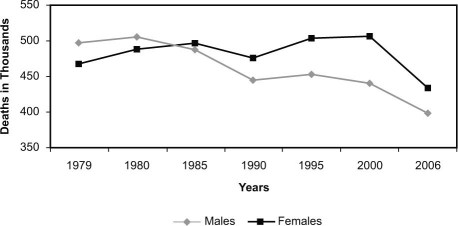
Historians will mark the past 50 years as a period of extraordinary progress in the understanding of cardiovascular disease (CVD), its treatment, and its prevention—spurred initially by an alarming epidemic of coronary artery disease in young American men in the middle of the 20th century. Public Health Service data later showed a 59% reduction in overall coronary death rates from 1950 to 1999, yet during that era the CVD mortality rate of women overtook that of men and continued to rise. Cardiovascular disease remains the most common cause of death in the United States today for both sexes.1,2 Women have not shared equally in the medical revolution: up to the turn of the millennium, women experienced adverse trending rather than improvement in mortality trends, as shown in Figure 1.3 In this article, we will examine the reasons for this disparity and consider how women's health is dependent upon research programs that allocate both the burdens and benefits of cardiovascular research equally.
Fig. 1 Cardiovascular Disease Mortality Trends for Males and Females in the United States: 1979–2006. Reproduced with permission.3
Cardiovascular disease is an equal-opportunity killer. Nonetheless, the typical CVD clinical trial comprises a population that is 85% male; those women who do participate are predominantly postmenopausal. Consider the Coronary Drug Project, the 1st major clinical trial funded in 1965 by the National Heart Institute, an antecedent of the National Heart, Lung, and Blood Institute.4 The Framingham population had shown a 10-year lag in female cardiovascular morbidity and mortality rates relative to those of men, a lag that was punctuated by the age of menopause and suggestive of a protective effect of estrogen.5 The ambitious Coronary Drug Project therefore included a randomized controlled trial of estrogen for the secondary prevention of coronary disease in an exclusively male post-myocardial infarction population. Although enrollment into that limb was curtailed early due to a higher mortality rate in the intervention group, liberal postmenopausal administration of estrogen to women continued thereafter, on the sole basis of observational data in women. Those early observational studies showed lower rates of coronary disease in postmenopausal users of estrogen replacement than in nonusers, but they distinguished poorly between causality and selection bias in subject populations. Lifestyle and other socioeconomic factors might have biased the data. Randomized controlled trials to formally test the hypothesis in women were not undertaken until 3 decades after the Coronary Drug Project. These largely took the form of the Women's Health Initiative and ultimately showed not only lack of benefit from estrogen replacement therapy, but actual risk.6 Thus, for 3 decades, futile and sometimes harmful hormonal therapy was widely prescribed to women, on the basis of flawed clinical-trial methodology.
Why were randomized controlled trials systematically avoided in women in the 20th century? Perhaps the most proximate cause was the contemporary revelation of horrific birth defects in children exposed to thalidomide in utero. The recognition that pharmaceutical intervention in pregnant women could result in fetal injury chilled testing of investigational new drugs in women of childbearing potential and laid the legislative groundwork for the modern U.S. Food and Drug Administration (FDA). It was also a contributing factor in the design of the Belmont Report, which provided the modern framework for the protection of human research subjects. Although neither endeavor proscribed research in women of reproductive potential, the clear recognition of fetal risk established a paternalistic approach to the design of clinical trials of the day. The uncertain effectiveness of the contraceptives of that era did not engender confidence in the ability to avoid fetal exposure to investigational products; however, FDA-approved drugs continued to be prescribed for premenopausal women, on the basis of testing in other populations.
Is the human male an appropriate surrogate for women in clinical trials? Obvious differences in size, anatomy, and hormones result in demonstrable gender differences in physiology, pharmacokinetics, and pharmacodynamics. Yet, enrollment of a gender-balanced study population in CVD in sufficient numbers to provide subgroup analysis by gender increases the cost and duration of randomized controlled trials. Furthermore, the female population is less homogeneous than the male, for women experience cyclical hormonal effects on metabolism and the cardiovascular system during reproductive years. Because of the heterogeneity in hormonal status among female subjects, one could argue that simple parity in enrollment in a clinical trial might be insufficient for adequate subgroup analysis. Clinical CVD trial enrollment might be further unbalanced by the ages of the participants, because later disease onset, later symptom recognition, or both are typical in affected women. Randomized controlled trials that exclude subjects over the age of 80 to 85 years are systematically biased against female enrollment.
