Abstract
Lead is a blue–gray and highly toxic divalent metal that occurs naturally in the earth's crust and is spread throughout the environment by various human activities. The efficacy of garlic (Allium sativum) to reduce hepatotoxicity induced by lead nitrate was evaluated experimentally in male mice. Oral treatment with lead nitrate at a dose of 50 mg/kg body weight daily for 40 days (1/45 of LD50) induced a significant increase in the levels of hepatic aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, acid phosphatase, cholesterol, lipid peroxidation, and lead nitrate. In parallel, hepatic protein levels in lead-exposed mice were significantly depleted. Lead nitrate exposure also produced detrimental effects on the redox status of the liver indicated by a significant decline in the levels of liver antioxidants such as superoxide dismutase, catalase, and glutathione. After exposure to lead nitrate (50 mg/kg body weight for 10 days), the animals received aqueous garlic extract (250 mg/kg body weight and 500 mg/kg body weight) and ethanolic garlic extract (100 mg/kg body weight and 250 mg/kg body weight), and partially restored the deranged parameters significantly. Histological examination of the liver also revealed pathophysiological changes in lead nitrate-exposed group and treatment with garlic improved liver histology. Our data suggest that garlic is a phytoantioxidant that can counteract the deleterious effects of lead nitrate.
Keywords: lead, Allium sativum, lipid peroxidation, marker enzymes, hepatotoxicity, metal estimation
Many metals play important roles in the functioning of enzymes, cell-signaling processes, and gene regulation. Lead is not known to have any biological role. Increasing concern has been expressed about the rapidly rising level of chemicals in the environment, particularly lead, which has well-known hazardous effects. In India, the range of sources of lead exposure is extensive and yet not well understood. This ubiquitous environmental pollutant enters the atmosphere from production of coal, oil, iron, steel, and batteries, as well as from smelters, solid waste, and tobacco smoke. Lead can disrupt biological systems by altering the molecular interactions, cell signaling, and ultimately cellular function. The toxic effect of lead nitrate is well documented in mammals, in which it leads to a broad range of physiological, biochemical, and behavioral dysfunctions (1). Lead exposure occurs mainly through the respiratory and gastrointestinal systems. Liver is a frequent target for many toxicants (2). Autopsy studies of lead-exposed humans indicate that among soft tissue, liver is the largest repository (33%) of lead, followed by kidney. Lead-induced hepatic damage is mostly rooted in lipid peroxidation (LPO) and disturbance of the prooxidant–antioxidant balance by generation of reactive oxygen species (ROS) (3, 4).
The currently approved treatment for lead intoxication is to give chelating agents, such as meso-2,3-dimercaptosuccinic acid (DMSA) and monoisoamyl DMSA (MiADMSA), which form an insoluble complex with lead and shield it from biological targets, thereby reducing its toxicity (5).
However, these chelators are potentially toxic (6) and often fail to remove lead from all body tissues (7). Moreover, because they are hydrophilic or lipophobic (8, 9), they cannot cross the cell membrane to capture intracellular lead. Thus, drugs with lipophilic properties are needed.
Recent trends in controlling and treating diseases favor natural antioxidants. The human diet, which contains many natural compounds, is essential in protecting the body against the development of diseases, and garlic (Allium sativum Linn.) has a broad spectrum of activities: antibacterial, anticarcinogenic, hypolipidemic, hypoglycemic, antifungal, antiatherosclerotic, and antioxidant (10). Garlic is used widely in foodstuffs and medicines. Its use is cost effective and so it is suitable for economically disadvantaged people or societies. The antioxidant activity of garlic is attributed to biologically active lipophilic sulfur-bearing compounds such as allicin, S-allyl-cysteine (SAC), diallyl-di-sul?de (DADS), and diallyl-sul?de (DAS). Processed garlic preparations typically contain different sulfur compounds. One can expect that the composition of processed garlic products depend on the processing method.
This study was conducted to determine if different garlic extracts (aqueous garlic extract (AGE) and ethanolic garlic extract (EGE)) can ameliorate hepatotoxicity induced in Swiss male albino mice by lead nitrate.
Material and methods
Plant material
The garlic plant (A. sativum) was obtained from the University medicinal plant garden, Banasthali, India. A botanist at our department identified it as a local variety.
Preparation of garlic extracts
Aqueous garlic extract (AGE)
Peeled garlic cloves (5 g) were crushed mechanically in a mortar–pestle for 1 min with 10 ml distilled water. The crushed material was decanted by pressing through cheese cloth (yield was 500 mg/ml). The extract was freshly prepared for the experiment whenever needed.
Ethanolic garlic extract (EGE)
The powered garlic bulbs (100 g) were extracted with ethanol (600 ml) in a Soxhlet extractor for 48 h at 60°C. After extraction, the solvent was evaporated to dryness at 50–55°C by using a rotary evaporator and the solid extract (yield was 7.1 g/kg) was stored at 4°C. It was dissolved in distilled water whenever needed for experiments.
Chemicals
Lead nitrate was purchased from Central Drug House (India). All other chemicals were of analytical grade and obtained from Sisco Research Laboratories (India), Qualigens (India/Germany), SD Fine Chemicals (India), HIMEDIA (India), and Central Drug House (India).
Animals
Male Swiss albino mice weighing 15–30 g (2–2.5 months) were obtained from Haryana Agricultural University, Hissar, India. The Animal Ethics Committee of Banasthali University, Banasthali, India approved the study. All experiments were conducted on adult male albino mice when they weighed 25–35 g (3–4 months old). They were housed in polypropylene cages in an air-conditioned room at 25±3°C, relative humidity of 50±5% and 12-h alternating light and dark cycles. The mice were provided with chow diet (Hindustan Lever Limited, India) and drinking water ad libitum.
Experimental design
The mice were divided into six groups of 12 mice each. Mice receiving lead nitrate were given 50 mg/kg body weight lead nitrate: 1.25 mg lead nitrate dissolved in 1 ml distilled water and given by gavage. This dose is equivalent to 1/45 of LD50 (11). The doses of garlic extracts were decided on the basis of experiments conducted in our own laboratory and on other published report (12).
Group 1 received 1 ml distilled water by oral gavage (control group).
Group 2 received lead nitrate daily for 40 days.
Groups 3 and 4 were given AGE 250 mg/kg body weight or 500 mg/kg body weight by oral gavage once daily for 30 days starting 11 days after the start of lead nitrate treatment until the end of the experiment.
