Abstract
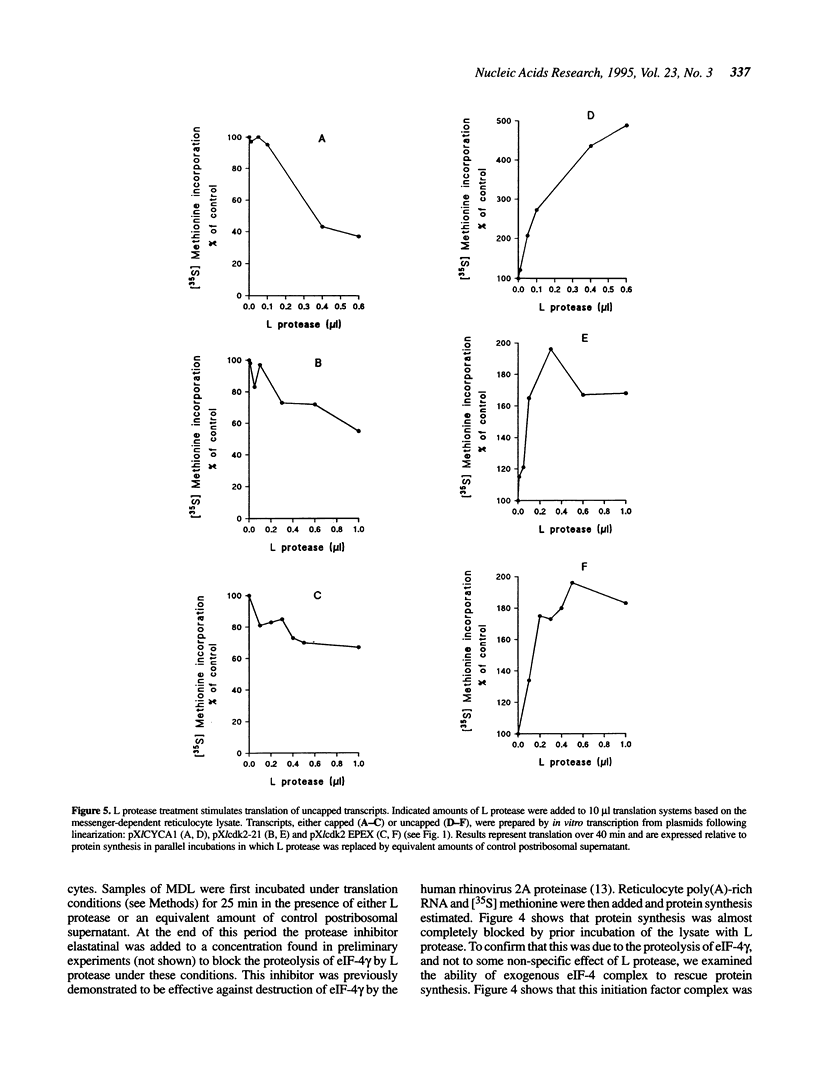
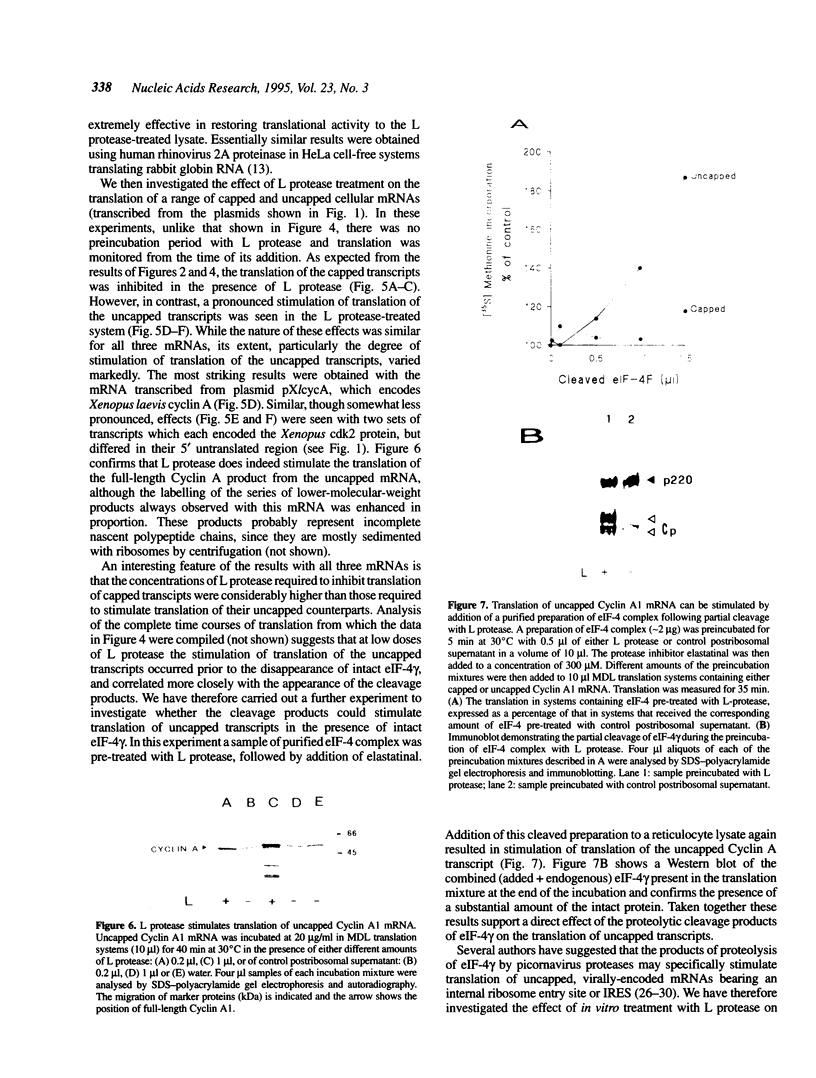
Infection of cells with the foot-and-mouth-disease virus, a member of the picornavirus family, results in the shut-off of host protein synthesis. A major contributory mechanism is the proteolytic destruction of the gamma subunit of the complex eIF-4, which functions in translation to promote the binding of the 43S ribosomal preinitiation complex to the 5' end of the cellular mRNA molecules bearing a 5' terminal cap structure. Picornavirus RNA molecules, which are uncapped, use a distinct mechanism for translational initiation, which can operate in the absence, or at low levels, of eIF-4. The proteolysis of eIF-4 gamma in cells infected by foot-and-mouth-disease virus results from expression of a virus-encoded cysteine proteinase known as Leader (or L) protease. We have used a transcription plasmid encoding this protease as a tool to deplete in vitro translation systems of eIF-4 gamma in order to elucidate in more detail the role of this polypeptide in the control of translation. Using in vitro transcribed mRNAs we have observed a marked contrast between capped and uncapped transcripts in the response of their translation to the proteolysis of eIF-4 gamma. Translation of capped mRNAs is, as expected, severely impaired, and is restored by addition of eIF-4 complex containing the intact gamma-subunit. On the other hand, translation of uncapped transcripts, normally inefficient, is substantially enhanced. The data suggest that the translation of uncapped mRNAs may be stimulated in this system by one or more of the proteolytic degradation products of eIF-4 gamma.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belsham G. J., Brangwyn J. K. A region of the 5' noncoding region of foot-and-mouth disease virus RNA directs efficient internal initiation of protein synthesis within cells: involvement with the role of L protease in translational control. J Virol. 1990 Nov;64(11):5389–5395. doi: 10.1128/jvi.64.11.5389-5395.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman A., Jackson R. J. Initiation of translation of human rhinovirus RNA: mapping the internal ribosome entry site. Virology. 1992 Jun;188(2):685–696. doi: 10.1016/0042-6822(92)90523-r. [DOI] [PubMed] [Google Scholar]
