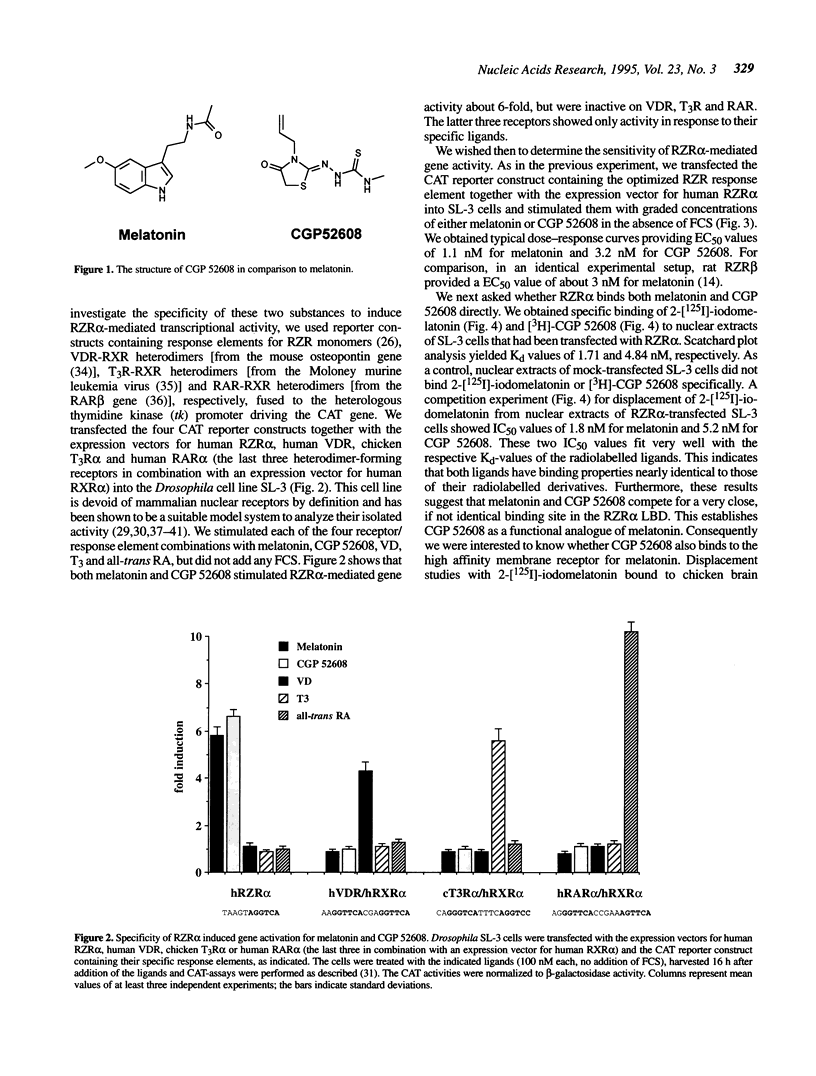
Abstract
Many important physiological functions are controlled by hormones via binding and activating members of the nuclear receptor superfamily. This group of structurally related transcription factors also includes a still growing number of orphan receptors for which no ligand is known so far. The identification of ligands for orphan receptors is a key to understanding their physiological role, as has been successfully shown for retinoid X receptors and the discovery of 9-cis retinoic acid as a specific ligand. We have discovered very recently that the pineal gland hormone melatonin is a specific ligand for the brain-specific nuclear receptor RZR beta. Here we report that the alpha-subtype of RZR, RZR alpha and its splicing variant ROR alpha 1, is also a nuclear receptor for melatonin with binding specificities in the low nanomolar range. In contrast to RZR beta, RZR/ROR alpha is expressed in many tissues and cells outside the brain. We found that RZR alpha and ROR alpha 1 vary in their constitutive transactivational activity and are activated to a different extent by melatonin. Furthermore, we identified a synthetic RZR-ligand, the thiazolidine dione CGP 52608. This compound is a functional analogue of melatonin at its nuclear receptor, but does not bind to the high affinity membrane receptor for melatonin. Therefore, this specific RZR-ligand may help to differentiate between nuclear and membrane signalling of melatonin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acuña-Castroviejo D., Pablos M. I., Menendez-Pelaez A., Reiter R. J. Melatonin receptors in purified cell nuclei of liver. Res Commun Chem Pathol Pharmacol. 1993 Nov;82(2):253–256. [PubMed] [Google Scholar]
- Becker-André M., André E., DeLamarter J. F. Identification of nuclear receptor mRNAs by RT-PCR amplification of conserved zinc-finger motif sequences. Biochem Biophys Res Commun. 1993 Aug 16;194(3):1371–1379. doi: 10.1006/bbrc.1993.1976. [DOI] [PubMed] [Google Scholar]
- Becker-André M., Wiesenberg I., Schaeren-Wiemers N., André E., Missbach M., Saurat J. H., Carlberg C. Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J Biol Chem. 1994 Nov 18;269(46):28531–28534. [PubMed] [Google Scholar]
- Blask D. E., Hill S. M. Effects of melatonin on cancer: studies on MCF-7 human breast cancer cells in culture. J Neural Transm Suppl. 1986;21:433–449. [PubMed] [Google Scholar]
- Carlberg C., Bendik I., Wyss A., Meier E., Sturzenbecker L. J., Grippo J. F., Hunziker W. Two nuclear signalling pathways for vitamin D. Nature. 1993 Feb 18;361(6413):657–660. doi: 10.1038/361657a0. [DOI] [PubMed] [Google Scholar]
- Carlberg C., Hooft van Huijsduijnen R., Staple J. K., DeLamarter J. F., Becker-André M. RZRs, a new family of retinoid-related orphan receptors that function as both monomers and homodimers. Mol Endocrinol. 1994 Jun;8(6):757–770. doi: 10.1210/mend.8.6.7935491. [DOI] [PubMed] [Google Scholar]