Groups 5 and 6 were given EGE at 100 mg/kg body weight or 250 mg/kg body weight by oral gavage once daily for 30 days starting 11 days after the start of lead nitrate treatment until the end of the experiment.
After administration of the last dose, the animals were given a rest overnight and the following day they were killed under light ether anesthesia. Livers were removed immediately, weighed, rinsed in ice-cold saline, blotted, and used for various biochemical assays, histological studies, and determination of lead concentration.
Half of each liver was processed for biochemical analysis and histological examination, and the other half was stored at −20°C before wet acid digestion with HNO3 for lead estimation.
Biochemical assays
Liver was minced and homogenized (10% w/v) in ice-cold 0.1 M sodium phosphate buffer (pH 7.4). The homogenate was centrifuged at 10,000 rpm for 15–20 min at 4°C twice to get the enzyme fraction. The supernatant was used for biochemical assays.
Lipid peroxidation (LPO)
LPO was estimated colorimetrically by measuring malondialdehyde (MDA) formation as described by Nwanjo and Ojiako (13). In brief, 0.1 ml of homogenate was treated with 2 ml of a 1:1:1 ratio of TBA–TCA–HCl (TBA 0.37%, TCA 15%, HCl 0.25 N) and placed in water bath at 65°C for 15 min, cooled, and centrifuged at 5,000 rpm for 10 min at room temperature. The optical density of the clear supernatant was measured at 535 nm against a reference blank. The MDA formed was calculated by using the molar extinction coefficient of thiobarbituric acid reactants (TBARS; 1.56×105 l/mole cm−1). The product of LPO was expressed as nmol of MDA formed per g of tissue.
Superoxide dismutase (SOD)
Hepatic SOD activity was assayed according to the method of Marklund and Marklund (14). For the control, 0.1 ml of 20 mM pyrogallol solution was added to 2.9 ml of Tris buffer and mixed, and reading was taken at 420 nm after 1.5 and 3.5 mins. The absorbance difference for 2 min was recorded and the concentration of pyrogallol was adjusted in such a way that the rate in change of absorbance per 2 min was approximately 0.020–0.023 optical density units.
Liver extract (200 µl) was treated with 10 µl of 25% triton X-100 and kept at 4°C for 30 min. To 2.8 ml of Tris buffer, 0.1 ml of treated sample was added and mixed, and the reaction was started by adding 0.1 ml of adjusted pyrogallol solution (as for control). Reading was taken at 420 nm after 1.5 and 3.5 mins and the difference in absorbance was recorded. The enzyme activity was expressed as U/ml of liver extract and 1 U of enzyme is defined as the enzyme activity that inhibits auto-oxidation of pyrogallol by 50%.
Catalase (CAT)
Catalase (CAT) activity was estimated following the method of Aebi (15). Liver extract (100 µl) was treated with ethanol (10 µl) and placed on an ice bath for 30 min. To this, 10 µl of 25% triton X-100 was added and again kept for 30 min on ice. To 200 µl phosphate buffer (0.1 M), 50 µl of treated liver extract and 250 µl of 0.066 M H2O2 (prepared in 0.1 M phosphate buffer, pH 7.0) were added in a cuvette. The decrease in optical density was measured at 240 nm for 60 s. The molar extinction coefficient of 43.6 cm−1 was used to determine CAT activity. One unit of activity is equal to the moles of H2O2 degraded/min/mg protein.
Glutathione (GSH)
Reduced glutathione (GSH) was determined by the method of Ellman (16). In brief, 1 ml of supernatant was taken after precipitating 0.5 ml of liver homogenate with 2 ml of 5% TCA. To this, 0.5 ml of Ellman's reagent (0.0198% DTNB in 1% sodium citrate) and 3 ml of phosphate buffer (1 M, pH 8.0) was added. The color developed was read at 412 nm. Reduced GSH concentration is measured by using a drawn standard curve and was expressed as mg/g of tissue.
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT)
Activities of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were assayed by the method of Reitman and Frankel (17). In brief, 0.2 ml of liver fraction and 0.5 ml of substrate solution (for AST: aspartate and 2-ketoglutarate; for ALT: alanine and 2-ketoglutarate) were incubated at 37°C for 60 min for AST and 30 min for ALT. After incubation, 0.5 ml of DNPH solution was added to arrest the reaction, which was kept for 20 min at room temperature. To this, 1 ml of 0.4 N NaOH was added and absorbance was read at 510 nm. Activities were expressed as IU/L.
Acid phosphatase (ACP) and alkaline phosphatase (ALP)
Activities of acid phosphatase (ACP) and alkaline phosphatase (ALP) were determined according to the protocol described in a laboratory practical manual (18). Substrate solution (3 ml) was incubated at 37°C for 15 min and then 0.5 ml liver homogenate was added. It was mixed well and immediately 0.05 ml of the mixture was removed and mixed with 9.5 ml of 0.085 N NaOH. This corresponded to zero time assay (blank). The remaining solution (substrate+enzyme) was incubated for 15 min at 37°C and then 0.5 ml was drawn and mixed with 9.5 ml of 0.085 N NaOH. Absorbance was measured at 405 nm against the reference blank. Specific activities were expressed as µmoles of p-nitrophenol formed per min per g tissue.
Protein
Protein content was determined by the method of Lowry et al. (19) and bovine serum albumin as a standard.
Cholesterol
Cholesterol was determined by the method of Zak (20) with cholesterol as a standard.
Lead nitrate
The accurately measured samples of liver (250 mg) were digested in 10 ml concentrated HNO3 by using Microwave Digestion System. After evaporation of HNO3, dried samples were dissolved in 10 ml of distilled water. Lead content was estimated using a Hydride Vapour Generation System (Model MHS-10, Perkin Elmer) fitted with a Flame Atomic Absorption Spectrometer (AAS, Perkin Elmer model A Analyst 100, Uberlingen, Germany) against suitable standards processed identically.
Histological studies
Liver was dissected from the animals and fixed in buffered 10% formalin at room temperature for 72 h. It was then thoroughly washed under running water and dehydrated in ascending grades of ethyl alcohol, cleared, and embedded in soft paraffin. Tissue sections of about 6 µm were cut, stained with hematoxylin and eosin, and examined with a light microscope.
Statistical analysis
Data are expressed as the mean±SEM. The data were analyzed by analysis of variance (ANOVA) followed by Tukey test using the Statistical Package for the Social Sciences (S.P.S.S. 11). The level of significance was set at p<0.05.
