% of the patients were < 45 years old. 45.8% were males and 54.2% were females, giving a male:female ratio of 0.84:1. There was no significant difference between females and males in age. Among the patients, 39.9% had previous attack, and 60.1% had a first ever stroke (FES). Patients with FES were younger than those with recurrent stroke (67.94 ± 11.66 versus 70.80 ± 10.19 years, respectively); the difference in age between the two groups was not significant (P = 0.11).
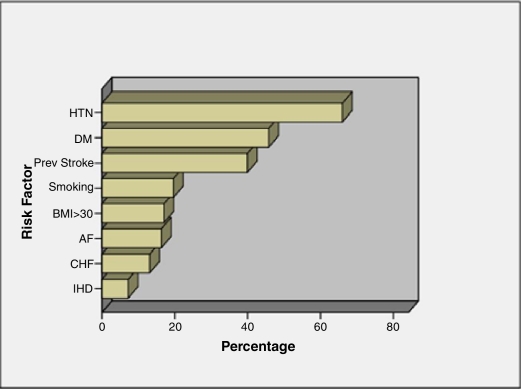
The prevalence of risk factors in the study sample was investigated (Figure 1). Patients had an average of 3.4 ± 1.2 risk factors (range 1 – 7) before the attack. HTN was the most prevalent risk factor (66%) of the patients, followed by DM (45.8%). Renal dysfunction was also common: 33.9% of the patients had an estimated crcl < 60 ml/min. Smoking was practiced by 19.6% of the patients, most of whom (99%) were males. AF was found in 16.3% of patients. No significant difference was found in the prevalence of risk factors between males and females, with the exception of smoking which was significantly associated with males. However, CHF and DM were more common in females, while HTN was more common in males.
Figure 1.
Prevalence of various risk factors in the stroke patients admitted to the hospital during the study period. HTN: hypertension, DM: diabetes mellitus, BMI: body mass index, AF: atrial fibrillation, CHF: congestive heart failure, IHD: ischemic heart disease
Twenty-six (17%) of the patients died during hospitalization. Of the 127 survivors, three left the hospital against medical advice and 124 continued treatment until discharge. Four variables were significantly associated with in-hospital mortality: history of previous stroke (P= 0.004), crcl at admission (P = 0.004), number of post-stroke complications (P = 0.001), and age (P = 0.043). Gender, HTN and DM were insignificantly associated with mortality. Multiple logistic regression analysis indicated that the number of post-stroke complications (P= 0.001) and previous stroke (P = 0.03) were significant independent predictors of in-hospital mortality. Table 1 summarizes the demographic and clinical characteristics of patients who survived versus those who died. Table 2 shows multiple logistic regression model using the enter method for predicting mortality after stroke.
Table 1.
Results of univariate analysis of 153 ischemic stroke patients according to vital status at discharge.
| Variable | Died N = 26 | Survived N = 127 | P value |
|---|---|---|---|
| Age | 73.11 ± 11.19 | 68.26 ± 11.01 | 0.043* |
| Creatinine clearance | 61.63 ± 41.97 | 90.64± 50.79 | 0.004* |
| Post-stroke complication | 1.73± 1.15 | 0.86 ± 0.92 | 0.001* |
| Gender | |||
| (Male) | 14 (53.8%) | 56 (44.1%) | |
| (Female) | 12 (42.6%) | 71 (55.9%) | 0.36 |
| HTN | 19 (73.1%) | 82 (64.6%) | 0.40 |
| DM | 15 (57.9%) | 55 (43.3%) | 0.18 |
| Previous stroke | 17 (65.4%) | 44 (34.6%) | 0.004 |
| Previous Anti-platelet | 19 (73.1%) | 74 (58.3%) | 0.159 |
| AF | 4 (15.4%) | 21 (16.5%) | 0.885 |
| Smoking | 7 (26.9%) | 23 (18.1%) | 0.302 |
| IHD | 3 (11.5%) | 8 (6.3%) | 0.346 |
| CHF | 3 (11.5%) | 17 (13.4%) | 0.799 |
| Obesity | 12 (46.1%) | 46 (36.2%) | 0.263 |
HTN: hypertension, DM: diabetes mellitus, AF: Atrial fibrillation, IHD: ischemic heart disease, CHF: congestive heart failure. Continuous variables were expressed as mean ± SD, while categorical variables were expressed as frequency and percentage. P value was calculated using independent t test for continuous variables and Chi-square test for categorical variables.
