Abstract
Several methods are available for delivering stem cells to the heart. Recent studies have highlighted the advantages of injecting the cells directly into the myocardium in order to increase myocardial retention of cells. A particular focus has been on percutaneous transendocardial injection, facilitated by electromechanical mapping.
The NOGA® XP Cardiac Navigation System has a multicomponent catheter that is designed to guide and deliver transendocardial injections via a transfemoral approach, without a guidewire. However, this method may not be feasible in some patients who have peripheral vascular disease. Herein, we describe the case of a 68-year-old man whose tortuous, sharply angled iliac arteries precluded a femoral approach to transendocardial injection. To overcome the anatomic and mechanical challenges, we used a brachial approach. We believe that this is the 1st report of using the brachial route for transendocardial injection, and that it can be a viable alternative to the transfemoral approach in selected patients.
Key words: Biological products/therapeutic use; coronary vessels/pathology; electrophysiologic techniques, cardiac/instrumentation/methods; heart diseases/therapy; imaging, three-dimensional; injections; myocardial infarction/pathology; regenerative medicine/methods; stem cell transplantation/methods; treatment outcome
Stem-cell-based intervention is a recent therapeutic innovation in cardiovascular medicine: progenitor cells are delivered to aid the structural and functional repair of diseased myocardium.1,2 Clinical success strongly depends upon the effective and targeted deployment of stem-cell-based products.3,4 Cells can be administered to the heart in several ways. Recent studies have highlighted the advantages of directly injecting cells into the myocardium, a technique that increases myocardial retention without the need to rely on the upregulation of inflammatory signals to assist transvascular cell migration and tissue invasion.5 A particular focus has been on percutaneous transendocardial injection facilitated by intramyocardial navigation.6
The current mainstay of this methodology is the NOGA® XP Cardiac Navigation System (Biologics Delivery Systems Group of Cordis Corporation, a Johnson & Johnson company; Irwindale, Calif). The NOGA XP system incorporates a multicomponent injection catheter and the real-time collection of spatial, electrophysiologic, and mechanical information to reconstruct the heart's endocardial surface in 3 dimensions.7 A left ventricular (LV) endocardial or electromechanical map is used to characterize the underlying tissue and to navigate the injection catheter so that the injections can be precisely targeted. The map is constructed by acquiring a series of points at multiple locations. These points are gated to a surface electrocardiogram. Ultra-low magnetic fields (10−1 to 10−6 T) are generated by a triangular magnetic pad placed under the patient. Each point sample contains information about local electrical activity: unipolar voltage (UniV), and local contractility or linear local shortening (LLS). The resulting 3-dimensional electromechanical map of the LV also distinguishes ischemic areas (which have low LLS and preserved UniV) from infarcted areas (low LLS and low UniV).8
In regard to transendocardial injections, the NOGA system is designed for a transfemoral approach without a guidewire. However, this route may not be feasible in some patients who have peripheral vascular disease. Herein, we describe a brachial approach to electromechanical mapping and NOGA-guided transendocardial injection.
Case Report
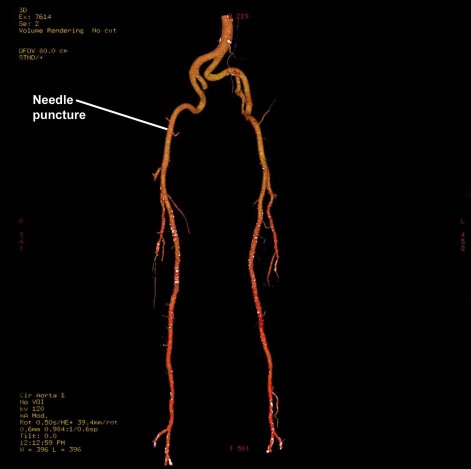
In October 2009, a 68-year-old man with a history of acute anteroapical myocardial infarction and ischemic dilated cardiomyopathy was admitted for elective electromechanical mapping and NOGA-guided transendocardial stem-cell injection. His risk factors included hypertension and hyperlipidemia. He presented with New York Heart Association (NYHA) functional class II dyspnea, despite optimal medical therapy that included β-blockers, angiotensin-converting enzyme inhibitors, and diuretics. A multigated acquisition scan showed reduced LV function (ejection fraction, 0.37), and an echocardiogram showed an LV end-diastolic diameter of 76 mm. Computed tomography revealed a normal aorta, but both iliac arteries were tortuous with sharp angles (Fig. 1). Coronary angiography and left ventriculography, performed with use of a standard Judkins catheter, revealed no significant coronary artery stenosis in the presence of an enlarged LV and reduced LV function.
Fig. 1 Computed tomography reveals a normal aorta and tortuous, sharply angled iliac arteries.