Results
Table 1 shows the activity of TBARS and antioxidant-related parameters in liver. Lead nitrate exposure was detrimental to the redox status of liver, as evidenced by a significant rise (p<0.001) in LPO level (61.0%) and significant depletion in CAT activity (55.7%), SOD activity (34.5%), and GSH content (78.9%) in mice treated with lead nitrate but no garlic extract (Group 2) compared with untreated animals.
Table 1.
Protective effect of garlic extracts on hepatic oxidative stress-related parameters and lead level in lead nitrate-exposed mice
| Control group | Treated mouse groups | |||||
|---|---|---|---|---|---|---|
| Parameter | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 |
| LPO | 110.07±1.37 | 177.29±1.98* (+61.0%) | 145.42±1.56a (−17.9%) | 131.37±2.04a (−25.9%) | 140.77±1.17a (−20.5%) | 118.5±1.86a (−33.1%) |
| SOD | 1.13±0.03 | 0.74±0.10* (−34.5%) | 0.83±0.01 (+12.1%) | 0.97±0.02b (+31.08%) | 0.79±0.01 (+6.7%) | 0.87±0.01 (+17.5%) |
| CAT | 78.92±1.08 | 34.96±0.73* (−55.7%) | 45.04±0.98a (+30.3%) | 65.65±1.04a (+89.9%) | 47.04±0.94a (+36.1%) | 70.55±1.11a (+104.1%) |
| GSH | 7.41±0.08 | 1.56±0.12* (−78.9%) | 4.02±0.05a (+157.6%) | 5.91±0.06a (+278.8%) | 3.91±0.06a (+150.6%) | 6.45±0.14a (+313.4%) |
| Lead level | 0.053±0.007 | 3.84±0.28* (+7145.2%) | 2.38±0.21a (−38.0%) | 1.14±0.19a (−70.3%) | 2.36±0.32a (−35.5%) | 1.26±0.38a (−67.18%) |
Note: LPO, lipid peroxidation (Malondialdehyde formed in nmole/gm); SOD, superoxide dismutase (U/ml of tissue extract); CAT, catalase (µmoles of H2O2 degraded per min per mg protein); GSH, reduced glutathione (mg/g tissue); lead, (µg/gm wet tissue).Values are mean±SEM, n=12.
p<0.001 compared to normal animals.
p<0.001 compared to lead nitrate-exposed animals.
p<0.01 compared to lead nitrate-exposed animals.
Compared to Group 2 (only PbNO3), Groups 3 and 4 showed a marked decrease in LPO level (17.9 and 25.9%) and a marked increase in CAT level (30.3 and 89.9%), and GSH content (157.6 and 278.8%) (p<0.001). In Group 4, there was a significant increase (31.08%; p<0.01) in the activity of SOD compared to Group 2, but SOD activity in Group 3 did not differ significantly from Group 2.
In Groups 5 and 6, the percent decrease (p<0.001) in hepatic LPO level was 20.5% and 33.1%, respectively, as compared with lead nitrate control values (Group 2). CAT (36.1 and 104.1%) and GSH (150.6 and 313.4%) activities were raised significantly (p<0.001) in Groups 5 and 6, respectively, when compared with lead nitrate-administered mice (Group 2), but no significant difference in SOD activity was observed in both Groups 5 and 6 treated animals.
Table 1 shows the lead accumulation pattern in the liver of different experimental groups. Administration of lead nitrate to Group 2 significantly increased (p<0.001) the level of lead nitrate in liver (3.84±0.28 µg/g wet tissue vs. 0.053±0.007 µg/g wet tissue in the negative control group).
In Groups 3 and 4, lead nitrate content reduced significantly (p<0.001) (38.02% and 70.31%, respectively), in comparison to Group 2. Hepatic lead nitrate content decreased significantly in Groups 5 and 6 (38.54% and 67.18%, p<0.001, respectively), when compared with Group 2.
Exposure to lead nitrate (50 mg/kg body weight) alone induced a significant augmentation (p<0.001) in AST (56.3%), ALT (105.9%), ACP (196.8%), ALP (170.9%), and total cholesterol (131.4%) levels, as compared to control animals. However, lead nitrate administration significantly decreased (p<0.001) total protein content (39.7%) in the liver when compared with the control group.
Compared to Group 2, which received only lead nitrate, Groups 3 and 4 showed significant declines (p<0.001) in the levels of AST (15.3% and 27.0%, respectively), ALT (31.8 and 38.6%), ACP (23.3 and 51.7%), ALP (25.9 and 34.5%), and total cholesterol (20.7 and 50.3%) accompanied by a significant increase (p<0.001) in total protein content (31.6 and 55.1%).
Compared to mice receiving only lead nitrate (Group 2), administration of EGE in Groups 5 and 6 significantly (p<0.001) reduced the augmentation of AST (by 17.4 and 29.0%), ALT (by 23.2 and 41.0%), ACP (by 34.9 and 56.1%), ALP (by 31.8 and 44.5%), and total cholesterol (by 33.6 and 43.9%). On the other hand, protein content in liver was significantly increased by 23.5% (p<0.02) and 49.4% (p<0.001), in Groups 5 and 6, respectively (Table 2).
Table 2.
Protective effect of garlic extract against lead nitrate-induced changes in some hepatic biochemical parameters in mice
| Control group | Treated mouse groups | |||||
|---|---|---|---|---|---|---|
| Parameter | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 |
| AST | 48.19±0.72 | 75.35±0.90* (+56.3%) | 63.78±0.93a (−15.3%) | 54.98±1.21a (−27.0%) | 62.17±1.26a (−17.4%) | 53.49±1.45a (−29.0%) |
| ALT | 33.52±1.06 | 69.05±1.06* (+105.9%) | 47.05±0.89a (−31.8%) | 42.34±0.91a (−38.6%) | 52.99±0.84a (−23.2%) | 40.7±0.85a (−41.0%) |
| ACP | 12.54±0.38 | 37.22±0.76* (+196.8%) | 28.53±0.74a (−23.3%) | 17.97±0.48a (−51.7%) | 24.21±0.77a (−34.9%) | 16.31±0.41a (−56.1%) |
| ALP | 17.92±0.71 | 48.56±2.10* (+170.9%) | 35.95±0.74a (−25.9%) | 31.79±1.05a (−34.5%) | 33.10±0.69a (−31.8%) | 26.94±1.18a (−44.5%) |
| Protein | 8.40±0.30 | 5.06±0.21* (−39.7%) | 6.66±0.20a (+31.6%) | 7.85±0.20a (+55.1%) | 6.25±0.13b (+23.5%) | 7.56±0.18a (+49.4%) |
| Cholesterol | 24.15±0.54 | 55.88±1.02* (+131.3%) | 44.27±1.01a (−20.7%) | 27.72±0.43a (−50.3%) | 37.07±0.52a (−33.6%) | 31.32±0.85a (−43.9%) |
Notes: AST, Aspartate transaminase (IU/L); ALAT, alanine transaminase (IU/L); ALP, alkaline phosphatase (µmoles of p-nitrophenol formed per min per g of tissue); ACP, acid phosphatase (µmoles of p-nitrophenol formed per min per g of tissue); protein (g/dl of tissue extract); cholesterol (mg/g tissue extract). Values are mean±SEM, n=12.
p<0.001 compared to normal animals.
p<0.001 compared to lead nitrate-exposed animals.
p<0.02 compared to lead nitrate-exposed animals.