Table 2.
Multiple logistic regression by the enter method for identifying predictors of mortality after ischemic stroke.
| Variable | Beta | St. error | P value | 95% CI |
|---|---|---|---|---|
| Age | .001 | 0.003 | 0.677 | [−0.005 – 0.007] |
| Creatinine clearance | −0.001 | 0.001 | 0.148 | [−0.002 – 0.000] |
| No. of complications | .099 | 0.029 | 0.001 | [0.041 – 0.156] |
| Previous stroke | .131 | 0.060 | 0.032 | [0.012 – 0.250] |
Medications prescribed for stroke survivors at discharge were analyzed. An average of 4.33 medications (SD = 1.5, range: 1–9) were prescribed per stroke survivor. The medications prescribed were from six different therapeutic drug classes. Seventy-four (92.5%) stroke survivors with a diagnosis of hypertension were discharged on antihypertensive medications. ACE-I were the most (n = 62, 50%) was the class of antihypertensive agents most frequently prescribed for stroke survivors. Medications prescribed at discharge were similar to those given at admission. The main exceptions were anti-coagulants, antibiotics, and anti-inflammatory agents, all of which were used at admission but were discontinued at discharge. Most patients (98%) were discharged with anti-platelets. There was no difference in the average number of medications prescribed for males and females at discharge (P= 0.36).
Discussion
We studied the characteristics of patients with ischemic stroke attacks at Al-watani hospital, Palestine. The mean age was higher than that reported in hospital-based studies from some other Arab countries but lower than that reported from developed countries [16, 17]. An important observation is the very low percentage (1.6%) of stroke patients who were less than 45 years old. The prevalence of stroke in young patients seems to vary between different ethnic groups of various geographical areas. For instance, only 3–5% of all strokes occurred in individuals under 45 years old in some countries, while in others they constituted as much as 19–30% [18–20]. The male:female (M:F) ratio of 0.84:1 in this study was not in agreement with previous studies on stroke from other countries. One study in a province of Saudi Arabia found a M:F ratio of 1.8:1. A second study among the National Guard community in Saudi Arabia found a M:F ratio of 2.2:1. A third study in Saudi Arabia found a ratio of 3.4:1 [21–23]. However, a hospital-based study in Saudi Arabia found a 1:1 male: female ratio in stroke patients in general [24]. The unexpected gender ratio in our study might indicate the presence of undiagnosed or uncontrolled stroke-related risk factors in women in Palestine.
The high prevalence of risk factors among the stroke victims indicates that control of these factors is important for the prevention of stroke. Thus, screening, modification, and better control of existing risk factors, such as HTN, DM, and cardiac diseases, should be the primary strategy for prevention of stroke. Prospective observational studies have established that the risk of primary stroke is strongly related to blood pressure (BP) [25]. Lowering diastolic BP by 5 mmHg or systolic BP by 10 mmHg reduces the risk of stroke by an estimated 38% [26]. In our study, most patients with HTN were given anti-hypertensive agents, particularly ACE-I, at discharge. About one-third of the patients had an estimated crcl < 60 ml/min. A recent study indicated that mild degrees of renal dysfunction are associated with increased risk of incident ischemic stroke or transient ischemic attack [27]. Recent studies have also established that patients with end stage renal disease (ESRD) have 5–10–fold higher risk of stroke compared to patients with non-ESRD [28, 29].
Results of in-hospital mortality among stroke patients in this study were also close to those reported in most Arab countries. Four variables were significant risk factors of in-hospital mortality in stroke patients, namely, history of previous stroke, number of post-stroke complications, creatinine clearance, and age. The influence of age on stroke outcome is still a matter of debate. While several studies showed a negative influence, other studies showed no influence [30–38]; our results resemble those that showed a negative influence of age on stroke outcome. It is difficult to establish whether age influences stroke outcome itself, or through other factors associated with age. Our finding regarding crcl as an independent predictor of early mortality in stroke patients is endorsed by some studies. For example, a study by Friedman in 1991 indicated that serum creatinine was an independent predictor of mortality among 492 stroke survivors followed up for a mean period of 18 months [39]. Another 7-year follow up study carried out by MacWalter et al. in 2002 indicated that patients with creatinine clearance calculated at admission of < 51 ml/min had a higher mortality rate [40]. Finally, a recent study by Fabjan et al. indicated that in patients with ischemic stroke, decreased crcl was associated with higher in-hospital mortality [41].
