Results from more than 40 observational studies conducted in women over 3 decades have repeatedly indicated the cardioprotective effects of postmenopausal hormone replacement therapy (HRT).1 Women taking estrogen in these observational trials experienced a consistent 30% to 50% reduction in cardiovascular (CV) events. The biologic benefit of estrogen has been attributed to improvements in lipid profiles and endothelial function.
Observational Trials
The largest of these observational trials, the Nurses' Health Study (NHS),2 initially monitored 28,263 healthy postmenopausal women and evaluated the risk of subsequent major cardiac events in those who were currently taking HRT (21.8%), those who had previously taken it (25.2%), and those who had never taken it (53%). Unopposed oral conjugated estrogen was used in over 71% of the hormone-treated participants, which suggests that a large number of the women had undergone hysterectomy. The trial reported a 51% reduction in all-cause death and a 40% reduction in CV deaths in current estrogen users, compared with those who had never used hormones. Women with the greatest CV risk burden also appeared to benefit the greatest from hormone use. The benefit was lost within 5 years of hormone cessation.
At 20 years' follow-up, with 70,533 nurse participants, the NHS again reported a 54% to 58% reduction in CV deaths in women who took standard doses of unopposed estrogen. The addition of progestin diminished the benefit of estrogen, with a demonstrated risk reduction of 36%. However, the risk of stroke was significantly increased both in women who received progestin (relative risk [RR], 1.45) and in women who took estrogen at doses of 0.625 mg or greater daily (RR, 1.35–1.63). In 2001, widespread confidence in the overall health benefits of hormone replacement by women and their physicians was such that 38% of postmenopausal women in the United States were using HRT.
Randomized Trials
Secondary Prevention
Data from randomized controlled clinical trials, beginning in 1998 with the Heart and Estrogen/Progestin Replacement Study (HERS), led to a dramatic revision of the hypothesis regarding the CV benefits of postmenopausal hormone replacement. A secondary-prevention trial, HERS studied 2,763 postmenopausal women (average age, 67 yr) with documented coronary heart disease (CHD). During the 1st year in which women were randomized to receive HRT, there was a reported 50% increase in CV death and nonfatal myocardial infarction (MI)3 (Fig. 1). At 6.8 years' follow-up in HERS-II, there was no CV benefit from HRT and no long-term difference in CV death or nonfatal MI among the 2,321 postmenopausal women randomized to receive either HRT or placebo. Venous thromboembolism was also increased by estrogen and progestin. Results from HERS suggested an early acceleration of CV risk from HRT in susceptible older postmenopausal women with established CHD.1
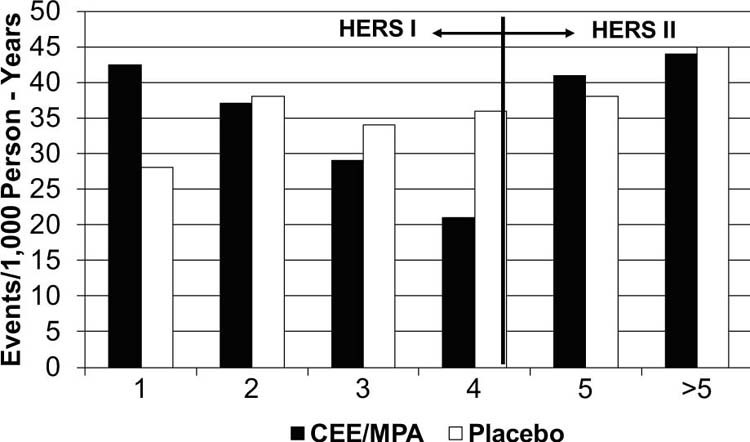
Fig. 1 Coronary heart disease events by year in the Heart and Estrogen/Progestin Replacement Study (HERS) and HERS II. Data from Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/Progestin Replacement Study follow-up (HERS II). JAMA 2002;288(1):49–57.
Relative hazard = 0.99 (95% confidence interval, 0.80–1.22); P for trend = 0.009
CEE/MPA = conjugated estrogens plus medroxyprogesterone acetate
Primary Prevention
The Women's Health Initiative (WHI) was a prospective, primary prevention trial of HRT in older, mostly healthy postmenopausal women (average age, 63 yr). Two parallel, randomized, double-blinded, placebo-controlled clinical trials were run concurrently to evaluate the CV risk (CV death and nonfatal MI) and overall safety (including the increased risk of breast cancer, pulmonary embolism, stroke, colon cancer, and hip fracture) of combined estrogen and progestin use among 16,608 women with a uterus, versus the use of estrogen alone in 10,739 women without a uterus. There was a marked discrepancy in CV risk between the 2 groups. Hysterectomized women were at greater CV risk, as shown by the higher CV event rates for women in the placebo cohort of that group (CV death rate, 16%; CV death/MI rate, 54%) in comparison with women with an intact uterus (CV death rate, 8%; CV death/MI rate, 33%).