Histological results
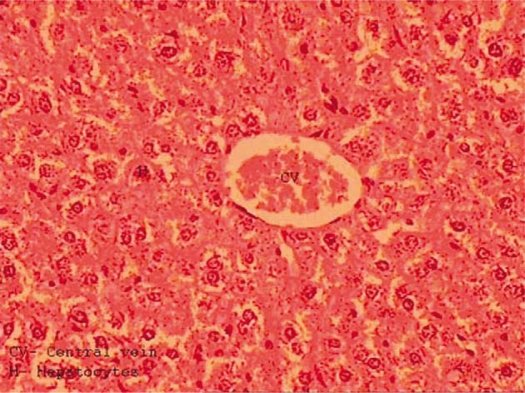
The liver of control mice showed normal hexagonadal or pentagonadal lobules with central veins and peripheral hepatic triads or tetrads embedded in connective tissue. Hepatocytes are arranged in trabecules running radially from the central vein and are separated by sinusoids containing Kupffer cells. They are regular and contain a large spheroidal nucleus (Fig. 1).
Fig. 1.
Transverse section of the liver of a control mouse showing hepatic cords arranged radially around the central vein and normal hepatocytes with centrally located nuclei.
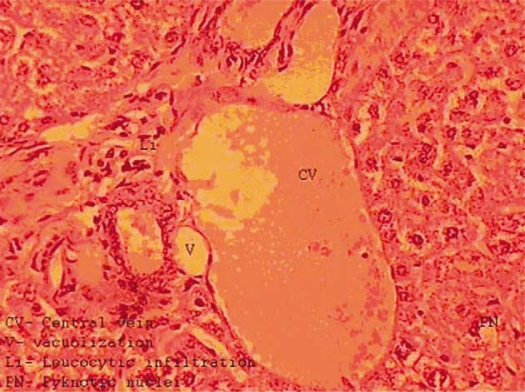
The livers of mice exposed to lead nitrate for 40 days revealed disruption of the normal structural organization of the hepatic lobules and loss of the characteristic cord-like arrangement of the normal liver cells. The central and portal veins were congested. Many hepatic cells were damaged and lost their characteristic appearance while others showed marked cytoplasmic vacuolization. The nuclei of these cells were pyknotic. The central vein and sinusoids between hepatocytes were dilated. Some leukocyte infiltration and fatty deposition were also evident (Fig. 2).
Fig. 2.
Transverse section of the liver of a mouse treated with lead nitrate showing congestion of central vein, vacuolization, leucocytic infiltration, pyknotic nuclei, and loss of radial arrangement of hepatocytes.
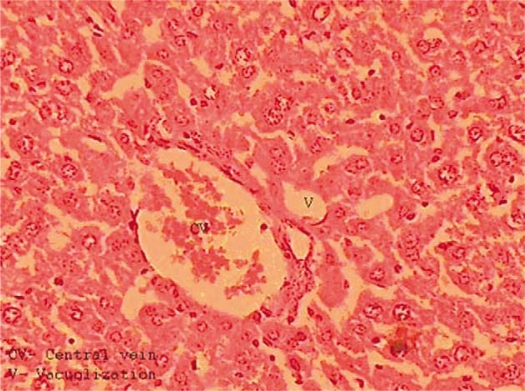
Animals treated with low doses of aqueous and EGEs (Groups 3 and 5) showed that the majority of these histopathological changes were diminished but some hepatocytes appeared with vacuolized cytoplasm and the central vein appeared congested (Fig. 3).
Fig. 3.
Liver section obtained from a mouse treated with lead nitrate followed by a low dose of garlic extract. Normal arrangement of hepatocytes is restored but a central vein is congested.
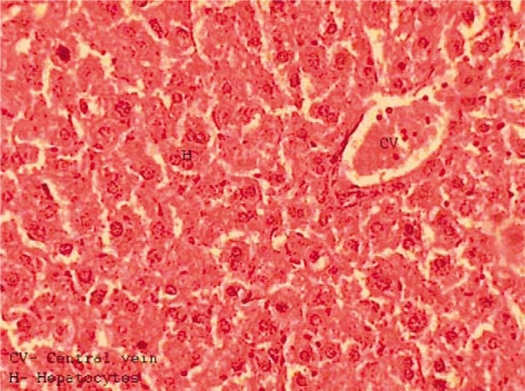
In the high dose groups (Groups 4 and 6), the liver restored most of its normal structure and was able to diminish the fibrosis, congestion, incidence of inflammatory cells infiltration, centrilobular hepatocytes swelling, hepatocytes vacuolization, fatty changes, and hemorrhagic clots (Fig. 4).
Fig. 4.
Liver section obtained from a mouse after treatment with lead nitrate and garlic showing improvement of hepatic tissue.
Discussion
Lead is a wide spread constituent of earth's crust (21). It can cause hypertension, developmental defects, neurological problems, renal dysfunction, and anemia. In recent years dietary plants with antioxidant property have been receiving considerable attention. It is believed that these plants can protect tissues against the damaging effect of free radicals (22). The role played by natural compounds in the modulation of the toxic effects of lead nitrate is little known. The present demonstrates the efficacy of A. sativum in treating lead nitrate toxicity.
Lead nitrate
Our results indicate a significant alternation in the peroxidative process following lead nitrate exposure. The increase in LPO level and decrease in the endogenous antioxidant enzymes SOD, CAT, and GSH by lead nitrate (Table 1) are consistent with earlier reports (23, 24).