Interestingly, we found that prior use of anti-platelets was not associated with decreased risk of mortality. A hospital-based study carried out in Kuwait found that non-se of anti-platelets is significantly associated with deleterious 30-day outcome [42]. One way to reduce early mortality in stroke patients is to develop a stroke unit supervised by a specialized neurologist. Admission of acute stroke patients into specialized hospitals seems to reduce the risk of in-hospital mortality [43]. Moreover, academic medical centers with vascular neurologists had lower rates of in-hospital mortality for patients with ischemic stroke [44]. It seems that mortality in patients with stroke does not depend only on patient-related factors but also on hospital characteristics.
Conclusion
Most of the patients in the study had risk factors commonly present in stroke patients. Better control of these risk factors might decrease the future incidence rate of stroke in Palestine. The number of post-stroke complications and previous stroke were significant independent predictors of in-hospital mortality. Medications prescribed at discharge for stroke survivors are consistent with the type of risk factors, especially HTN, present in patient's medical files.
References
- 1.Chong JY, Sacco RL. Epidemiology of stroke in young adults: race/ethnic differences. J Thromb Thrombolysis. 2005;20(2):77–83. doi: 10.1007/s11239-005-3201-9. [DOI] [PubMed] [Google Scholar]
- 2.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–43. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 3.Pandey DK, Gorelick PB. Epidemiology of stroke in African Americans and Hispanic Americans. Med Clin North Am. 2005;89(4):739–52. doi: 10.1016/j.mcna.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Klatsky AL, Friedman GD, Sidney S, Kipp H, Kubo A, Armstrong MA. Risk of hemorrhagic stroke in Asian American ethnic groups. Neuroepidemiology. 2005;25(1):26–31. doi: 10.1159/000085310. [DOI] [PubMed] [Google Scholar]
- 5.White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111(10):1327–31. doi: 10.1161/01.CIR.0000157736.19739.D0. [DOI] [PubMed] [Google Scholar]
- 6.Deleu D, Hamad AA, Kamram S, El Siddig A, Al Hail H, Hamdy SM. Ethnic variations in risk factor profile, pattern and recurrence of non-cardioembolic ischemic stroke. Arch Med Res. 2006;37(5):655–62. doi: 10.1016/j.arcmed.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Hamad A, Hamad A, Sokrab TE, Momeni S, Mesraoua B, Lingren A. Stroke in Qatar: a one-year, hospital-based study. J Stroke Cerebrovasc Dis. 2001;10(5):236–41. doi: 10.1053/jscd.2001.30382. [DOI] [PubMed] [Google Scholar]
- 8.El Sayed MM, Adeuja AO, El-Nahrawy E, Olaish MA. Characteristics of stroke in Hofuf, Saudi Arabia. Ann Saudi Med. 1999;19(1):27–31. doi: 10.5144/0256-4947.1999.27. [DOI] [PubMed] [Google Scholar]
- 9.Egnerova A, Khogali M. Cerebrovascular diseases in Kuwait: A descriptive study. Saudi Medical Journal. 1987;8:27. [Google Scholar]
- 10.El Zunni S, Ahmed M, Prakash PS, Hassan KM. Stroke: Incidence and pattern in Benghazi, Libya. Ann Saudi Med. 1995;15(4):367–69. doi: 10.5144/0256-4947.1995.367. [DOI] [PubMed] [Google Scholar]
- 11.Mrabet A, Attia-Romdhane N, Ben Hamida M, Gharbi N, Le Noan H, Hentati R, et al. Epidemiologic aspects of cerebrovascular accidents in Tunisia. Rev Neurol (Paris) 1990;146(4):297–301. [PubMed] [Google Scholar]
- 12.Tuhrim S. Stroke risk factors. CNS Spectr. 2000;5(3):70–4. doi: 10.1017/s1092852900012980. [DOI] [PubMed] [Google Scholar]