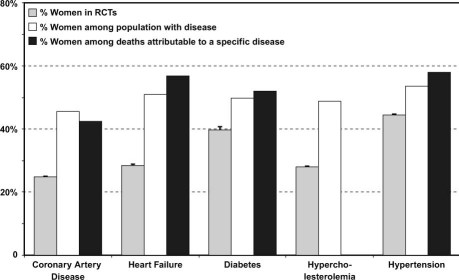
Despite the remedial effort of the Women's Health Initiative to overcome gender disparities in CVD research, women continue to constitute a minority of study populations, as shown in Figure 2.7–9 A 1992 General Accounting Office (GAO) Report on Women's Health found extensive underrepresentation of women in all phases of drug trials and systematic exclusion of women of childbearing potential. The follow-up GAO report in 2001 showed some interim improvement insofar as women accounted for 52% of enrollment in investigational new drug trials, but their participation in Phase I and II studies was only 22%. These early investigational new drug trials, which serve the crucial role of dose-ranging and initial safety testing, become the basis for dosing in pivotal clinical trials and prescription guidelines once a drug is approved. Underrepresentation of women early in product development can result in unrecognized toxicity, ineffectiveness, drug interactions, and unexpected outcomes when the product is made available to larger populations. In this manner, women treated (after FDA approval) on the basis of dose-ranging trials that were performed predominantly in men and postmenopausal women can bear the onus of untested therapy—the outcome of which is detected only during the course of post-marketing surveillance. Examples of such missed opportunities include the variant outcomes by gender in acute coronary syndrome trials and the greater susceptibility of women in general to drug-related QT prolongation.
Fig. 2 Percentages of women in randomized controlled trials (RCTs), compared with the percentages of women among the populations with given diseases and the percentages of women among deaths attributable to those diseases. The upper 95th percentile of the confidence interval for the proportion of women in RCTs is shown by the whisker. Hyperlipidemia is defined as low-density-lipoprotein cholesterol >130 mg/dL. The proportion of women among deaths due to hyperlipidemia is not reported in Heart Disease and Stroke Statistics (American Heart Association). Reproduced with permission.9
In order to share in both the individual and the community rewards of cardiovascular research, women must also contribute to the research process. The principle of distributive justice mandates that both the burdens and benefits be fairly allocated, in such a manner that the population tested is representative of the population affected by the disease. Women in their reproductive years and even pregnant women do experience cardiovascular illness and require treatment. We still lack adequate information on how to apply current standards of care to younger women, in particular. With careful methodology, women of reproductive potential and even those who are pregnant should be included in trials of interventions for illnesses that affect them and will continue to affect women in generations to come. Without sharing the risks of participation in well-designed, randomized, controlled trials, they cannot share in their rewards. In 2006, women accounted for 52.1% of CVD deaths.3 It is time for research efforts to mirror the impact of CVD on women, so that we can address ourselves equitably to prevention and treatment.
Footnotes
Address for reprints: Anne Hamilton Dougherty, MD, FACC, Vice President, Human Research Programs; and Director, Cardiac Electrophysiology, UT Health Science Center, 6431 Fannin St., MSB 1.246, Houston, TX 77030. E-mail: Anne.H.Dougherty@uth.tmc.edu
Presented at the Risk, Diagnosis and Treatment of Cardiovascular Disease in Women symposium; Denton A. Cooley Auditorium, Texas Heart Institute, Houston; 11 September 2010.
References
- 1.Fox CS, Evans JC, Larson MG, Kannel WB, Levy D. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: the Framingham Heart Study. Circulation 2004;110(5):522–7. [DOI] [PubMed]
- 2.Writing Group Members, Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, et al. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 2010;121(7):e46–e215. [DOI] [PubMed]
- 3.Women and Cardiovascular Diseases – Statistics [homepage on the Internet; cited 2011 Mar 8]. Available from: http://www.americanheart.org/downloadable/heart/1260905040318FS10WM10.pdf.
- 4.The Coronary Drug Project. Findings leading to discontinuation of the 2.5-mg day estrogen group. The coronary Drug Project Research Group. JAMA 1973;226(6):652–7. [PubMed]
- 5.Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J 1986;111(2):383–90. [DOI] [PubMed]
- 6.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998;280 (7):605–13. [DOI] [PubMed]
- 7.Harris DJ, Douglas PS. Enrollment of women in cardiovascular clinical trials funded by the National Heart, Lung, and Blood Institute. N Engl J Med 2000;343(7):475–80. [DOI] [PubMed]
- 8.Kim ES, Menon V. Status of women in cardiovascular clinical trials. Arterioscler Thromb Vasc Biol 2009;29(3):279–83. [DOI] [PubMed]
- 9.Melloni C, Berger JS, Wang TY, Gunes F, Stebbins A, Pieper KS, et al. Representation of women in randomized clinical trials of cardiovascular disease prevention. Circ Cardiovasc Qual Outcomes 2010;3(2):135–42. [DOI] [PubMed]