Cellular systems are well protected from ROS-induced cell injuries by an array of defenses composed of various antioxidants with different functions. Whenever the ROS present in the cellular system overpower these defense systems, they cause oxidative stress or cell injury, leading to the development of diseases. It has been revealed that lead toxicity leads to free radical damage via two separate pathways: (1) the generation of ROS, including hydroperoxides, singlet oxygen, and hydrogen peroxide and (2) the direct depletion of antioxidant reserves (25).
The cell membrane is the main target of the oxidative damage produced by xenobiotics, including heavy metals (26). This is mainly due to changes in polyunsaturated fatty acids having double bonds, largely present in the phospholipids of membranes (27). Lead is known to produce oxidative damage by enhancing peroxidation of membrane lipids, and LPO is a deleterious process carried out solely by free radicals. In fact, LPO is an outcome of the chain of events involving initiation, propagation, and termination reactions (28). Unchecked peroxidative decomposition of membrane lipids is catastrophic for living systems. The lipid peroxides produced are degraded into a variety of products, including alkanals, hydroxyl alkanals, ketones, and alkenes (26). All these products inactivate cell constituents by oxidation or cause oxidative stress by undergoing radical chain reactions ultimately leading to loss of membrane integrity. LPO can also adversely affect the function of membrane-bound proteins, such as enzymes and receptors. Several studies have focused on the possible toxic effects of lead on membrane components and identified a correlation between these effects and lead-induced oxidative damage. Yiin and Lin (29) demonstrated a marked enhancement in MDA concentration following incubation of linoic, linolenic, and arachidonic acid with lead. According to Caylak et al. (30), lead might have a direct peroxidative activity or act indirectly by providing conditions suitable for LPO. Direct peroxidative activity of lead may be associated with ROS generation, such as H2O2, atomic oxygen, and hydroxyl radicals (31). Usually, the deleterious effects of oxidative stress are counteracted by endogenous antioxidant enzymes, mainly SOD, CAT, and GSH (32).
In the present study, the activities of SOD, CAT, and GSH antioxidants were reduced by lead nitrate, thus exposing the tissues to peroxidative damage. CAT and SOD are metalloproteins accomplishing their antioxidant functions by enzymatically detoxifying peroxides (−OOH), H2O2, and O2·. These antioxidant enzymes depend on various essential trace elements and prosthetic groups for proper molecular structure and enzymatic activity. The pathogenesis of lead toxicity is multifactorial, as lead directly interrupts enzyme activation, competitively inhibits trace mineral absorption, and binds to sulfhydryl proteins (interrupting structural protein synthesis) (27).
New findings revealed that GSH depletion is another important mechanism of lead toxicity. GSH is a tripeptide-containing cysteine with a reactive –SH group and reductive potency. GSH is an important cellular antioxidant defense system against free radical overproduction, and decreasing its cellular concentration impairs cellular defenses against oxidative stress (33). It can act as a non-enzymatic antioxidant by direct interaction of the –SH group with ROS, or it can be involved in the enzymatic detoxification reactions for ROS as a cofactor or a coenzyme (34). It possesses carboxylic acid groups, an amino group, a –SH group, and two peptide linkages as sites for reactions of metals. Lead binds exclusively to the –SH group, which decreases the GSH levels and can interfere with the antioxidant activity of GSH (4).
Liver enzymes such as AST, ALT, ACP, and ALP are marker enzymes for liver function and integrity (35). These enzymes are usually elevated in acute hepatotoxicity or mild hepato-cellular injury, but tend to decrease with prolonged intoxication due to liver damage (36). In our study, administration of lead nitrate led to a significant rise in total cholesterol level, AST, ALT, ACP, and ALP activities, and conversely decreased total protein level (Table 2). These results are in accordance with our previous findings (37).
The increased levels of AST and ALT have been attributed to the damaged structural integrity of the liver. Lead causes cell lysis by affecting the K+–Ca2+ channels. Cytoskeleton alterations induce increased susceptibility to lysis (38).
Administration of lead nitrate also causes assimilation of fat in the liver, leading to increased ACP activity. This may be also due to the lysosomal imbalance resulting in the destruction of the intact membranes (39). ALP has been reported to be the marker enzyme for plasma membrane (40) and is required in certain amounts for proper functioning of organs (41). Increase in the ACP and ALP activities indicated the increased permeability, damage, and/or necrosis of cells.
In our study, decrease in total protein levels was observed in liver tissue, which is in agreement with El-Zayat et al. (42), who found a decrease in hepatic total protein content in response to lead intoxication. The inhibitory role of lead in protein synthesis may be due to its damaging effect on DNA and RNA (43). Lead is associated with DNA damage through base pair mutation, deletion, or oxygen radical attack on DNA. Moreover, Pb2+ disturbs intracellular Ca2+ homeostasis (44) and damages the endoplasmic reticulum, which in turn results in reduction of protein synthesis.
In the present work, lead nitrate intake increased the mean values of cholesterol significantly in liver tissue. In agreement with our data, other workers also demonstrated that lead intake is associated with significant increase in plasma cholesterol (45). It was found that administration of lead to rats elevated plasma low-density lipoprotein(LDL) and reduced plasma high-density lipoprotein (HDL) (46). Lead nitrate-mediated development of hypercholesterolemia involves the activation of cholesterol biosynthetic enzymes (i.e. 3-hydroxy-3methyglutaryl-CoA reductase, farnesyl diphosphate synthase, and squalene synthase, CYP51) and the simultaneous suppression of cholesterol-catabolic enzymes such as 7α-hydroxylase (47).
We observed that lead exposure produced pronounced hepatic histopathology evidenced by histological alternations in liver, including focal necrosis with hepatocyte vacuolization and swelling, pyknotic nuclei, and dilation of central vein and sinusoids. These findings are in support with Shalan et al. (43). In accordance with our findings, El Sokkary et al. (48) also showed that liver of lead-treated rats revealed remarkable degenerative alterations. Lead hepatotoxicity led to vacuolization of the cells, polymorphism of the nuclei, and decrease in glycogen content of the hepatocytes (49, 50).
Allium sativum
Garlic and garlic extracts, used for millennia in folk medicine, are reported to provide protection against free radical damage in the body through their antioxidant activities (51). The role of garlic organosulfur compounds in free radical scavenging has been investigated by numerous investigations. Allicin is a major component of garlic organosulfurs and its antioxidant properties has already been confirmed. In addition to allicin, other garlic organosulfurs, such as alliin, allyl cysteine, allyl disulfide, and diallyl disulfide, possess antioxidant properties and can neutralize several types of ROS (52). The consequence of synergism between various compounds is responsible for the antioxidant activity of garlic. The chemical reactions of garlic principles may be represented as follows:
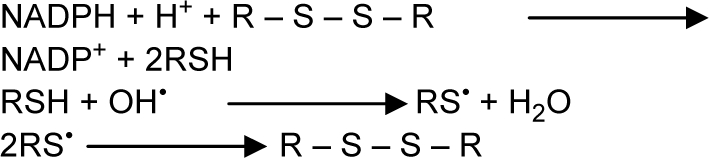
Garlic extract was found to scavenge hydroxyl radical (53), superoxide anion (53), and modulate LPO (54).