- Carlberg C. RXR-independent action of the receptors for thyroid hormone, retinoid acid and vitamin D on inverted palindromes. Biochem Biophys Res Commun. 1993 Sep 30;195(3):1345–1353. doi: 10.1006/bbrc.1993.2191. [DOI] [PubMed] [Google Scholar]
- Carlberg C., Saurat J. H., Siegenthaler G. 9-cis-retinoic acid is a natural antagonist for the retinoic acid receptor response pathway. Biochem J. 1993 Oct 15;295(Pt 2):343–346. doi: 10.1042/bj2950343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubocovich M. L., Shankar G., Mickel M. 2-[125I]iodomelatonin labels sites with identical pharmacological characteristics in chicken brain and chicken retina. Eur J Pharmacol. 1989 Mar 21;162(2):289–299. doi: 10.1016/0014-2999(89)90292-6. [DOI] [PubMed] [Google Scholar]
- Ebisawa T., Karne S., Lerner M. R., Reppert S. M. Expression cloning of a high-affinity melatonin receptor from Xenopus dermal melanophores. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6133–6137. doi: 10.1073/pnas.91.13.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman B. M., Chen J., Blumberg B., Kliewer S. A., Henshaw R., Ong E. S., Evans R. M. Cross-talk among ROR alpha 1 and the Rev-erb family of orphan nuclear receptors. Mol Endocrinol. 1994 Sep;8(9):1253–1261. doi: 10.1210/mend.8.9.7838158. [DOI] [PubMed] [Google Scholar]
- Giguère V., Tini M., Flock G., Ong E., Evans R. M., Otulakowski G. Isoform-specific amino-terminal domains dictate DNA-binding properties of ROR alpha, a novel family of orphan hormone nuclear receptors. Genes Dev. 1994 Mar 1;8(5):538–553. doi: 10.1101/gad.8.5.538. [DOI] [PubMed] [Google Scholar]
- Glass C. K. Differential recognition of target genes by nuclear receptor monomers, dimers, and heterodimers. Endocr Rev. 1994 Jun;15(3):391–407. doi: 10.1210/edrv-15-3-391. [DOI] [PubMed] [Google Scholar]
- Heyman R. A., Mangelsdorf D. J., Dyck J. A., Stein R. B., Eichele G., Evans R. M., Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992 Jan 24;68(2):397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- Hill S. M., Blask D. E. Effects of the pineal hormone melatonin on the proliferation and morphological characteristics of human breast cancer cells (MCF-7) in culture. Cancer Res. 1988 Nov 1;48(21):6121–6126. [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Mangelsdorf D. J., Evans R. M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992 Jan 30;355(6359):446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D. N., Dubocovich M. L. Melatonin receptors. Annu Rev Pharmacol Toxicol. 1991;31:549–568. doi: 10.1146/annurev.pa.31.040191.003001. [DOI] [PubMed] [Google Scholar]