- Bu X., Haas D. W., Hagedorn C. H. Novel phosphorylation sites of eukaryotic initiation factor-4F and evidence that phosphorylation stabilizes interactions of the p25 and p220 subunits. J Biol Chem. 1993 Mar 5;268(7):4975–4978. [PubMed] [Google Scholar]
- Buckley B., Ehrenfeld E. The cap-binding protein complex in uninfected and poliovirus-infected HeLa cells. J Biol Chem. 1987 Oct 5;262(28):13599–13606. [PubMed] [Google Scholar]
- Devaney M. A., Vakharia V. N., Lloyd R. E., Ehrenfeld E., Grubman M. J. Leader protein of foot-and-mouth disease virus is required for cleavage of the p220 component of the cap-binding protein complex. J Virol. 1988 Nov;62(11):4407–4409. doi: 10.1128/jvi.62.11.4407-4409.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison D., Milburn S. C., Edery I., Sonenberg N., Hershey J. W. Inhibition of HeLa cell protein synthesis following poliovirus infection correlates with the proteolysis of a 220,000-dalton polypeptide associated with eucaryotic initiation factor 3 and a cap binding protein complex. J Biol Chem. 1982 Dec 25;257(24):14806–14810. [PubMed] [Google Scholar]
- Hambidge S. J., Sarnow P. Translational enhancement of the poliovirus 5' noncoding region mediated by virus-encoded polypeptide 2A. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10272–10276. doi: 10.1073/pnas.89.21.10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- Kirchweger R., Ziegler E., Lamphear B. J., Waters D., Liebig H. D., Sommergruber W., Sobrino F., Hohenadl C., Blaas D., Rhoads R. E. Foot-and-mouth disease virus leader proteinase: purification of the Lb form and determination of its cleavage site on eIF-4 gamma. J Virol. 1994 Sep;68(9):5677–5684. doi: 10.1128/jvi.68.9.5677-5684.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Nicklin M. J., Toyoda H., Etchison D., Wimmer E. Poliovirus proteinase 2A induces cleavage of eucaryotic initiation factor 4F polypeptide p220. J Virol. 1987 Sep;61(9):2711–2718. doi: 10.1128/jvi.61.9.2711-2718.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamphear B. J., Panniers R. Cap binding protein complex that restores protein synthesis in heat-shocked Ehrlich cell lysates contains highly phosphorylated eIF-4E. J Biol Chem. 1990 Apr 5;265(10):5333–5336. [PubMed] [Google Scholar]
- Lamphear B. J., Yan R., Yang F., Waters D., Liebig H. D., Klump H., Kuechler E., Skern T., Rhoads R. E. Mapping the cleavage site in protein synthesis initiation factor eIF-4 gamma of the 2A proteases from human Coxsackievirus and rhinovirus. J Biol Chem. 1993 Sep 15;268(26):19200–19203. [PubMed] [Google Scholar]