We observed that administration of A. sativum partly restored the altered levels of SOD, CAT, and GSH antioxidant molecules, which confirmed that garlic contains antioxidant compounds and protects tissues against lead nitrate-induced oxidative stress. Thiols (SH-group) are thought to play a pivotal role in protecting cells against ROS (55). Augmentation of GSH and its related enzymes is a major mechanism of garlic organosulfur hepatoprotection.
We also found that levels of liver marker enzymes (AST, ALT, ACP, and ALP) were decreased significantly by treatment with A. sativum extract. The observed decrease in these enzymes showed that garlic preserves the structural integrity of the tissues from the toxic effect of lead nitrate. Organosulfur components (as diallyl sulfide) present in garlic exhibit protective effects against toxicants (56).
Total restoration of protein levels in liver of mice treated with A. sativum extract indicated the ability of garlic extracts to stimulate the regeneration of tissues. It increased protein synthesis in damaged tissues and led to improvement in the functional status of the cells.
A. sativum treatment decreased total cholesterol level in liver. The mechanism of action was first suggested by Sodimu et al. (57), who indicated that garlic prevented an increase of cholesterol, triglyceride, and total lipids by inactivation of thiol group enzymes such as HMG-CoA reductase and CoASH, the rate-limiting enzyme for cholesterol biosynthesis, and the multi-enzyme complex for fatty acid biosynthesis.
Results from the present investigation also revealed that the use of garlic extracts along with lead nitrate can reduce lead accumulation in tissue. Sulfur-containing amino acids such as cysteine have already been reported for their chemoprophylactic use in lead toxicosis (58). The efficiency of garlic was perhaps due to the presence of these sulfur-containing amino acids and compounds having free carboxyl (C=O) and amino (NH2) groups in their structures. These biologically active compounds might have chelated lead and enhanced its excretion from the body, resulting in reduced lead accumulation in tissues. The mechanism of A. sativum-mediated chelation of lead nitrate might include formation of ionic bonds between sulfur-containing compounds and lead. Garlic components with sulfur moieties have been documented to act as active Lewis acids with electron affinity and therefore have a tendency to form compounds with positively charged ions (59). In contrast, lead is a highly electropositive metalloid exhibiting ionic states of +2 and is an active Lewis base. It thus possesses an affinity for negative ions and forms stable compounds with them. In the reaction, major organosulfur compounds, which act as oxidants (e.g. allicin and SAC) (60) are probably reduced.
Pb2+ acts as Lewis base and highly electropositive
Chelation of lead
Moreover, garlic also contains biologically active lipophilic sulfur-bearing compounds such as allicin, SAC, DADS, and DAS, and these compounds can easily permeate through phospholipid membranes (61) and reduce intracellular lead. This can explain the considerable reduction of hepatic lead burden following administration of A. sativum. Our study confirms earlier reports suggesting that garlic can reduce lead in some tissues in mice (62).
A. sativum extracts at both dose levels produced protective effects on histological structure of the liver against lead toxicity. When garlic extracts were administered with lead, the liver retained its normal architecture and was also able to diminish fibrosis, congestion, and hepatocyte vacuolation. These results are in accordance with Banerjee et al. (12) to some extent.
Conclusion
We show that garlic treatment partly mitigates lead nitrate-induced changes in hepato-chemical parameters. This could be due to its antioxidant nature, which combines free radical scavenging with metal chelating properties. The healing effect of garlic was also confirmed by histological observations, which suggest that the garlic extract was effective in bringing about functional improvement of hepatocytes.
Garlic can be given as a dietary supplement to human populations exposed to environmental toxicants and can provide protection against toxic effects without being appreciably harmful itself. Moreover, efforts are needed for the choice of appropriate dose, duration of treatment, and possible side-effects on major organs.
Acknowledgements
The authors are thankful to the authorities of Banasthali University for providing support to this study.
Conflict of interest and funding
The authors have not received any funding or benefits from industry to conduct this study.
References
- 1.Courtois E, Marques M, Barrientos A. Lead-induced down-regulation of soluble guanylate cyclase in isolated rat aortic segments mediated by reactive oxygen species and cyclo-oxygenase-2. J Am Soc Nephrol. 2003;14:1464–70. doi: 10.1097/01.asn.0000064947.14997.69. [DOI] [PubMed] [Google Scholar]
- 2.Meyer SA, Kulkarni AP. Hepatotoxicity. In: Hodgson E, Smart RC, editors. Introduction to biochemical toxicology. Vol. 3. New York: John Wiley; 2001. pp. 487–90. [Google Scholar]
- 3.Gurer H, Ercal N. Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic Biol Med. 2000;29:927–45. doi: 10.1016/s0891-5849(00)00413-5. [DOI] [PubMed] [Google Scholar]
- 4.Bechara EJH. Lead poisoning and oxidative stress. Free Radic Biol Med. 2004;36:S22. [Google Scholar]
- 5.Flora SJ, Saxena G, Gautam P, Kaur P, Gill KD. Response of lead-induced oxidative stress and alterations in biogenic amines in different rat brain regions to combined administration of DMSA and MiADMSA. Chem Biol Interact. 2007;170:209–20. doi: 10.1016/j.cbi.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Flora SJS, Saxena G, Mehta A. Reversal of lead-induced neuronal apoptosis by chelation treatment in rats: role of ROS and intracellular Ca2+ . J Pharmacol Exp Ther. 2007;322:108–16. doi: 10.1124/jpet.107.121996. [DOI] [PubMed] [Google Scholar]