- Laudet V., Hänni C., Coll J., Catzeflis F., Stéhelin D. Evolution of the nuclear receptor gene superfamily. EMBO J. 1992 Mar;11(3):1003–1013. doi: 10.1002/j.1460-2075.1992.tb05139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J. M., Zhang X. K., Graupner G., Lee M. O., Hermann T., Hoffmann B., Pfahl M. Formation of retinoid X receptor homodimers leads to repression of T3 response: hormonal cross talk by ligand-induced squelching. Mol Cell Biol. 1993 Dec;13(12):7698–7707. doi: 10.1128/mcb.13.12.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Levin A. A., Sturzenbecker L. J., Kazmer S., Bosakowski T., Huselton C., Allenby G., Speck J., Kratzeisen C., Rosenberger M., Lovey A. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature. 1992 Jan 23;355(6358):359–361. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Ong E. S., Dyck J. A., Evans R. M. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990 May 17;345(6272):224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- Menendez-Pelaez A., Poeggeler B., Reiter R. J., Barlow-Walden L., Pablos M. I., Tan D. X. Nuclear localization of melatonin in different mammalian tissues: immunocytochemical and radioimmunoassay evidence. J Cell Biochem. 1993 Dec;53(4):373–382. doi: 10.1002/jcb.240530415. [DOI] [PubMed] [Google Scholar]
- Morgan P. J., Barrett P., Howell H. E., Helliwell R. Melatonin receptors: localization, molecular pharmacology and physiological significance. Neurochem Int. 1994 Feb;24(2):101–146. doi: 10.1016/0197-0186(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Noda M., Vogel R. L., Craig A. M., Prahl J., DeLuca H. F., Denhardt D. T. Identification of a DNA sequence responsible for binding of the 1,25-dihydroxyvitamin D3 receptor and 1,25-dihydroxyvitamin D3 enhancement of mouse secreted phosphoprotein 1 (SPP-1 or osteopontin) gene expression. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9995–9999. doi: 10.1073/pnas.87.24.9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley B. W., Conneely O. M. Orphan receptors: in search of a unifying hypothesis for activation. Mol Endocrinol. 1992 Sep;6(9):1359–1361. doi: 10.1210/mend.6.9.1331771. [DOI] [PubMed] [Google Scholar]
- Pothier F., Ouellet M., Julien J. P., Guérin S. L. An improved CAT assay for promoter analysis in either transgenic mice or tissue culture cells. DNA Cell Biol. 1992 Jan-Feb;11(1):83–90. doi: 10.1089/dna.1992.11.83. [DOI] [PubMed] [Google Scholar]
- Power R. F., Conneely O. M., O'Malley B. W. New insights into activation of the steroid hormone receptor superfamily. Trends Pharmacol Sci. 1992 Aug;13(8):318–323. doi: 10.1016/0165-6147(92)90099-r. [DOI] [PubMed] [Google Scholar]
- Reiter R. J. The melatonin rhythm: both a clock and a calendar. Experientia. 1993 Aug 15;49(8):654–664. doi: 10.1007/BF01923947. [DOI] [PubMed] [Google Scholar]
- Retnakaran R., Flock G., Giguère V. Identification of RVR, a novel orphan nuclear receptor that acts as a negative transcriptional regulator. Mol Endocrinol. 1994 Sep;8(9):1234–1244. doi: 10.1210/mend.8.9.7838156. [DOI] [PubMed] [Google Scholar]