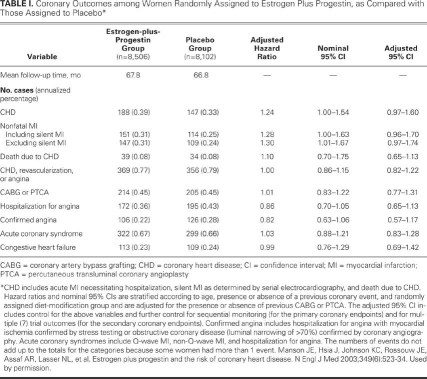
The trial of combined estrogen and progestin in women with a uterus (the lower-risk cohort) was halted early at a mean follow-up of 5.2 years because of an increased risk of invasive breast cancer in those randomly assigned to receive estrogen plus progestin, compared with those assigned to placebo.4 Estrogen plus progestin caused an increased risk of coronary events, stroke, breast cancer, and pulmonary embolism. A “global index” found these harmful outcomes to outweigh the decreased risk of hip fracture and colon cancer. A 2-fold increase in dementia among older (>65 yr) hormone-using WHI participants was later reported.5 In adjusted analyses, women assigned to receive combined estrogen and progestin had a 24% higher risk of CHD (total cases of CHD, 335), compared with women randomly assigned to placebo (total cases of CHD, 257) (Table I). The hazard ratio (HR) for the combined CHD risk was greatest during the 1st year of combined hormone treatment (HR, 1.81), and the HR did not fall below 1 until year 6. The lower HR after year 6 generated great interest that longer-term HRT may actually be cardioprotective. However, the lower HR after year 6 probably is related to lower numbers of CV events in later years—possibly due to a shift toward early events in susceptible women treated with HRT, higher placebo event rates, and decreased drug compliance. Furthermore, the risk of breast cancer increases with a longer duration of combined hormone treatment, contributing to excess overall risk.
TABLE I. Coronary Outcomes among Women Randomly Assigned to Estrogen Plus Progestin, as Compared with Those Assigned to Placebo*
The estrogen-only arm of the WHI completed almost 7 years of follow-up and was terminated 8 months before its scheduled completion because of an increased incidence of stroke (12 cases per 10,000 person-years) in patients randomized to receive 0.625 mg of unopposed estrogen (HR, 1.39).6 Stroke risk emerged early and persisted through follow-up. There was a 33% increase in venous thromboembolism rates in the estrogen-onlytreatment group (28 vs 21 cases per 10,000 person-years). The risk of breast cancer was 23% lower in the estrogen-only group, compared with the placebo group (94 vs 124 cases per 10,000 person-years, P = 0.06). Despite a higher-risk cohort, with 23% minority participation in the estrogen-only arm of the WHI as compared with the combined estrogen and progestin WHI cohort in women with an intact uterus, the absolute number of CHD events (CV death, MI, and total CHD) was lower in the group assigned to receive estrogen alone (54, 132, and 186 per 10,000 person-years vs 59, 153, and 212 per 10,000 person-years; P = not significant). Overall global risk was well balanced between the groups. Findings from the WHI triggered the U.S. Food and Drug Administration to require a black-box warning of harm for postmenopausal HRT. Medical guidelines were changed to recommend that HRT not be used to prevent disease and that, when HRT was used to treat vasomotor symptoms, it be used at the lowest effective dose for the shortest possible time.
Discrepancies between Observational and Randomized Trials
Discrepancies among findings of observational and randomized trials of HRT led to questions concerning the integrity and validity of the observational studies. The disparate benefits seen in the large observational studies are due, at least in part, to selection factors confounded by participants' baseline health and behaviors. The observational studies included women who began HRT at the onset of menopause—at an average age of 51 years, when their menopause symptoms were greatest—and who continued treatment for many years without interruption. However, in an effort to reduce crossovers in treatment assignment because of menopause symptoms, the randomized controlled trials of HRT were conducted in women who were no longer symptomatic. The women in these trials averaged in age from 63 to 67 years, and many of them began taking the study hormones more than 10 years after onset of menopause. The timing of HRT initiation in relation to the state of their vasculature might have been an additional source of discrepancy in the results of the observational and randomized trials.7 In younger women, estrogen might slow the development of atherosclerosis by improving lipid profile and vascular endothelial function, but in older women, it may trigger CHD events through prothrombotic and inflammatory mechanisms when advanced lesions are present.