- 13.Bodo M, Thuróczy G, Pánczél G, Sipos K, Iliás L, Szonyi P, et al. Prevalence of stroke/cardiovascular risk factors in rural Hungary--a cross-sectional descriptive study. Ideggyogy Sz. 2008;61(3–4):87–96. [PubMed] [Google Scholar]
- 14.Hankey GJ. Preventable stroke and stroke prevention. J Thromb Haemost. 2005;3(8):1638–45. doi: 10.1111/j.1538-7836.2005.01427.x. [DOI] [PubMed] [Google Scholar]
- 15.Sacco RL. Risk factors and outcomes for ischemic stroke. Neurology. 1995;45(2 Suppl 1):S10–4. [PubMed] [Google Scholar]
- 16.Bahou Y, Hamid H, Raqab MZ. Ischemic stroke in Jordan 2000 to 2002: a two-year, hospital-based study. J Stroke Cerebrovasc Dis. 2004;13(2):81–4. doi: 10.1016/j.jstrokecerebrovasdis.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Silvestrelli G, Corea F, Paciaroni M, Palmerini F, Parnetti L, Gallai V. The Perugia hospital-based stroke registry: report of the 2nd year. Clin Exp Hypertens. 2002;24(7–8):485–91. doi: 10.1081/ceh-120015324. [DOI] [PubMed] [Google Scholar]
- 18.Srinivasan K. Ischemic cerebrovascular disease in the young. Two common causes in India. Stroke. 1984;15(4):733–5. doi: 10.1161/01.str.15.4.733. [DOI] [PubMed] [Google Scholar]
- 19.Radhakrishnan K, Ashok PP, Sridharan R, Mousa ME. Stroke in the young: incidence and pattern in Benghazi, Libya. Acta Neurol Scand. 1986;73(4):434–8. doi: 10.1111/j.1600-0404.1986.tb03301.x. [DOI] [PubMed] [Google Scholar]
- 20.Khan FY. Risk factors of young ischemic stroke in Qatar. Clin Neurol Neurosurg. 2007;109(9):770–3. doi: 10.1016/j.clineuro.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Al-Rajeh S, Larbi EB, Bademosi O, Awada A, Yousef A, al-Freihi H, et al. Stroke register: experience from the eastern province of Saudi Arabia. Cerebrovasc Dis. 1998;8(2):86–9. doi: 10.1159/000015823. [DOI] [PubMed] [Google Scholar]
- 22.al Rajeh S, Awada A, Niazi G, Larbi E. Stroke in a Saudi Arabian National Guard community. Analysis of 500 consecutive cases from a population-based hospital. Stroke. 1993;24(11):1635–9. doi: 10.1161/01.str.24.11.1635. [DOI] [PubMed] [Google Scholar]
- 23.Qari FA. Profile of stroke in a teaching university hospital in the western region. Saudi Med J. 2000;21(11):1030–3. [PubMed] [Google Scholar]
- 24.El Sayed MM, Adeuja AO, El-Nahrawy E, Olaish MA. Characteristics of stroke in Hofuf, Saudi Arabia. Ann Saudi Med. 1999;19(1):27–31. doi: 10.5144/0256-4947.1999.27. [DOI] [PubMed] [Google Scholar]
- 25.MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, et al. Blood pressure, stroke, and coronary heart disease: part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. The Lancet. 1990;335:765–74. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 26.MacMahon S, Rodgers A. Blood pressure, antihypertensive treatment and stroke risk. Journal of Hypertension. 1994;12(10):S5–S14. [PubMed] [Google Scholar]
- 27.Hojs Fabjan T, Hojs R, Tetickovic E, Pecovnik Balon B. Ischaemic stroke--impact of renal dysfunction on in-hospital mortality. Eur J Neurol. 2007;14(12):1351–6. doi: 10.1111/j.1468-1331.2007.01976.x. [DOI] [PubMed] [Google Scholar]
- 28.Seliger S, Gillen D, Kestenbaum B, Ball A, Wasse H, Stehman-Breen C. A comparison of stroke rates among dialysis and general populacion. Journal of the American Society of Nephrology. 2002;13:438a. [Google Scholar]
- 29.Seliger SL, Gillen DL, Tirschwell D, Wasse H, Kestenbaum BR, Stehman-Breen CO. Risk factors for incident stroke among patients with end-stage renal disease. Journal of the American Society of Nephrology. 2003;14:2623–31. doi: 10.1097/01.asn.0000088722.56342.a8. [DOI] [PubMed] [Google Scholar]