- Liebig H. D., Ziegler E., Yan R., Hartmuth K., Klump H., Kowalski H., Blaas D., Sommergruber W., Frasel L., Lamphear B. Purification of two picornaviral 2A proteinases: interaction with eIF-4 gamma and influence on in vitro translation. Biochemistry. 1993 Jul 27;32(29):7581–7588. doi: 10.1021/bi00080a033. [DOI] [PubMed] [Google Scholar]
- Lloyd R. E., Grubman M. J., Ehrenfeld E. Relationship of p220 cleavage during picornavirus infection to 2A proteinase sequencing. J Virol. 1988 Nov;62(11):4216–4223. doi: 10.1128/jvi.62.11.4216-4223.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macadam A. J., Ferguson G., Fleming T., Stone D. M., Almond J. W., Minor P. D. Role for poliovirus protease 2A in cap independent translation. EMBO J. 1994 Feb 15;13(4):924–927. doi: 10.1002/j.1460-2075.1994.tb06336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina M., Domingo E., Brangwyn J. K., Belsham G. J. The two species of the foot-and-mouth disease virus leader protein, expressed individually, exhibit the same activities. Virology. 1993 May;194(1):355–359. doi: 10.1006/viro.1993.1267. [DOI] [PubMed] [Google Scholar]
- Merrick W. C. Mechanism and regulation of eukaryotic protein synthesis. Microbiol Rev. 1992 Jun;56(2):291–315. doi: 10.1128/mr.56.2.291-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J., Golsteyn R., Hill C. S., Hunt T. The A- and B-type cyclin associated cdc2 kinases in Xenopus turn on and off at different times in the cell cycle. EMBO J. 1990 Sep;9(9):2865–2875. doi: 10.1002/j.1460-2075.1990.tb07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley S. J., Dever T. E., Etchison D., Traugh J. A. Phosphorylation of eIF-4F by protein kinase C or multipotential S6 kinase stimulates protein synthesis at initiation. J Biol Chem. 1991 Mar 15;266(8):4669–4672. [PubMed] [Google Scholar]
- Morley S. J., Rau M., Kay J. E., Pain V. M. Increased phosphorylation of eukaryotic initiation factor 4 alpha during early activation of T lymphocytes correlates with increased initiation factor 4F complex formation. Eur J Biochem. 1993 Nov 15;218(1):39–48. doi: 10.1111/j.1432-1033.1993.tb18349.x. [DOI] [PubMed] [Google Scholar]
- Morley S. J. Signal transduction mechanisms in the regulation of protein synthesis. Mol Biol Rep. 1994 May;19(3):221–231. doi: 10.1007/BF00986964. [DOI] [PubMed] [Google Scholar]