Timing Hypothesis
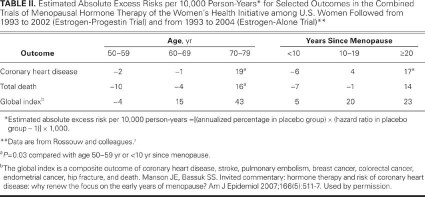
A secondary analysis of the combined WHI groups examined the effect of HRT on CV risk by age or years since menopause. Stroke risk, although the absolute numbers were small, was not influenced by years since menopause, drug regimen, or presence of vasomotor symptoms. A gradient of elevated CV risk was noted with increasing years since menopause (Table II) (RR = 0.76, 1.1, and 1.28 for women who were <10, 10–19, and ≥20 yr past menopause at study entry, respectively; P trend=0.02).7 Although a subgroup analysis could not identify any group with reduced CHD risk, the total mortality rate was lower in women aged 50 to 59 years who received HRT, compared with those who did not (total cases, 69 vs 95; HR=0.7).
TABLE II. Estimated Absolute Excess Risks per 10,000 Person-Years* for Selected Outcomes in the Combined Trials of Menopausal Hormone Therapy of the Women's Health Initiative among U.S. Women Followed from 1993 to 2002 (Estrogen-Progestin Trial) and from 1993 to 2004 (Estrogen-Alone Trial)**
In a substudy of the WHI estrogen-only cohort, coronary artery calcification was evaluated as a marker of total coronary plaque burden in 1,064 women aged 50 to 59 years, after a mean of 7.4 years of follow-up.8 Mean coronary artery calcium scores were lower in women receiving unopposed estrogen, compared with those receiving placebo (mean score, 83.1 vs 123.1; P = 0.02). When adjusted multivariate analysis was restricted to participants with >80% adherence to study treatment for at least 5 years, the distribution of coronary artery calcium scores was significantly lower in the group receiving estrogen alone (P = 0.002). These data on younger women enrolled in the WHI study provide reassurance that estrogen is unlikely to impart an adverse risk of coronary events in postmenopausal women who are considering HRT for the relief of vasomotor symptoms.
Conclusions
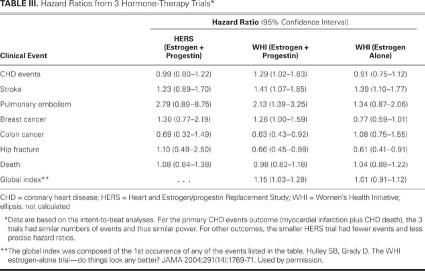
Hormone therapy after menopause does not reduce the risk of CHD and should not be used for primary or secondary CHD prevention (Table III). Rates of stroke and venous thromboembolism are consistently increased in users of hormones after menopause. Screening for stroke risk factors is advised before initiating hormones in all postmenopausal women. In addition, the use of postmenopausal hormones increases the risk of dementia. Breast cancer and CHD events are increased by estrogen plus progestin treatment in women with an intact uterus. For women with postmenopausal vasomotor symptoms who are considering hormone treatment, the cardiovascular risk within the first 10 years after menopause is minimal. If HRT is used, it should be at the lowest effective dose for the least amount of time.
TABLE III. Hazard Ratios from 3 Hormone-Therapy Trials*
Footnotes
Address for reprints: Stephanie A. Coulter, MD, 6720 Bertner Ave., Houston, TX 77030, E-mail: scoulter@texasheart.org
Presented at the Risk, Diagnosis and Treatment of Cardiovascular Disease in Women symposium; Denton A. Cooley Auditorium, Texas Heart Institute, Houston; 11 September 2010.
References
- 1.Manson JE, Martin KA. Clinical practice. Postmenopausal hormone-replacement therapy. N Engl J Med 2001;345(1):34–40. [DOI] [PubMed]
- 2.Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med 2000;133(12):933–41. [DOI] [PubMed]
- 3.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) [published erratum appears in JAMA 2002;288(9):1064]. JAMA 2002;288(1):49–57. [DOI] [PubMed]
- 4.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 2002;288(3):321–33. [DOI] [PubMed]
- 5.Hulley SB, Grady D. The WHI estrogen-alone trial–do things look any better? JAMA 2004;291(14):1769–71. [DOI] [PubMed]
- 6.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 2004; 291(14):1701–12. [DOI] [PubMed]
- 7.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause [published erratum appears in JAMA 2008;299(12):1426]. JAMA 2007;297(13):1465–77. [DOI] [PubMed]
- 8.Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med 2007;356(25):2591–602. [DOI] [PubMed]