- 30.Ahlsiö B, Britton M, Murray V, Theorell T. Disablement and quality of life after stroke. Stroke. 1984;15(5):886–90. doi: 10.1161/01.str.15.5.886. [DOI] [PubMed] [Google Scholar]
- 31.Kotila M, Waltimo O, Niemi ML, Laaksonen R, Lempinen M. The profile of recovery from stroke and factors influencing outcome. Stroke. 1984;15(6):1039–44. doi: 10.1161/01.str.15.6.1039. [DOI] [PubMed] [Google Scholar]
- 32.Wade DT, Wood VA, Hewer RL. Recovery after stroke: the first three months. J Neurol Neurosurg Psychiatry. 1985;48:7–13. doi: 10.1136/jnnp.48.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kojima S, Omura T, Wakamatsu W, Kishi M, Yamazaki T, Iida M, Komachi Y. Prognosis and disability of stroke patients after 5 years in Akita, Japan. Stroke. 1990;21:72–7. doi: 10.1161/01.str.21.1.72. [DOI] [PubMed] [Google Scholar]
- 34.Dennis MS, Burn JPS, Sandercock PAG, Bamford J, Wade DT, Warlow CP. Long-term survival after first ever stroke: the Oxfordshire community stroke project. Stroke. 1993;24:796–800. doi: 10.1161/01.str.24.6.796. [DOI] [PubMed] [Google Scholar]
- 35.Fiorelli M, Alpérovitch A, Argentino C, Sacchetti ML, Toni D, Sette G, Cavalletti C, Gori MC, Fieschi C. Prediction of long-term outcome in the early hours following acute ischemic stroke. Arch Neurol. 1995;52:250–5. doi: 10.1001/archneur.1995.00540270038017. [DOI] [PubMed] [Google Scholar]
- 36.Feigenson JS, McDowell FH, Meese P, McCarthy ML, Greenberg SD. Factors influencing outcome and length of stay in a stroke rehabilitation unit, Part I: analysis of 248 unscreened patients: medical and functional prognostic indications. Stroke. 1977;8:651–6. doi: 10.1161/01.str.8.6.651. [DOI] [PubMed] [Google Scholar]
- 37.Wade DT, Hewer RL, Wood VA. Stroke: the influence of age upon outcome. Age Ageing. 1984;13:357–62. doi: 10.1093/ageing/13.6.357. [DOI] [PubMed] [Google Scholar]
- 38.Asplund K, Carlberg B, Sundström G. Stroke in the elderly. Observations in a population based sample of hospitalized patients. Cerebrovasc Dis. 1992;2:152–7. [Google Scholar]
- 39.Friedman PJ. Serum creatinine: an independent predictor of survival after stroke. J Intern Med. 1991;229(2):175–9. doi: 10.1111/j.1365-2796.1991.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 40.MacWalter RS, Wong SY, Wong KY, Stewart G, Fraser CG, Fraser HW, Ersoy Y, Ogston SA, Chen R. Does renal dysfunction predict mortality after acute stroke? A 7-year follow-up study. Stroke. 2002;33(6):1630–5. doi: 10.1161/01.str.0000016344.49819.f7. [DOI] [PubMed] [Google Scholar]
- 41.Fabjan TH, Hojs R, Tetickovic E, Balon BP. Ischaemic stroke-impact of renal dysfunction on in-hospital mortality. Eur J Neurol. 2007;14(12):1351–6. doi: 10.1111/j.1468-1331.2007.01976.x. [DOI] [PubMed] [Google Scholar]
- 42.Al-Shammri S, Shahid Z, Ghali A, Mehndiratta MM, Swaminathan TR, Chadha G, Sharma PN, Akanji AO. Risk factors, subtypes and outcome of ischaemic stroke in Kuwait--a hospital-based study. Med Princ Pract. 2003;12(4):218–23. doi: 10.1159/000072287. [DOI] [PubMed] [Google Scholar]
- 43.Baptista MV, van Melle G, Bogousslavsky J. Prediction of in-hospital mortality after first-ever stroke: the Lausanne Stroke Registry. J Neurol Sci. 1999;166(2):107–14. doi: 10.1016/s0022-510x(99)00117-3. [DOI] [PubMed] [Google Scholar]
- 44.Gillum LA, Johnston SC. Characteristics of academic medical centers and ischemic stroke outcomes. Stroke. 2001;32(9):2137–42. doi: 10.1161/hs0901.094260. [DOI] [PubMed] [Google Scholar]