- Rubio A., Menendez-Pelaez A., Reiter R. J. Thyroxine 5'-deiodinase type II activity in chick pineal and Harderian gland: nyctohemeral rhythmicity and its regulation by noradrenergic input. J Pineal Res. 1993 Mar;14(2):53–59. doi: 10.1111/j.1600-079x.1993.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Schmitt J., Stunnenberg H., Vennström B. Repression of transcription mediated at a thyroid hormone response element by the v-erb-A oncogene product. Nature. 1989 Jul 20;340(6230):242–244. doi: 10.1038/340242a0. [DOI] [PubMed] [Google Scholar]
- Schräder M., Becker-André M., Carlberg C. Thyroid hormone receptor functions as monomeric ligand-induced transcription factor on octameric half-sites. Consequences also for dimerization. J Biol Chem. 1994 Mar 4;269(9):6444–6449. [PubMed] [Google Scholar]
- Schräder M., Bendik I., Becker-André M., Carlberg C. Interaction between retinoic acid and vitamin D signaling pathways. J Biol Chem. 1993 Aug 25;268(24):17830–17836. [PubMed] [Google Scholar]
- Schräder M., Müller K. M., Becker-André M., Carlberg C. Response element selectivity for heterodimerization of vitamin D receptors with retinoic acid and retinoid X receptors. J Mol Endocrinol. 1994 Jun;12(3):327–339. doi: 10.1677/jme.0.0120327. [DOI] [PubMed] [Google Scholar]
- Schräder M., Müller K. M., Carlberg C. Specificity and flexibility of vitamin D signaling. Modulation of the activation of natural vitamin D response elements by thyroid hormone. J Biol Chem. 1994 Feb 25;269(8):5501–5504. [PubMed] [Google Scholar]
- Schräder M., Wyss A., Sturzenbecker L. J., Grippo J. F., LeMotte P., Carlberg C. RXR-dependent and RXR-independent transactivation by retinoic acid receptors. Nucleic Acids Res. 1993 Mar 11;21(5):1231–1237. doi: 10.1093/nar/21.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D. J., Sharp P. A., Wahli W. W., Keller M. J. A high-efficiency HeLa cell nuclear transcription extract. DNA. 1988 Jan-Feb;7(1):47–55. doi: 10.1089/dna.1988.7.47. [DOI] [PubMed] [Google Scholar]
- Sugden D. Melatonin: binding site characteristics and biochemical and cellular responses. Neurochem Int. 1994 Feb;24(2):147–157. doi: 10.1016/0197-0186(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Yu V. C., Delsert C., Andersen B., Holloway J. M., Devary O. V., När A. M., Kim S. Y., Boutin J. M., Glass C. K., Rosenfeld M. G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991 Dec 20;67(6):1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Hoffmann B., Tran P. B., Graupner G., Pfahl M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature. 1992 Jan 30;355(6359):441–446. doi: 10.1038/355441a0. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Lehmann J., Hoffmann B., Dawson M. I., Cameron J., Graupner G., Hermann T., Tran P., Pfahl M. Homodimer formation of retinoid X receptor induced by 9-cis retinoic acid. Nature. 1992 Aug 13;358(6387):587–591. doi: 10.1038/358587a0. [DOI] [PubMed] [Google Scholar]
- de Thé H., Vivanco-Ruiz M. M., Tiollais P., Stunnenberg H., Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990 Jan 11;343(6254):177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]