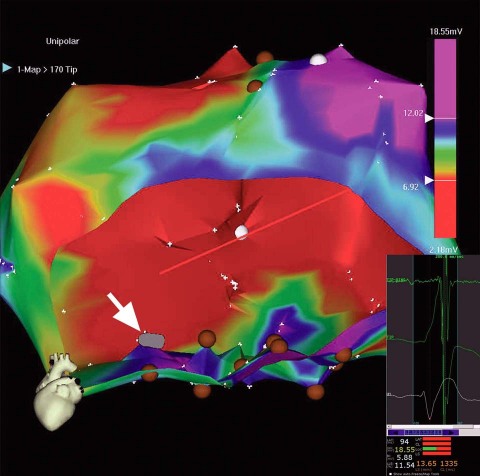
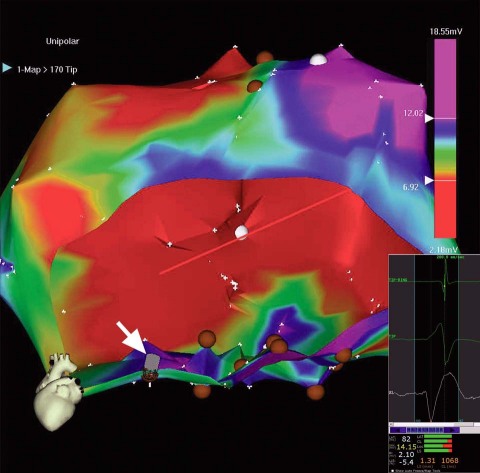
The conventional right femoral approach was initially chosen for electromechanical mapping. However, at the outset, the tortuous, sharply angled right iliac artery made it quite difficult to advance the mapping catheter to the LV and then to manipulate it. A larger D curve and a NOGASTAR® mapping catheter (Cordis) were used to try to obtain a diagnostic electromechanical map. A target area for cell delivery possibly could have been delineated with much difficulty after prolonged manipulation. However, despite the use of longer and larger 9F, 10F, and 11F sheaths, stable catheter positioning for effective transendocardial injection was not achieved, and each sheath kinked repeatedly (Fig. 2). It was therefore decided to proceed by way of a brachial approach. An 8F sheath was introduced into the right brachial artery, and a Myostar® catheter (Cordis) was inserted into the LV without major difficulty (Fig. 3). Thirteen transendocardial injections of stem-cell product were readily delivered to the designated areas.
Fig. 2 Unipolar voltage map (color range, 6.7–12 mV). Scarred areas (unipolar voltages, <6.7 mV), in red, are excluded from injection. To prevent intramyocardial injections from entering the blood pool of the left ventricular cavity, the catheter tip (gray icon, indicated with white arrow) must be perpendicular to the left ventricular wall; here, it is parallel to that wall.
Inset (lower right): The trajectory of unstable catheter-tip movement (catheter flipping away) during a single cardiac cycle. The predominantly red bars at bottom are NOGA stability criteria that confirm an unstable catheter position.
Fig. 3 Unipolar voltage map (color range, 6.7–12 mV). Scarred areas (unipolar voltages, <6.7 mV), in red, are excluded from injection. The injection catheter's tip is stable, perpendicular to the left ventricular wall (gray icon, indicated with white arrow). Injections were performed chiefly in the inferior zone (brown circles).
Inset (lower right): Stable catheter position (closed loop) during a single cardiac cycle. Predominantly green bars at bottom show good NOGA stability.
The patient developed hematuria due to the prolonged use of heparin during the procedure. The postprocedural level of troponin I was 1.053 μg/L, and the brain natriuretic peptide level was 365.38 pg/mL. With clinical monitoring and in-hospital care, the patient's postprocedural course was uneventful, and he was discharged from the hospital on the 5th day after the procedure. Six months later, he was in NYHA functional class I and in stable condition.
Discussion
The intramyocardial delivery of therapeutic substances can be achieved by means of direct injection after open thoracotomy (transepicardial) or by use of catheter-based techniques (transendocardial) with electromechanical mapping or fluoroscopic guidance.3,4 Electromechanical mapping enables clear delineation of the target area and highly precise deployment of the therapeutic product (within a 2-mm radius).7 This delivery platform has proved to be feasible in the presence of chronic ischemic heart disease and in subacute myocardial infarction (within 10 days after infarction). Myocardial retention rates have been similar to those of a direct transepicardial approach.7–10 Typically, the transendocardial approach is safe, although catheter-related mechanical complications have been reported.7–10 The Euroinject One trial investigators11 reported the following sequelae: pericardial tamponade in 2 of 80 patients; and, in 1 patient each, high-degree atrioventricular block, ST-segment-elevation myocardial infarction, and embolic events. The sequelae were all thought to be related to the procedure and independent of the transendocardially injected compound. These findings emphasize the importance of technical considerations, especially in the early stages of implementing new technology. Likewise, sequelae may be minimized by carefully selecting patients and using different imaging methods during preprocedural evaluation.