- Pause A., Méthot N., Svitkin Y., Merrick W. C., Sonenberg N. Dominant negative mutants of mammalian translation initiation factor eIF-4A define a critical role for eIF-4F in cap-dependent and cap-independent initiation of translation. EMBO J. 1994 Mar 1;13(5):1205–1215. doi: 10.1002/j.1460-2075.1994.tb06370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheper G. C., Voorma H. O., Thomas A. A. Eukaryotic initiation factors-4E and -4F stimulate 5' cap-dependent as well as internal initiation of protein synthesis. J Biol Chem. 1992 Apr 15;267(11):7269–7274. [PubMed] [Google Scholar]
- Sommergruber W., Ahorn H., Klump H., Seipelt J., Zoephel A., Fessl F., Krystek E., Blaas D., Kuechler E., Liebig H. D. 2A proteinases of coxsackie- and rhinovirus cleave peptides derived from eIF-4 gamma via a common recognition motif. Virology. 1994 Feb;198(2):741–745. doi: 10.1006/viro.1994.1089. [DOI] [PubMed] [Google Scholar]
- Sonenberg N. Measures and countermeasures in the modulation of initiation factor activities by viruses. New Biol. 1990 May;2(5):402–409. [PubMed] [Google Scholar]
- Thomas A. A., Scheper G. C., Kleijn M., De Boer M., Voorma H. O. Dependence of the adenovirus tripartite leader on the p220 subunit of eukaryotic initiation factor 4F during in vitro translation. Effect of p220 cleavage by foot-and-mouth-disease-virus L-protease on in vitro translation. Eur J Biochem. 1992 Jul 15;207(2):471–477. doi: 10.1111/j.1432-1033.1992.tb17073.x. [DOI] [PubMed] [Google Scholar]
- Whetter L. E., Day S. P., Elroy-Stein O., Brown E. A., Lemon S. M. Low efficiency of the 5' nontranslated region of hepatitis A virus RNA in directing cap-independent translation in permissive monkey kidney cells. J Virol. 1994 Aug;68(8):5253–5263. doi: 10.1128/jvi.68.8.5253-5263.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff E. E., Hershey J. W., Ehrenfeld E. Eukaryotic initiation factor 3 is required for poliovirus 2A protease-induced cleavage of the p220 component of eukaryotic initiation factor 4F. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9529–9533. doi: 10.1073/pnas.87.24.9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R., Rychlik W., Etchison D., Rhoads R. E. Amino acid sequence of the human protein synthesis initiation factor eIF-4 gamma. J Biol Chem. 1992 Nov 15;267(32):23226–23231. [PubMed] [Google Scholar]