- 7.Cory-Slechta DA, Weiss B, Cox C. Mobilization and redistribution of lead over the course of calcium disodium ethylene diamine tetra acetate chelation therapy. J Pharmacol Exp Ther. 1987;243:804–13. [PubMed] [Google Scholar]
- 8.Ding GS, Liang YY. Antidotal effects of dimercaptosuccinic acid. J Appl Toxicol. 1991;11:7–14. doi: 10.1002/jat.2550110103. [DOI] [PubMed] [Google Scholar]
- 9.Bosque MA, Domingo JL, Corbella J, Jones MM, Singh PK. Developmental toxicity evaluation of monoisoamyl meso-2,3-dimercaptosuccinate in mice. J Toxicol Environ Health. 1994;42:443–50. doi: 10.1080/15287399409531894. [DOI] [PubMed] [Google Scholar]
- 10.Hassan HA, El-Agmy SM, Gaur RL, Fernando A, Raj MH, Ouhtit A. In vivo evidence of hepato- and reno-protective effect of garlic oil against sodium nitrite-induced oxidative stress. Int J Biol Sci. 2009;5:249–55. doi: 10.7150/ijbs.5.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plastunov B, Zub S. Lipid peroxidation processes and antioxidant defense under lead intoxication and iodine-deficient in experiment. An Univ Mariae Curie Sklodowska Lublin-polonia. 2008;21:215–7. [Google Scholar]
- 12.Banerjee SK, Maulik M, Manchanda SC, Dinda AK, Das TK, Maulik SK. Garlic-induced alteration in rat liver and kidney morphology and associated changes in endogenous antioxidant status. Food Chem Toxicol. 2001;39:793–7. doi: 10.1016/s0278-6915(01)00018-7. [DOI] [PubMed] [Google Scholar]
- 13.Nwanjo HU, Ojiako OA. Effect of vitamins E and C on exercise induced oxidative stress. Global J Pure Appl Sci. 2005;12:199–202. [Google Scholar]
- 14.Marklund S, Marklund G. Involvement of superoxide anion radical in the autooxidation of pyrogallol and convenient assay for SOD. Eur J Biochem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 15.Aebi HE. Catalase. In: Bergmeyer HU, Bergmeyer J, Grabl M, editors. Methods of enzymatic analysis. Vol. 3. Weinheim: Velag Chemie Gmbh; 1993. pp. 273–86. [Google Scholar]
- 16.Ellman GC. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 17.Reitman S, Frankel AS. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminase. Am J Clin Pathol. 1957;28:53–6. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 18.Sadashivam S, Manickam A. Biochemical methods. Vol. 2. India: 1996. pp. 121–4. [Google Scholar]
- 19.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurements with the folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 20.Zak B. Cholesterol methodologies: a review. Clin Chem. 1977;23:1201–14. [PubMed] [Google Scholar]
- 21.Herbert LN. History of lead poisoning in the world. In: Abraham M, George, editors. Proceedings of the International Conference on Lead Poisoning, Prevention and Treatment; 8–10, February; 1999. pp. 17–25. [Google Scholar]
- 22.Osawa T, Kato Y. Protective role of antioxidative food factors in oxidative stress caused by hyperglycemia. Ann NY Acad Sci. 2005;1043:440–51. doi: 10.1196/annals.1333.050. [DOI] [PubMed] [Google Scholar]
- 23.Mohammad IK, Mahdi AA, Raviraja A, Najmul I, Iqbal A, Thuppil V. Oxidative stress in painters exposed to low lead levels. Arh Hig Rada Toksikol. 2008;59:161–9. doi: 10.2478/10004-1254-59-2008-1883. [DOI] [PubMed] [Google Scholar]
- 24.Adanaylo VN, Oteiza PI. Lead intoxication: antioxidant defenses and oxidative damage in rat brain. Toxicology. 1999;135:77–85. doi: 10.1016/s0300-483x(99)00051-7. [DOI] [PubMed] [Google Scholar]
- 25.Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress. Part 1. Mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. 2001;1:529–39. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- 26.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Oxford: Clarendon; 1989. [Google Scholar]
- 27.Slater TS. Free radical mechanism in tissue injury. Biochem J. 1985;222:1–25. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halliwell B. Mechanisms involved in the generation of free radicals. Pathol Biol. 1996;44:6–13. [PubMed] [Google Scholar]
- 29.Yiin SJ, Lin TH. Lead-catalyzed peroxidation of essential unsaturated fatty acid. Biol Trace Elem Res. 1995;50:167–72. doi: 10.1007/BF02789419. [DOI] [PubMed] [Google Scholar]
- 30.Caylak E, Halifeoiglu I, Aydin S, Telo S, Bulmus O, Celik H. The effects of sulfur-containing compounds on total antioxidant capacity levels of liver, kidney and brain in lead-exposed rats. Turkiye Klinikleri J Med Sci. 2007;27:823–8. [Google Scholar]
- 31.Ding Y, Gonick HC, Vaziri ND. Lead promotes hydroxyl radical generation and lipid peroxidation in cultured aortic endothelial cells. Am J Hypertens. 2000;13:552–5. doi: 10.1016/s0895-7061(99)00226-5. [DOI] [PubMed] [Google Scholar]
- 32.Winterbourn CC. Superoxide as an intracellular sink. Free Radic Biol Med. 1993;14:85–90. doi: 10.1016/0891-5849(93)90512-s. [DOI] [PubMed] [Google Scholar]
- 33.Dickinson DA, Moellering DR, Iles KE, Patel RP, Levonen AL, Wigley A, et al. Cytoprotection against oxidative stress and the regulation of glutathione synthesis. Biol Chem. 2003;384:527–37. doi: 10.1515/BC.2003.061. [DOI] [PubMed] [Google Scholar]
- 34.Gürer H, Ozgünes H, Neal R, Spitz DR, Erçal N. Antioxidant effects of N-acetylcysteine and succimer in red blood cells from lead exposed rats. Toxicology. 1998;128:181–9. doi: 10.1016/s0300-483x(98)00074-2. [DOI] [PubMed] [Google Scholar]
- 35.Adaramoye OA, Osaimoje DO, Akinsanya AM, Nneji CM, Fafunso MA, Ademowo OG. Changes in antioxidant status and biochemical indices after acute administration of artemether, artemether–lumefan-trine and halofantrine in rats. J Compilation Basic Clin Pharmacol Toxicol. 2008;102:412–8. doi: 10.1111/j.1742-7843.2008.00211.x. [DOI] [PubMed] [Google Scholar]
- 36.Cornelius CE. Biochemical evaluation of hepatic function in dogs. J Am Anim Hosp Assoc. 1979;15:25–9. [Google Scholar]