NOGA mapping is designed for the transfemoral approach, which precludes its use in patients who have peripheral vascular disease with calcified or tortuous iliac or femoral arteries. Patients who have undergone LV electromechanical mapping or received transendocardial injections have typically been good candidates for the femoral approach, and this is an issue of clinical relevance. Because NOGA mapping and injection catheters are advanced into the LV without a guidewire, manipulating them can be difficult—especially when arteries are tortuous. Manipulation can be even more challenging when a stiffer injection catheter is used. To date, there has been no formal recommendation on alternative approaches in patients with peripheral vascular tortuosity.
In our patient, the unsuitable femoral approach necessitated an alternative. Our preprocedural evaluations with appropriate imaging enabled us to consider the brachial route as a safe alternative. The potential for the brachial route might be supported based on investigations of other new interventions (such as percutaneous aortic valve replacement) in which even larger catheters are used: the 18F CoreValve® catheter (Medtronic, Inc.; Minneapolis, Minn) or the Edwards SAPIEN 22F (Edwards Lifesciences LLC; Irvine, Calif) have both been used in transfemoral and subclavian approaches.
Success in the case of this patient, who was otherwise a candidate for stem-cell myocardial intervention, suggests that brachial access can expand the use of NOGA-guided LV mapping and catheter-based delivery of stem-cell therapy. In patients with tortuous iliac or femoral arteries, the brachial route could be taken at the outset of the procedure in order to avoid procedural complications.
Stem-cell therapy for ischemic heart disease requires various delivery options. Our experience might provide clinical researchers with the confidence to pursue nonfemoral approaches to transendocardial injections when necessary, thus providing stem-cell therapy to a subset of patients who would otherwise be excluded because of peripheral vascular disease.
Footnotes
Address for reprints: Miodrag C. Ostojic, MD, PhD, Head, Department of Cardiology, University Institute for Cardiovascular Diseases, Clinical Center of Serbia, 8 Koste Todorovic, 11000 Belgrade, Serbia. E-mail: mostojic2003@yahoo.com
This study was supported by grant #41022 from the National Ministry of Science & Technology, Republic of Serbia.
References
- 1.Nelson T, Behfar A, Terzic A. Stem cells: biologics for regeneration. Clin Pharmacol Ther 2008;84(5):620–3. [DOI] [PubMed]
- 2.Nelson TJ, Behfar A, Yamada S, Martinez-Fernandez A, Terzic A. Stem cell platforms for regenerative medicine. Clin Transl Sci 2009;2(3):222–7. [DOI] [PMC free article] [PubMed]
- 3.Bartunek J, Sherman W, Vanderheyden M, Fernandez-Aviles F, Wijns W, Terzic A. Delivery of biologics in cardiovascular regenerative medicine. Clin Pharmacol Ther 2009;85(5):548–52. [DOI] [PubMed]
- 4.Gersh BJ, Simari RD, Behfar A, Terzic CM, Terzic A. Cardiac cell repair therapy: a clinical perspective. Mayo Clin Proc 2009;84(10):876–92. [DOI] [PMC free article] [PubMed]
- 5.Perin EC, Silva GV, Assad JA, Vela D, Buja LM, Sousa AL, et al. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J Mol Cell Cardiol 2008;44(3):486–95. [DOI] [PubMed]
- 6.Psaltis PJ, Worthley SG. Endoventricular electromechanical mapping-the diagnostic and therapeutic utility of the NOGA XP Cardiac Navigation System. J Cardiovasc Transl Res 2009; 2(1):48–62. [DOI] [PubMed]
- 7.Psaltis PJ, Zannettino AC, Gronthos S, Worthley SG. Intramyocardial navigation and mapping for stem cell delivery. J Cardiovasc Transl Res 2010;3(2):135–46. [DOI] [PubMed]
- 8.Perin EC, Lopez J. Methods of stem cell delivery in cardiac diseases. Nat Clin Pract Cardiovasc Med 2006;3 Suppl 1: S110–3. [DOI] [PubMed]
- 9.Dib N, Dinsmore J, Lababidi Z, White B, Moravec S, Campbell A, et al. One-year follow-up of feasibility and safety of the first U.S., randomized, controlled study using 3-dimensional guided catheter-based delivery of autologous skeletal myoblasts for ischemic cardiomyopathy (CAuSMIC study). JACC Cardiovasc Interv 2009;2(1):9–16. [DOI] [PubMed]
- 10.Krause K, Jaquet K, Schneider C, Haupt S, Lioznov MV, Otte KM, Kuck KH. Percutaneous intramyocardial stem cell injection in patients with acute myocardial infarction: first-in-man study. Heart 2009;95(14):1145–52. [DOI] [PubMed]
- 11.Kastrup J, Jorgensen E, Ruck A, Tagil K, Glogar D, Ruzyllo W, et al. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris. A randomized double-blind placebo-controlled study: the Euroinject One trial. J Am Coll Cardiol 2005;45(7):982–8. [DOI] [PubMed]