- 37.Sharma A, Sharma V, Kansal L. Therapeutic Effects of Allium sativum on lead-induced biochemical changes in soft tissues of Swiss albino mice. Phcog Mag. 2009;5:364–71. [Google Scholar]
- 38.Raquel ES, Ines AG, Juan LC. Lead effects on structural and functional cellular parameters in human red cells from a prenatal hematopoiesis stage. Biometals. 1997;10:331–5. doi: 10.1023/a:1018384702582. [DOI] [PubMed] [Google Scholar]
- 39.Abraham P, Wilfred G. Lysosomal enzymes in the pathogenesis of carbon tetrachloride induced injury to the testis and the rat. Ind J Pharmacol. 2000;32:250–1. [Google Scholar]
- 40.Wright PJ, Plummer DT. The use of urinary enzyme measurement to detect renal damage caused by nephritic compounds. Biochem Pharmacol. 1974;23:65–73. doi: 10.1016/0006-2952(74)90314-1. [DOI] [PubMed] [Google Scholar]
- 41.Brain RI, Kay KO. Kidney phosphatase II: the enzyme in disease. Biochem J. 1927;21:1104–8. doi: 10.1042/bj0211104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Zayat EM, El-Ymany NA, Kamel ZH. Combined supplementation of zinc and vitamin C as protective agents against lead toxicity in growing male albino rats. 1 liver functions. J Egypt Ger Soc Zool. 1996;20:115–39. [Google Scholar]
- 43.Shalan MG, Mostafa MS, Hassouna MM, El-Nabi SE, El-Refaie A. Amelioration of lead toxicity on rat liver with vitamin C and silymarin supplements. Toxicology. 2005;206:1–15. doi: 10.1016/j.tox.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 44.Simons T. Lead–calcium interactions in cellular lead toxicity. Neurotoxicology. 1993;14:77–86. [PubMed] [Google Scholar]
- 45.Kasperczyk S, Birkner E, Kasperczyk A, Kasperczyk J. Lipids, lipid peroxidation and 7-ketocholesterol in workers exposed to lead. Hum Exp Toxicol. 2005;24:287–95. doi: 10.1191/0960327105ht528oa. [DOI] [PubMed] [Google Scholar]
- 46.Bashandy SA. Beneficial effect of combined administration of vitamin C and vitamin E in amelioration of chronic lead hepatotoxicity. Egyp J Hosp Med. 2006;23:371–84. [Google Scholar]
- 47.Kojima M, Nemoto K, Murai U, Yoshimura N, Ayabe Y, Degawa M. Altered gene expression of hepatic lanosterol 14x-demethylase (CYP51) in lead nitrate-treated rats. Arch Toxicol. 2002;76:398–403. doi: 10.1007/s00204-002-0365-3. [DOI] [PubMed] [Google Scholar]
- 48.El Sokkary GH, Abdel-Rahman GH, Kamel ES. Melatonin protects against lead induced hepatic and renal toxicity in male rats. Toxicology. 2005;231:25–33. doi: 10.1016/j.tox.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Foulkes EC. Metals and biological membranes. In: Chang L, editor. Toxicology of metals. Boca Raton, FL: CRC; 1996. pp. 133–43. [Google Scholar]
- 50.Pereira R, Pereira ML, Ribeiro R, Goncalves F. Wildlife animals as sentinels to human health due to environmental exposure to heavy metals; Abstract Book. Proceeding of the 11th annual meeting of Europe Society Environmental Toxicology and Chemistry; 6–10 May 2001; Madrid. Europe: SETAC; [Google Scholar]
- 51.Omurtag GZ, Güranlioglu FD, Sehirli O, Arbak S, Uslu B, Gedik N, et al. Protective effect of aqueous garlic extract against naphthalene-induced oxidative stress in mice. J Pharm Pharmacol. 2005;57:623–30. doi: 10.1211/0022357055939. [DOI] [PubMed] [Google Scholar]
- 52.Chung LY. The antioxidant properties of garlic compounds: allyl cysteine, alliin, allicin, and allyl disulfide. J Med Food. 2006;9:205–13. doi: 10.1089/jmf.2006.9.205. [DOI] [PubMed] [Google Scholar]
- 53.Kim KM, Chun SB, Koo MS. Differential regulation of NO availability from macrophages and endothelial cells by the garlic components S-allyl cystiene. Free Radic Biol Med. 2001;30:747–56. doi: 10.1016/s0891-5849(01)00460-9. [DOI] [PubMed] [Google Scholar]
- 54.Saravanan G, Prakash J. Effect of garlic (Allium sativum) on lipid peroxidation in experimental myocardial infarction in rats. J Ethinopharmacol. 2004;94:155–8. doi: 10.1016/j.jep.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 55.Fatma MEl, Mokhtar IY, Fatma MER. Ameliorating effect of curcumin on sodium arsenite-induced oxidative damage and lipid peroxidation in different rat organs. Food ChemToxicol. 2009;47:249–54. doi: 10.1016/j.fct.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 56.Kwak MK, Kim SG, Kwak JY, Novak RF, Kim ND. Inhibition of cytochrome P4502E1 expression by organosulfur compounds allylsulfide, allylmercaptan and allylmethyl-sulfide in rats. Biochem Pharmacol. 1993;47:531–9. doi: 10.1016/0006-2952(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 57.Sodimu O, Joseph PK, Augusti KT. Certain biochemical effects of garlic oil on rats maintained on high fat–high cholesterol diet. Experientia. 1984;40:78–80. doi: 10.1007/BF01959111. [DOI] [PubMed] [Google Scholar]
- 58.Tandon SK, Sharma BC, Singh S. Chelation in metal intoxication XXVII: chelating agents containing vicinal thioether groups as antidotes of lead toxicity. Drug Chem Toxicol. 1988;11:71–84. doi: 10.3109/01480548809038657. [DOI] [PubMed] [Google Scholar]
- 59.Block E. The chemistry of garlic and onions. Sci Am. 1985;252:114–9. doi: 10.1038/scientificamerican0385-114. [DOI] [PubMed] [Google Scholar]
- 60.Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive components. J Nutr. 2001;131:955–62. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]
- 61.Miron T, Rabinkov A, Mirelman D, Miron T, Rabinkov A, Mirelman D, Wilchek M, Weiner L. The mode of action of allicin: its ready permeability through phospholipid membranes may contribute to its biological activity. Biochim Biophys Acta. 2000;1463:20–30. doi: 10.1016/s0005-2736(99)00174-1. [DOI] [PubMed] [Google Scholar]
- 62.Badiei K, Pourjaafar M, Nowrooziasl A. Effect of dried garlic powder Allium sativum on lead content of different tissues following subclinical lead poisoning in goats. Ira J Vet Res. 2005;6:10–4. [Google Scholar]